In Vitro and In Planta Antagonistic Effect of Endophytic Bacteria on Blight Causing Xanthomonas axonopodis pv. punicae: A Destructive Pathogen of Pomegranate
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Isolation of Bacterial Endophytes from Field Grown Plants
2.3. Isolation of Endophytes from In Vitro Grown Explants
2.4. In Vitro Evaluation of Endophytes against Xap
2.5. Challenge Inoculation and In Vivo Evaluation of Endophytes against Xap
- Set-I, where Xap and endophyte culture broths were sprayed at the same time.
- Set-II, where Xap was sprayed 8 days earlier than endophytes.
- Set-III, where endophyte culture broths were sprayed 8 days earlier than the Xap.
2.6. Reconfirmation of X. axonopodis pv. Punicae Identity Isolated from Challenged Inoculated Host Plants
2.7. Estimation of Disease Incidence and Severity
2.8. 16S rDNA-Based Identification of Endophytes and Submission to the National Agriculturally Important Microbial Culture Collection
2.9. Physiological and Biochemical Analysis of Host Pomegranate Plants
2.10. Statistical Analysis
3. Results
3.1. Endophyte Isolation
3.2. In Vitro Evaluation of Endophytes against Xap
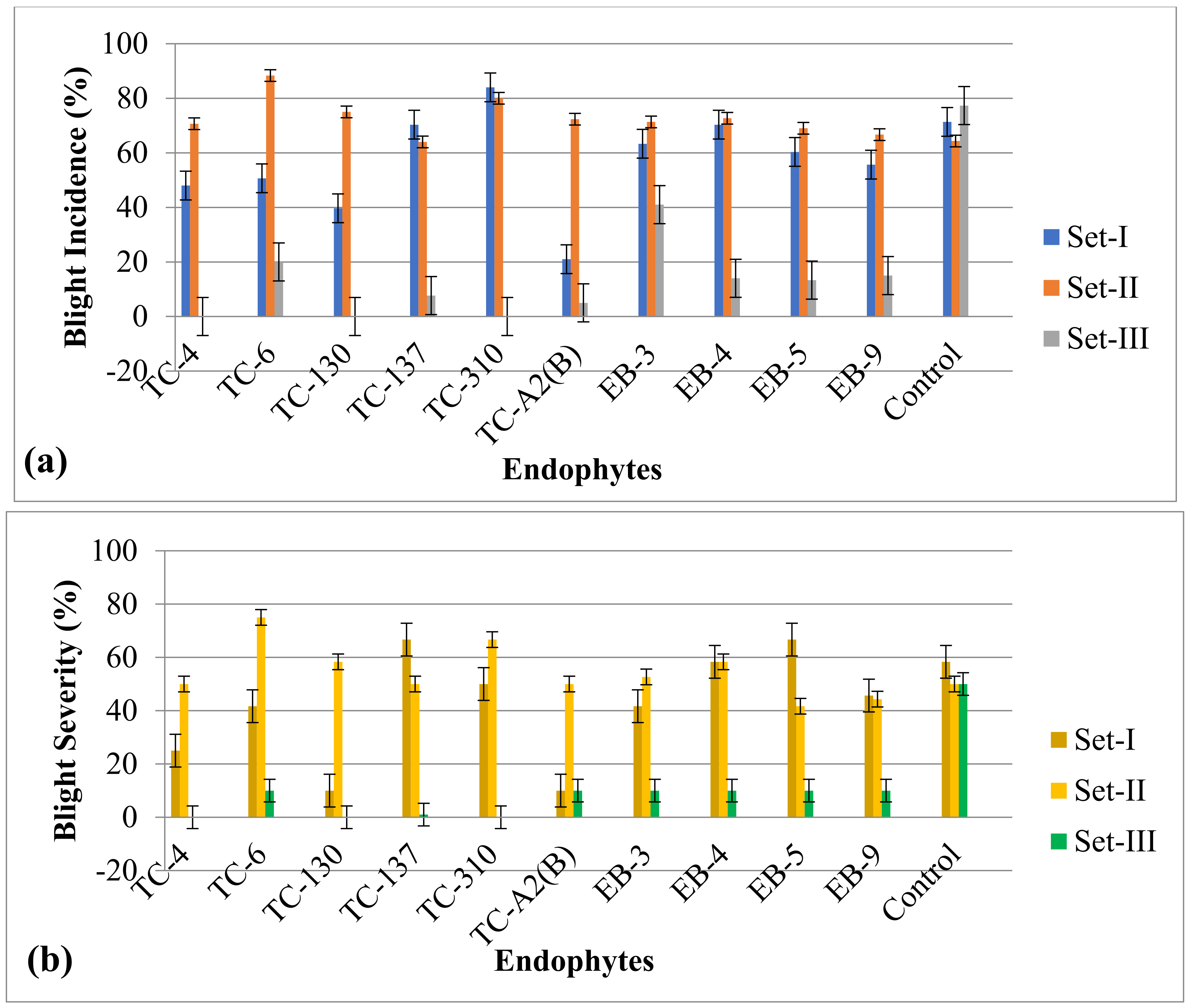
3.3. In Vivo Evaluation of Selected Endophytes against Xap
3.4. Identification of Promising Endophytes
3.5. Host Pomegranate Plant Response to External Application of Promising Endophytes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonesi, M.; Tundis, R.; Sicari, V.; Loizzo, M.R. The juice of pomegranate (Punica granatum L.): Recent studies on its bioactivities. In Quality Control in the Beverage Industry; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 459–489. ISBN 9780128166819. [Google Scholar] [CrossRef]
- Moriguchi, T.M.; Omura, N.; Natsuta, N.; Kozaki, I. In vitro adventitious shoot formation from anthers of pomegranate. HortScience 1987, 22, 947–948. [Google Scholar] [CrossRef]
- Halvorsen, B.L.; Holte, K.; Myhrstad, M.; Barikmo, I.; Hvattum, E.; Remberg, S.F.; Wold, A.-B.; Haffner, K.; Baugerød, H.; Andersen, L.F.; et al. A systematic screening of total antioxidants in dietary plants. J. Nutr. 2002, 132, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Tzulker, R.; Glazer, I.; Bar-Ilan, I.; Holland, D.; Aviram, M.; Amir, R. Antioxidant activity, polyphenol content, and related compounds in different fruit juices and homogenates prepared from 29 different pomegranate accessions. J. Agric. Food Chem. 2007, 55, 9559–9570. [Google Scholar] [CrossRef] [PubMed]
- Longtin, R. The Pomegranate: Nature’s power fruit? J. Natl. Cancer Inst. 2003, 95, 346–348. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, S.; Sithul, H.; Muraleedharan, P.; Azeez, J.H.; Sreeharshan, S. Pomegranate fruit as a rich source of biologically active compounds. BioMed Res. Int. 2014, 2014, 686921. [Google Scholar] [CrossRef]
- Holland, D.; Hatib, K.; Bar-Ya’akov, I. Pomegranate: Botany, Horticulture, Breeding. In Horticultural Reviews; Janick, J., Ed.; Wiley and Sons: Hoboken, NJ, USA, 2009; Volume 35, pp. 127–191. [Google Scholar]
- da Silva, J.A.T.; Rana, T.S.; Narzary, D.; Verma, N.; Meshram, D.T.; Ranade, S.A. Pomegranate biology and biotechnology: A review. Sci. Hortic. 2013, 160, 85–107. [Google Scholar] [CrossRef]
- Kim, N.D.; Mehta, R.; Yu, W.; Neeman, I.; Livney, T.; Amichay, A.; Poirier, D.; Nicholls, P.; Kirby, A.; Jiang, W.; et al. Chemopreventive and adjuvant therapeutic potential of pomegranate (Punica granatum) for human breast cancer. Breast Cancer Res. Treat. 2002, 71, 203–217. [Google Scholar] [CrossRef]
- Sánchez-Lamar, A.; Fonseca, G.; Fuentes, J.L.; Cozzi, R.; Cundari, E.; Fiore, M.; Ricordy, R.; Perticone, P.; Degrassi, F.; De Salvia, R. Assessment of the genotoxic risk of Punica granatum L. (Punicaceae) whole fruit extracts. J. Ethnopharmacol. 2008, 115, 416–422. [Google Scholar] [CrossRef]
- Sharma, J.; Ramchandra, K.K.; Sharma, D.; Meshram, D.T.; Ashis Maity, N.N. Pomegranate: Cultivation, Marketing and Utilization; Technical Bulletin No. NRCP/2014/1; ICAR-NRCP: Solapur, India, 2014; 88p. [Google Scholar]
- Hingroni, M.K.; Mehta, P.P. Bacterial leaf spot of pomegranate. Indian Phytopathol. 1952, 5, 55–56. [Google Scholar]
- Chand, R.; Kishun, R. Systemic movement of Xanthomonas campestris pv. punicae (Hingorani and Singh) dye from leaf to node in Pomegranate. Int. J. Trop. Plant Dis. 1993, 11, 85–90. [Google Scholar]
- Kumar, A.; Sharma, J.; Munjal, V.; Sakthivel, K.; Thalor, S.K.; Mondal, K.K.; Chinchure, S.; Gharate, R. Polyphasic phenotypic and genetic analysis reveals clonal nature of Xanthomonas axonopodis pv. punicae causing pomegranate bacterial blight. Plant Pathol. 2020, 69, 347–359. [Google Scholar]
- Akhtar, M.A.; Bhatti, M.H.R. Occurrence of bacterial leaf spot of pomegranate in Pakistan. Pak. J. Agric. Res. 1992, 13, 95–97. [Google Scholar]
- Petersen, Y.; Mansvelt, E.L.; Venter, E.; Langenhoven, W.E. Detection of (Xanthomonas axanopodis pv. punicae) causing bacterial blight on pomegranate in South Africa. Australas. Plant Pathol. 2010, 39, 544–546. [Google Scholar]
- Icoz, S.M.; Polat, I.; Sulu, G.; Yilmaz, M.; Unlu, A.; Soylu, S.; Bozkurt, I.A.; Baysal, Ö. First report of bacterial blight of pomegranate caused by (Xanthomas axanopodis pv. punicae) in Turkey. Plant Dis. 2014, 98, 1427. [Google Scholar] [CrossRef]
- Hallmann, J.; Quadt-Hallmann, A.; Mahaffee, W.F.; Kloepper, J.W. Endophytic bacteria in agricultural crops. Can. J. Microbiol. 1997, 43, 895–914. [Google Scholar] [CrossRef]
- Aravind, R.; Kumar, A.; Eapen, S.; Ramana, K. Endophytic bacterial flora in root and stem tissues of black pepper (Piper nigrum L.) genotype: Isolation, identification and evaluation against Phytophthora capsici. Lett. Appl. Microbiol. 2009, 48, 58–64. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Schippers, B.; Bakker, P.A.H.M. Proposed elimination of the term endorhizosphere. Phytopathology 1992, 82, 726–727. [Google Scholar]
- Sturz, A.V.; Christie, B.R.; Nowak, J. Bacterial endophytes: Potential role in developing sustainable system of crop production. Crit. Rev. Plant Sci. 2000, 19, 1–30. [Google Scholar] [CrossRef]
- Adhikari, T.B.; Joseph, C.M.; Phillips, D.A.; Nelson, M. Evaluation of bacteria isolated from rice for plant growth promotion and biological control of seedling diseases of rice. Can. J. Microbiol. 2001, 47, 916–924. [Google Scholar] [CrossRef]
- Tjamos, E.C.; Tsitsigiannis, T.I.; Tjamos, S.E.; Antoniou, P.; Katinakis, P. Selection and screening of endorhizosphere bacteria from solarised soils as biocontrol agents against Verticillium dahliae of solanaceous hosts. Eur. J. Plant Pathol. 2004, 110, 35–44. [Google Scholar] [CrossRef]
- Khare, E.; Mishra, J.; Arora, N.K. Multifaceted interactions between endophytes and plant: Developments and prospects. Front. Microbiol. 2018, 9, 2732. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Hallmann, J. Control of plant pathogenic fungi with bacterial endophytes. In Microbial Root Endophytes; Schulz, B.J.E., Boyle, C.J.C., Sieber, T.N., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 53–69. [Google Scholar]
- Karn, M.; Sharma, S.K.; Sharma, A.; Pal, J. Isolation and characterization of endophytes against bacterial blight of pomegranate. Int. J. Bio-Resour. Stress Manag. 2022, 13, 683–692. [Google Scholar] [CrossRef]
- Ahmed, M.; Muzaffer, H.; Manoj, K.; Sanjana, K. Isolation of microbial endophytes from some ethnomedicinal plants of Jammu and Kashmir. J. Nat. Prod. Plant Resour. 2012, 2, 215–220. [Google Scholar]
- Schulz, B.; Guske, S.; Dammann, U.; Boyle, C. Endophyte-host interactions II. Defining symbiosis of the endophyte-host interaction. Symbiosis 1998, 25, 213–227. [Google Scholar]
- Sharma, J.; Sharma, K.K.; Kumar, A.; Mondal, K.K.; Thalor, S.; Maity, A.; Gharate, R.; Chinchure, S.; Jadhav, V.T. Pomegranate bacterial blight: Symptomatology and rapid inoculation technique for (Xanthomonas axanopodis pv. punicae). J. Plant Pathol. 2017, 99, 109–119. [Google Scholar]
- Kalyan, K.M.; Jyotsana, S.; Richa, S.; Geeta, V. The reliable and rapid polymerase chain reaction (PCR) diagnosis for Xanthomonas axonopodis pv. punicae in pomegranate. Afr. J. Microbiol. Res. 2012, 6, 5950–5956. [Google Scholar]
- Ahuja, D.B.; Singh, N.; Sharma, J.; Rao, C.N.; Das, A.K.; Thangavelu, R.; Thangavelu, R.; Raghuvanshi, K.S.; Pardeshi, S.; Suroshe, S.; et al. Implementation of electronic–pest (e-pest) surveillance. In Pests of Fruit Trees (Citrus, Banana, Mango, Pomegranate and Sapota) E-Pest Surveillance and Pest Management Advisory; Ahuja, D.B., Chattopadhyay, C., Eds.; ICAR-NRCIPM: New Delhi, India; Department of Horticulture, Commissionerate of Agriculture: Pune, India, 2015; pp. 85–101. [Google Scholar]
- Singh, N.V.; Abburi, V.L.; Ramajayam, D.; Kumar, R.; Chandra, R.; Sharma, K.K.; Sharma, J.; Babu, K.D.; Pal, R.K.; Mundewadikar, D.M.; et al. Genetic diversity and association mapping of bacterial blight and other horticulturally important traits with microsatellite markers in pomegranate from India. Mol. Genet. Genom. 2015, 290, 1393–1402. [Google Scholar] [CrossRef]
- Doddaraju, P.; Kumar, P.; Gunnaiah, R.; Gowda, A.A.; Lokesh, V.; Pujer, P.; Manjunatha, G. Reliable and early diagnosis of bacterial blight in pomegranate caused by Xanthomonas axonopodis pv. punicae using sensitive PCR techniques. Sci. Rep. 2019, 9, 10097. [Google Scholar] [CrossRef]
- Kumar, A.; Sarma, Y.R.; Anandaraj, M. Evaluation genetic diversity of Ralstonia solanacearum causing bacterial wilt of ginger using Rep-PCR and RFLP-PCR. Curr. Sci. 2004, 87, 1555–1561. [Google Scholar]
- Yu, J.; Zhou, X.F.; Yang, S.J.; Liu, W.H.; Hu, X.F. Design and application of specific 16S rDNA-targeted primers for assessing endophytic diversity in (Dendrobium officinale) using nested PCR-DGGE. Appl. Microbiol. Biotechnol. 2013, 97, 9825–9836. [Google Scholar] [CrossRef]
- Altshull, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSIBLAST:A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Weatherley, P.E. Studies in water relation of cotton plant I. Field measurement of water deficits in leaves. New Physiol. 1950, 49, 81–97. [Google Scholar] [CrossRef]
- Malik, C.P.; Singh, M.B. Plant Enzymology and Histo-Enzymology; Kalyani Publishers: New Delhi, India, 1980; 286p. [Google Scholar]
- Barnes, J.D.; Balaguer, L.; Maurigue, E.; Elvira, S.; Davison, A.W. A reappraisal of the use of DMSO for the extraction and determination of chlorophyll ‘a’ and ‘b’ in lichens and higher plants. Environ. Exp. Bot. 1992, 32, 87–99. [Google Scholar] [CrossRef]
- Sharma, J.; Manjunatha, N.; Pokhare, S.S.; Patil, P.G.; Agarrwal, R.; Chakranarayan, M.G.; Aralimar, A.; Devagire, P.; Marathe, R.A. Genetic diversity and streptomycin sensitivity in Xanthomonas axonopodis pv . punicae causing oily spot disease in pomegranates. Horticulturae 2022, 16, 8–441. [Google Scholar] [CrossRef]
- De Silva, N.I.; Brooks, S.; Lumyong, S.; Hyde, K.D. Use of endophytes as biocontrol agents. Fungal Biol. Rev. 2019, 33, 133–148. [Google Scholar] [CrossRef]
- Arnold, A.E.; Herre, E.A. Canopy cover and leaf age affect colonization by tropical fungal endophytes: Ecological pattern and process in Theobroma cacao (Malvaceae). Mycologia 2003, 95, 388–398. [Google Scholar] [CrossRef]
- Ryan, R.P.; Germaine, K.; Franks, A.; Ryan, D.J.; Dowling, D.N. Bacterial endophytes: Recent developments and applications. FEMS Microbiol. Lett. 2008, 278, 1–9. [Google Scholar] [CrossRef]
- Afzal, I.; Shinwari, Z.K.; Sikandar, S.; Shahzad, S. Plant beneficial endophytic bacteria: Mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 2019, 221, 36–49. [Google Scholar] [CrossRef]
- Quambusch, M.; Winkelmann, T. Bacterial endophytes in plant tissue culture: Mode of action, detection, and control. In Plant Cell Culture Protocols; Humana Press: New York, NY, USA, 2018; pp. 69–88. [Google Scholar]
- Queiroz, E.G.; Degenhardt, J.; Quoirin, M.; Silva, K.D. Endophytic bacteria associated with tissue culture and leaves of Plinia peruviana. Pesqui. Agropecuária Bras. 2020, 18, 55. [Google Scholar] [CrossRef]
- Nagendran, K.; Karthikeyan, G.; Peeran, M.F.; Raveendran, M.; Prabakar, K.; Raguchander, T. Management of bacterial leaf blight disease in rice with endophytic bacteria. World Appl. Sci. J. 2013, 28, 2229–2241. [Google Scholar]
- Basu, P.K.; Wallen, V.R. Factors affecting virulence and pigment production of Xanthomonas phaseoli var. fuscans. Can. J. Bot. 1967, 45, 2367–2374. [Google Scholar] [CrossRef]
- Xie, J.; Wu, Y.Y.; Zhang, T.Y.; Zhang, M.Y.; Peng, F.; Lin, B.; Zhang, Y.X. New antimicrobial compounds produced by endophytic (Penicillium janthinellum) isolated from Panaxnoto ginseng as potential inhibitors of FtsZ. Fitoterapia 2018, 131, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xu, Y.; Lai, X.; Shan, C.; Deng, Z.; Ji, Y. Screening and characterization of endophytic Bacillus and Paenibacillus strains from medicinal plant (Lonicera japonica) for use as potential plant growth promoters. Braz. J. Microbiol. 2015, 46, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hafeez, S.; Rafique, M.; Mumtaz, M.Z.; Subhani, Z.; Holatko, J.; Hammerschmiedt, T.; Malicek, O.; Mustafa, A.; Kintl, A.; et al. Plant-endophyte mediated improvement in physiological and bio-protective abilities of marigold (Tagetes patula). Front. Plant Sci. 2022, 13, 993130. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.B.; Shashikala, J.; Krishnamurthy, Y.L. Diversity of fungal endophytes in shrubby medicinal plants of Malnad region, Western Ghats, Southern India. Fungal Ecol. 2008, 1, 89–93. [Google Scholar] [CrossRef]
- Huang, C.L.; Wang, Y.Z. New records of endophytic fungi associated with the Araucariaceae in Taiwan. Collect. Res. 2011, 24, 87–95. [Google Scholar]
- Hammerschmidt, L.; Wray, V.; Lin, W.; Kamilova, E.; Proksch, P.; Aly, A.H. New styrylpyrones from the fungal endophyte Penicillium glabrum isolated from Punica granatum. Phytochem. Lett. 2012, 5, 600–603. [Google Scholar] [CrossRef]
- Mussi-Dias, V.; Araújo, A.C.O.; Silveira, S.F.; Rocabado, J.M.A.; Araújo, K.L. Endophytic fungi associated with medicinal plants. Rev. Bras. Plantas Med. 2012, 14, 261–266. [Google Scholar] [CrossRef][Green Version]
- Vahedi-Darmiyan, M.B.; Jahani, M.; Mirazee, M.R.; Asgari, B. A noteworthy record of endophytic (Quambalaria cyanescens) from (Punica granatum) in Iran. Czech Mycol. 2017, 69, 113–123. [Google Scholar] [CrossRef]
- Atay, M.; Kara, M.; Uysal, A.; Soylu, S.; Kurt, Ş.; Soylu, E.M. In vitro antifungal activities of endophytic bacterial isolates against postharvest heart rot disease agent Alternaria alternata in pomegranate fruits. In Proceedings of the IV Balkan Symposium on Fruit Growing, Istanbul, Turkey, 14–18 September 2019; pp. 309–314. [Google Scholar]
- Daungfu, O.; Youpensuk, S.; Lumyong, S. Endophytic Bacteria Isolated from Citrus Plants for Biological Control of Citrus Canker in Lime Plants. Trop. Life Sci. Res. 2019, 30, 73–88. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, J.; Zhang, H.; Ji, G.; Zeng, L.; Li, Y.; Yu, C.; Fernando, W.G.D.; Chen, W. Bacterial blight induced shifts in endophytic microbiome of rice leaves and the enrichment of specific bacterial strains with pathogen antagonism. Front. Plant Sci. 2020, 11, 963. [Google Scholar] [CrossRef]
| Variety/Genotype | CFU Per Gram of Tissue | Number of Isolates | ||
|---|---|---|---|---|
| Stem/Shoot Tip/Nodal Segments | Leaves | Roots | ||
| Ganesh | 8 × 107 (EB1) | 2.67 × 107 (EB2) | 7.33 × 107 (EB3) | 3 |
| Bhagawa | 2.16 × 107 (EB9, EB11, TC 1, 2, 4, 6, 7, 9, 10, 130, 137, 310, A2B, B) | 2.56 × 107 (EB4, EB5, EB10) | 3.6 × 107 (EB6, EB7, EB8) | 20 |
| Nana | 7 × 106 (EB12) | - | - | 1 |
| Daru | 5 × 106 (EB14) | - | 3.33 × 106 (EB13) | 2 |
| Endophytes | Percent Growth Inhibition of Xap over Control *,$ | Fuscan Production by Xap |
|---|---|---|
| EB1 | 31.58 ± 2.21 f,g,h,i # | 0 |
| EB2 | 30.42 ± 0.42 h,i,j | 1 |
| EB3 | 36.77 ± 2.02 e,f,g | 0 |
| EB4 | 34.83 ± 1.82 f,g,h | 0 |
| EB5 | 35.24 ± 1.42 f,g,h | 0 |
| EB6 | 32.45 ± 1.42 f,g,h,i | 1 |
| EB7 | 29.93 ± 1.92 h,i,j | 0 |
| EB8 | 25.06 ± 0.48 j,k | 2 |
| EB9 | 42.42 ± 1.48 d,e | 1 |
| EB10 | 26.86 ± 1.62 i,j,k | 2 |
| EB11 | 26.92 ± 0.90 i,j,k | 3 |
| EB12 | 25.03 ± 0.97 j,k | 2 |
| EB13 | 32.42 ± 1.89 f,g,h,i | 0 |
| EB14 | 25.06 ± 0.48 j,k | 3 |
| TC-1 | 15.65 ± 1.25 l | 3 |
| TC-2 | 22.32 ± 2.28 k | 2 |
| TC-4 | 51.12 ± 2.16 a,b | 1 |
| TC-6 | 56.35 ± 2.66 a | 0 |
| TC-7 | 37.91 ± 0.84 d,e,f | 2 |
| TC-9 | 43.00 ± 0.32 c,d | 1 |
| TC-10 | 29.53 ± 3.66 h,i,j | 2 |
| TC-130 | 48.75 ± 2.20 b,c | 1 |
| TC-137 | 48.61 ± 4.68 b,c | 0 |
| TC-310 | 49.40 ± 4.14 b | 0 |
| TC-A2B | 49.84 ± 3.18 b | 0 |
| TC-B | 31.03 ± 1.95 g,h,i,j | 2 |
| Control | 0.00 ± 0.0 m | 3 |
| CV (%) | 10.78 | -- |
| SE (m) | 2.12 | -- |
| SE (d) | 2.99 | -- |
| Critical difference (p ≤ 0.05) | 6.02 | -- |
| Isolate | Query Coverage (bp) | Identity (%) | Sequence Homology | Gene Bank Accession Number | NAIMCC * Accession Number |
|---|---|---|---|---|---|
| TC6 | 1426 | 100.00 | Bacillus subtilis | KY575578 | NAIMCC-B-03179 |
| TC7 | 1414 | 99.08 | Burkholderia stabilis | KY575579 | -- |
| TC130 | 1414 | 100.00 | Bacillus licheniformis | KY575581 | -- |
| TC 137 | -- | -- | Bacillus subtilis | OP999332 | -- |
| TC310 | 1424 | 99.51 | Bacillus tequilensis | KY575582 | NAIMCC-B-03180 |
| EB4 | 1418 | 99.86 | Bacillus tequilensis | KY575583 | -- |
| EB6 | 1427 | 99.09 | Lysinibacillus macroides | KY575584 | -- |
| EB9 | 1420 | 99.93 | Bacillus subtilis | KY575585 | -- |
| Treatment | Relative Leaf Water Content (%) | Total Phenol Content (mg Catechol Equivalent/100 g FW) | SPAD $ Value | Total Leaf Chlorophyll (mg/g FW) |
|---|---|---|---|---|
| EB-3 | 85.87 ± 0.39 | 43.33 ± 1.76 b,c | 68.74 ± 2.37 | 2.23 ± 0.09 a,b |
| EB-4 | 85.45 ± 0.89 | 44.00 ± 2.31 b,c | 66.31 ± 2.78 | 2.18 ± 0.07 b,c |
| EB-5 | 84.48 ± 0.54 | 52.67 ± 8.51 a,b | 66.12 ± 2.90 | 2.11 ± 0.04 b,c |
| EB-9 | 85.88 ± 0.64 | 55.33 ± 5.81 a,b | 68.24 ± 3.70 | 2.45 ± 0.13 a |
| TC-6 | 86.09 ± 1.44 | 45.33 ± 3.33 b,c | 60.64 ± 1.55 | 2.10 ± 0.07 b,c |
| TC-9 | 83.99 ± 1.71 | 61.33 ± 2.67 a | 63.93 ± 2.45 | 2.18 ± 0.03 b,c |
| TC-310 | 86.09 ± 1.44 | 35.33 ± 2.40 c | 66.13 ± 1.52 | 2.28 ± 0.10 a,b |
| Control | 80.66 ± 1.68 | 36.00 ± 2.31 c | 57.36 ± 1.49 | 1.96 ± 0.08 c |
| CV (%) | 2.45 | 15.74 | 6.58 | 6.39 |
| SE (m) | 1.20 | 4.24 | 2.46 | 0.08 |
| SE (d) | 1.68 | 6.00 | 3.48 | 0.11 |
| Critical difference (CD) at p ≤ 0.05 | NS | 12.83 | NS | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, N.V.; Sharma, J.; Dongare, M.D.; Gharate, R.; Chinchure, S.; Nanjundappa, M.; Parashuram, S.; Patil, P.G.; Babu, K.D.; Mundewadikar, D.M.; et al. In Vitro and In Planta Antagonistic Effect of Endophytic Bacteria on Blight Causing Xanthomonas axonopodis pv. punicae: A Destructive Pathogen of Pomegranate. Microorganisms 2023, 11, 5. https://doi.org/10.3390/microorganisms11010005
Singh NV, Sharma J, Dongare MD, Gharate R, Chinchure S, Nanjundappa M, Parashuram S, Patil PG, Babu KD, Mundewadikar DM, et al. In Vitro and In Planta Antagonistic Effect of Endophytic Bacteria on Blight Causing Xanthomonas axonopodis pv. punicae: A Destructive Pathogen of Pomegranate. Microorganisms. 2023; 11(1):5. https://doi.org/10.3390/microorganisms11010005
Chicago/Turabian StyleSingh, Nripendra Vikram, Jyotsana Sharma, Manjushri Dinkar Dongare, Ramakant Gharate, Shivkumar Chinchure, Manjunatha Nanjundappa, Shilpa Parashuram, Prakash Goudappa Patil, Karuppannan Dhinesh Babu, Dhananjay Morteppa Mundewadikar, and et al. 2023. "In Vitro and In Planta Antagonistic Effect of Endophytic Bacteria on Blight Causing Xanthomonas axonopodis pv. punicae: A Destructive Pathogen of Pomegranate" Microorganisms 11, no. 1: 5. https://doi.org/10.3390/microorganisms11010005
APA StyleSingh, N. V., Sharma, J., Dongare, M. D., Gharate, R., Chinchure, S., Nanjundappa, M., Parashuram, S., Patil, P. G., Babu, K. D., Mundewadikar, D. M., Salutgi, U., Tatiya, M., Kumar, A., & Marathe, R. A. (2023). In Vitro and In Planta Antagonistic Effect of Endophytic Bacteria on Blight Causing Xanthomonas axonopodis pv. punicae: A Destructive Pathogen of Pomegranate. Microorganisms, 11(1), 5. https://doi.org/10.3390/microorganisms11010005






