Antibacterial Activity of a Natural Clay Mineral against Burkholderia cepacia Complex and Other Bacterial Pathogens Isolated from People with Cystic Fibrosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Clay Mineral Sample
2.3. Antimicrobial Susceptibility Assay
2.4. Preparation of Aqueous Suspensions and Leachate of Clay
2.5. Antibacterial Assay with Clay Suspensions or Aqueous Leachates
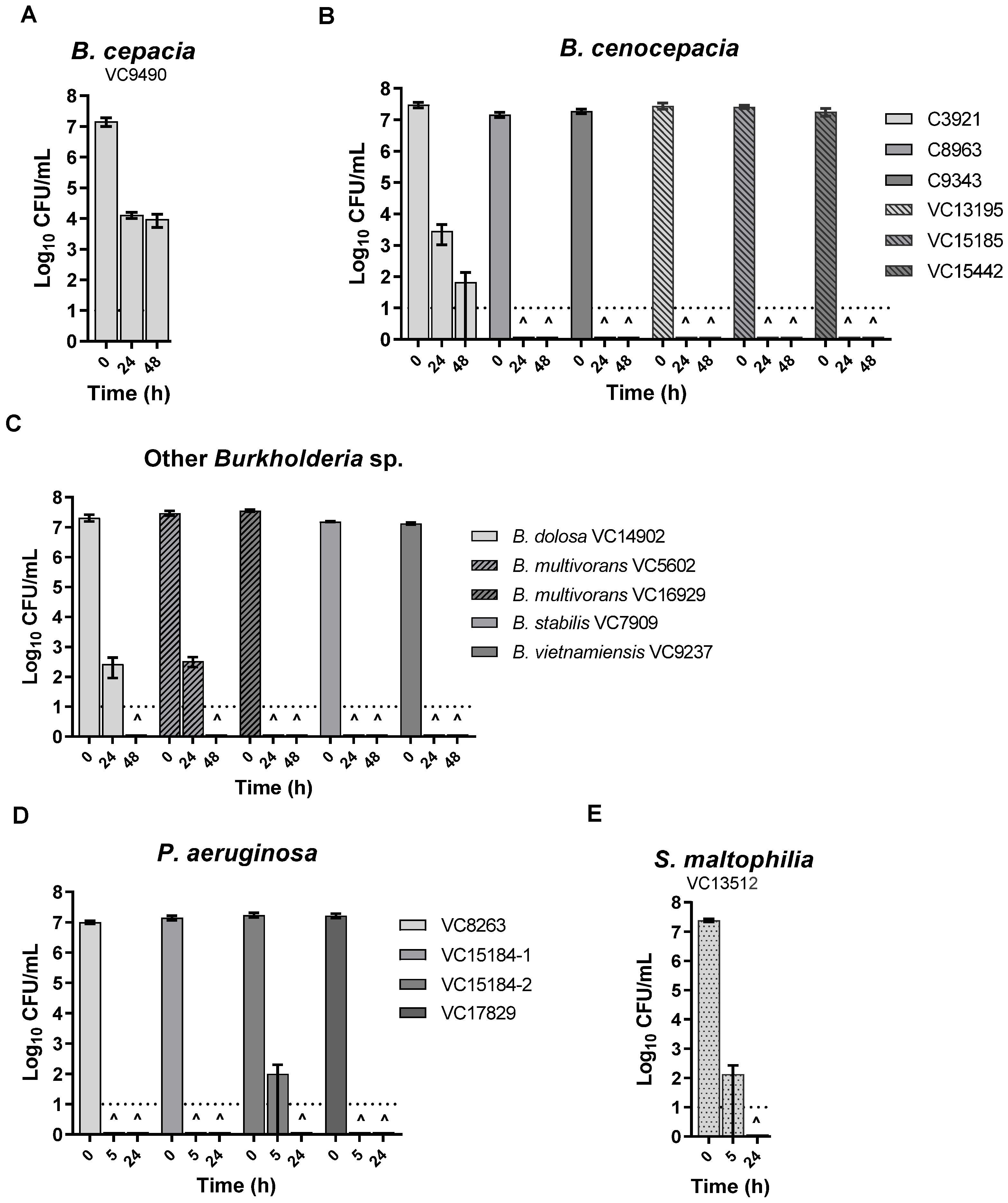
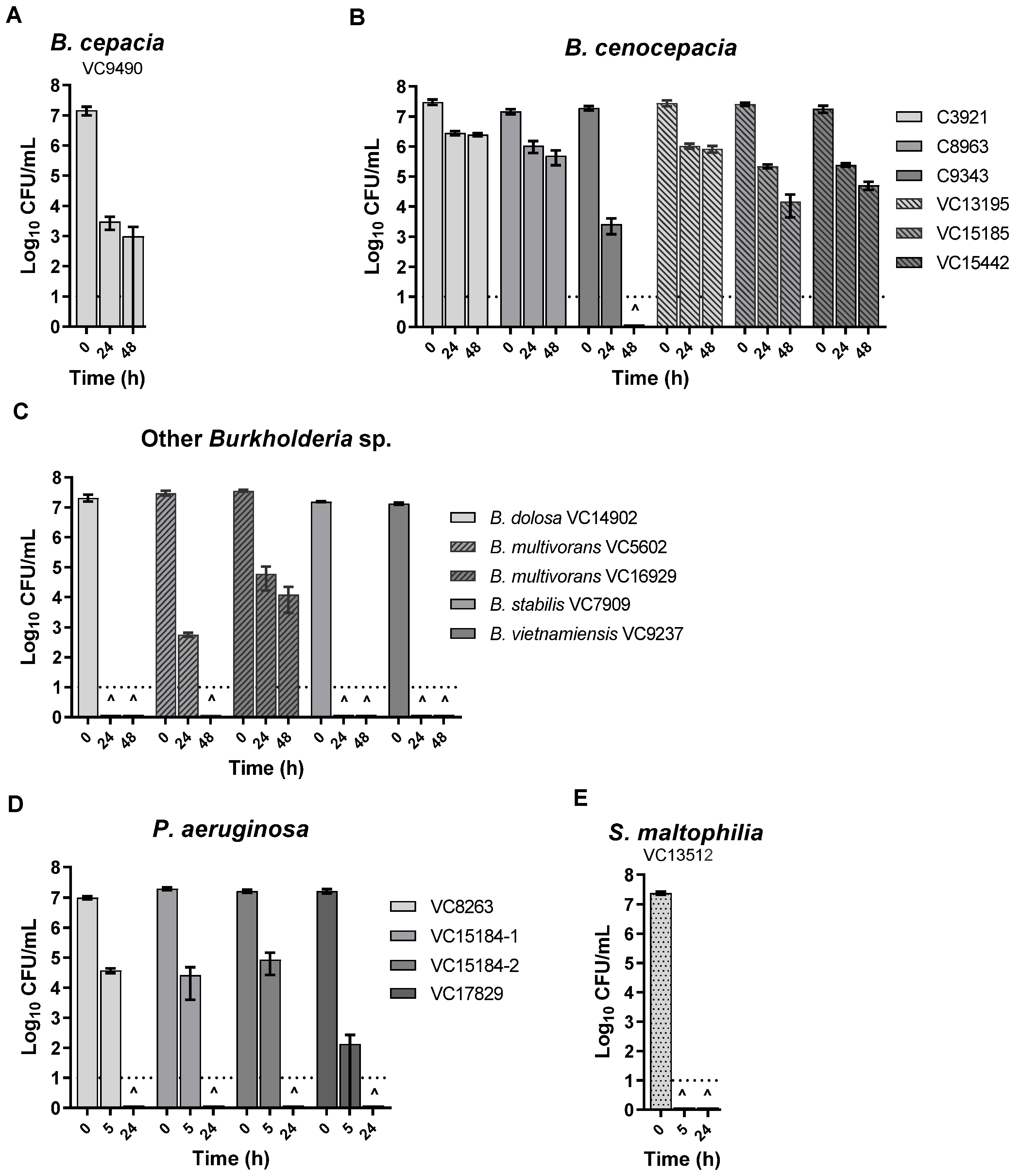
3. Results
3.1. All the CF Isolates Exhibited Extensively Drug-Resistant (XDR) or MDR Profiles
3.2. KC Aqueous Suspensions and Leachate Showed Potent Antibacterial Activity against All the Isolates
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grant, S.S.; Hung, D.T. Persistent bacterial infections, antibiotic tolerance, and the oxidative stress response. Virulence 2013, 4, 273–283. [Google Scholar] [CrossRef]
- Exner, M.; Bhattacharya, S.; Christiansen, B.; Gebel, J.; Goroncy-Bermes, P.; Hartemann, P.; Heeg, P.; Ilschner, C.; Kramer, A.; Larson, E.; et al. Antibiotic resistance: What is so special about multidrug-resistant Gram-negative bacteria? GMS Hyg. Infect. Control 2017, 12, Doc05. [Google Scholar] [CrossRef] [PubMed]
- Goetz, D.; Ren, C.L. Review of Cystic Fibrosis. Pediatr. Ann. 2019, 48, e154–e161. [Google Scholar] [CrossRef] [PubMed]
- Ratjen, F.; Bell, S.C.; Rowe, S.M.; Goss, C.H.; Quittner, A.L.; Bush, A. Cystic fibrosis. Nat. Rev. Dis. Prim. 2015, 1, 15010. [Google Scholar] [CrossRef] [PubMed]
- Lyczak, J.B.; Cannon, C.L.; Pier, G.B. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 2002, 15, 194–222. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.L.; Burns, J.L.; Ramsey, B.W. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2003, 168, 918–951. [Google Scholar] [CrossRef] [PubMed]
- Andersen, D.H. Cystic fibrosis of the pancreas and its relation to celiac disease. Am. J. Dis. Child. 1938, 56, 344–399. [Google Scholar] [CrossRef]
- Kerem, B.S.; Zielenski, J.; Markiewicz, D.; Bozon, D.; Gazit, E.; Yahav, J.; Kennedy, D.; Riordan, J.R.; Collins, F.S.; Rommens, J.M. Identification of mutations in regions corresponding to the two putative nucleotide (ATP)-binding folds of the cystic fibrosis gene. Proc. Natl. Acad. Sci. USA 1990, 87, 8447–8451. [Google Scholar] [CrossRef]
- Spoonhower, K.A.; Davis, P.B. Epidemiology of Cystic Fibrosis. Clin. Chest Med. 2016, 37, 1–8. [Google Scholar] [CrossRef]
- Scotet, V.; L’Hostis, C.; Férec, C. The Changing Epidemiology of Cystic Fibrosis: Incidence, Survival and Impact of the CFTR Gene Discovery. Genes 2020, 11, 589. [Google Scholar] [CrossRef]
- De Boeck, K. Cystic fibrosis in the year 2020: A disease with a new face. Acta Paediatr. 2020, 109, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Shen, Y.; Zheng, J. A review of cystic fibrosis: Basic and clinical aspects. Anim. Model. Exp. Med. 2021, 4, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Cutting, G.R. Cystic fibrosis genetics: From molecular understanding to clinical application. Nat. Rev. Genet. 2015, 16, 45–56. [Google Scholar] [CrossRef]
- Regard, L.; Martin, C.; Chassagnon, G.; Burgel, P.R. Acute and chronic non-pulmonary complications in adults with cystic fibrosis. Expert Rev. Respir. Med. 2019, 13, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Garcia, B.; Flume, P.A. Pulmonary Complications of Cystic Fibrosis. Semin. Respir. Crit. Care Med. 2019, 40, 804–809. [Google Scholar] [CrossRef]
- Filkins, L.M.; O’Toole, G.A. Cystic Fibrosis Lung Infections: Polymicrobial, Complex, and Hard to Treat. PLoS Pathog. 2015, 11, e1005258. [Google Scholar] [CrossRef]
- Davies, J.C. Pseudomonas aeruginosa in cystic fibrosis: Pathogenesis and persistence. Paediatr. Resp. Rev. 2002, 3, 128–134. [Google Scholar] [CrossRef]
- Lipuma, J.J. The changing microbial epidemiology in cystic fibrosis. Clin. Microbiol. Rev. 2010, 23, 299–323. [Google Scholar] [CrossRef]
- Leitão, J.H.; Sousa, S.A.; Ferreira, A.S.; Ramos, C.G.; Silva, I.N.; Moreira, L.M. Pathogenicity, virulence factors, and strategies to fight against Burkholderia cepacia complex pathogens and related species. Appl. Microbiol. Biotechnol. 2010, 87, 31–40. [Google Scholar] [CrossRef]
- Sfeir, M.M. Burkholderia cepacia complex infections: More complex than the bacterium name suggest. J. Infect. 2018, 77, 166–170. [Google Scholar] [CrossRef]
- Ragupathi, N.K.D.; Veeraraghavan, B. Accurate identification and epidemiological characterization of Burkholderia cepacia complex: An update. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 7. [Google Scholar] [CrossRef]
- Jin, Y.; Zhou, J.; Zhou, J.; Hu, M.; Zhang, Q.; Kong, N.; Ren, H.; Liang, L.; Yue, J. Genome-based classification of Burkholderia cepacia complex provides new insight into its taxonomic status. Biol. Direct 2020, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Laraya-Cuasay, L.; Lipstein, M.; Huang, N. Pseudomonas cepacia in the respiratory flora of patients with cystic fibrosis (CF). Pediatr. Res. 1977, 11, 502. [Google Scholar] [CrossRef]
- Vandamme, P.; Holmes, B.; Vancanneyt, M.; Coenye, T.; Hoste, B.; Coopman, R.; Revets, H.; Lauwers, S.; Gillis, M.; Kersters, K.; et al. Occurrence of multiple genomovars of Burkholderia cepacia in cystic fibrosis patients and proposal of Burkholderia multivorans sp. nov. Int. J. Syst. Evol. Bacteriol. 1997, 47, 1188–1200. [Google Scholar] [CrossRef]
- Tablan, O.C.; Chorba, T.L.; Schidlow, D.V.; White, J.W.; Hardy, K.A.; Gilligan, P.H.; Morgan, W.M.; Carson, L.A.; Martone, W.J.; Jason, J.M. Pseudomonas cepacia colonization in patients with cystic fibrosis: Risk factors and clinical outcome. J. Pediatr. 1985, 107, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Eberl, L.; Vandamme, P. Members of the genus Burkholderia: Good and bad guy. F1000Research 2016, 5, 1007. [Google Scholar] [CrossRef] [PubMed]
- Lipuma, J.J. Update on the Burkholderia cepacia complex. Curr. Opin. Pulm. Med. 2005, 11, 528–533. [Google Scholar] [CrossRef]
- Mahenthiralingam, E.; Urban, T.A.; Goldberg, J.B. The multifarious, multireplicon Burkholderia cepacia complex. Nat. Rev. Microbiol. 2005, 3, 144–156. [Google Scholar] [CrossRef]
- Speert, D.P. Advances in Burkholderia cepacia complex. Paediatr. Respir. Rev. 2002, 3, 230–235. [Google Scholar] [CrossRef]
- Leitão, J.H.; Feliciano, J.R.; Sousa, S.A.; Guerreiro, T.P.A.I. Burkholderia cepacia Complex Infections Among Cystic Fibrosis Patients: Perspectives and Challenges. In Progress in Understanding Cystic Fibrosis; IntechOpen: London, UK, 2017; pp. 73–99. [Google Scholar] [CrossRef]
- LiPuma, J.J. Burkholderia cepacia. Management issues and new insights. Clin. Chest Med. 1998, 19, 473–486. [Google Scholar] [CrossRef]
- Scoffone, V.C.; Chiarelli, L.R.; Trespidi, G.; Mentasti, M.; Riccardi, G.; Buroni, S. Burkholderia cenocepacia Infections in Cystic Fibrosis Patients: Drug Resistance and Therapeutic Approaches. Front. Microbiol. 2017, 8, 1592. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.C.; Mall, M.A.; Gutierrez, H.; Macek, M.; Madge, S.; Davies, J.C.; Burgel, P.R.; Tullis, E.; Castaños, C.; Castellani, C.; et al. The future of cystic fibrosis care: A global perspective. Lancet Respir. Med. 2020, 8, 65–124. [Google Scholar] [CrossRef]
- Hirche, T.O.; Knoop, C.; Hebestreit, H.; Shimmin, D.; Solé, A.; Elborn, J.S.; Ellemunter, H.; Aurora, P.; Hogardt, M.; Wagner, T.O.; et al. Practical guidelines: Lung transplantation in patients with cystic fibrosis. Pulm. Med. 2014, 2014, 621342. [Google Scholar] [CrossRef]
- De Soyza, A.; McDowell, A.; Archer, L.; Dark, J.H.; Elborn, S.J.; Mahenthiralingam, E.; Gould, K.; Corris, P.A. Burkholderia cepacia complex genomovars and pulmonary transplantation outcomes in patients with cystic fibrosis. Lancet 2001, 358, 1780–1781. [Google Scholar] [CrossRef]
- Mahenthiralingam, E.; Baldwin, A.; Dowson, C.G. Burkholderia cepacia complex bacteria: Opportunistic pathogens with important natural biology. J. Appl. Microbiol. 2008, 104, 1539–1551. [Google Scholar] [CrossRef]
- Leitão, J.H.; Sousa, S.A.; Cunha, M.V.; Salgado, M.J.; Melo-Cristino, J.; Barreto, M.C.; Sá-Correia, I. Variation of the antimicrobial susceptibility profiles of Burkholderia cepacia complex clonal isolates obtained from chronically infected cystic fibrosis patients: A five-year survey in the major Portuguese treatment center. Eur. J. Clin. Microbiol. Infect. Dis. 2008, 27, 1101–1111. [Google Scholar] [CrossRef]
- Rhodes, K.A.; Schweizer, H.P. Antibiotic resistance in Burkholderia species. Drug Resist. Update 2016, 28, 82–90. [Google Scholar] [CrossRef]
- Lord, R.; Jones, A.M.; Horsley, A. Antibiotic treatment for Burkholderia cepacia complex in people with cystic fibrosis experiencing a pulmonary exacerbation. Cochrane Database Syst. Rev. 2020, 4, CD009529. [Google Scholar] [CrossRef]
- Regan, K.H.; Bhatt, J. Eradication therapy for Burkholderia cepacia complex in people with cystic fibrosis. Cochrane Database Syst. Rev. 2019, 4, CD009876. [Google Scholar] [CrossRef]
- Zlosnik, J.E.; Zhou, G.; Brant, R.; Henry, D.A.; Hird, T.J.; Mahenthiralingam, E.; Chilvers, M.A.; Wilcox, P.; Speert, D.P. Burkholderia species infections in patients with cystic fibrosis in British Columbia, Canada. 30 years’ experience. Ann. Am. Thorac. Soc. 2015, 12, 70–78. [Google Scholar] [CrossRef]
- Conway, B.A.; Venu, V.; Speert, D.P. Biofilm formation and acyl homoserine lactone production in the Burkholderia cepacia complex. J. Bacteriol. 2002, 184, 5678–5685. [Google Scholar] [CrossRef]
- Davies, J.C.; Bilton, D. Bugs, biofilms, and resistance in cystic fibrosis. Respir. Care 2009, 54, 628–640. [Google Scholar] [CrossRef]
- Ciofu, O.; Tolker-Nielsen, T.; Jensen, P.Ø.; Wang, H.; Høiby, N. Antimicrobial resistance, respiratory tract infections and role of biofilms in lung infections in cystic fibrosis patients. Adv. Drug Deliv. Rev. 2015, 85, 7–23. [Google Scholar] [CrossRef]
- Waters, V.; Smyth, A. Cystic fibrosis microbiology: Advances in antimicrobial therapy. J. Cyst. Fibros. 2015, 14, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Silver, L.L. Challenges of antibacterial discovery. Clin. Microbiol. Rev. 2011, 24, 71–109. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.E.; Behroozian, S. An ancient solution to a modern problem. Mol. Microbiol. 2020, 113, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Carretero, M.I. Clay minerals and their beneficial effect upon human health. A review. Appl. Clay Sci. 2002, 21, 155–163. [Google Scholar] [CrossRef]
- Williams, L.B.; Holland, M.; Eberl, D.D.; Brunet, T.; de Courrsou, L.B. Killer clays! Natural antibacterial clay minerals. Miner. Soc. Bull. 2004, 139, 3–8. [Google Scholar]
- Gomes, C.S.F.; Silva, J.B.P. Minerals and clay minerals in medical geology. Appl. Clay Sci. 2007, 36, 4–21. [Google Scholar] [CrossRef]
- Carretero, M.I.; Gomes, C.S.F.; Tateo, F. Clays and human health. In Handbook of Clay Science, Developments in Clay Science; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 1, pp. 717–741. ISBN 978-0-08-044183-2. [Google Scholar]
- Gomes, C. Healing and edible clays: A review of basic concepts, benefits and risks. Environ. Geochem. Health 2018, 40, 1739–1765. [Google Scholar] [CrossRef]
- Velde, B. Geology of clays. In Origin and Mineralogy of Clays, Clays and the Environment; Velde, B., Ed.; Springer: Berlin, Germany, 1995; pp. 1–7. [Google Scholar]
- Brigatti, M.F.; Galán, E.; Theng, B.K.G. Structure and Mineralogy of Clay Minerals. In Handbook of Clay Science, Developments in Clay Science; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 1, pp. 21–81. ISBN 978-0-08-044183-2. [Google Scholar]
- Uddin, F. Clays, nanoclays, and montmorillonite minerals. Met. Mater. Trans. A 2008, 39, 2804–2814. [Google Scholar] [CrossRef]
- World Health Organization. Draft Report of the 5th WHO Advisory Group Meeting on Buruli Ulcer; Study group report on Buruli Ulcer treatment with clay; World Health Organization: Geneva, Switzerland, 2002.
- Williams, L.B.; Haydel, S.E.; Giese, R.F.; Eberl, D.D. Chemical and mineralogical characteristics of French green clays used for healing. Clays Clay Miner. 2008, 56, 437–452. [Google Scholar] [CrossRef] [PubMed]
- Haydel, S.E.; Remenih, C.M.; Williams, L.B. Broad-spectrum in vitro antibacterial activities of clay minerals against antibiotic-susceptible and antibiotic-resistant bacterial pathogens. J. Antimicrob. Chemother. 2008, 61, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.B.; Metge, D.W.; Eberl, D.D.; Harvey, R.W.; Turner, A.G.; Prapaipong, P.; Poret-Peterson, A.T. What makes a natural clay antibacterial? Environ. Sci. Technol. 2011, 45, 3768–3773. [Google Scholar] [CrossRef]
- Mpuchane, S.F.; Ekosse, G.I.E.; Gashe, B.A.; Morobe, I.; Coetzee, S.H. Mineralogy of southern Africa medicinal and cosmetic clays and their effects on the growth of selected test microorganisms. Fresenius Environ. Bull. 2008, 17, 547–557. [Google Scholar] [CrossRef]
- Morrison, K.D.; Misra, R.; Williams, L.B. Unearthing the antibacterial mechanism of medicinal clay: A geochemical approach to combating antibiotic resistance. Sci. Rep. 2016, 6, 19043. [Google Scholar] [CrossRef] [PubMed]
- Hauser, E.A. Kisameet Bay Clay Deposit. In Problems of Clay and Laterite Genesis Symposium at Annual Meeting of the American Institute of Mining and Metallurgical Engineers, St. Louis, MO, USA, 19–22 February 1951; The American Institute of Mining and Metallurgical Engineers: St. Louis, MO, USA, 1952; pp. 178–190. [Google Scholar]
- Ure, W.; Harris, J.A. Curative properties of rare earths found in B.C. peloid deposits. Bull. Vanc. Med. Assoc. 1946, 22, 230–237. [Google Scholar] [PubMed]
- Svensson, S.L.; Behroozian, S.; Xu, W.; Surette, M.G.; Li, L.; Davies, J. Kisameet Glacial Clay: An Unexpected Source of Bacterial Diversity. mBio 2017, 8, e00590-17. [Google Scholar] [CrossRef] [PubMed]
- Behroozian, S. Antimicrobial Properties of Kisameet Clay, A Natural Clay Mineral from British Columbia, Canada. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2019. Available online: http://open.library.ubc.ca/collections/ubctheses/24/items/1.0380811 (accessed on 4 September 2019).
- Behroozian, S.; Svensson, S.L.; Li, L.Y.; Davies, J.E. Broad-Spectrum Antimicrobial and Antibiofilm Activity of a Natural Clay Mineral from British Columbia, Canada. mBio 2020, 11, e02350-20. [Google Scholar] [CrossRef]
- Behroozian, S.; Svensson, S.L.; Davies, J. Kisameet clay exhibits potent antibacterial activity against the ESKAPE pathogens. mBio 2016, 7, e01842-15. [Google Scholar] [CrossRef]
- Speert, D.P.; Campbell, M.E.; Henry, D.A.; Milner, R.; Taha, F.; Gravelle, A.; Davidson, A.G.; Wong, L.T.; Mahenthiralingam, E. Epidemiology of Pseudomonas aeruginosa in cystic fibrosis in British Columbia, Canada. Am. J. Respir. Crit. Care Med. 2002, 166, 988–993. [Google Scholar] [CrossRef]
- Speert, D.P.; Henry, D.; Vandamme, P.; Corey, M.; Mahenthiralingam, E. Epidemiology of Burkholderia cepacia complex in patients with cystic fibrosis, Canada. Emerg. Infect. Dis. 2002, 8, 181–187. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Hudzicki, J. Kirby-Bauer Disk Diffusion Susceptibility Test Protocol. American Society for Microbiology. 2009. Available online: https://asm.org/Protocols/Kirby-Bauer-Disk-Diffusion-Susceptibility-Test-Pro. (accessed on 19 June 2016).
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Miller, R.R.; Hird, T.J.; Tang, P.; Zlosnik, J.E. Whole-Genome Sequencing of Three Clonal Clinical Isolates of B. cenocepacia from a Patient with Cystic Fibrosis. PLoS ONE 2015, 10, e0143472. [Google Scholar] [CrossRef] [PubMed]
- Zlosnik, J.E.; Speert, D.P. The role of mucoidy in virulence of bacteria from the Burkholderia cepacia complex: A systematic proteomic and transcriptomic analysis. J. Infect. Dis. 2010, 202, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.N.; Santos, P.M.; Santos, M.R.; Zlosnik, J.E.; Speert, D.P.; Buskirk, S.W.; Bruger, E.L.; Waters, C.M.; Cooper, V.S.; Moreira, L.M. Long-Term Evolution of Burkholderia multivorans during a Chronic Cystic Fibrosis Infection Reveals Shifting Forces of Selection. mSystems 2016, 1, e00029-16. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.; Beaudoin, T.; Yau, Y.C.; Caraher, E.; Zlosnik, J.E.; Speert, D.P.; LiPuma, J.J.; Tullis, E.; Waters, V. Activity of Tobramycin against Cystic Fibrosis Isolates of Burkholderia cepacia Complex Grown as Biofilms. Antimicrob. Agents Chemother. 2016, 60, 348–355. [Google Scholar] [CrossRef]
- Veyssier, P.; Bryskier, A. Aminocyclitol aminoglycosides. In Antimicrobial Agents; Bryskier, A., Ed.; ASM Press: Washington, DC, USA, 2005; pp. 453–469. [Google Scholar]
- Ramirez, M.S.; Tolmasky, M.E. Aminoglycoside modifying enzymes. Drug Resist. Update 2010, 13, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Van Dalem, A.; Herpol, M.; Echahidi, F.; Peeters, C.; Wybo, I.; De Wachter, E.; Vandamme, P.; Piérard, D. In Vitro Susceptibility of Burkholderia cepacia Complex Isolated from Cystic Fibrosis Patients to Ceftazidime-Avibactam and Ceftolozane-Tazobactam. Antimicrob. Agents Chemother. 2018, 62, e00590-18. [Google Scholar] [CrossRef]
- Zeiser, E.T.; Becka, S.A.; Wilson, B.M.; Barnes, M.D.; LiPuma, J.J.; Papp-Wallace, K.M. “Switching Partners”: Piperacillin-Avibactam Is a Highly Potent Combination against Multidrug-Resistant Burkholderia cepacia Complex and Burkholderia gladioli Cystic Fibrosis Isolates. J. Clin. Microbiol. 2019, 57, e00181-19. [Google Scholar] [CrossRef] [PubMed]
- El-Halfawy, O.M.; Naguib, M.M.; Valvano, M.A. Novel antibiotic combinations proposed for treatment of Burkholderia cepacia complex infections. Antimicrob. Resist. Infect. Control 2017, 6, 120. [Google Scholar] [CrossRef]
- Vasireddy, L.; Bingle, L.; Davies, M.S. Antimicrobial activity of essential oils against multidrug-resistant clinical isolates of the Burkholderia cepacia complex. PLoS ONE 2018, 13, e0201835. [Google Scholar] [CrossRef]
- Maida, I.; Lo Nostro, A.; Pesavento, G.; Barnabei, M.; Calonico, C.; Perrin, E.; Chiellini, C.; Fondi, M.; Mengoni, A.; Maggini, V.; et al. Exploring the Anti-Burkholderia cepacia Complex Activity of Essential Oils: A Preliminary Analysis. Evid.-Based Complement. Altern. Med. 2014, 2014, 573518. [Google Scholar] [CrossRef] [PubMed]
- Waters, V.; Yau, Y.; Beaudoin, T.; Wettlaufer, J.; Tom, S.K.; McDonald, N.; Rizvi, L.; Klingel, M.; Ratjen, F.; Tullis, E. Pilot trial of tobramycin inhalation powder in cystic fibrosis patients with chronic Burkholderia cepacia complex infection. J. Cyst. Fibros. 2017, 16, 492–495. [Google Scholar] [CrossRef]
- Nichols, D.P.; Durmowicz, A.G.; Field, A.; Flume, P.A.; VanDevanter, D.R.; Mayer-Hamblett, N. Developing Inhaled Antibiotics in Cystic Fibrosis: Current Challenges and Opportunities. Ann. Am. Thorac. Soc. 2019, 16, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Semler, D.D.; Goudie, A.D.; Finlay, W.H.; Dennis, J.J. Aerosol phage therapy efficacy in Burkholderia cepacia complex respiratory infections. Antimicrob. Agents Chemother. 2014, 58, 4005–4013. [Google Scholar] [CrossRef] [PubMed]
- Pradenas, G.A.; Ross, B.N.; Torres, A.G. Burkholderia cepacia Complex Vaccines: Where Do We Go from here? Vaccines 2016, 4, 10. [Google Scholar] [CrossRef]
- Le Moigne, V.; Gaillard, J.L.; Herrmann, J.L. Vaccine strategies against bacterial pathogens in cystic fibrosis patients. Med. Mal. Infect. 2016, 46, 4–9. [Google Scholar] [CrossRef]
- Drevinek, P.; Mahenthiralingam, E. Burkholderia cenocepacia in cystic fibrosis: Epidemiology and molecular mechanisms of virulence. Clin. Microbiol. Infect. 2010, 16, 821–830. [Google Scholar] [CrossRef]
- Limmathurotsakul, D.; Golding, N.; Dance, D.A.; Messina, J.P.; Pigott, D.M.; Moyes, C.L.; Rolim, D.B.; Bertherat, E.; Day, N.P.; Peacock, S.J.; et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat. Microbiol. 2016, 1, 15008. [Google Scholar] [CrossRef] [PubMed]
- Gassiep, I.; Armstrong, M.; Norton, R. Human Melioidosis. Clin. Microbiol. Rev. 2020, 33, e00006-19. [Google Scholar] [CrossRef] [PubMed]
- Nunvar, J.; Capek, V.; Fiser, K.; Fila, L.; Drevinek, P. What matters in chronic Burkholderia cenocepacia infection in cystic fibrosis: Insights from comparative genomics. PLoS Pathog. 2017, 13, e1006762. [Google Scholar] [CrossRef] [PubMed]
- Bish, D.L. Studies of Clays and Clay Minerals Using X-ray Powder Diffraction and the Rietveld Method. United States. 1993. Available online: http://www.osti.gov/servlets/purl/10192067 (accessed on 26 June 2015).
| Amikacin (10) | Gentamicin (30) | Kanamycin (30) | Neomycin (30) | Spectinomycin (100) * | Streptomycin (10) | Tobramycin (10) | Ertapenem (10) | Imipenem (10) | Meropenem (10) | Cephalothin (30) | Cephazolin (30) | Cefotetan (30) | Cefoxitin (30) | Ceftazidime (30) | Cefixime (5) | Cefpodoxime (10) | Ceftriaxone (30) | Cefotaxime (30) | Amoxicillin-clavulanic acid (30) | Ampicillin (10) | Piperacilin (100) | Colistin (10) | Polymyxin B (300) | Ciprofloxacin (5) | Levofloxacin (5) | Nalidixic acid (30) | Sulfamethoxazole- trimethoprim (25) | Sulfadiazine (250) | Trimethoprim (5) | Doxycycline (30) | Tetracycline (30) | Chloramphenicol (30) | Nitrofurantoin (300) | |||||
| No. | Isolate | Strain | Year | Source | Aminoglycosides | Carbapenems | 1st, 2nd, 3rd Generation Cephalosporins | Penicillins | Polypeptides | Quinolones | Sulfonamides | Tetracyclines | ||||||||||||||||||||||||||
| 1 | Burkholderia cepacia | VC9490 | 1999 | Sputum | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | |||||
| 2 | Burkholderia cenocepacia | C3921 a [73,74] | 1990 | Sputum | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | |||||
| 3 | Burkholderia cenocepacia | C8963 a [73,74] | 2000 | Resp e | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ||||||
| 4 | Burkholderia cenocepacia | C9343 a [73,74] | 2000 | Resp | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ||||||||
| 5 | Burkholderia cenocepacia | VC13195 b | 2006 | Resp | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | |
| 6 | Burkholderia cenocepacia | VC15185 b | 2010 | Resp | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| 7 | Burkholderia cenocepacia | VC15442 b | 2010 | Blood f | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | |
| 8 | Burkholderia dolosa | VC14902 | 2009 | Sputum | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ||||||||||||||
| 9 | Burkholderia multivorans | VC5602 c [75] | 1993 | Sputum | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ||||||||||||||||
| 10 | Burkholderia multivorans | VC16929 c | 2013 | Sputum | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | |||||||||||
| 11 | Burkholderia stabilis | VC7909 | 1993 | Sputum | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ||||||||
| 12 | Burkholderia vietnamiensis | VC9237 [76] | 1998 | Resp | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ||||||||||||||||||
| 13 | Pseudomonas aeruginosa | VC8263 [68] | 1997 | Resp | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ||||||||||||||||
| 14 | Pseudomonas aeruginosa | VC15184-1 d | 2010 | Resp | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | |||||||||||
| 15 | Pseudomonas aeruginosa | VC15184-2 d | 2010 | Resp | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | |||||||||||
| 16 | Pseudomonas aeruginosa | VC17829 [68] | 2015 | Sputum | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ||||||||||||
| 17 | Stenotrophomonas maltophilia | VC13512 | 2006 | Sputum | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behroozian, S.; Zlosnik, J.E.A.; Xu, W.; Li, L.Y.; Davies, J.E. Antibacterial Activity of a Natural Clay Mineral against Burkholderia cepacia Complex and Other Bacterial Pathogens Isolated from People with Cystic Fibrosis. Microorganisms 2023, 11, 150. https://doi.org/10.3390/microorganisms11010150
Behroozian S, Zlosnik JEA, Xu W, Li LY, Davies JE. Antibacterial Activity of a Natural Clay Mineral against Burkholderia cepacia Complex and Other Bacterial Pathogens Isolated from People with Cystic Fibrosis. Microorganisms. 2023; 11(1):150. https://doi.org/10.3390/microorganisms11010150
Chicago/Turabian StyleBehroozian, Shekooh, James E. A. Zlosnik, Wanjing Xu, Loretta Y. Li, and Julian E. Davies. 2023. "Antibacterial Activity of a Natural Clay Mineral against Burkholderia cepacia Complex and Other Bacterial Pathogens Isolated from People with Cystic Fibrosis" Microorganisms 11, no. 1: 150. https://doi.org/10.3390/microorganisms11010150
APA StyleBehroozian, S., Zlosnik, J. E. A., Xu, W., Li, L. Y., & Davies, J. E. (2023). Antibacterial Activity of a Natural Clay Mineral against Burkholderia cepacia Complex and Other Bacterial Pathogens Isolated from People with Cystic Fibrosis. Microorganisms, 11(1), 150. https://doi.org/10.3390/microorganisms11010150






