A Plasmid Carrying blaIMP-56 in Pseudomonas aeruginosa Belonging to a Novel Resistance Plasmid Family
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of P. aeruginosa PE52
2.2. Genome Sequencing
2.3. Plasmid Analysis
2.4. Plasmid Characterization
2.5. Comparative Analysis of Plasmids Obtained from GenBank and pPE52IMP
3. Results
3.1. Structural Features of the pPE52IMP Plasmid
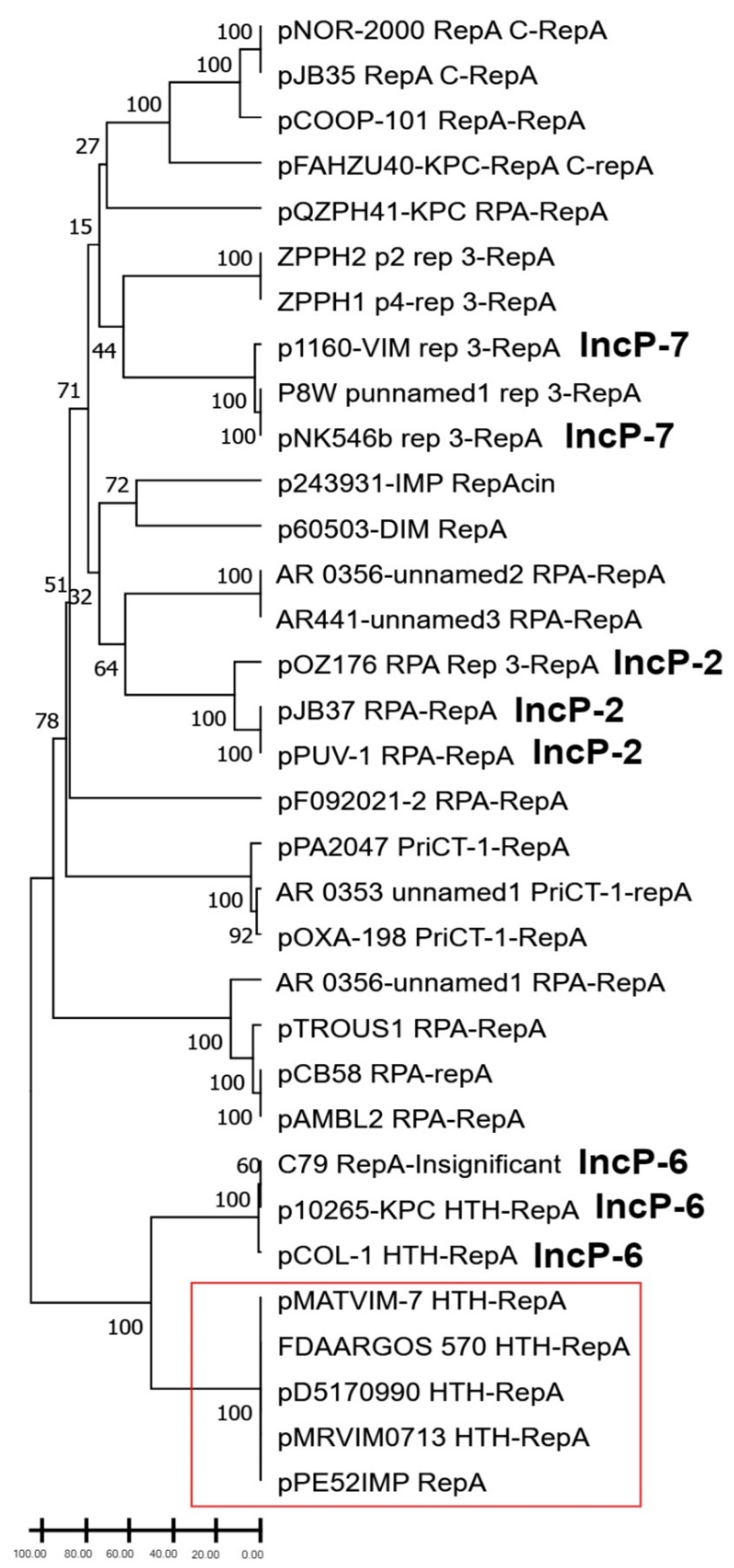
3.2. Phylogenetic Analysis of RepA
3.3. Comparative Analysis of pPE52IMP and Plasmids with Same RepA and Similar Structure
3.4. Plasmids with Similar Backbone as pPE52IMP Present in Other Bacterial Genera
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moradali, M.F.; Ghods, S.; Rehm, B.H.A. Pseudomonas aeruginosa lifestyle: A paradigm for adaptation, survival, and persistence. Front. Cell. Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.Y.; Wang, Q.; Sun, Q.L.; Chen, G.X.; Zhang, R. A novel plasmid carrying carbapenem-resistant gene blakpc-2 in Pseudomonas aeruginosa. Infect. Drug Resist. 2019, 12, 1285–1288. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Ruiz-Garbajosa, P.; Cantón, R. Epidemiology of antibiotic resistance in Pseudomonas aeruginosa. Implications for empiric and definitive therapy. Rev. Esp. Quimioter. 2017, 30 (Suppl. 1), 8–12. [Google Scholar] [PubMed]
- Botelho, J.; Grosso, F.; Peixe, L. Antibiotic resistance in Pseudomonas aeruginosa—Mechanisms, epidemiology and evolution. Drug Resist. Updat. 2019, 44, 100640. [Google Scholar] [CrossRef] [PubMed]
- Yoon, E.J.; Jeong, S.H. Mobile carbapenemase genes in Pseudomonas aeruginosa. Front. Microbiol. 2021, 12, 614058. [Google Scholar] [CrossRef]
- Naas, T.; Oueslati, S.; Bonnin, R.A.; Dabos, M.L.; Zavala, A.; Dortet, L.; Retailleau, P.; Iorga, B.I. Beta-lactamase database (BLDB)–structure and function. J. Enzyme Inhib. Med. Chem. 2017, 32, 917–919. [Google Scholar] [CrossRef]
- Hanson, N.D.; Hossain, A.; Buck, L.; Moland, E.S.; Thomson, K.S. First occurrence of a Pseudomonas aeruginosa isolate in the United States producing an IMP metallo-β-lactamase, IMP-18. Antimicrob. Agents Chemother. 2006, 50, 2272–2273. [Google Scholar] [CrossRef]
- Garza-Ramos, U.; Tinoco, P.; Silva-Sanchez, J.; Morfin-Otero, R.; Rodriguez-Noriega, E.; Leon-Garnica, G.; Sader, H.S.; Jones, R.N. Metallo-β-lactamase IMP-18 is located in a class 1 integron (In96) in a clinical isolate of Pseudomonas aeruginosa from Mexico. Int. J. Antimicrob. Agents 2008, 31, 78–80. [Google Scholar] [CrossRef]
- Sánchez-Martinez, G.; Garza-Ramos, U.J.; Reyna-Flores, F.L.; Gaytán-Martínez, J.; Lorenzo-Bautista, I.G.; Silva-Sanchez, J. In169, A New Class 1 Integron that Encoded blaIMP-18 in a Multidrug-Resistant Pseudomonas aeruginosa Isolate from Mexico. Arch. Med. Res. 2010, 41, 235–239. [Google Scholar] [CrossRef]
- Wolter, D.J.; Khalaf, N.; Robledo, I.E.; Vázquez, J.G.; Santé, I.M.; Aquino, E.E.; Goering, R.V.; Hanson, N.D. Surveillance of carbapenem-resistant Pseudomonas aeruginosa isolates from Puerto Rican Medical Center Hospitals: Dissemination of KPC and IMP-18 β-lactamases. Antimicrob. Agents Chemother. 2009, 53, 1660–1664. [Google Scholar] [CrossRef]
- Hocquet, D.; Plésiat, P.; Dehecq, B.; Mariotte, P.; Talon, D.; Bertrand, X. Nationwide investigation of extended-spectrum β-lactamases, metallo-β-lactamases, and extended-spectrum oxacillinases produced by ceftazidime-resistant Pseudomonas aeruginosa strains in France. Antimicrob. Agents Chemother. 2010, 54, 3512–3515. [Google Scholar] [CrossRef]
- Martínez, T.; Vázquez, G.J.; Aquino, E.E.; Ramírez-Ronda, R.; Robledo, I.E. First report of a Pseudomonas aeruginosa clinical isolate co-harbouring KPC-2 and IMP-18 carbapenemases. Int. J. Antimicrob. Agents 2012, 39, 542–543. [Google Scholar] [CrossRef]
- López-García, A.; del Carmen Rocha-Gracia, R.; Bello-López, E.; Juárez-Zelocualtecalt, C.; Sáenz, Y.; Castañeda-Lucio, M.; López-Pliego, L.; Cristina González-Vázquez, M.; Torres, C.; Ayala-Nuñez, T.; et al. Characterization of antimicrobial resistance mechanisms in carbapenem-resistant Pseudomonas aeruginosa carrying IMP variants recovered from a Mexican hospital. Infect Drug Resist 2018, 11, 1523–1536. [Google Scholar] [CrossRef]
- Zhao, W.H.; Hu, Z.Q. IMP-type metallo-β-lactamases in Gram-negative bacilli: Distribution, phylogeny, and association with integrons. Crit. Rev. Microbiol. 2011, 37, 214–226. [Google Scholar] [CrossRef]
- Hong, D.J.; Bae, I.K.; Jang, I.H.; Jeong, S.H.; Kang, H.K.; Lee, K. Epidemiology and characteristics of metallo-ß-lactamase-producing Pseudomonas aeruginosa. Infect Chemother. 2015, 47, 81–97. [Google Scholar] [CrossRef]
- Xiong, J.; Alexander, D.C.; Jennifer, H.M.; Dérasp, M.; Low, D.E.; Jamieson, F.B.; Roy, P.H. Complete sequence of pOZ176, a 500-kilobase IncP-2 plasmid encoding imp-9-mediated carbapenem resistance, from outbreak isolate Pseudomonas aeruginosa 96. Antimicrob. Agents Chemother. 2013, 57, 3775–3782. [Google Scholar] [CrossRef]
- San Millan, A.; Toll-Riera, M.; Escudero, J.A.; Cantón, R.; Coque, T.M.; Craig MacLean, R. Sequencing of plasmids pAMBL1 and pAMBL2 from Pseudomonas aeruginosa reveals a blaVIM-1 amplification causing high-level carbapenem resistance. J. Antimicrob. Chemother. 2015, 70, 3000–3003. [Google Scholar] [CrossRef]
- Feng, W.; Zhou, D.; Wang, Q.; Luo, W.; Zhang, D.; Sun, Q.; Tong, Y.; Chen, W.; Sun, F.; Xia, P. Dissemination of IMP-4-encoding pIMP-HZ1-related plasmids among Klebsiella pneumoniae and Pseudomonas aeruginosa in a Chinese teaching hospital. Sci. Rep. 2016, 6, 33419. [Google Scholar] [CrossRef]
- Botelho, J.; Grosso, F.; Peixe, L. Characterization of the pJB12 plasmid from Pseudomonas aeruginosa reveals Tn6352, a novel putative transposon associated with mobilization of the blaVIM-2-harboring In58 integron. Antimicrob. Agents Chemother. 2017, 61, e02532-16. [Google Scholar] [CrossRef]
- Elena, A.; Quinteros, M.; di Conza, J.; Gutkind, G.; Cejas, D.; Radice, M.A. Full characterization of an IncR plasmid harboring qnrS1 recovered from a VIM-11-producing Pseudomonas aeruginosa. Rev. Argent. Microbiol. 2020, 52, 298–304. [Google Scholar] [CrossRef]
- Dai, X.; Zhou, D.; Xiong, W.; Feng, J.; Luo, W.; Luo, G.; Wang, H.; Sun, F.; Zhou, X. The IncP-6 plasmid p10265-KPC from Pseudomonas aeruginosa carries a novel ΔISEc33-associated blaKPC-2 gene cluster. Front. Microbiol. 2016, 7, 310. [Google Scholar] [CrossRef]
- Yuan, M.; Guan, H.; Sha, D.; Cao, W.; Song, X.; Che, J.; Kan, B.; Li, J. Characterization of blaKPC-2-carrying plasmid pR31-kpc from a Pseudomonas aeruginosa strain isolated in china. Antibiotics 2021, 10, 1234. [Google Scholar] [CrossRef]
- Shintani, M.; Sanchez, Z.K.; Kimbara, K. Genomics of microbial plasmids: Classification and identification based on replication and transfer systems and host taxonomy. Front. Microbiol. 2015, 6, 242. [Google Scholar] [CrossRef]
- Carattoli, A.; Bertini, A.; Villa, L.; Falbo, V.; Hopkins, K.L.; Threlfall, E.J. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 2005, 63, 219–228. [Google Scholar] [CrossRef]
- Bertini, A.; Poirel, L.; Mugnier, P.D.; Villa, L.; Nordmann, P.; Carattoli, A. Characterization and PCR-based replicon typing of resistance plasmids in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2010, 54, 4168–4177. [Google Scholar] [CrossRef]
- Alvarado, A.; Garcillán-Barcia, M.P.; de la Cruz, F. A Degenerate Primer MOB Typing (DPMT) Method to Classify Gamma-Proteobacterial Plasmids in Clinical and Environmental Settings. PLoS ONE 2012, 7, e40438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Meng, L.; Guan, C.; Zhou, Y.; Peng, J.; Liang, H. Genomic characterisation of clinical Pseudomonas aeruginosa isolate PAG5 with a multidrug-resistant megaplasmid from China. J. Glob. Antimicrob. Resist. 2020, 21, 130–131. [Google Scholar] [CrossRef] [PubMed]
- SNPsaurus|GENOMES to GENOTYPES. Available online: https://www.snpsaurus.com/ (accessed on 31 January 2021).
- Andrews, S. FastQC—A Quality Control Tool for High Throughput Sequence Data. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 28 February 2021).
- BLAST: Basic Local Alignment Search Tool. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 6 June 2021).
- Vielva, L.; de Toro, M.; Lanza, V.F.; de La Cruz, F. PLACNETw: A web-based tool for plasmid reconstruction from bacterial genomes. Bioinformatics 2017, 33, 3796–3798. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef]
- Proksee. Available online: https://proksee.ca/projects/new (accessed on 6 August 2021).
- Li, X.; Xie, Y.; Liu, M.; Tai, C.; Sun, J.; Deng, Z.; Ou, H.Y. OriTfinder: A web-based tool for the identification of origin of transfers in DNA sequences of bacterial mobile genetic elements. Nucleic Acids Res. 2018, 46, W229–W234. [Google Scholar] [CrossRef]
- Garcillán-Barcia, M.P.; Redondo-Salvo, S.; Vielva, L.; de la Cruz, F. MOBscan: Automated Annotation of MOB Relaxases. Methods Mol. Biol. 2020, 2075, 295–308. [Google Scholar] [CrossRef]
- Carattoli, A.; Zankari, E.; Garciá-Fernández, A.; Larsen, M.V.; Lund, O.; Villa, L.; Aarestrup, F.M.; Hasman, H. In Silico detection and typing of plasmids using PlasmidFinder and Plasmid Multilocus Sequence Typing. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef]
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef]
- Finn, R.D.; Mistry, J.; Tate, J.; Coggill, P.; Heger, A.; Pollington, J.E.; Gavin, O.L.; Gunasekaran, P.; Ceric, G.; Forslund, K.; et al. The Pfam protein families database. Nucleic Acids Res. 2009, 38, D211–D222. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Darling, A.C.E.; Mau, B.; Blattner, F.R.; Perna, N.T. Mauve: Multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 2004, 14, 1394–1403. [Google Scholar] [CrossRef]
- Sullivan, M.J.; Petty, N.K.; Beatson, S.A. Easyfig: A genome comparison visualizer. Bioinformatics 2011, 27, 1009–1010. [Google Scholar] [CrossRef]
- Villa, L.; García-Fernández, A.; Fortini, D.; Carattoli, A. Replicon sequence typing of IncF plasmids carrying virulence and resistance determinants. J. Antimicrob. Chemother. 2010, 65, 2518–2529. [Google Scholar] [CrossRef]
- Nagshetty, K.; Shilpa, B.M.; Patil, S.A.; Shivannavar, C.T.; Manjula, N.G. An Overview of Extended Spectrum Beta Lactamases and Metallo Beta Lactamases. Adv. Microbiol. 2021, 11, 37–62. [Google Scholar] [CrossRef]
- Oliver, A.; Mulet, X.; López-Causapé, C.; Juan, C. The increasing threat of Pseudomonas aeruginosa high-risk clones. Drug Resist. Updat. 2015, 21–22, 41–59. [Google Scholar] [CrossRef]
- Tenover, F.C.; Nicolau, D.P.; Gill, C.M. Carbapenemase-producing Pseudomonas aeruginosa –an emerging challenge. Emerg. Microbes Infect. 2022, 11, 811–814. [Google Scholar] [CrossRef]
- Bassetti, M.; Vena, A.; Croxatto, A.; Righi, E.; Guery, B. How to manage Pseudomonas aeruginosa infections. Drugs Context 2018, 7, 212527. [Google Scholar] [CrossRef]
- Poirel, L.; Naas, T.; Nicolas, D.; Collet, L.; Bellais, S.; Cavallo, J.D.; Nordmann, P. Characterization of VIM-2, a carbapenem-hydrolyzing metallo-β-lactamase and its plasmid- and integron-borne gene from a Pseudomonas aeruginosa clinical isolate in France. Antimicrob. Agents Chemother. 2000, 44, 891–897. [Google Scholar] [CrossRef]
- Yano, H.; Kuga, A.; Okamoto, R.; Kitasato, H.; Kobayashi, T.; Inoue, M. Plasmid-encoded metallo-β-lactamase (IMP-6) conferring resistance to carbapenems, especially meropenem. Antimicrob. Agents Chemother. 2001, 45, 1343–1348. [Google Scholar] [CrossRef]
- Kung, V.L.; Ozer, E.A.; Hauser, A.R. The Accessory Genome of Pseudomonas aeruginosa. Microbiol. Mol. Biol. Rev. 2010, 74, 621–641. [Google Scholar] [CrossRef] [PubMed]
- Nishida, H. Comparative Analyses of Base Compositions, DNA Sizes, and Dinucleotide Frequency Profiles in Archaeal and Bacterial Chromosomes and Plasmids. Int. J. Evol. Biol. 2012, 2012, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Norman, A.; Hansen, L.H.; Sørensen, S.J. Conjugative plasmids: Vessels of the communal gene pool. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2275–2289. [Google Scholar] [CrossRef] [PubMed]
- Kawalek, A.; Wawrzyniak, P.; Bartosik, A.A.; Jagura-Burdzy, G. Rules and exceptions: The role of chromosomal ParB in DNA segregation and other cellular processes. Microorganisms 2020, 8, 105. [Google Scholar] [CrossRef]
- Funnell, B.E. ParB partition proteins: Complex formation and spreading at bacterial and plasmid centromeres. Front. Mol. Biosci. 2016, 3, 44. [Google Scholar] [CrossRef]
- Adamczyk, M.; Dolowy, P.; Jonczyk, M.; Thomas, C.M.; Jagura-Burdzy, G. The kfrA gene is the first in a tricistronic operon required for survival of IncP-1 plasmid R751. Microbiology 2006, 152, 1621–1637. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, M.; Lewicka, E.; Szatkowska, R.; Nieznanska, H.; Ludwiczak, J.; Jasiński, M.; Dunin-Horkawicz, S.; Sitkiewicz, E.; Swiderska, B.; Goch, G.; et al. Revealing biophysical properties of KfrA-type proteins as a novel class of cytoskeletal, coiled-coil plasmid-encoded proteins. BMC Microbiol. 2021, 21, 32. [Google Scholar] [CrossRef]
- Lewicka, E.; Mitura, M.; Steczkiewicz, K.; Kieracinska, J.; Skrzynska, K.; Adamczyk, M.; Jagura-Burdzy, G. Unique Properties of the Alpha-Helical DNA-Binding Protein KfrA Encoded by the IncU Incompatibility Group Plasmid RA3 and Its Host-Dependent Role in Plasmid Maintenance. Appl. Environ. Microbiol. 2021, 87, e01771-20. [Google Scholar] [CrossRef]
- Fernández-García, L.; Blasco, L.; Lopez, M.; Bou, G.; García-Contreras, R.; Wood, T.; Tomas, M. Toxin-antitoxin systems in clinical pathogens. Toxins 2016, 8, 227. [Google Scholar] [CrossRef]
- Yang, Q.E.; Walsh, T.R. Toxin-antitoxin systems and their role in disseminating and maintaining antimicrobial resistance. FEMS Microbiol. Rev. 2017, 41, 343–353. [Google Scholar] [CrossRef]
- Smillie, C.; Garcillán-Barcia, M.P.; Francia, M.V.; Rocha, E.P.; de la Cruz, F. Mobility of Plasmids. Microbiol. Mol. Biol. Rev. 2010, 74, 1092–2172. [Google Scholar] [CrossRef]
- Costa, T.R.D.; Harb, L.; Khara, P.; Zeng, L.; Hu, B.; Christie, P.J. Type IV secretion systems: Advances in structure, function, and activation. Mol. Microbiol. 2021, 115, 436–452. [Google Scholar] [CrossRef]
- Adamczyk, M.; Jagura-Burdzy, G. Spread and survival of promiscuous IncP-1 plasmids. Acta Biochim. Pol. 2003, 50, 425–453. [Google Scholar] [CrossRef]
- Nascimento, A.M.A.; Chartone-Souza, E.; Nascimento, A.M.A.; Chartone-Souza, E. Operon mer: Bacterial resistance to mercury and potential for bioremediation of contaminated environments. Genet. Mol. Res. 2003, 2, 92–101. [Google Scholar] [PubMed]
- Ng, S.P.; Davis, B.; Palombo, E.A.; Bhave, M. A Tn5051-like mer-containing transposon identified in a heavy metal tolerant strain Achromobacter sp. AO22. BMC Res. Notes 2009, 2, 38. [Google Scholar] [CrossRef] [PubMed]
- Orlek, A.; Stoesser, N.; Anjum, M.F.; Doumith, M.; Ellington, M.J.; Peto, T.; Crook, D.; Woodford, N.; Sarah Walker, A.; Phan, H.; et al. Plasmid classification in an era of whole-genome sequencing: Application in studies of antibiotic resistance epidemiology. Front. Microbiol. 2017, 8, 182. [Google Scholar] [CrossRef]
- Garcillán-Barcia, M.P.; de la Cruz, F. Ordering the bestiary of genetic elements transmissible by conjugation. Mob. Genet Elements 2013, 3, e24263. [Google Scholar] [CrossRef]
- Salgado-Camargo, A.D.; Castro-Jaimes, S.; Gutierrez-Rios, R.M.; Lozano, L.F.; Altamirano-Pacheco, L.; Silva-Sanchez, J.; Pérez-Oseguera, Á.; Volkow, P.; Castillo-Ramírez, S.; Cevallos, M.A. Structure and Evolution of Acinetobacter baumannii Plasmids. Front. Microbiol. 2020, 11, 1283. [Google Scholar] [CrossRef]
- Thomas, C.M. Plasmid Incompatibility. In Molecular Life Sciences; Bell, E., Ed.; Springer: New York, NY, USA, 2014; pp. 1–3. [Google Scholar]
- Norberg, P.; Bergström, M.; Jethava, V.; Dubhashi, D.; Hermansson, M. The IncP-1 plasmid backbone adapts to different host bacterial species and evolves through homologous recombination. Nat. Commun. 2011, 2, 268. [Google Scholar] [CrossRef]
- Naas, T.; Bonnin, R.A.; Cuzon, G.; Villegas, M.V.; Nordmann, P. Complete sequence of two KPC-harbouring plasmids from Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2013, 68, 1757–1762. [Google Scholar] [CrossRef]
- Jain, A.; Srivastava, P. Broad host range plasmids. FEMS Microbiol. Lett. 2013, 348, 87–96. [Google Scholar] [CrossRef]
- Bonnin, R.A.; Nordmann, P.; Carattoli, A.; Poirel, L. Comparative genomics of IncL/M-type plasmids: Evolution by acquisition of resistance genes and insertion sequences. Antimicrob. Agents Chemother. 2013, 57, 674–676. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Martínez, J.; Rocha-Gracia, R.d.C.; Bello-López, E.; Cevallos, M.A.; Castañeda-Lucio, M.; López-García, A.; Sáenz, Y.; Jiménez-Flores, G.; Cortés-Cortés, G.; Lozano-Zarain, P. A Plasmid Carrying blaIMP-56 in Pseudomonas aeruginosa Belonging to a Novel Resistance Plasmid Family. Microorganisms 2022, 10, 1863. https://doi.org/10.3390/microorganisms10091863
Gómez-Martínez J, Rocha-Gracia RdC, Bello-López E, Cevallos MA, Castañeda-Lucio M, López-García A, Sáenz Y, Jiménez-Flores G, Cortés-Cortés G, Lozano-Zarain P. A Plasmid Carrying blaIMP-56 in Pseudomonas aeruginosa Belonging to a Novel Resistance Plasmid Family. Microorganisms. 2022; 10(9):1863. https://doi.org/10.3390/microorganisms10091863
Chicago/Turabian StyleGómez-Martínez, Jessica, Rosa del Carmen Rocha-Gracia, Elena Bello-López, Miguel Angel Cevallos, Miguel Castañeda-Lucio, Alma López-García, Yolanda Sáenz, Guadalupe Jiménez-Flores, Gerardo Cortés-Cortés, and Patricia Lozano-Zarain. 2022. "A Plasmid Carrying blaIMP-56 in Pseudomonas aeruginosa Belonging to a Novel Resistance Plasmid Family" Microorganisms 10, no. 9: 1863. https://doi.org/10.3390/microorganisms10091863
APA StyleGómez-Martínez, J., Rocha-Gracia, R. d. C., Bello-López, E., Cevallos, M. A., Castañeda-Lucio, M., López-García, A., Sáenz, Y., Jiménez-Flores, G., Cortés-Cortés, G., & Lozano-Zarain, P. (2022). A Plasmid Carrying blaIMP-56 in Pseudomonas aeruginosa Belonging to a Novel Resistance Plasmid Family. Microorganisms, 10(9), 1863. https://doi.org/10.3390/microorganisms10091863








