Evaluation of Two Different CMV-Immunoglobulin Regimens for Combined CMV Prophylaxis in High-Risk Patients following Lung Transplant
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Immunosuppressive Protocol
2.3. CMV Prophylaxis
2.3.1. Antiviral Regimen
2.3.2. Adjunctive CMV-Ig Regimens
- Label use or short regimen (SR-Ig) regimen, used in one center and given according to the Summary of Product Characteristics (SmPC), namely one 150 IU/kg dose on the day of the transplant, then six 100 IU/kg additional doses given at 2, 7, 14, 21, 35, 56, and 77 days post-transplantation [20].
- Off-label dosage or extended regimen (ER-Ig), used in the other 2 hospitals and consisting of 2 mL/kg (200 UI/kg) on days 1, 4, 8, 15, and 30 post-transplant, then monthly for 1 year thereafter [21].
2.4. Variables and Outcomes Assessed
2.5. Statistical Analysis
3. Results
3.1. Antiviral CMV Prophylaxis
3.2. Incidence and Type of CMV Episodes
3.3. Risk Factors for CMV Infection
3.4. Incidence of Acute Cellular and Chronic Allograft Rejection
3.5. Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chambers, D.C.; Yusen, R.D.; Cherikh, W.S.; Goldfarb, S.B.; Kucheryavaya, A.Y.; Khusch, K.; Levvey, B.J.; Lund, L.H.; Meiser, B.; Rossano, J.W.; et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-fourth Adult Lung And Heart-Lung Transplantation Report-2017; Focus Theme: Allograft ischemic time. J. Heart Lung Transplant. 2017, 36, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.A.; Brennan, D.C.; Yusen, R.D.; Olsen, M.A. Incidence, Risk Factors and Outcomes of Delayed-onset Cytomegalovirus Disease in a Large Retrospective Cohort of Lung Transplant Recipients. Transplantation 2015, 99, 1658–1666. [Google Scholar] [CrossRef] [PubMed]
- Ljungman, P.; Boeckh, M.; Hirsch, H.H.; Josephson, F.; Lundgren, J.; Nichols, G.; Pikis, A.; Razonable, R.R.; Miller, V.; Griffiths, P.D. Definitions of Cytomegalovirus Infection and Disease in Transplant Patients for Use in Clinical Trials. Clin. Infect. Dis. 2017, 64, 87–91. [Google Scholar] [CrossRef] [PubMed]
- McDevitt, L.M. Etiology and impact of cytomegalovirus disease on solid organ transplant recipients. Am. J. Health Syst. Pharm. 2006, 63, S3–S9. [Google Scholar] [CrossRef]
- Kotton, C.N. Cytomegalovirus in Solid Organ Transplant Recipients: Prevention, Diagnosis, and Treatment. In Emerging Transplant Infections: Clinical Challenges and Implications; Morris, M.I., Kotton, C.N., Wolfe, C.R., Eds.; Springer International Publishing: Cham, Swizterland, 2021; pp. 547–571. [Google Scholar]
- Hakimi, Z.; Aballéa, S.; Ferchichi, S.; Scharn, M.; Odeyemi, I.A.; Toumi, M.; Saliba, F. Burden of cytomegalovirus disease in solid organ transplant recipients: A national matched cohort study in an inpatient setting. Transpl. Infect. Dis. 2017, 19, e12732. [Google Scholar] [CrossRef]
- Hakimi, N.; Dorey, J.; Hakimi, Z.; Aballea, S.; Odeyemi, I.I.; Toumi, M. PIN77 Elicitation of Health-Related Quality of Life Concepts Associated With Cytomegalovirus in Transplant Recipients. Value Health 2012, 15, A399. [Google Scholar] [CrossRef]
- Kotton, C.N.; Kumar, D.; Caliendo, A.M.; Huprikar, S.; Chou, S.; Danziger-Isakov, L.; Humar, A.; The Transplantation Society International CMV Consensus Group. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation. Transplantation 2018, 102, 900–931. [Google Scholar] [CrossRef]
- Razonable, R.R.; Humar, A. Cytomegalovirus in solid organ transplant recipients—Guidelines of the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13512. [Google Scholar] [CrossRef]
- Chambers, D.C.; Perch, M.; Zuckermann, A.; Cherikh, W.S.; Harhay, M.O.; Hayes, D., Jr.; Hsich, E.; Khush, K.K.; Potena, L.; Sadavarte, A.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-eighth adult lung transplantation report - 2021; Focus on recipient characteristics. J. Heart Lung Transplant. 2021, 40, 1060–1072. [Google Scholar] [CrossRef]
- Torre-Cisneros, J.; Aguado, J.M.; Caston, J.J.; Almenar, L.; Alonso, A.; Cantisán, S.; Carratalá, J.; Cervera, C.; Cordero, E.; Fariñas, M.C.; et al. Management of cytomegalovirus infection in solid organ transplant recipients: SET/GESITRA-SEIMC/REIPI recommendations. Transplant. Rev. 2016, 30, 119–143. [Google Scholar] [CrossRef]
- Rosenheck, J.P.; Botros, M.M.; Nunley, D.N. Cytomegalovirus in Lung Transplant. OBM Transplantation 2021, 05, 145. [Google Scholar] [CrossRef]
- Hammond, S.P.; Martin, S.T.; Roberts, K.; Gabardi, S.; Fuhlbrigge, A.L.; Camp, P.C.; Goldberg, H.J.; Marty, F.M.; Baden, L.R. Cytomegalovirus disease in lung transplantation: Impact of recipient seropositivity and duration of antiviral prophylaxis. Transpl. Infect. Dis. 2013, 15, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Hodson, E.M.; Jones, C.A.; Strippoli, G.F.; Webster, A.C.; Craig, J.C. Immunoglobulins, vaccines or interferon for preventing cytomegalovirus disease in solid organ transplant recipients. Cochrane Database Syst. Rev. 2007, Cd005129. [Google Scholar] [CrossRef] [PubMed]
- Bonaros, N.; Mayer, B.; Schachner, T.; Laufer, G.; Kocher, A. CMV-hyperimmune globulin for preventing cytomegalovirus infection and disease in solid organ transplant recipients: A meta-analysis. Clin. Transplant. 2008, 22, 89–97. [Google Scholar] [CrossRef]
- Barten, M.J.; Baldanti, F.; Staus, A.; Hüber, C.M.; Glynou, K.; Zuckermann, A. Effectiveness of Prophylactic Human Cytomegalovirus Hyperimmunoglobulin in Preventing Cytomegalovirus Infection following Transplantation: A Systematic Review and Meta-Analysis. Life (Basel) 2022, 12, 361. [Google Scholar] [CrossRef]
- Zuk, D.M.; Humar, A.; Weinkauf, J.G.; Lien, D.C.; Nador, R.G.; Kumar, D. An international survey of cytomegalovirus management practices in lung transplantation. Transplantation 2010, 90, 672–676. [Google Scholar] [CrossRef]
- Le Page, A.K.; Jager, M.M.; Kotton, C.N.; Simoons-Smit, A.; Rawlinson, W.D. International survey of cytomegalovirus management in solid organ transplantation after the publication of consensus guidelines. Transplantation 2013, 95, 1455–1460. [Google Scholar] [CrossRef]
- Gavaldà, J.; Monforte, V.; Len, Ó. Prevención de la enfermedad por citomegalovirus en el trasplante de pulmón. Enferm. Infecc. Microbiol. Clin. 2021, 29, 46–51. [Google Scholar] [CrossRef]
- Biotest (UK) Ltd. Cytotect CP Biotest 100 U/ml solution for infusion. Summary of Product Characteristics. Updated 19-July-2022. Available online: https://www.medicines.org.uk/emc/product/11030/smpc/print (accessed on 5 September 2022).
- Lopez Garcia-Gallo, C.; García Fadul, C.; Laporta, R.; Portero, F.; Millan, I.; Ussetti, P. Cytomegalovirus Immunoglobulin for Prophylaxis and Treatment of Cytomegalovirus Infection in the (Val)Ganciclovir Era: A Single-Center Experience. Ann. Transplant. 2015, 20, 661–666. [Google Scholar] [CrossRef]
- Stewart, S.; Fishbein, M.C.; Snell, G.I.; Berry, G.J.; Boehler, A.; Burke, M.M.; Glanville, A.; Gould, F.K.; Magro, C.; Marboe, C.C.; et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J. Heart Lung Transplant. 2007, 26, 1229–1242. [Google Scholar] [CrossRef]
- Verleden, G.M.; Glanville, A.R.; Lease, E.D.; Fisher, A.J.; Calabrese, F.; Corris, P.A.; Ensor, C.R.; Gottlieb, J.; Hachem, R.R.; Lama, V.; et al. Chronic lung allograft dysfunction: Definition, diagnostic criteria, and approaches to treatment-A consensus report from the Pulmonary Council of the ISHLT. J. Heart Lung Transplant. 2019, 38, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Glanville, A.R.; Verleden, G.M.; Todd, J.L.; Benden, C.; Calabrese, F.; Gottlieb, J.; Hachem, R.R.; Levine, D.; Meloni, F.; Palmer, S.M.; et al. Chronic lung allograft dysfunction: Definition and update of restrictive allograft syndrome-A consensus report from the Pulmonary Council of the ISHLT. J. Heart Lung Transplant. 2019, 38, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Chambers, D.C.; Cherikh, W.S.; Harhay, M.O.; Hayes, D., Jr.; Hsich, E.; Khush, K.K.; Meiser, B.; Potena, L.; Rossano, J.W.; Toll, A.E.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult lung and heart-lung transplantation Report-2019; Focus theme: Donor and recipient size match. J. Heart Lung Transplant. 2019, 38, 1042–1055. [Google Scholar] [CrossRef] [PubMed]
- Kruger, R.M.; Paranjothi, S.; Storch, G.A.; Lynch, J.P.; Trulock, E.P. Impact of prophylaxis with cytogam alone on the incidence of CMV viremia in CMV-seropositive lung transplant recipients. J. Heart Lung Transplant. 2003, 22, 754–763. [Google Scholar] [CrossRef]
- Zamora, M.R.; Nicolls, M.R.; Hodges, T.N.; Marquesen, J.; Astor, T.; Grazia, T.; Weill, D. Following universal prophylaxis with intravenous ganciclovir and cytomegalovirus immune globulin, valganciclovir is safe and effective for prevention of CMV infection following lung transplantation. Am. J. Transplant. 2004, 4, 1635–1642. [Google Scholar] [CrossRef]
- Ruttmann, E.; Geltner, C.; Bucher, B.; Ulmer, H.; Höfer, D.; Hangler, H.B.; Semsroth, S.; Margreiter, R.; Laufer, G.; Müller, L.C. Combined CMV Prophylaxis Improves Outcome and Reduces the Risk for Bronchiolitis Obliterans Syndrome (BOS) after Lung Transplantation. Transplantation 2006, 81, 1415–1420. [Google Scholar] [CrossRef]
- Ranganathan, K.; Worley, S.; Michaels, M.G.; Arrigan, S.; Aurora, P.; Ballmann, M.; Boyer, D.; Conrad, C.; Eichler, I.; Elidemir, O.; et al. Cytomegalovirus immunoglobulin decreases the risk of cytomegalovirus infection but not disease after pediatric lung transplantation. J. Heart Lung Transplant. 2009, 28, 1050–1056. [Google Scholar] [CrossRef]
- Czer, L.S.; Ruzza, A.; Vespignani, R.; Rafiei, M.; Pixton, J.R.; Awad, M.; De Robertis, M.; Wong, A.V.; Trento, A. Prophylaxis of cytomegalovirus disease in mismatched patients after heart transplantation using combined antiviral and immunoglobulin therapy. Transplant. Proc. 2011, 43, 1887–1892. [Google Scholar] [CrossRef]
- Mitsani, D.; Nguyen, M.H.; Kwak, E.J.; Silveira, F.P.; Vadnerkar, A.; Pilewski, J.; Crespo, M.; Toyoda, Y.; Bermudez, C.; Clancy, C.J. Cytomegalovirus disease among donor-positive/recipient-negative lung transplant recipients in the era of valganciclovir prophylaxis. J. Heart Lung Transplant. 2010, 29, 1014–1020. [Google Scholar] [CrossRef]
- Khurana, M.P.; Lodding, I.P.; Mocroft, A.; Sørensen, S.S.; Perch, M.; Rasmussen, A.; Gustafsson, F.; Lundgren, J.D. Risk Factors for Failure of Primary (Val)ganciclovir Prophylaxis Against Cytomegalovirus Infection and Disease in Solid Organ Transplant Recipients. Open Forum Infect Dis 2019, 6, ofz215. [Google Scholar] [CrossRef]
- Gift, T.; Palafox, J.; Jones, K.; Imburgia, T.; Astor, T. CMV Viremia after Discontinuation of Valganciclovir Prophylaxis in Lung Transplant Recipients. J. Heart Lung Transplant. 2020, 39, S485. [Google Scholar] [CrossRef]
- Märtson, A.G.; Edwina, A.E.; Kim, H.Y.; Knoester, M.; Touw, D.J.; Sturkenboom, M.G.G.; Alffenaar, J.C. Therapeutic Drug Monitoring of Ganciclovir: Where Are We? Ther. Drug Monit. 2022, 44, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Marty, F.M.; Ljungman, P.; Chemaly, R.F.; Maertens, J.; Dadwal, S.S.; Duarte, R.F.; Haider, S.; Ullmann, A.J.; Katayama, Y.; Brown, J.; et al. Letermovir Prophylaxis for Cytomegalovirus in Hematopoietic-Cell Transplantation. N. Engl. J. Med. 2017, 377, 2433–2444. [Google Scholar] [CrossRef]
- Veit, T.; Munker, D.; Barton, J.; Milger, K.; Kauke, T.; Meiser, B.; Michel, S.; Zoller, M.; Nitschko, H.; Keppler, O.T.; et al. Letermovir in lung transplant recipients with cytomegalovirus infection: A retrospective observational study. Am. J. Transplant. 2021, 21, 3449–3455. [Google Scholar] [CrossRef] [PubMed]
- Saullo, J.L.; Baker, A.W.; Snyder, L.D.; Reynolds, J.M.; Zaffiri, L.; Eichenberger, E.M.; Ferrari, A.; Steinbrink, J.M.; Maziarz, E.K.; Bacchus, M.; et al. Cytomegalovirus prevention in thoracic organ transplantation: A single-center evaluation of letermovir prophylaxis. J. Hear Lung Transplant. 2022, 41, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Singha, A.; Burcham, P.; Logan, A.; El Boghdadly, Z.; Howsare, M.; Nunley, D.; Lustberg, M.; Keller, B. Letermovir for Cytomegalovirus Prophylaxis in Lung Transplant Patients with Valganciclovir-Induced Leukopenia. Transplantology 2021, 2, 129–139. [Google Scholar] [CrossRef]
- Veit, T.; Munker, D.; Kauke, T.; Zoller, M.; Michel, S.; Ceelen, F.; Schiopu, S.; Barton, J.; Arnold, P.; Milger, K.; et al. Letermovir for difficult to treat cytomegalovirus infection in lung transplant recipients. Transplantation 2020, 104, 410–414. [Google Scholar] [CrossRef]
- Aryal, S.; Katugaha, S.B.; Cochrane, A.; Brown, A.W.; Nathan, S.D.; Shlobin, O.A.; Ahmad, K.; Marinak, L.; Chun, J.; Fregoso, M.; et al. Single-center experience with use of letermovir for CMV prophylaxis or treatment in thoracic organ transplant recipients. Transpl. Infect. Dis. 2019, 21, e13166. [Google Scholar] [CrossRef]
- Avery, R.K.; Alain, S.; Alexander, B.D.; Blumberg, E.A.; Chemaly, R.F.; Cordonnier, C.; Duarte, R.F.; Florescu, D.F.; Kamar, N.; Kumar, D.; et al. Maribavir for Refractory Cytomegalovirus Infections With or Without Resistance Post-Transplant: Results From a Phase 3 Randomized Clinical Trial. Clin. Infect. Dis. 2022, 75, 690–701. [Google Scholar]
- El Chami, H.; Hassoun, P.M. Immune and inflammatory mechanisms in pulmonary arterial hypertension. Prog. Cardiovasc. Dis. 2012, 55, 218–228. [Google Scholar] [CrossRef]
- Tomaszewski, M.; Bębnowska, D.; Hrynkiewicz, R.; Dworzyński, J.; Niedźwiedzka-Rystwej, P.; Kopeć, G.; Grywalska, E. Role of the Immune System Elements in Pulmonary Arterial Hypertension. J. Clin. Med. 2021, 10, 3757. [Google Scholar] [CrossRef]
- van Uden, D.; Koudstaal, T.; van Hulst, J.A.C.; Vink, M.; van Nimwegen, M.; van den Toorn, L.M.; Chandoesing, P.P.; van den Bosch, A.E.; Kool, M.; Hendriks, R.W.; et al. Peripheral Blood T Cells of Patients with IPAH Have a Reduced Cytokine-Producing Capacity. Int. J. Mol. Sci. 2022, 23, 6508. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewski, M.; Grywalska, E.; Tomaszewski, A.; Błaszczak, P.; Kurzyna, M.; Roliński, J.; Kopeć, G. Overexpression of PD-1 on Peripheral Blood Lymphocytes in Patients with Idiopathic Pulmonary Arterial Hypertension and Its Association with High Viral Loads of Epstein-Barr Virus and Poor Clinical Parameters. J. Clin. Med. 2020, 9, 1966. [Google Scholar] [CrossRef] [PubMed]
- de Melo Silva, J.; Pinheiro-Silva, R.; Dhyani, A.; Pontes, G.S. Cytomegalovirus and Epstein-Barr Infections: Prevalence and Impact on Patients with Hematological Diseases. BioMed Res. Int. 2020, 2020, 1627824. [Google Scholar] [CrossRef]
- Maple, P.A.C. Cytomegalovirus and Epstein-Barr Virus Associations with Neurological Diseases and the Need for Vaccine Development. Vaccines 2020, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Organización Nacional de Trasplantes (ONT), Ministerio de Sanidad. Gobierno de España. Actividad de donación y trasplante pulmonar. España. 2021. Available online: http://www.ont.es/infesp/Memorias/ (accessed on 20 October 2022).
| All Recipients (n = 124) | CMV-Ig Short Regimen (n = 62) | CMV-Ig Extended Regimen (n = 62) | p-Value | |
|---|---|---|---|---|
| Number of centres, n | 3 | 1 | 2 | |
| Age *, years, median (IQR) | 51.6 (38.0–60.8) | 56.1 (43.0–62.1) | 47.4 (34.3–57.3) | 0.013 |
| Sex, male, n (%) | 74 (59.7) | 37 (59.7) | 37 (59.7) | 1.000 |
| Bilateral lung transplantation, n (%) | 103 (83.1) | 51 (82.3) | 52 (83.9) | 0.811 |
| Indication for lung transplant, n (%) | 0.428 | |||
| COPD | 29 (23.4) | 14 (22.6) | 15 (24.2) | |
| ILD | 40 (32.3) | 25 (40.3) | 15 (24.2) | |
| Bronchiectasis | 30 (24.2) | 12 (19.4) | 18 (29.0) | |
| PAH | 13 (10.5) | 6 (9.7) | 7 (11.3) | |
| Lung retransplantation | 1 (0.8) | 0 (0) | 1 (1.6) | |
| Other | 11 (8.9) | 5 (8.1) | 6 (9.7) | |
| Induction therapy **, n (%) | 101 (81.5) | 39 (62.9) | 62 (100) | <0.001 |
| Maintenance therapy, n (%) | ||||
| Calcineurin inhibitors | 0.619 | |||
| Tacrolimus | 120 (96.8) | 59 (95.2) | 61 (98.4) | |
| Cyclosporine | 4 (3.2) | 3 (4.8) | 1 (1.6) | |
| Anti-metabolites | <0.001 | |||
| MMF/MPS | 115 (92.7) | 62 (100) | 53 (85.5) | |
| Azathioprine | 9 (7.3) | 0 (0) | 9 (14.5) | |
| Corticoids | 123 (99.2) | 62 (100) | 61 (98.4) | 0.315 |
| Azithromycin | 86 (69.4) | 47 (75.8) | 39 (62.9) | 0.172 |
| mTOR inhibitor | 37 (29.8) | 6 (9.7) | 31 (50.0) | <0.001 |
| CMV antiviral prophylaxis, n (%) | ||||
| GCV/VGCV | 100 | 100 | 100 | 1.000 |
| Other (leflunomide) | 2 (1.6) | 0 (0) | 2 (3.2) | 0.496 |
| Follow-up, years, median (IQR) | 3.65 (2.0–6.9) | 3.50 (1.5–6.7) | 4.10 (2.9–7.1) | 0.236 |
| All Recipients (n = 124) | CMV-Ig Short Regimen (n = 62) | CMV-Ig Extended Regimen (n = 62) | p-Value | |
|---|---|---|---|---|
| VGCV duration, months, median (IQR) | 12 (10–12) | 12 (12–13) | 12 (7–12) | 0.003 |
| Premature VGCV prophylaxis discontinuation, n (%) | 31 (25) | 5 (8.1) | 26 (41.9) | <0.001 |
| VGCV duration, months, median (IQR) among those who discontinued | 7 (5–10) | 10 (7–12) | 7 (4.8–10) | 0.291 |
| Indication for discontinuation, n (%) | ||||
| Myelotoxicity | 13 (41.9) | 1 (20.0) | 12 (46.9) | 0.284 |
| Renal failure | 8 (25.8) | 1 (20.0) | 7 (26.9) | |
| CMV infection | 7 (22.6) | 3 (60.0) | 4 (15.4) | |
| Gastrointestinal distress | 2 (6.5) | 0 (0.0) | 2 (7.7) | |
| Non-adherence | 1 (3.2) | 0 (0.0) | 1 (3.8) | |
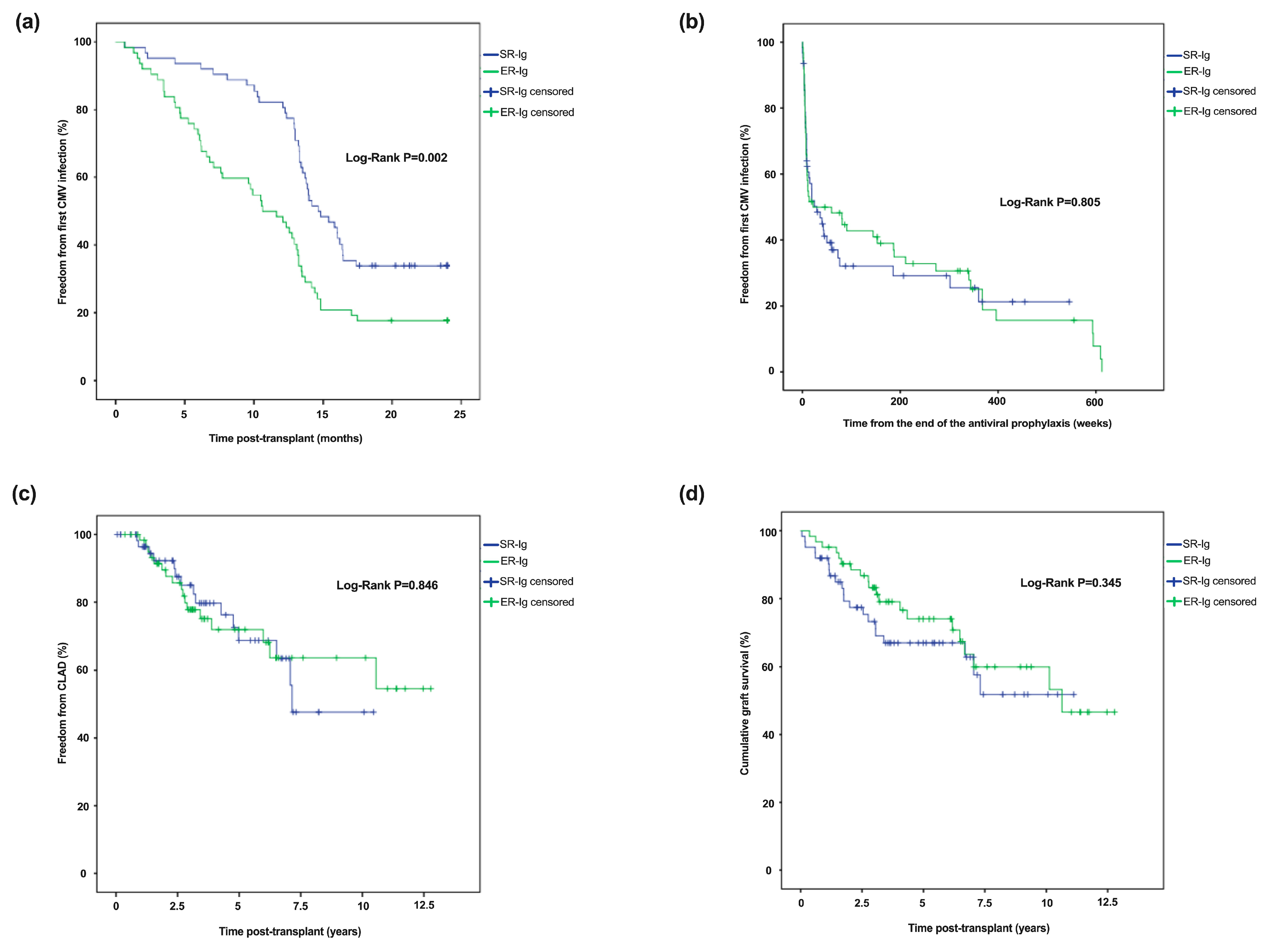
| CMV infection, n (%) | 92 (74) | 42 (67.7) | 50 (80.6) | 0.150 |
| First CMV infection | ||||
| Time from transplant, months, median (IQR) | 13.30 (7.81–18.33) | 14.75 (12.93–21.28) | 11.13 (5.56–14.65) | <0.001 |
| Time from the end of antiviral CMV prophylaxis, weeks, median (IQR) | 7.43 (4.43–14.0) | 8.57 (5.03–20.60) | 6.85 (4.21–10.28) | 0.209 |
| Tissue-invasive disease, n (%) | 11 (12) | 5 (11.9) | 6 (12.0) | 0.989 |
| Pneumonitis | 4 (36.4) | 2 (40) | 2 (33.3) | 0.569 |
| Gastritis | 2 (18.2) | 0 (0.0) | 2 (33.3) | |
| Colitis | 2 (18.2) | 1 (20.0) | 1 (16.7) | |
| Hepatitis | 2 (18.2) | 1 (20.0) | 1 (16.7) | |
| Colitis and hepatitis | 1 (9.1) | 1 (20.0) | 0 (0.0) | |
| Number of CMV infections, median (IQR) | 2 (1–3) | 1 (1–2) | 2 (1–3) | <0.001 |
| With IV treatment | 1 (1–1) | 1 (1–1) | 1 (1–1.25) | 0.325 |
| With oral treatment | 1 (1–2) | 1 (1–2) | 1 (1–2) | 0.091 |
| Acute rejections, n, median (IQR) | 1 (1–2) | 1 (1–2) | 1.5 (1–2) | 0.902 |
| Severity grade, n, median (IQR) | ||||
| A1-A2 | 1 (1–2) | 1 (1–2) | 1 (1–2) | 0.919 |
| A23-A4 | 1 (1–1.25) | 1 (1–2) | 1 (1–1) | 0.454 |
| Treated with corticosteroids megadose, n (IQR) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 0.830 |
| Treated with oral corticosteroids, n (IQR) | 1 (1–1) | 1 (1–1) | 1 (1–1) | 1.000 |
| CLAD, n (%) | 32 (25.8) | 15 (24.2) | 17 (27.4) | 0.838 |
| BOS | 29 (90.6) | 14 (93.3) | 15 (88.2) | 0.621 |
| RAS | 3 (9.4) | 1 (6.7) | 2 (11.8) | |
| Time from transplant, years, median (IQR) | 3.16 (1.70–6.15) | 3.18 (1.38–5.68) | 3.15 (2.01–6.21) | 0.055 |
| Death, n (%) | 41 (33.1) | 21 (33.9) | 20 (32.2) | 0.849 |
| Variables | Crude OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value |
|---|---|---|---|---|
| Age at transplant | 0.964 (0.933–0.997) | 0.034 | - | - |
| Months of VGCV prophylaxis | 1.039 (0.944–1.144) | 0.436 | ||
| Type of transplant Unilateral Bilateral | 0.383 (0.144–1.022) Ref. | 0.055 | ||
| Immunosuppression induction Yes No | 1.711 (0.647–4.526) Ref. | 0.279 | ||
| Indication for lung transplant COPD DILD Bronchiectasis PAH Other | Ref. 0.791 (0.277–2.259) 1.048 (0.332–3.302) 4.571 (0.508–41.114) 3.810 (0.417–34.763) | 0.662 0.937 0.175 0.236 | ||
| Acute rejection | 0.999 (0.591–1.690) | 0.997 | ||
| Acute rejection severity A1-A2 A3-A4 | 0.607 0.050 | 0.165 0.063 | ||
| Acute rejection treated with corticosteroids megadose | 0.974 (0.555–1.710) | 0.926 | ||
| Premature VGCV prophylaxis discontinuation Yes No | 4.229 (1.189–15.043) Ref. | 0.026 | 4.083 (1.146–14.552) | 0.030 |
| CMV-Ig schedule SR-Ig ER-Ig | Ref. 1.984 (0.870–4.527) | 0.104 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mora, V.M.; Ussetti, P.; de Pablo, A.; Iturbe, D.; Laporta, R.; Alonso, R.; Aguilar, M.; Quezada, C.A.; Cifrián, J.M. Evaluation of Two Different CMV-Immunoglobulin Regimens for Combined CMV Prophylaxis in High-Risk Patients following Lung Transplant. Microorganisms 2023, 11, 32. https://doi.org/10.3390/microorganisms11010032
Mora VM, Ussetti P, de Pablo A, Iturbe D, Laporta R, Alonso R, Aguilar M, Quezada CA, Cifrián JM. Evaluation of Two Different CMV-Immunoglobulin Regimens for Combined CMV Prophylaxis in High-Risk Patients following Lung Transplant. Microorganisms. 2023; 11(1):32. https://doi.org/10.3390/microorganisms11010032
Chicago/Turabian StyleMora, Víctor M., Piedad Ussetti, Alicia de Pablo, David Iturbe, Rosalía Laporta, Rodrigo Alonso, Myriam Aguilar, Carlos A. Quezada, and José M. Cifrián. 2023. "Evaluation of Two Different CMV-Immunoglobulin Regimens for Combined CMV Prophylaxis in High-Risk Patients following Lung Transplant" Microorganisms 11, no. 1: 32. https://doi.org/10.3390/microorganisms11010032
APA StyleMora, V. M., Ussetti, P., de Pablo, A., Iturbe, D., Laporta, R., Alonso, R., Aguilar, M., Quezada, C. A., & Cifrián, J. M. (2023). Evaluation of Two Different CMV-Immunoglobulin Regimens for Combined CMV Prophylaxis in High-Risk Patients following Lung Transplant. Microorganisms, 11(1), 32. https://doi.org/10.3390/microorganisms11010032






