Detection of Acanthamoeba from Acanthamoeba Keratitis Mouse Model Using Acanthamoeba-Specific Antibodies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Immunocytochemistry
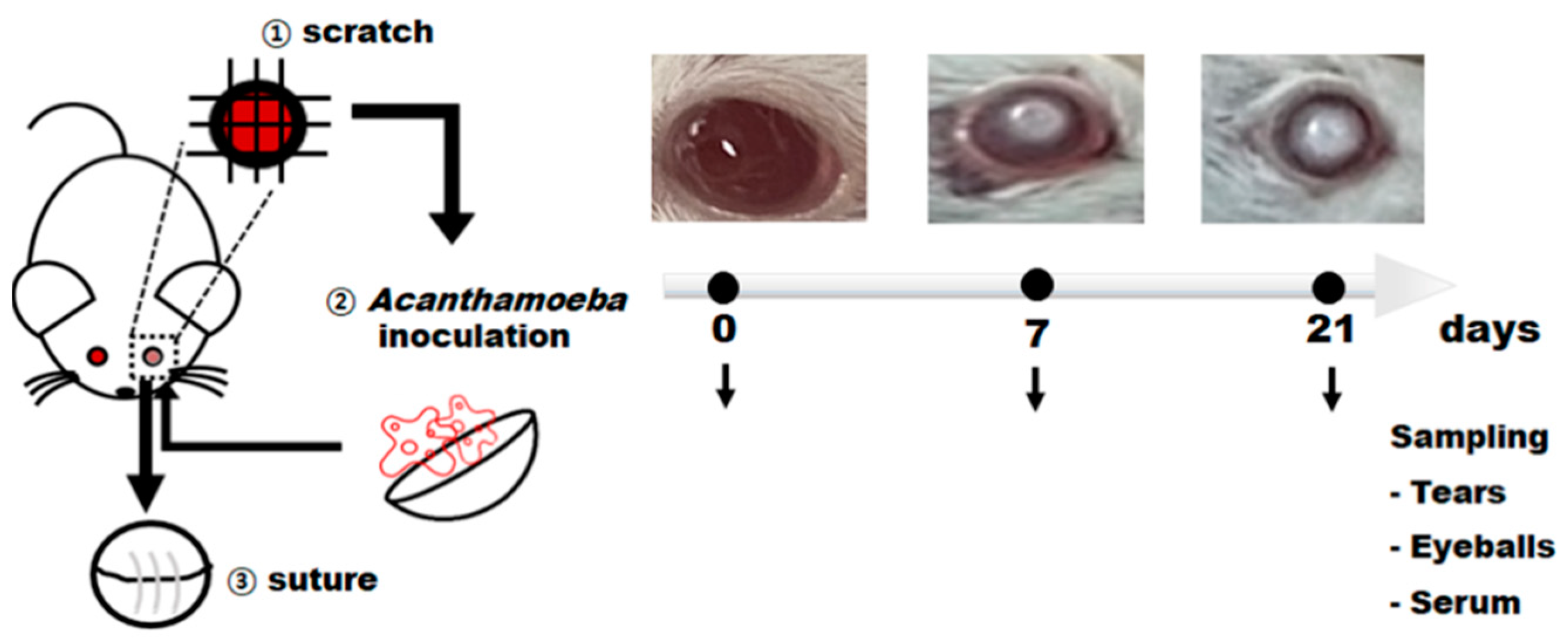
2.3. Production of Acanthamoeba keratitis Mouse Model
2.4. Detection of Acanthamoeba Antigens Using ACAP and PBP Antibodies
2.5. Anti-Acanthamoeba Antibody Responses in AK Mouse Model
2.6. Statistical Analysis
3. Results
3.1. Confirming the Specificities of ACAP and PBP Antibodies
3.2. Production of AK Mouse Model
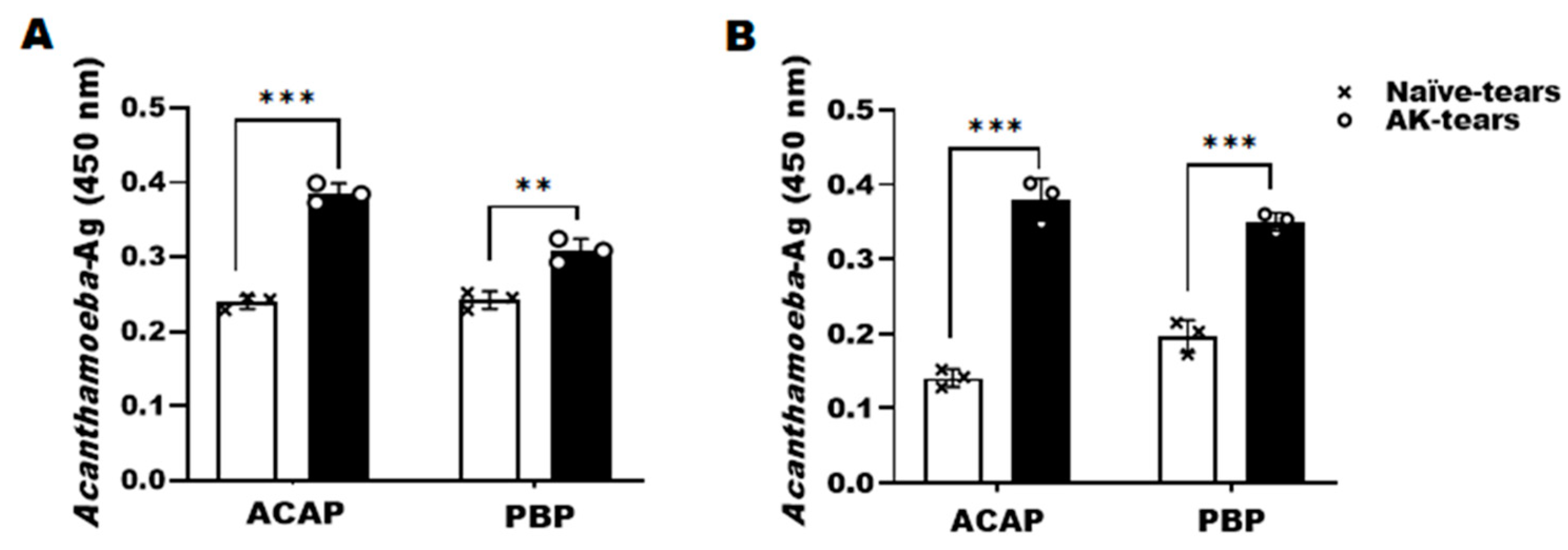
3.3. Detection of Acanthamoeba Antigens from Tears of AK Mouse Models Using ACAP and PBP Antibodies
3.4. Detection of Acanthamoeba Antigens from Eyeball Lysates of AK Mouse Models Using ACAP and PBP Antibodies
3.5. Evaluation of Anti-Acanthamoeba Serum IgG, IgA, and IgM Antibodies in the AK Mouse Model
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Page, M.A.; Mathers, W.D. Acanthamoeba keratitis: A 12-year experience covering a wide spectrum of presentations, diagnoses, and outcomes. J. Ophthalmol. 2013, 2013, 670242. [Google Scholar] [CrossRef]
- Tananuvat, N.; Techajongjintana, N.; Somboon, P.; Wannasan, A. The First Acanthamoeba keratitis Case of Non-Contact Lens Wearer with HIV Infection in Thailand. Korean J. Parasitol. 2019, 57, 505–511. [Google Scholar] [CrossRef]
- Kilvington, S.; Gray, T.; Dart, J.; Morlet, N.; Beeching, J.R.; Frazer, D.G.; Matheson, M. Acanthamoeba keratitis: The role of domestic tap water contamination in the United Kingdom. Invest. Ophthalmol. Vis. Sci. 2004, 45, 165–169. [Google Scholar] [CrossRef]
- Raghavan, A.; Baidwal, S.; Venkatapathy, N.; Rammohan, R. The Acanthamoeba-Fungal Keratitis Study. Am. J. Ophthalmol. 2019, 201, 31–36. [Google Scholar] [CrossRef]
- Connelly, L.; Anijeet, D.; Alexander, C.L. A descriptive case of persistent Acanthamoeba keratitis: Raising awareness of this complex ocular disease. Access Microbiol. 2020, 2, 3. [Google Scholar] [CrossRef]
- Szentmáry, N.; Daas, L.; Shi, L.; Laurik, K.L.; Lepper, S.; Milioti, G.; Seitz, B. Acanthamoeba keratitis-Clinical signs, differential diagnosis and treatment. J. Curr. Ophthalmol. 2018, 31, 16–23. [Google Scholar] [CrossRef]
- Sharma, S.; Athmanathan, S.; Ata-Ur-Rasheed, M.; Garg, P.; Rao, G.N. Evaluation of immunoperoxidase staining technique in the diagnosis of Acanthamoeba keratitis. Indian J. Ophthalmol. 2001, 49, 181–186. [Google Scholar]
- Goh, J.W.Y.; Harrison, R.; Hau, S.; Alexander, C.L.; Tole, D.M.; Avadhanam, V.S. Comparison of In Vivo Confocal Microscopy, PCR and Culture of Corneal Scrapes in the Diagnosis of Acanthamoeba Keratitis. Cornea 2018, 37, 480–485. [Google Scholar] [CrossRef]
- Cerva, L. Acanthamoeba culbertsoni and Naegleria fowleri: Occurrence of antibodies in man. J. Hyg. Epidemiol. Microbiol. Immunol. 1989, 33, 99–103. [Google Scholar]
- Cursons, R.T.; Brown, T.J.; Keys, E.A.; Moriarty, K.M.; Till, D. Immunity to pathogenic free-living amoebae: Role of humoral antibody. Infect. Immun. 1980, 29, 401–407. [Google Scholar] [CrossRef]
- Alizadeh, H.; Apte, S.; El-Agha, M.S.; Li, L.; Hurt, M.; Howard, K.; Cavanagh, H.D.; McCulley, J.P.; Niederkorn, J.Y. Tear IgA and serum IgG antibodies against Acanthamoeba in patients with Acanthamoeba keratitis. Cornea 2001, 20, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Leher, H.; Zaragoza, F.; Taherzadeh, S.; Alizadeh, H.; Niederkorn, J.Y. Monoclonal IgA antibodies protect against Acanthamoeba keratitis. Exp. Eye Res. 1999, 69, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Leher, H.F.; Alizadeh, H.; Taylor, W.M.; Shea, A.S.; Silvany, R.S.; Van Klink, F.; Jager, M.J.; Niederkorn, J.Y. Role of mucosal IgA in the resistance to Acanthamoeba keratitis. Invest. Ophthalmol. Vis. Sci. 1998, 39, 2666–2673. [Google Scholar]
- Said, N.A.; Shoeir, A.T.; Panjwani, N.; Garate, M.; Cao, Z. Local and systemic humoral immune response during acute and chronic Acanthamoeba keratitis in rabbits. Curr. Eye Res. 2004, 29, 429–439. [Google Scholar] [CrossRef]
- Walochnik, J.; Obwaller, A.; Haller-Schober, E.M.; Aspöck, H. Anti-Acanthamoeba IgG, IgM, and IgA immunoreactivities in correlation to strain pathogenicity. Parasitol. Res. 2001, 87, 651–656. [Google Scholar] [CrossRef]
- Naveed, A.K.; John, G.; Katherine, P.T.; Victoria, C.H.; Graham, S.T.; Timothy, A.P. Isolation of Acanthamoeba-Specific Antibodies from a Bacteriophage Display Library. J. Clin. Microbiol. 2000, 38, 2374–2377. [Google Scholar] [CrossRef]
- Turner, M.L.; Cockerell, E.J.; Brereton, H.M.; Badenoch, P.R.; Tea, M.; Coster, D.J.; Williams, K.A. Antigens of selected Acanthamoeba species detected with monoclonal antibodies. Int. J. Parasitol. 2005, 35, 981–990. [Google Scholar] [CrossRef]
- Kang, A.Y.; Park, A.Y.; Shin, H.J.; Khan, N.A.; Maciver, S.K.; Jung, S.Y. Production of a monoclonal antibody against a mannose-binding protein of Acanthamoeba culbertsoni and its localization. Exp. Parasitol. 2018, 192, 19–24. [Google Scholar] [CrossRef]
- Park, A.Y.; Kang, A.Y.; Jung, S.Y. Protective effects of a monoclonal antibody to a mannose-binding protein of Acanthamoeba culbertsoni. Biomed. Sci. Lett. 2018, 24, 435–438. [Google Scholar] [CrossRef]
- Kim, D.Y.; Son, D.H.; Matin, A.; Jung, S.Y. Production of a monoclonal antibody against a galactose-binding protein of Acanthamoeba castellanii and its cytotoxicity. Parasitol. Res. 2021, 120, 3845–3850. [Google Scholar] [CrossRef]
- Park, S.M.; Lee, H.A.; Chu, K.B.; Quan, F.S.; Kim, S.J.; Moon, E.K. Production of a polyclonal antibody against inosine-uridine preferring nucleoside hydrolase of Acanthamoeba castellanii and its access to diagnosis of Acanthamoeba keratitis. PLoS ONE 2020, 15, e0239867. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.A.; Chu, K.B.; Kim, M.J.; Quan, F.S.; Kong, H.H.; Moon, E.K. Chorismate mutase peptide antibody enables specific detection of Acanthamoeba. PLoS ONE 2021, 16, e0250342. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Chu, K.B.; Lee, H.A.; Quan, F.S.; Kong, H.H.; Moon, E.K. Detection of Acanthamoeba spp. using carboxylesterase antibody and its usage for diagnosing Acanthamoeba-keratitis. PLoS ONE 2022, 17, e0262223. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Lee, H.A.; Quan, F.S.; Kong, H.H.; Moon, E.K. Characterization of a Peptide Antibody Specific to the Adenylyl Cyclase-Associated Protein of Acanthamoeba castellanii. Korean J. Parasitol. 2022, 60, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Quan, F.S.; Kong, H.H.; Kim, J.H.; Moon, E.K. Specific Detection of Acanthamoeba species using Polyclonal Peptide Antibody Targeting the Periplasmic Binding Protein of A. castellanii. Korean J. Parasitol. 2022, 60, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Bowers, B.; Korn, E.D. The fine structure of Acanthamoeba castellanii (Neff strain). II. Encystment. J. Cell. Biol. 1969, 41, 786–805. [Google Scholar] [CrossRef]
- Kang, H.; Sohn, H.J.; Park, A.Y.; Ham, A.J.; Lee, J.H.; Oh, Y.H.; Chwae, Y.J.; Kim, K.; Park, S.; Yang, H.; et al. Establishment of an Acanthamoeba keratitis mouse model confirmed by amoebic DNA amplification. Sci. Rep. 2021, 11, 4183. [Google Scholar] [CrossRef]
- Cooper, E.; Cowmeadow, W.; Elsheikha, H.M. Should Veterinary Practitioners Be Concerned about Acanthamoeba Keratitis? Parasitologia 2021, 1, 12–19. [Google Scholar] [CrossRef]
- Feng, X.; Zheng, W.; Wang, Y.; Zhao, D.; Jiang, X.; Lv, S. A Rabbit Model of Acanthamoeba Keratitis That Better Reflects the Natural Human Infection. Anat. Rec. 2015, 298, 1509–1517. [Google Scholar] [CrossRef]
- Marciano-Cabral, F.; Cabral, G. Acanthamoeba spp. as agents of Disease in Humans. Clin. Microbiol. Rev. 2003, 16, 273–307. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-J.; Ham, A.-J.; Park, A.-Y.; Sohn, H.-J.; Shin, H.-J.; Quan, F.-S.; Kong, H.-H.; Moon, E.-K. Detection of Acanthamoeba from Acanthamoeba Keratitis Mouse Model Using Acanthamoeba-Specific Antibodies. Microorganisms 2022, 10, 1711. https://doi.org/10.3390/microorganisms10091711
Kim M-J, Ham A-J, Park A-Y, Sohn H-J, Shin H-J, Quan F-S, Kong H-H, Moon E-K. Detection of Acanthamoeba from Acanthamoeba Keratitis Mouse Model Using Acanthamoeba-Specific Antibodies. Microorganisms. 2022; 10(9):1711. https://doi.org/10.3390/microorganisms10091711
Chicago/Turabian StyleKim, Min-Jeong, A-Jeong Ham, A-Young Park, Hae-Jin Sohn, Ho-Joon Shin, Fu-Shi Quan, Hyun-Hee Kong, and Eun-Kyung Moon. 2022. "Detection of Acanthamoeba from Acanthamoeba Keratitis Mouse Model Using Acanthamoeba-Specific Antibodies" Microorganisms 10, no. 9: 1711. https://doi.org/10.3390/microorganisms10091711
APA StyleKim, M.-J., Ham, A.-J., Park, A.-Y., Sohn, H.-J., Shin, H.-J., Quan, F.-S., Kong, H.-H., & Moon, E.-K. (2022). Detection of Acanthamoeba from Acanthamoeba Keratitis Mouse Model Using Acanthamoeba-Specific Antibodies. Microorganisms, 10(9), 1711. https://doi.org/10.3390/microorganisms10091711






