Colletotrichum truncatum Causing Anthracnose of Tomato (Solanum lycopersicum L.) in Malaysia
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Fungal Isolation
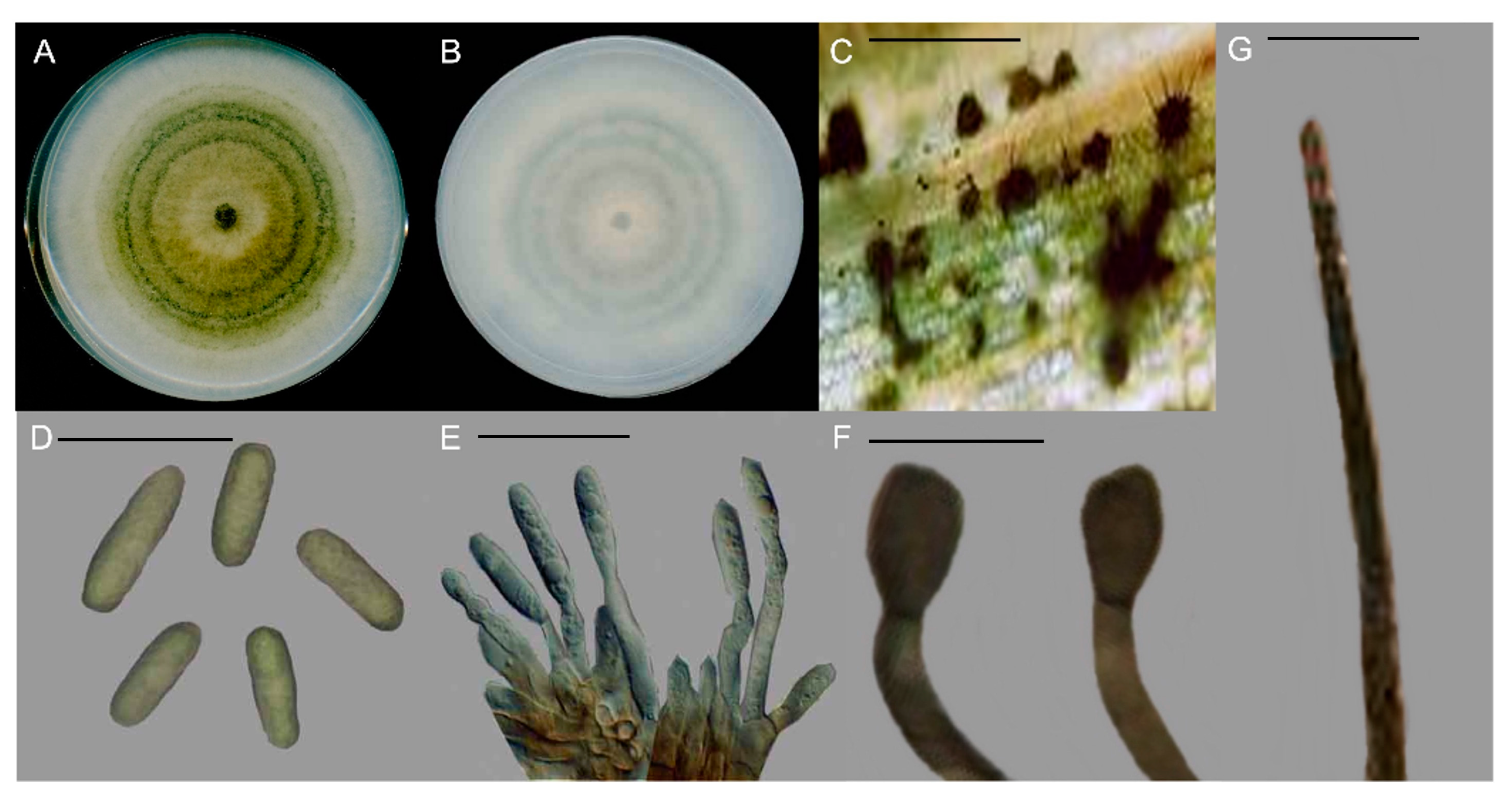
2.2. Morphological Characteristics
2.3. Extraction of Genomic DNA, PCR Amplification, and DNA Sequencing
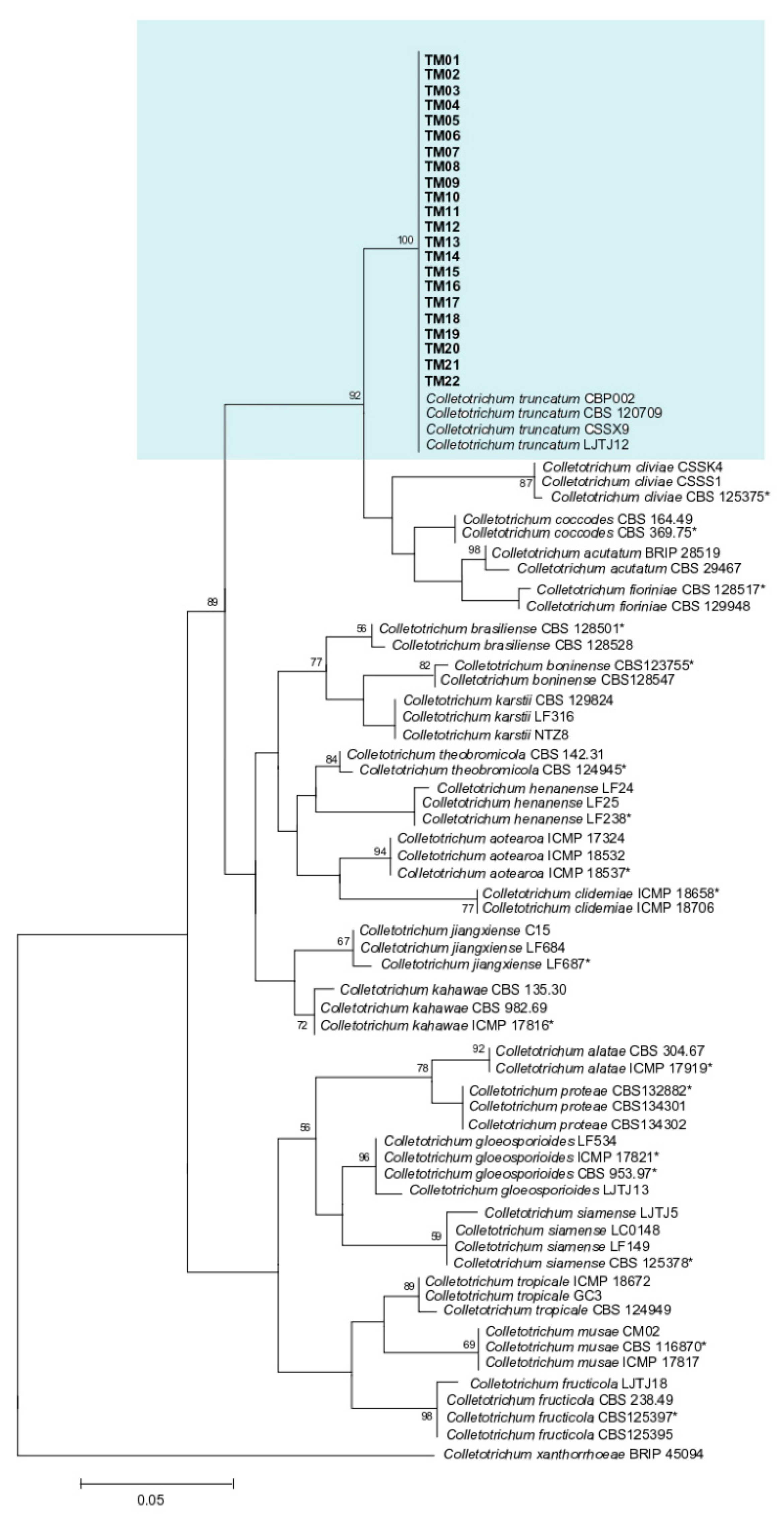
2.4. Sequences Alignment, BLAST, and Phylogenetic Analysis
2.5. Pathogenicity Assays
3. Results
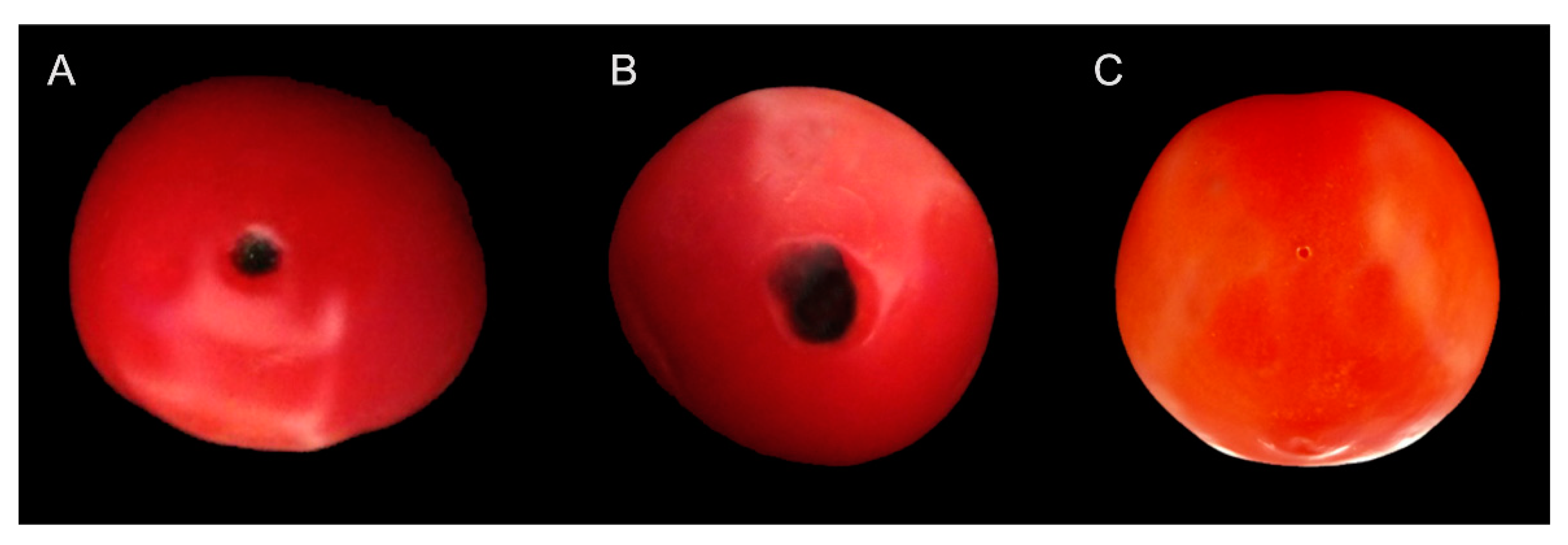
3.1. Disease Survey
3.2. Fungal Isolation and Morphological Characterisation
3.3. Molecular Identification and Phylogenetic Analysis
3.4. Pathogenicity Assays
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Panthee, D.R.; Chen, F. Genomics of fungal disease resistance in tomato. Curr. Genomics. 2010, 11, 30–39. [Google Scholar] [CrossRef]
- Cannon, P.F.; Damm, U.; Johnton, P.R.; Weir, B.S. Colletotrichum-current status and future directions. Stud. Mycol. 2012, 73, 181–213. [Google Scholar] [CrossRef]
- Garrido, C.; Carbu, N.; Fernandez-Acero, F.J.; Vallejo, I.; Cantoral, J.M. Phylogenetic relationships and genome organization of Colletotrichum acutatum causing anthracnose in strawberry. Eur. J. Plant Pathol. 2009, 125, 397–411. [Google Scholar] [CrossRef]
- Liao, C.Y.; Chen, M.Y.; Chen, Y.K.; Wang, T.C.; Sheu, Z.M.; Kuo, K.C.; Chang, P.F.L.; Chung, K.R.; Lee, M.H. Characterization of three Colletotrichum acutatum isolates from Capsicum spp. Eur. J. Plant Pathol. 2012, 133, 599–608. [Google Scholar] [CrossRef]
- Sezer, A.; Dolar, F.S. Colletotrichum acutatum, a new pathogen of hazelnut. J. Phytopathol. 2012, 160, 428–430. [Google Scholar] [CrossRef]
- Riccardo, B.; Amby, D.B.; Zapparata, A.; Sarrocco, S.; Vannacci, G.; Le Floch, G.; Harrison, R.J.; Holub, E.; Sukno, S.A.; Sreenivasaprasad, S.; et al. Gene family expansions and contractions are associated with host range in plant pathogens of the genus Colletotrichum. BMC Genomics 2016, 17, 555. [Google Scholar]
- Sato, T. Studies on the taxonomy and identification of plant pathogenic fungi, based on morphology and phylogenetic analyses, and fungal pathogenicity focused on host specificity. J. Gen. Plant Pathol. 2016, 82, 332–337. [Google Scholar] [CrossRef]
- Wiesner-Hanks, T.; Nelson, R. Multiple disease resistance in plants. Annu. Rev. Phytopathol. 2016, 54, 229–252. [Google Scholar] [CrossRef]
- Cai, L.; Hyde, K.D.; Taylor, P.W.J.; Weir, B.S.; Waller, J.M.; Abang, M.M.; Zhang, J.Z.; Yang, Y.L.; Phoulivong, S.; Liu, Z.Y.; et al. A polyphasic approach for studying Colletotrichum. Fungal Divers. 2009, 39, 183–204. [Google Scholar]
- Sharma, G.; Pinnaka, A.K.; Shenoy, B.D. Resolving the Colletotrichum siamense species complex using ApMat marker. Fungal Divers. 2015, 71, 247–264. [Google Scholar] [CrossRef]
- Cannon, P.F.; Bridge, P.D.; Monte, E. Linking the Past, Present, and Future of Colletotrichum Systematics. In Colletotrichum: Host specificity, Pathology, and Host-Pathogen Interaction; Prusky, D., Freeman, S., Dickman, M., Eds.; APS Press: St. Paul, MN, USA, 2000; pp. 1–20. [Google Scholar]
- Photita, W.; Taylor, P.W.J.; Ford, R.; Lumyong, P.; McKenzie, H.C.; Hyde, K.D. Morphological and molecular characterization of Colletotrichum species from herbaceous plants in Thailand. Fungal Divers. 2005, 18, 117–133. [Google Scholar]
- Liu, F.; Weir, B.S.; Damm, U.; Crous, P.W.; Wang, Y.; Liu, B.; Wang, M.; Zhang, M.; Cai, L. Unravelling Colletotrichum species associated with Camellia: Employing ApMat and GS loci to resolve species in the C. gloeosporioides complex. Persoonia 2015, 35, 63–86. [Google Scholar] [CrossRef] [PubMed]
- Weir, B.S.; Johnston, P.R.; Damm, U. The Colletotrichum gloeosporioides species complex. Stud. Mycol. 2012, 73, 115–180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Su, Y.-Y.; Cai, L. An optimized protocol of single spore isolation for fungi. Cryptogam. Mycol. 2013, 34, 349–356. [Google Scholar] [CrossRef]
- Cabrera, L.; Rojas, P.; Rojas, S.; Pardo-De la Hoz, C.J.; Mideros, M.F.; Danies, G.; Lopez-Kleine, L.; Jimenez, P.; Restrepo, S. Most Colletotrichum species associated with tree tomato (Solanum betaceum) and mango (Mangifera indica) crops are not host-specific. Plant Pathol. 2018, 67, 1022–1030. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Snisky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Glass, N.L.; Donaldson, G. Development of primer sets designed for use with PCR to amplify conserved genes from filamentous Ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Cigelnik, E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet. Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef]
- Templeton, M.D.; Rikkerink, E.H.; Solon, S.L.; Crowhurst, R.N. Cloning and molecular characterization of the glyceraldehyde-3-phosphate dehydrogenase-encoding gene and cDNA from the plant pathogenic fungus Glomerella cingulata. Gene 1992, 122, 225–230. [Google Scholar] [CrossRef]
- Carbone, I.; Kohn, L. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Baroncelli, R.; Sarrocco, S.; Zapparata, A.; Tavarini, S.; Angelini, L.G.; Vannacci, G. Characterization and epidemiology of Colletotrichum acutatum sensu lato (C. chrysanthemi) causing Carthamus tinctorius anthracnose. Plant Pathol. 2015, 64, 375–384. [Google Scholar] [CrossRef]
- Hyde, K.D.; Cai, L.; McKenzie, E.H.C.; Yang, Y.L.; Zhang, J.Z.; Prihastuti, H. Colletotrichum: A catalogue of confusion. Fungal Divers. 2009, 39, 1–17. [Google Scholar]
- Schena, L.; Mosca, S.; Cacciola, S.O.; Faedda, R.; Sanzani, S.M.; Agosteo, G.E.; Sergeeva, V.; Magnano di San Lio, G. Species of the Colletotrichum gloeosporioides and C. boninense complexes associated with olive anthracnose. Plant Pathol. 2014, 63, 437–446. [Google Scholar] [CrossRef]
- Rashid, T.S.; Sijam, K.; Kadir, J.; Saud, H.M.; Awla, H.K.; Hata, E.M. First report of tomato anthracnose caused by Colletotrichum boninense in Malaysia. J. Plant Pathol. 2015, 97, 209–220. [Google Scholar]
- Diao, Y.Z.; Zhang, C.; Lin, D.; Liu, X.L. First report of Colletotrichum truncatum causing anthracnose of tomato in China. Dis. Notes 2014, 98, 687. [Google Scholar] [CrossRef] [PubMed]
- Saini, T.J.; Gupta, S.G.; Anandalakshmi, R. Detection of tomato anthracnose caused by Colletotrichum truncatum in India. Australas. Plant Dis. Notes 2017, 12, 48. [Google Scholar] [CrossRef]
- Villafana, R.T.; Ramdass, A.C.; Rampersad, S.N. First Report of Colletotrichum truncatum causing anthracnose in tomato fruit in Trinidad. Dis. Notes 2018, 102, 1857. [Google Scholar] [CrossRef]
- Bincader, S.; Pongpisutta, R.; Rattanakreetakul, C. Diversity of Colletotrichum species causing anthracnose disease from Mango cv. Nam Dork Mai See Tong based on ISSR-PCR. Indian J. Agric. Res. 2022, 56, 81–90. [Google Scholar] [CrossRef]
- de Silva, D.D.; Groenewald, J.Z.; Crous, P.W.; Ades, P.K.; Nasruddin, A.; Mongkolporn, O.; Taylor, P.W. Identification, prevalence and pathogenicity of Colletotrichum species causing anthracnose of Capsicum annuum in Asia. IMA Fungus 2019, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Luo, C.-X.; Wu, H.-J.; Peng, B.; Kang, B.-S.; Liu, L.-M.; Zhang, M.; Gu, Q.-S. Colletotrichum species associated with anthracnose disease of watermelon (Citrullus lanatus) in China. J. Fungi 2022, 8, 790. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, Y.; Yu, H.; Zhou, J.; Hu, M.; Liu, A.; Wu, J.; Wang, H.; Zhang, C. Colletotrichum Spp. Diversity between leaf anthracnose and crown rot from the same strawberry plant. Front. Microbiol. 2022, 13, 860694. [Google Scholar] [CrossRef]
- Li, M.; Feng, W.; Yang, J.; Gao, Z.; Zhang, Z.; Zhang, W.; Wang, S.; Wang, W.; Gong, D.; Hu, M. First report of anthracnose caused by Colletotrichum siamense on avocado fruits in China. Crop Prot. 2022, 155, 105922. [Google Scholar] [CrossRef]
- Licciardello, G.; Moral, J.; Strano, M.C.; Caruso, P.; Sciara, M.; Bella, P.; Sorrentino, G.; Di Silvestro, S. Characterization of Colletotrichum strains associated with olive anthracnose in Sicily. Phytopathol. Mediterr. 2022, 61, 139–151. [Google Scholar] [CrossRef]
- Pacheco-Esteva, M.C.; Soto-Castro, D.; Vásquez-López, A.; Tovar-Pedraza, J.M. First report of Colletotrichum siamense causing papaya anthracnose in Mexico. J. Plant Pathol. 2022, 104, 1175. [Google Scholar] [CrossRef]
- Goh, K.S.; Balasubramaniam, J.; Sani, S.F.; Alam, M.W.; Ismail, N.A.; Gleason, M.L.; Rosli, H. First report of Colletotrichum scovillei causing anthracnose on watermelon (Citrullus lanatus) in Malaysia. Dis. Notes 2022, 106, 2521. [Google Scholar] [CrossRef] [PubMed]
- Khoo, Y.W.; Tan, H.T.; Khaw, Y.S.; Li, S.-F.; Chong, K.P. First report of anthracnose on ‘Purple Dream’ Solanum melongenain Malaysia caused by Colletotrichum siamense. Plant Dis. 2022, 106. [Google Scholar] [CrossRef]
- Mahmodi, F.; Kadir, J.; Puteh, A. Genetic diversity and pathogenic variability of Colletotrichum truncatum causing anthracnose of pepper in Malaysia. J. Phytopathol. 2014, 162, 456–465. [Google Scholar] [CrossRef]
- Vijaya, S.I.; Anuar, I.S.M.; Zakaria, L. Characterization and pathogenicity of Colletotrichum truncatum causing stem anthracnose of red-fleshed dragon fruit (Hylocereus polyrhizus) in Malaysia. J. Phytopathol. 2015, 163, 67–71. [Google Scholar] [CrossRef]
- Zakaria, L.; Sahak, S.; Zakaria, M.; Salleh, B. Characterisation of Colletotrichum species associated with anthracnose of banana. Trop. Life Sci. Res. 2009, 20, 119–125. [Google Scholar]
- Zĭvković, S.; Stojanović, S.; Ivanović, Z.; Trkulja, N.; Dolovac, N.; Aleksić, G.; Jelica Balaž, J. Morphological and molecular identification of Colletotrichum acutatum from tomato fruit. Pestic. Phytomed. 2010, 25, 231–239. [Google Scholar] [CrossRef]

| Gene | Primer | Sequence (5′–3′) | Reference |
|---|---|---|---|
| ITS | ITS1 ITS4 | TCCGTAGGTGAACCTGCGG TCCTCCGCTTATTGATATGC | [17] |
| tub2 | Bt2a Bt2b | GGTAACCAAATCGGTGCTGCTTTC ACCCTCAGTGTAGTGACCCTTGGC | [18,19] |
| gapdh | GDF1 GDR1 | GCCGTCAACGACCCCTTCATTGA GGGTGGAGTCGTACTTGAGCATGT | [20] |
| act | ACT-512F ACT-783R | ATGTGCAAGGCCGGTTTCGC TACGAGTCCTTCTGGCCCAT | [21] |
| cal | CAL-228F CAL-737R | GAGTTCAAGGAGGCCTTCTCCC CATCTTTCTGGCCATCATGG | [21] |
| Species | Isolate | Host | Location | GenBank Accession Number | ||||
|---|---|---|---|---|---|---|---|---|
| ITS | tub2 | gapdh | act | cal | ||||
| Colletotrichum truncatum | TM01 | Solanum lycopersicum | Malaysia | OP456600 | OP495634 | OP495656 | OP495678 | OP495700 |
| TM02 | Solanum lycopersicum | Malaysia | OP456601 | OP495635 | OP495657 | OP495679 | OP495701 | |
| TM03 | Solanum lycopersicum | Malaysia | OP456602 | OP495636 | OP495658 | OP495680 | OP495702 | |
| TM04 | Solanum lycopersicum | Malaysia | OP456603 | OP495637 | OP495659 | OP495681 | OP495703 | |
| TM05 | Solanum lycopersicum | Malaysia | OP456604 | OP495638 | OP495660 | OP495682 | OP495704 | |
| TM06 | Solanum lycopersicum | Malaysia | OP456605 | OP495639 | OP495661 | OP495683 | OP495705 | |
| TM07 | Solanum lycopersicum | Malaysia | OP456606 | OP495640 | OP495662 | OP495684 | OP495706 | |
| TM08 | Solanum lycopersicum | Malaysia | OP456607 | OP495641 | OP495663 | OP495685 | OP495707 | |
| TM09 | Solanum lycopersicum | Malaysia | OP456608 | OP495642 | OP495664 | OP495686 | OP495708 | |
| TM10 | Solanum lycopersicum | Malaysia | OP456609 | OP495643 | OP495665 | OP495687 | OP495709 | |
| TM11 | Solanum lycopersicum | Malaysia | OP456610 | OP495644 | OP495666 | OP495688 | OP495710 | |
| TM12 | Solanum lycopersicum | Malaysia | OP456611 | OP495645 | OP495667 | OP495689 | OP495711 | |
| TM13 | Solanum lycopersicum | Malaysia | OP456612 | OP495646 | OP495668 | OP495690 | OP495712 | |
| TM14 | Solanum lycopersicum | Malaysia | OP456613 | OP495647 | OP495669 | OP495691 | OP495713 | |
| TM15 | Solanum lycopersicum | Malaysia | OP456614 | OP495648 | OP495670 | OP495692 | OP495714 | |
| TM16 | Solanum lycopersicum | Malaysia | OP456615 | OP495649 | OP495671 | OP495693 | OP495715 | |
| TM17 | Solanum lycopersicum | Malaysia | OP456616 | OP495650 | OP495672 | OP495694 | OP495716 | |
| TM18 | Solanum lycopersicum | Malaysia | OP456617 | OP495651 | OP495673 | OP495695 | OP495717 | |
| TM19 | Solanum lycopersicum | Malaysia | OP456618 | OP495652 | OP495674 | OP495696 | OP495718 | |
| TM20 | Solanum lycopersicum | Malaysia | OP456619 | OP495653 | OP495675 | OP495697 | OP495719 | |
| TM21 | Solanum lycopersicum | Malaysia | OP456620 | OP495654 | OP495676 | OP495698 | OP495720 | |
| TM22 | Solanum lycopersicum | Malaysia | OP456621 | OP495655 | OP495677 | OP495699 | OP495721 | |
| CBP002 | Brassica parachinensis | China | KF030677 | KF240819 | KF300886 | KF158412 | KF114851 | |
| CBS 120709 | Capsicum frutescens | India | GQ485593 | GQ849429 | GQ856753 | GQ856783 | GQ849453 | |
| CSSX9 | Hymenocallis americana | China | GQ485594 | GQ849436 | GQ856752 | GQ856772 | GQ849461 | |
| LJTJ12 | Capsicum sp. | China | KP748203 | KP823843 | KP823782 | KP823765 | KP823834 | |
| Colletotrichum acutatum | BRIP 28519 | Carica papaya | Australia | FJ972601 | FJ907443 | FJ972580 | FJ907428 | FJ917510 |
| CBS 29467 | Carica papaya | Australia | FJ972610 | FJ907444 | FJ972581 | FJ907429 | FJ917511 | |
| Colletotrichum alatae | CBS 304.67 | Dioscorea alata | Nigeria | JX010191 | JX010449 | JX010011 | JX009470 | JX009739 |
| ICMP 17919 * | Dioscorea alata | India | JX010190 | JX010383 | JX009990 | JX009471 | JX009738 | |
| Colletotrichum aotearoa | ICMP 17324 | Kunzea ericoides | New Zealand | JX010198 | JX010418 | JX009991 | JX009538 | JX009619 |
| ICMP 18532 | Vitex lucens | New Zealand | JX010220 | JX010421 | JX009906 | JX009544 | JX009614 | |
| ICMP 18537 * | Coprosma sp. | New Zealand | JX010205 | JX010420 | JX010005 | JX009564 | JX009611 | |
| Colletotrichum boninense | CBS 123755 * | Crinum asiaticum | Japan | JQ005153 | JQ005588 | JQ005240 | JQ005501 | JQ005674 |
| CBS 128547 | Camellia sp. | New Zealand | JQ005159 | JQ005593 | JQ005249 | JQ005507 | JQ005680 | |
| Colletotrichum brasiliense | CBS 128501 * | Passiflora edulis | Brazil | JQ005235 | JQ005669 | JQ005322 | JQ005583 | JQ005756 |
| CBS 128528 | Passiflora edulis | Brazil | JQ005234 | JQ005668 | JQ005321 | JQ005582 | JQ005755 | |
| Colletotrichum clidemiae | ICMP 18658 * | Clidemia hirta | USA | JX010265 | JX010438 | JX009989 | JX009537 | JX009645 |
| ICMP 18706 | Vitis sp. | USA | JX010274 | JX010439 | JX009909 | JX009476 | JX009639 | |
| Colletotrichum cliviae | CBS 125375 * | Clivia miniate | China | JX519223 | JX519249 | JX546611 | JX519240 | KX957765 |
| CSSK4 | Clivia miniate | China | GQ485607 | GQ849440 | GQ856756 | GQ856777 | GQ849464 | |
| CSSS1 | Clivia miniate | China | GU109479 | GU085869 | GU085868 | GU085861 | GU085864 | |
| Colletotrichum coccodes | CBS 164.49 | Solanum tuberosum | Netherlands | HM171678 | KU821197 | HM171672 | HM171666 | HM171669 |
| CBS 369.75 * | Solanum tuberosum | Netherlands | HM171679 | KU821198 | HM171673 | HM171667 | HM171670 | |
| Colletotrichum fioriniae | CBS 128517 * | Fiorinia externa | USA | JQ948292 | JQ949943 | JQ948622 | JQ949613 | MN895526 |
| CBS 129948 | Tulipa sp. | UK | JQ948344 | JQ949995 | JQ948674 | JQ949665 | MN895531 | |
| Colletotrichum fructicola | CBS 238.49 | Ficus habrophylla | Germany | JX010181 | JX010400 | JX009923 | JX009495 | JX009671 |
| CBS 125395 | Theobroma cacao | Panama | JX010172 | JX010408 | JX009992 | JX009543 | JX009666 | |
| CBS 125397 * | Tetragastris panamensis | Panama | JX010173 | JX010409 | JX010032 | JX009581 | JX009674 | |
| LJTJ18 | Capsicum sp. | China | KP748209 | KP823856 | KP823788 | KP823744 | KP823814 | |
| Colletotrichum gloeosporioides | CBS 953.97 * | Citrus sinensis | Italy | GQ485605 | GQ849434 | GQ856762 | GQ856782 | GQ849452 |
| ICMP 17821 * | Citrus sinensis | Italy | JX010152 | JX010445 | JX010056 | JX009531 | JX009731 | |
| LF534 | Camellia sinensis | China | KJ955158 | KJ955305 | KJ954859 | KJ954434 | KJ954710 | |
| LJTJ13 | Capsicum sp. | China | KP748204 | KP823863 | KP823783 | KP823751 | KP823821 | |
| Colletotrichum henanense | LF24 | Cirsium japonicum | China | KM610182 | KM610184 | KM610178 | KM610172 | KM610176 |
| LF25 | Cirsium japonicum | China | KM610183 | KM610185 | KM610179 | KM610173 | KM610177 | |
| LF238 * | Camellia sinensis | China | KJ955109 | KJ955257 | KJ954810 | KM023257 | KJ954662 | |
| Colletotrichum jiangxiense | C15 | Citrus sinensis | China | MT318946 | MT602355 | MT602358 | MT602346 | KJ954701 |
| LF684 | Camellia sinensis | China | KJ955198 | KJ955345 | KJ954899 | KJ954469 | KJ954749 | |
| LF687 * | Camellia sinensis | China | KJ955201 | KJ955348 | KJ954902 | KJ954471 | KJ954752 | |
| Colletotrichum kahawae | CBS 135.30 | Coffea sp. | Kenya | JX010235 | JX010432 | JX010037 | JX009554 | JX009640 |
| CBS 982.69 | Coffea arabica | Angola | JX010234 | JX010435 | JX010040 | JX009474 | JX009638 | |
| ICMP 17816 * | Coffea arabica | Kenya | JX010231 | JX010444 | JX010012 | JX009452 | JX009642 | |
| Colletotrichum karstii | CBS 129824 | Musa sp. | Colombia | JQ005215 | JQ005649 | JQ005302 | JQ005563 | JQ005736 |
| LF316 | Camellia sinensis | China | KJ955125 | KJ955273 | KJ954826 | KJ954405 | KY971492 | |
| NTZ8 | Nandina domestica | China | MH152578 | MH152594 | MH152586 | MH152582 | MH152598 | |
| Colletotrichum musae | CBS 116870 * | Musa sp. | USA | JX010146 | HQ596280 | JX010050 | JX009433 | JX009742 |
| CM02 | Musa x paradisiaca | Brazil | MH746945 | MH746949 | MH746948 | MH622522 | MH746946 | |
| ICMP 17817 | Musa sapientum | Kenya | JX010142 | JX010395 | JX010015 | JX009432 | JX009689 | |
| Colletotrichum proteae | CBS 132882 * | Protea sp. | South Africa | KC297079 | KC297101 | KC297009 | KC296940 | KC296960 |
| CBS 134301 | Protea sp. | South Africa | KC842385 | KC842387 | KC842373 | KC842373 | KC842375 | |
| CBS 134302 | Protea sp. | South Africa | KC842386 | KC842388 | KC842380 | KC842374 | KC842376 | |
| Colletotrichum siamense | CBS 125378 * | Hymenocallis americana | China | JX010278 | JX010410 | JX010019 | JX009441 | JX009709 |
| LC0148 | Camellia sp. | China | KJ955078 | KJ955227 | KJ954779 | KJ954360 | KJ954631 | |
| LF149 | Camellia sp. | China | KJ955089 | KJ955238 | KJ954790 | KJ954371 | KJ954642 | |
| LJTJ5 | Capsicum sp. | China | KP748195 | KP823868 | KP823756 | KP823775 | KP823825 | |
| Colletotrichum theobromicola | CBS 142.31 | Fragaria x ananassa | USA | JX010286 | JX010373 | JX010024 | JX009516 | JX009592 |
| CBS 124945 * | Theobroma cacao | Panama | JX010294 | JX010447 | JX010006 | JX009444 | JX009591 | |
| Colletotrichum tropicale | CBS 124949 | Theobroma cacao | Panama | JX010264 | JX010407 | JX010007 | JX009489 | JX009719 |
| GC3 | Vitis sp. | Taiwan | MT555315 | MT648526 | MT648519 | MT648522 | MT062402 | |
| ICMP 18672 | Litchi chinensis | Japan | JX010275 | JX010396 | JX010020 | JX009480 | JX009722 | |
| Colletotrichum xanthorrhoeae | BRIP 45094 | Xanthorrhoea sp. | Australia | JX010261 | JX010448 | JX009927 | JX009478 | JX009653 |
| Fungal Species | Isolate Code | * Lesion Area (cm2) |
|---|---|---|
| C. truncatum | TM01 | 1.12 ± 0.17 ab |
| TM02 | 1.09 ± 0.07 ab | |
| TM03 | 1.25 ± 0.12 a | |
| TM04 | 1.21 ± 0.19 a | |
| TM05 | 1.07 ± 0.05 ab | |
| TM06 | 1.46 ± 0.17 a | |
| TM07 | 1.19 ± 0.11 a | |
| TM08 | 1.36 ± 0.21 a | |
| TM09 | 1.28 ± 0.07 a | |
| TM10 | 1.34 ± 0.11 a | |
| TM11 | 1.03 ± 0.13 ab | |
| TM12 | 1.15 ± 0.19 a | |
| TM13 | 1.27 ± 0.14 a | |
| TM14 | 1.04 ± 0.13 ab | |
| TM15 | 1.23 ± 0.21 a | |
| TM16 | 1.41 ± 0.09 a | |
| TM17 | 1.16 ± 0.11 a | |
| TM18 | 1.34 ± 0.19 a | |
| TM19 | 1.10 ± 0.05 ab | |
| TM20 | 1.19 ± 0.13 a | |
| TM21 | 1.26 ± 0.05 a | |
| TM22 | 1.07 ± 0.10 ab | |
| Control | 0 ± 0 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahriar, S.A.; Husna, A.; Paul, T.T.; Eaty, M.N.K.; Quamruzzaman, M.; Siddique, A.B.; Rahim, M.A.; Ahmmed, A.N.F.; Uddain, J.; Siddiquee, S. Colletotrichum truncatum Causing Anthracnose of Tomato (Solanum lycopersicum L.) in Malaysia. Microorganisms 2023, 11, 226. https://doi.org/10.3390/microorganisms11010226
Shahriar SA, Husna A, Paul TT, Eaty MNK, Quamruzzaman M, Siddique AB, Rahim MA, Ahmmed ANF, Uddain J, Siddiquee S. Colletotrichum truncatum Causing Anthracnose of Tomato (Solanum lycopersicum L.) in Malaysia. Microorganisms. 2023; 11(1):226. https://doi.org/10.3390/microorganisms11010226
Chicago/Turabian StyleShahriar, Saleh Ahmed, Asmaul Husna, Terna Tersoo Paul, Most. Nurjahan Khatun Eaty, Md Quamruzzaman, Abu Bakar Siddique, Md Abdur Rahim, Abu Noman Faruq Ahmmed, Jasim Uddain, and Shafiquzzaman Siddiquee. 2023. "Colletotrichum truncatum Causing Anthracnose of Tomato (Solanum lycopersicum L.) in Malaysia" Microorganisms 11, no. 1: 226. https://doi.org/10.3390/microorganisms11010226
APA StyleShahriar, S. A., Husna, A., Paul, T. T., Eaty, M. N. K., Quamruzzaman, M., Siddique, A. B., Rahim, M. A., Ahmmed, A. N. F., Uddain, J., & Siddiquee, S. (2023). Colletotrichum truncatum Causing Anthracnose of Tomato (Solanum lycopersicum L.) in Malaysia. Microorganisms, 11(1), 226. https://doi.org/10.3390/microorganisms11010226










