Epiphytic and Endophytic Fungi Colonizing Seeds of Two Poaceae Weed Species and Fusarium spp. Seed Degradation Potential In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Seed Collection
2.2. Isolation of Fungi
2.3. Identification of Fungi
2.4. Artificial Inoculation of Seeds
2.5. Statistical Analysis
3. Results
3.1. The Diversity of Epiphytic Seed-Borne Fungi Is Greater Than That of the Endophytic Seed-Borne Fungi
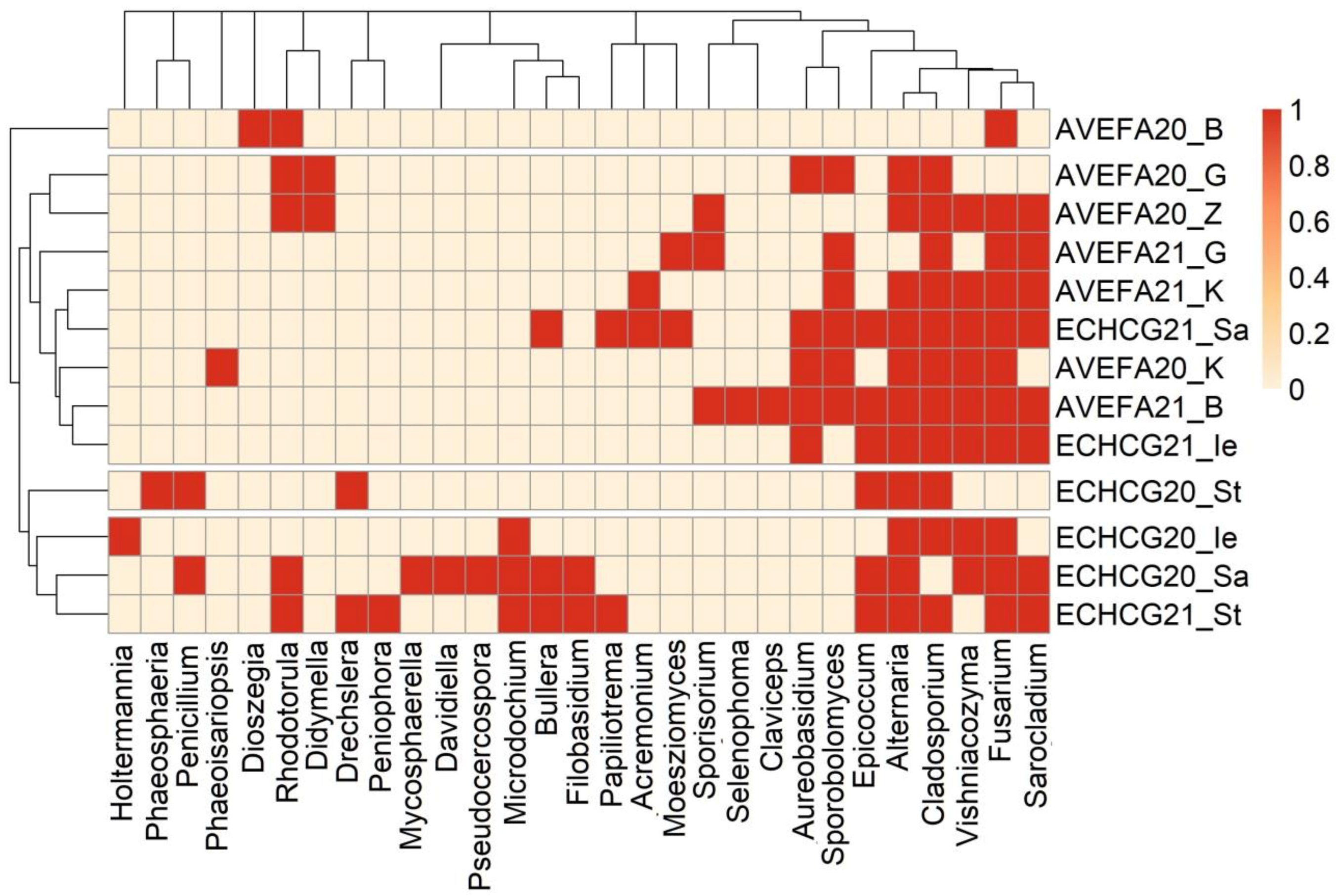
3.2. Taxonomic Composition of Seed-Borne Fungi Differs between the Two Species
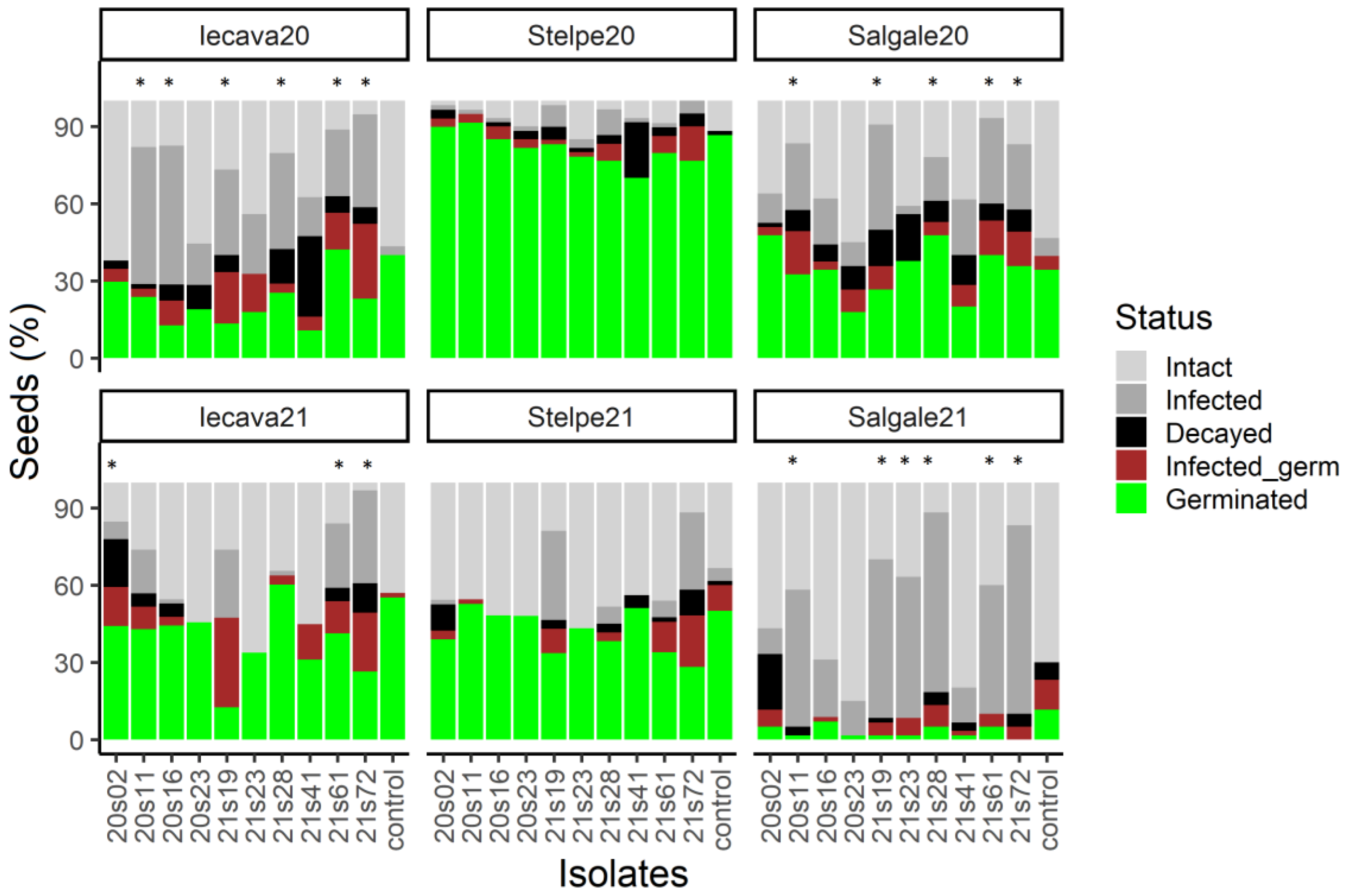
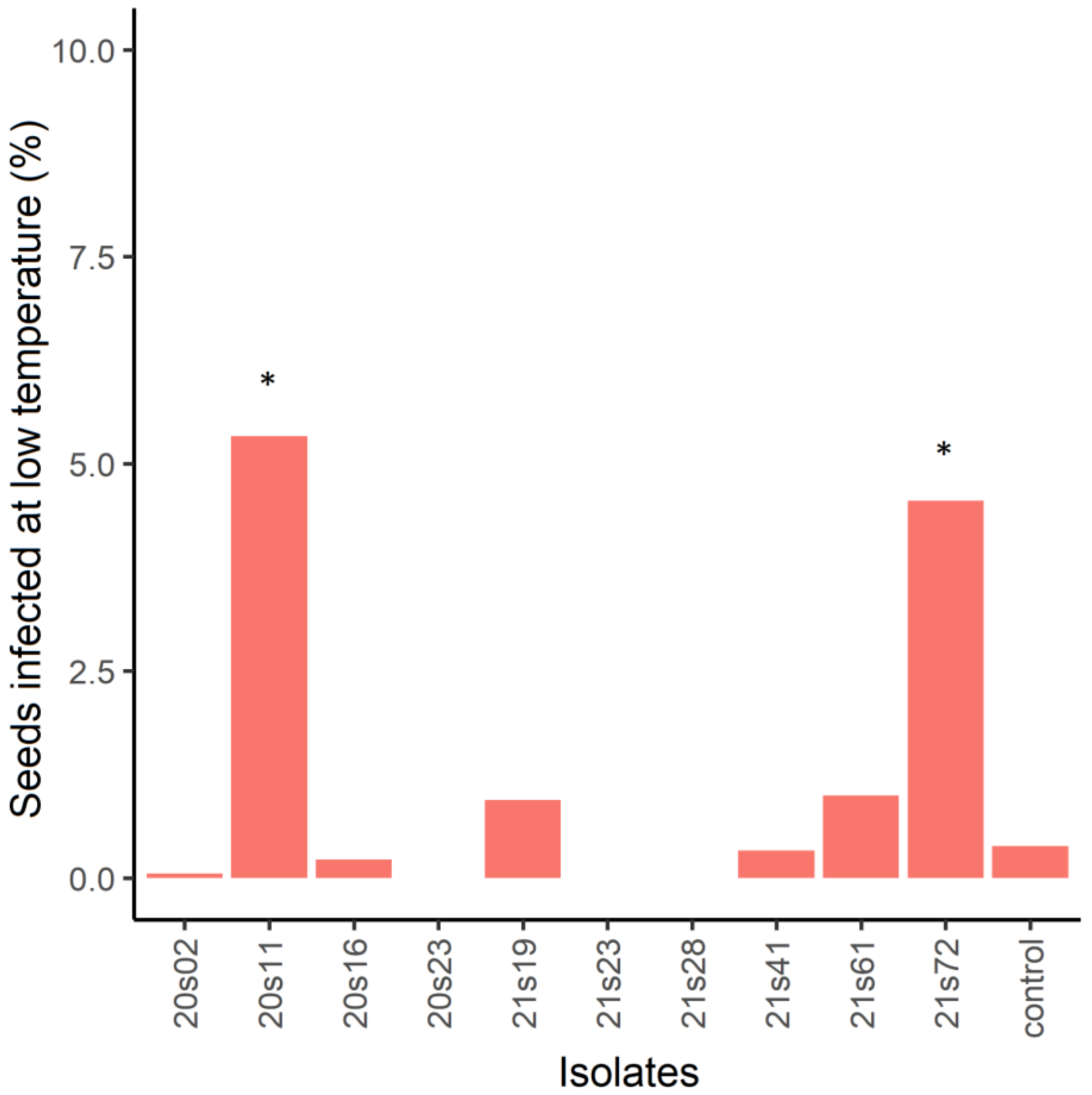
3.3. F. Sporotrichioides and F. Culmorum Had the Highest Potential to Reduce the Number of Intact E. Crus-Galli Seeds
4. Discussion
4.1. Taxonomic Composition of Seed Fungal Communities
4.2. Seed-Inoculation Experiment
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Kudsk, P. Advances in Integrated Weed Management; Burleigh Dodds Science Publishing: Cambridge, UK, 2022; pp. 1–452. [Google Scholar]
- Klaedtke, S.; Jacques, M.A.; Raggi, L.; Préveaux, A.; Bonneau, S.; Negri, V.; Chable, V.; Barret, M. Terroir is a key driver of seed-associated microbial assemblages. Environ. Microbiol. 2016, 18, 1792–1804. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, S.; de Cates, C.; Hodgson, J.; Morley, N.J.; Sutton, B.C.; Gange, A.C. Vertical transmission of fungal endophytes is widespread in forbs. Ecol. Evol. 2014, 4, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.B. The seed microbiome: Origins, interactions, and impacts. Plant Soil 2018, 422, 7–34. [Google Scholar] [CrossRef]
- Pollard, A.T. Seeds vs fungi: An enzymatic battle in the soil seedbank. Seed Sci. Res. 2018, 28, 197–214. [Google Scholar] [CrossRef]
- Dalling, J.W.; Davis, A.S.; Schutte, B.J.; Arnold, A.E. Seed survival in soil: Interacting effects of predation, dormancy and the soil microbial community. J. Ecol. 2011, 99, 89–95. [Google Scholar] [CrossRef]
- Davis, A.S.; Fu, X.; Schutte, B.J.; Berhow, M.A.; Dalling, J.W. Interspecific variation in persistence of buried weed seeds follows trade-offs among physiological, chemical, and physical seed defenses. Ecol. Evol. 2016, 6, 6836–6845. [Google Scholar] [CrossRef]
- Hendry, G.A.F.; Thompson, K.; Moss, C.J.; Edwards, E.; Thorpe, P.C. Seed persistence: A correlation between seed longevity in the soil and ortho-dihydroxyphenol concentration. Funct. Ecol. 1994, 8, 658–664. [Google Scholar] [CrossRef]
- Wagner, M.; Mitschunas, N. Fungal effects on seed bank persistence and potential applications in weed biocontrol: A review. Basic Appl. Ecol. 2008, 9, 191–203. [Google Scholar] [CrossRef]
- Gardarin, A.; Duerr, C.; Mannino, M.R.; Busset, H.; Colbach, N. Seed mortality in the soil is related to seed coat thickness. Seed Sci. Res. 2010, 20, 243–256. [Google Scholar] [CrossRef]
- Nolde, S.B.; Vassilevski, A.A.; Rogozhin, E.A.; Barinov, N.A.; Balashova, T.A.; Samsonova, O.V.; Baranov, Y.V.; Feofanov, A.V.; Egorov, T.A.; Arseniev, A.S.; et al. Disulfide-stabilized helical hairpin structure and activity of a novel antifungal peptide EcAMP1 from seeds of barnyard grass (Echinochloa crus-galli). J. Biol. Chem. 2011, 286, 25145–25153. [Google Scholar] [CrossRef] [PubMed]
- Hoefnagels, M.H.; Linderman, R.G. Biological suppression of seedborne Fusarium spp. during cold stratification of Douglas fir seeds. Plant Dis. 1999, 83, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Suárez, J.O.; Villada, D.; Oria de Rueda, J.A.; Alves-Santos, F.M.; Diez, J.J. Effects of Lactarius deliciosus and Rhizopogon roseolus ectomycorrhizal fungi on seeds and seedlings of Scots and stone pines inoculated with Fusarium oxysporum and Fusarium verticillioides. For. Chron. 2018, 94, 126–134. [Google Scholar] [CrossRef]
- Meyer, S.E.; Franke, J.L.; Baughman, O.W.; Beckstead, J.; Geary, B. Does Fusarium-caused seed mortality contribute to Bromus tectorum stand failure in the Great Basin? Weed Res. 2014, 54, 511–519. [Google Scholar] [CrossRef]
- De Luna, L.Z.; Kennedy, A.C.; Hansen, J.C.; Paulitz, T.C.; Gallagher, R.S.; Fuerst, E.P. Mycobiota on wild oat (Avena fatua L.) seed and their caryopsis decay potential. Plant Health Prog. 2011, 12, 20. [Google Scholar] [CrossRef]
- Mohler, C.L.; Dykeman, C.; Nelson, E.B.; Ditommaso, A. Reduction in weed seedling emergence by pathogens following the incorporation of green crop residue. Weed Res. 2012, 52, 467–477. [Google Scholar] [CrossRef]
- Garibaldi, A.; Gilardi, G.; Pasquali, M.; Kejji, S.; Gullino, M.L. Seed transmission of Fusarium oxysporum of Eruca vesicaria and Diplotaxis muralis. Z. Pflanzenk. Pflanzen.—J. Plant Dis. Protect. 2004, 4, 345–350. [Google Scholar]
- Masi, M.; Meyer, S.; Pescitelli, G.; Cimmino, A.; Clement, S.; Peacock, B.; Evidente, A. Phytotoxic activity against Bromus tectorum for secondary metabolites of a seed-pathogenic Fusarium strain belonging to the F. tricinctum species complex. Nat. Prod. Res. 2017, 31, 2768–2777. [Google Scholar] [CrossRef]
- Mancini, V.; Murolo, S.; Romanazzi, G. Diagnostic methods for detecting fungal pathogens on vegetable seeds. Plant Pathol. 2016, 65, 691–703. [Google Scholar] [CrossRef]
- Mortensen, K.; Hsiao, A.I. Fungal infestation of seeds from seven populations of wild oats (Avena fatua L.) with different dormancy and viability characteristics. Weed Res. 1987, 27, 297–304. [Google Scholar] [CrossRef]
- Donayre, D.K.M.; Dalisay, T.U.; Bayot, R.G.; Baltazar, A.M. Diversity and tissue specificity of endophytic fungi in barnyard grass (Echinochloa glabrescens Munro ex Hook. F.). Asia Life Sci. 2014, 23, 725–741. [Google Scholar]
- Heap, I. The International Herbicide-Resistant Weed Database. Available online: www.weedscience.org (accessed on 25 July 2022).
- Gallandt, E.R. How can we target the weed seedbank? Weed Sci. 2006, 54, 588–596. [Google Scholar] [CrossRef]
- Haring, S.C.; Flessner, M.L. Improving soil seed bank management. Pest Manag. Sci. 2018, 74, 2412–2418. [Google Scholar] [CrossRef]
- Maun, M.A.; Barrett, S.C.H. The biology of Canadian weeds. 77. Echinochloa crus-galli (L.) Beauv. Can. J. Plant Sci. 1986, 66, 739–759. [Google Scholar] [CrossRef]
- Collado, J.; Platas, G.; Paulus, B.; Bills, G.F. High-throughput culturing of fungi from plant litter by a dilution-to-extinction technique. FEMS Microbiol. Ecol. 2007, 60, 521–533. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols. A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Abarenkov, K.; Somervuo, P.; Nilsson, R.H.; Kirk, P.M.; Huotari, T.; Abrego, N.; Ovaskainen, O. Protax-fungi: A web-based tool for probabilistic taxonomic placement of fungal internal transcribed spacer sequences. New Phytol. 2018, 220, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Sampietro, D.A.; Marín, P.; Iglesias, J.; Presello, D.A.; Vattuone, M.A.; Catalán, C.A.N.; Jaen, M.G. A molecular based strategy for rapid diagnosis of toxigenic Fusarium species associated to cereal grains from Argentina. Fungal Biol. 2010, 114, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Purahong, W.; Alkadri, D.; Nipoti, P.; Pisi, A.; Lemmens, M.; Prodi, A. Validation of a modified Petri-dish test to quantify aggressiveness of Fusarium graminearum in durum wheat. Eur. J. Plant Pathol. 2012, 132, 381–391. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Package ‘Vegan’. Community Ecology Package; 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 19 December 2022).
- Kolde, R.; Kolde, M.R. Package ‘Pheatmap’. R Package 1; R Package, 2018. Available online: https://CRAN.R-project.org/package=pheatmap (accessed on 19 December 2022).
- Hothorn, T.; Bretz, F.; Westfall, P.; Heiberger, R.M.; Schuetzenmeister, A.; Scheibe, S.; Hothorn, M.T. Package ‘Multcomp’, Simultaneous Inference in General Parametric Models; Project for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Chen, H. VennDiagram: Generate High-Resolution Venn and Euler Plots, R package version 1.7.3; R package, 2022. Available online: https://CRAN.R-project.org/package=VennDiagram (accessed on 19 December 2022).
- Simonin, M.; Briand, M.; Chesneau, G.; Rochefort, A.; Marais, C.; Sarniguet, A.; Barret, M. Seed microbiota revealed by a large-scale meta-analysis including 50 plant species. New Phytol. 2022, 234, 1448–1463. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Kreutzer, B.; Graiss, W. Mycological investigations of seeds of cultivated grasses. Bodenkultur 2008, 59, 95–100. [Google Scholar]
- Mohammed, Y.M.; Badawy, M.E. Potential of phytopathogenic fungal isolates as a biocontrol agent against some weeds. Egypt. J. Biol. Pest Control 2020, 30, 92. [Google Scholar] [CrossRef]
- Mohammad, R.S.M. Evaluation of Alternaria alternata causing leaf spot of barnyardgrass grown in rice fields. Afr. J. Microbiol. Res. 2012, 6, 4481–4488. [Google Scholar]
- Hietaniemi, V.; Rämö, S.; Yli-Mattila, T.; Jestoi, M.; Peltonen, S.; Kartio, M.; Sieviläinen, E.; Koivisto, T.; Parikka, P. Updated survey of Fusarium species and toxins in Finnish cereal grains. Food Addit. Contam. A Chem. Anal. Control. Expo. Risk Assess 2016, 33, 831–848. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.W.; Okubara, P.A.; Sullivan, T.S.; Madden, B.J.; Johnson, K.L.; Charlesworth, M.C.; Fuerst, E.P. Proteome-wide response of dormant caryopses of the weed, Avena fatua, after colonization by a seed-decay isolate of Fusarium avenaceum. Phytopathology 2022, 112, 1103–1117. [Google Scholar] [CrossRef]
- Cobo-Diaz, J.F.; Legrand, F.; Le Floch, G.; Picot, A. Influence of maize residues in shaping soil microbiota and Fusarium spp. communities. Microb. Ecol. 2022, 83, 702–713. [Google Scholar] [CrossRef]
- Motlagh, M.R.S. Evaluation of Epicoccum purpurascens as biological control agent of Echinochloa spp. in rice fields. J. Food Agric. Environ. 2011, 9, 394–397. [Google Scholar]
- Giraldo, A.; Gené, J.; Sutton, D.A.; Madrid, H.; De Hoog, G.S.; Cano, J.; Decock, C.; Crow, P.W.; Guarro, J. Phylogeny of Sarocladium (Hypocreales). Pers. Mol. Phylogeny Evol. Fungi 2015, 34, 10–24. [Google Scholar] [CrossRef]
- Błaszczyk, L.; Waśkiewicz, A.; Gromadzka, K.; Mikołajczak, K.; Chełkowski, J. Sarocladium and Lecanicillium associated with maize seeds and their potential to form selected secondary metabolites. Biomolecules 2021, 11, 98. [Google Scholar] [CrossRef]
- Zalar, P.; Gostinčar, C.; De Hoog, G.S.; Uršič, V.; Sudhadham, M.; Gunde-Cimerman, N. Redefinition of Aureobasidium pullulans and its varieties. Stud. Mycol. 2008, 61, 21–38. [Google Scholar] [CrossRef]
- Prashanthi, S.K.; Kulkarni, S. Aureobasidium pullulans, a potential mycoherbicide for biocontrol of eupatorium [Chromolaena odorata (L.) King and Robinson] weed. Curr. Sci. 2005, 88, 18–21. [Google Scholar]
- Wachowska, U.; Głowacka, K. Antagonistic interactions between Aureobasidium pullulans and Fusarium culmorum, a fungal pathogen of winter wheat. Biocontrol 2014, 59, 635–645. [Google Scholar] [CrossRef]
- Al-Sadi, A.M. Bipolaris sorokiniana-induced black point, common root rot, and spot blotch diseases of wheat: A review. Front. Cell. Infect. Microbiol. 2021, 11, 584899. [Google Scholar] [CrossRef] [PubMed]
- Liljeroth, E.; Bryngelsson, T. Seed treatment of barley with Idriella bolleyi causes systemically enhanced defence against root and leaf infection by Bipolaris sorokiniana. Biocontrol. Sci. Technol. 2002, 12, 235–249. [Google Scholar] [CrossRef]
- Abdelhalim, M.; Brurberg, M.B.; Hofgaard, I.S.; Rognli, O.A.; Tronsmo, A.M. Pathogenicity, host specificity and genetic diversity in Norwegian isolates of Microdochium nivale and Microdochium majus. Eur. J. Plant Pathol. 2020, 156, 885–895. [Google Scholar] [CrossRef]
- Gavrilova, O.P.; Orina, A.S.; Kessenikh, E.D.; Gustyleva, L.K.; Savelieva, E.I.; Gogina, N.N.; Gagkaeva, T.Y. Diversity of physiological and biochemical characters of Microdochium fungi. Chem. Biodivers. 2020, 17, e2000294. [Google Scholar] [CrossRef]
- Zhang, W.; Krohn, K.; Draeger, S.; Schulz, B. Bioactive isocoumarins isolated from the endophytic fungus Microdochium bolleyi. J. Nat. Prod. 2008, 71, 1078–1081. [Google Scholar] [CrossRef] [PubMed]
- Kruse, J.; Doehlemann, G.; Kemen, E.; Thines, M. Asexual and sexual morphs of Moesziomyces revisited. IMA Fungus 2017, 8, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Triolet, M.; Edel-Hermann, V.; Gautheron, N.; Mondy, S.; Reibel, C.; André, O.; Guillemin, J.P.; Steinberg, C. Weeds harbor an impressive diversity of fungi, which offers possibilities for biocontrol. Appl. Environ. Microbiol. 2022, 88, e0217721. [Google Scholar] [CrossRef]
- Golubev, W.; Ikeda, R.; Shinoda, T.; Nakase, T. Antifungal activity of Bullera alba (Hanna) Derx. Mycoscience 1997, 38, 25–29. [Google Scholar] [CrossRef]
- Skadsen, R.W.; Hohn, T.M. Use of Fusarium graminearum transformed with gfp to follow infection patterns in barley and Arabidopsis. Physiol. Mol. Plant Pathol. 2004, 64, 45–53. [Google Scholar] [CrossRef]
- Gianinetti, A.; Finocchiaro, F.; Maisenti, F.; Satsap, D.K.; Morcia, C.; Ghizzoni, R.; Terzi, V. The caryopsis of red-grained rice has enhanced resistance to fungal attack. J. Fungi 2018, 4, 71. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Nan, Z.; Zhang, X.; Hou, F.; Christensen, M.; Baskin, C. Does dormancy protect seeds against attack by the pathogenic fungus Fusarium tricinctum in a semiarid grassland of Northwest China? Plant Soil 2018, 422, 155–168. [Google Scholar] [CrossRef]
- Müller, M.E.H.; Urban, K.; Köppen, R.; Siegel, D.; Korn, U.; Koch, M. Mycotoxins as antagonistic or supporting agents in the interaction between phytopathogenic Fusarium and Alternaria fungi. World Mycotoxin J. 2015, 8, 311–321. [Google Scholar] [CrossRef]


| Plant Species | Location Name | Coordinates | Date | Crop | ||
|---|---|---|---|---|---|---|
| 2020 | 2021 | 2020 | 2021 | |||
| A. fatua | Bebri | 56°44′ N 25°27′ E | 05.08. | 01.08. | Spring wheat | Spring wheat |
| A. fatua | Gaigalava | 56°43′ N 26°07′ E | 05.08. | 01.08. | Spring wheat | Spring wheat |
| A. fatua | Krimunas | 56°32′ N 23°27′ E | 13.08. | 04.08. | Peas/spring barley | Winter wheat |
| A. fatua | Ziras | 57°10′ N 21°35′ E | 12.08. | n/a | Spring barley | n/a |
| E. crus-galli | Iecava | 56°59′ N 24°25′ E | 05.09. | 23.08. | Zucchini | Maize |
| E. crus-galli | Salgale | 56°37′ N 23°58′ E | 01.09. | 25.08. | Spring oats | Potato |
| E. crus-galli | Stelpe | 56°52′ N 24°55′ E | 05.09. | 23.08. | Spring wheat | Spring wheat |
| Frequency of Occurrence | Fungal Genera/Species | ||||
|---|---|---|---|---|---|
| A. fatua | E. crus-galli | ||||
| Endosphere | Seed Surface | Endosphere | Seed Surface | Protax-Fungi | NCBI |
| 0.00 | 0.14 | 0.00 | 0.17 | Acremonium | Acremonium sclerotigenum |
| 0.43 | 0.43 | 0.83 | 0.83 | Alternaria | Alternaria |
| 0.00 | 0.43 | 0.00 | 0.33 | Aureobasidium pullulans | Aureobasidium melanogenum |
| 0.00 | 0.00 | 0.00 | 0.50 | Bullera alba | Bullera alba |
| 0.14 | 0.71 | 0.17 | 0.83 | Cladosporium | Cladosporium |
| 0.00 | 0.14 | 0.00 | 0.00 | Claviceps | Claviceps |
| 0.00 | 0.00 | 0.33 | 0.00 | Drechslera | Bipolaris sorokiniana |
| 0.00 | 0.00 | 0.17 | 0.00 | Davidiella | Davidiella |
| 0.29 | 0.00 | 0.00 | 0.00 | Didymella | Didymella pomorum |
| 0.00 | 0.14 | 0.00 | 0.00 | Dioszegia | Dioszegia hungarica |
| 0.14 | 0.00 | 0.83 | 0.17 | Epicoccum | Epicoccum nigrum |
| 0.00 | 0.00 | 0.00 | 0.33 | Filobasidium magnum/oeirense | Filobasidium magnum/oeirense |
| 0.71 | 0.43 | 0.50 | 0.83 | Fusarium | Fusarium |
| 0.00 | 0.00 | 0.00 | 0.17 | Holtermannia | Holtermanniella |
| 0.00 | 0.00 | 0.00 | 0.50 | Microdochium bolleyi | Microdochium bolleyi |
| 0.0 | 0.14 | 0.17 | 0.17 | Moeszyomyces bullatus | Moeszyomyces bullatus/Pseudozyma aphidis |
| 0.00 | 0.00 | 0.00 | 0.17 | Mycosphaerella | Mycosphaerella |
| 0.00 | 0.00 | 0.00 | 0.33 | Papiliotrema | Papiliotrema baii |
| 0.00 | 0.00 | 0.00 | 0.33 | Penicillium | Penicillium |
| 0.00 | 0.00 | 0.00 | 0.17 | Peniophora | Peniophora |
| 0.00 | 0.14 | 0.00 | 0.00 | Phaeoisariopsis | Cladosporium |
| 0.00 | 0.00 | 0.17 | 0.00 | Phaeosphaeria | Parastagonospora |
| 0.00 | 0.00 | 0.00 | 0.17 | Pseudocercospora | Cladosporium |
| 0.14 | 0.43 | 0.00 | 0.33 | Rhodotorula | Rhodotorula glutinis/mucilaginosa/babjevae |
| 0.00 | 0.00 | 0.00 | 0.17 | Sampaiozyma ingeniosa * | Rhodotorula ingeniosa |
| 0.14 | 0.57 | 0.33 | 0.50 | Sarocladium strictum | Sarocladium strictum |
| 0.00 | 0.14 | 0.00 | 0.00 | Selenophoma | Aureobasidium pullulans/subglaciale |
| 0.00 | 0.43 | 0.00 | 0.00 | Sporisorium | Sporisorium graminicola |
| 0.00 | 0.71 | 0.00 | 0.17 | Sporobolomyces | Sporobolomyces roseus/ruberrimus |
| 0.00 | 0.57 | 0.00 | 0.67 | Vishniacozyma tephrensis/unknown/carnescens | Vishniacozyma tephrensis/victoriae/carnescens |
| Isolates | Year of Isolation | Plant Species | Plant Population | Habitat | Species |
|---|---|---|---|---|---|
| 20s02 | 2020 | A. fatua | Bebri | Epiphyte | F. avenaceum |
| 20s11 | 2020 | A. fatua | Krimunas | Epiphyte | F. sporotrichioides |
| 20s16 | 2020 | E. crus-galli | Iecava | Epiphyte | Fusarium sp. |
| 20s23 | 2020 | E. crus-galli | Salgale | Epiphyte | Fusarium sp. |
| 21s19 | 2021 | E. crus-galli | Stelpe | Endophyte | F. culmorum |
| 21s23 | 2021 | E. crus-galli | Iecava | Endophyte | F. avenaceum |
| 21s28 | 2021 | E. crus-galli | Stelpe | Endophyte | F. avenaceum |
| 21s41 | 2021 | E. crus-galli | Stelpe | Epiphyte | F. avenaceum |
| 21s61 | 2021 | A. fatua | Gaigalava | Endophyte | F. sporotrichioides |
| 21s72 | 2021 | A. fatua | Bebri | Epiphyte | F. sporotrichioides |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ņečajeva, J.; Borodušķe, A.; Nikolajeva, V.; Seņkovs, M.; Kalniņa, I.; Roga, A.; Skinderskis, E.; Fridmanis, D. Epiphytic and Endophytic Fungi Colonizing Seeds of Two Poaceae Weed Species and Fusarium spp. Seed Degradation Potential In Vitro. Microorganisms 2023, 11, 184. https://doi.org/10.3390/microorganisms11010184
Ņečajeva J, Borodušķe A, Nikolajeva V, Seņkovs M, Kalniņa I, Roga A, Skinderskis E, Fridmanis D. Epiphytic and Endophytic Fungi Colonizing Seeds of Two Poaceae Weed Species and Fusarium spp. Seed Degradation Potential In Vitro. Microorganisms. 2023; 11(1):184. https://doi.org/10.3390/microorganisms11010184
Chicago/Turabian StyleŅečajeva, Jevgenija, Anete Borodušķe, Vizma Nikolajeva, Māris Seņkovs, Ineta Kalniņa, Ance Roga, Edmunds Skinderskis, and Dāvids Fridmanis. 2023. "Epiphytic and Endophytic Fungi Colonizing Seeds of Two Poaceae Weed Species and Fusarium spp. Seed Degradation Potential In Vitro" Microorganisms 11, no. 1: 184. https://doi.org/10.3390/microorganisms11010184
APA StyleŅečajeva, J., Borodušķe, A., Nikolajeva, V., Seņkovs, M., Kalniņa, I., Roga, A., Skinderskis, E., & Fridmanis, D. (2023). Epiphytic and Endophytic Fungi Colonizing Seeds of Two Poaceae Weed Species and Fusarium spp. Seed Degradation Potential In Vitro. Microorganisms, 11(1), 184. https://doi.org/10.3390/microorganisms11010184






