Analysis of the Specific Immune Response after the Third Dose of mRNA COVID-19 Vaccines in Organ Transplant Recipients: Possible Spike-S1 Reactive IgA Signature in Protection from SARS-CoV-2 Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Recruitment and Clinical Sample Collection
2.2. Detection of SARS-CoV-2 Anti-Spike Immunoglobulins
2.3. SARS-CoV-2 ELISpot Assay
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
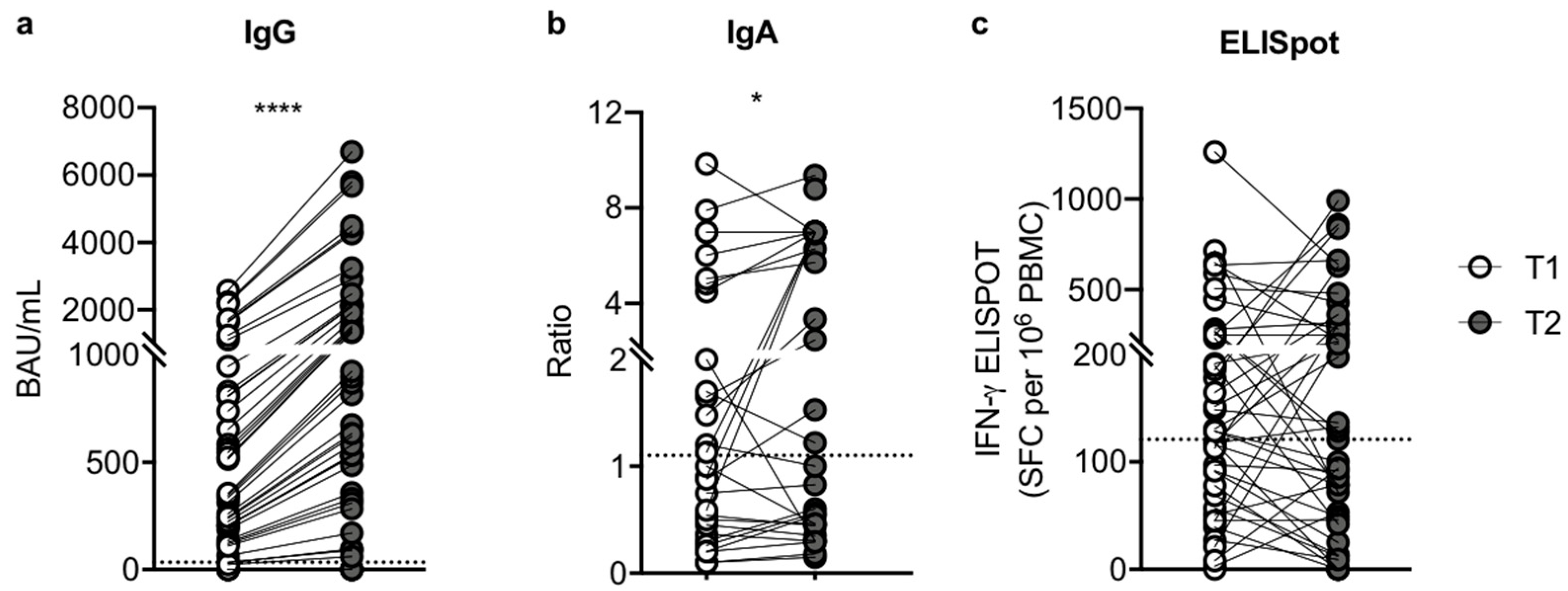
3.2. The Third Dose of Pfizer-BioNTech BNT162b2 mRNA Vaccine Induces an Improvement in Humoral Response
3.3. The Magnitude of Vaccine-Specific T Cell Responses
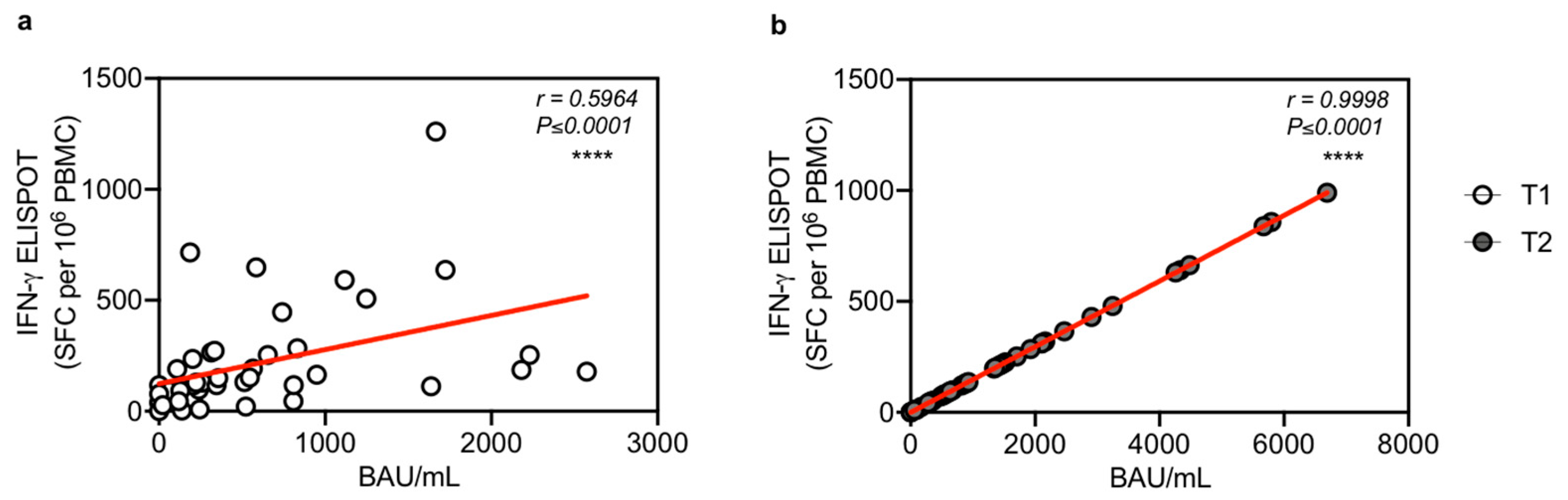
3.4. T Cell Response Correlates with IgG Antibody Levels after the Third Vaccination Dose
3.5. Clinical Outcome after the Third Dose
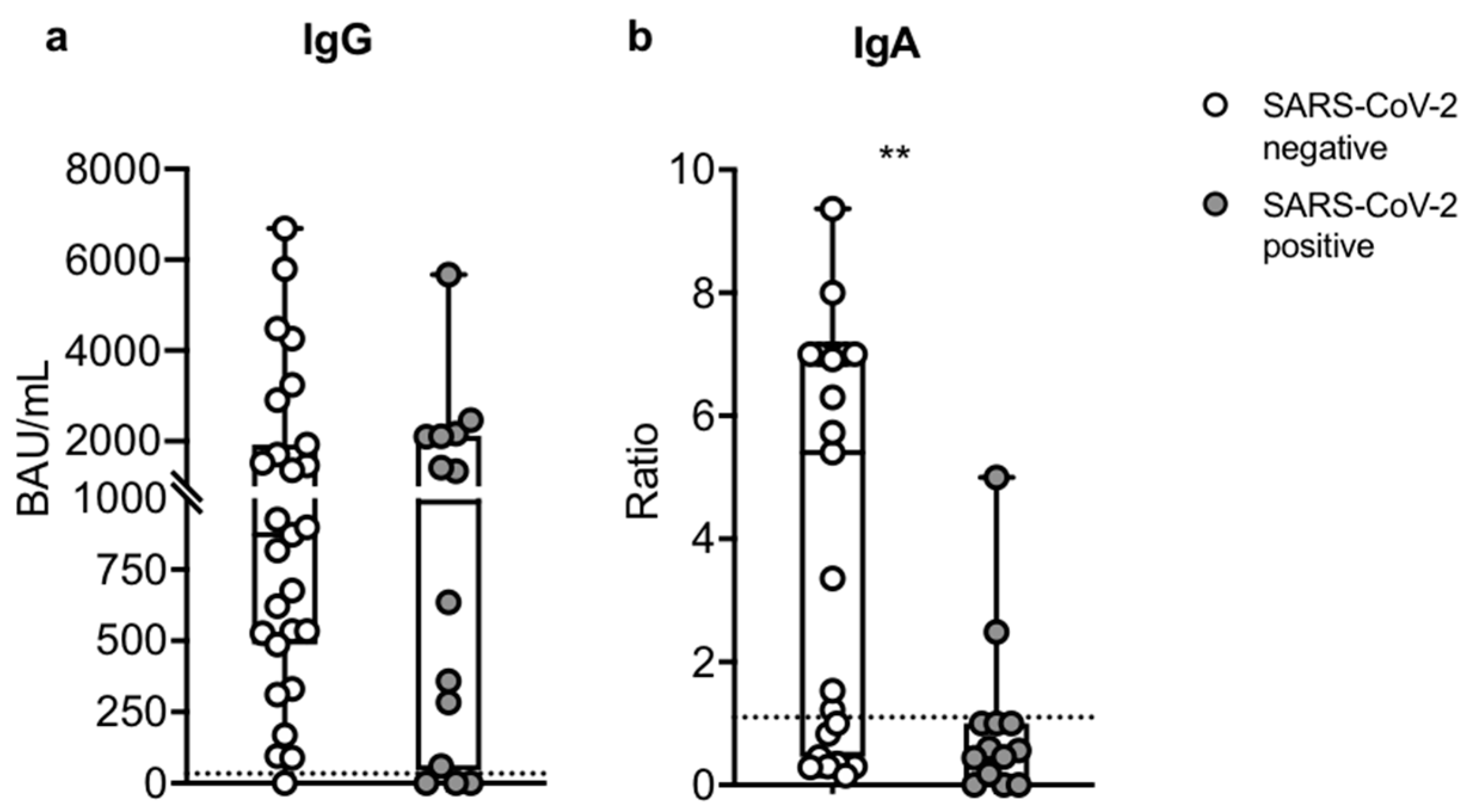
3.6. Humoral Assessment after Breakthrough Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernan, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef]
- Lauring, A.S.; Tenforde, M.W.; Chappell, J.D.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; et al. Clinical severity of, and effectiveness of mRNA vaccines against, COVID-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: Prospective observational study. BMJ 2022, 376, e069761. [Google Scholar] [CrossRef] [PubMed]
- Young-Xu, Y.; Korves, C.; Roberts, J.; Powell, E.I.; Zwain, G.M.; Smith, J.; Izurieta, H.S. Coverage and Estimated Effectiveness of mRNA COVID-19 Vaccines Among US Veterans. JAMA Netw. Open 2021, 4, e2128391. [Google Scholar] [CrossRef] [PubMed]
- Grupper, A.; Rabinowich, L.; Schwartz, D.; Schwartz, I.F.; Ben-Yehoyada, M.; Shashar, M.; Katchman, E.; Halperin, T.; Turner, D.; Goykhman, Y.; et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am. J. Transplant. 2021, 21, 2719–2726. [Google Scholar] [CrossRef] [PubMed]
- Guarino, M.; Cossiga, V.; Esposito, I.; Furno, A.; Morisco, F. Effectiveness of SARS-CoV-2 vaccination in liver transplanted patients: The debate is open! J. Hepatol. 2022, 76, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Marinaki, S.; Adamopoulos, S.; Degiannis, D.; Roussos, S.; Pavlopoulou, I.D.; Hatzakis, A.; Boletis, I.N. Immunogenicity of SARS-CoV-2 BNT162b2 vaccine in solid organ transplant recipients. Am. J. Transplant. 2021, 21, 2913–2915. [Google Scholar] [CrossRef]
- Marion, O.; Del Bello, A.; Abravanel, F.; Couat, C.; Faguer, S.; Esposito, L.; Hebral, A.L.; Izopet, J.; Kamar, N. Safety and Immunogenicity of Anti-SARS-CoV-2 Messenger RNA Vaccines in Recipients of Solid Organ Transplants. Ann. Intern. Med. 2021, 174, 1336–1338. [Google Scholar] [CrossRef]
- Miele, M.; Busa, R.; Russelli, G.; Sorrentino, M.C.; Di Bella, M.; Timoneri, F.; Mularoni, A.; Panarello, G.; Vitulo, P.; Conaldi, P.G.; et al. Impaired anti-SARS-CoV-2 humoral and cellular immune response induced by Pfizer-BioNTech BNT162b2 mRNA vaccine in solid organ transplanted patients. Am. J. Transplant. 2021, 21, 2919–2921. [Google Scholar] [CrossRef] [PubMed]
- Rincon-Arevalo, H.; Choi, M.; Stefanski, A.L.; Halleck, F.; Weber, U.; Szelinski, F.; Jahrsdorfer, B.; Schrezenmeier, H.; Ludwig, C.; Sattler, A.; et al. Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci. Immunol. 2021, 6, eabj1031. [Google Scholar] [CrossRef]
- Schramm, R.; Costard-Jackle, A.; Rivinius, R.; Fischer, B.; Muller, B.; Boeken, U.; Haneya, A.; Provaznik, Z.; Knabbe, C.; Gummert, J. Poor humoral and T-cell response to two-dose SARS-CoV-2 messenger RNA vaccine BNT162b2 in cardiothoracic transplant recipients. Clin. Res. Cardiol. 2021, 110, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Haddadin, Z.; Krueger, K.; Thomas, L.D.; Overton, E.T.; Ison, M.; Halasa, N. Alternative strategies of posttransplant influenza vaccination in adult solid organ transplant recipients. Am. J. Transplant. 2021, 21, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Hirzel, C.; Kumar, D. Influenza vaccine strategies for solid organ transplant recipients. Curr. Opin. Infect. Dis. 2018, 31, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, M.; Kampouri, E.; Manuel, O. Influenza in solid organ transplant recipients: Epidemiology, management, and outcomes. Expert Rev. Anti-Infect. Ther. 2020, 18, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Galmiche, S.; Luong Nguyen, L.B.; Tartour, E.; de Lamballerie, X.; Wittkop, L.; Loubet, P.; Launay, O. Immunological and clinical efficacy of COVID-19 vaccines in immunocompromised populations: A systematic review. Clin. Microbiol. Infect. 2022, 28, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Del Bello, A.; Abravanel, F.; Marion, O.; Couat, C.; Esposito, L.; Lavayssiere, L.; Izopet, J.; Kamar, N. Efficiency of a boost with a third dose of anti-SARS-CoV-2 messenger RNA-based vaccines in solid organ transplant recipients. Am. J. Transplant. 2022, 22, 322–323. [Google Scholar] [CrossRef] [PubMed]
- Peled, Y.; Ram, E.; Lavee, J.; Segev, A.; Matezki, S.; Wieder-Finesod, A.; Halperin, R.; Mandelboim, M.; Indenbaum, V.; Levy, I.; et al. Third dose of the BNT162b2 vaccine in heart transplant recipients: Immunogenicity and clinical experience. J. Heart Lung Transplant. 2022, 41, 148–157. [Google Scholar] [CrossRef]
- Boekel, L.; Steenhuis, M.; Hooijberg, F.; Besten, Y.R.; van Kempen, Z.L.E.; Kummer, L.Y.; van Dam, K.P.J.; Stalman, E.W.; Vogelzang, E.H.; Cristianawati, O.; et al. Antibody development after COVID-19 vaccination in patients with autoimmune diseases in the Netherlands: A substudy of data from two prospective cohort studies. Lancet. Rheumatol. 2021, 3, e778. [Google Scholar] [CrossRef]
- Deepak, P.; Kim, W.; Paley, M.A.; Yang, M.; Carvidi, A.B.; Demissie, E.G.; El-Qunni, A.A.; Haile, A.; Huang, K.; Kinnett, B.; et al. Effect of Immunosuppression on the Immunogenicity of mRNA Vaccines to SARS-CoV-2: A Prospective Cohort Study. Ann. Intern. Med. 2021, 174, 1572–1585. [Google Scholar] [CrossRef] [PubMed]
- Massarweh, A.; Eliakim-Raz, N.; Stemmer, A.; Levy-Barda, A.; Yust-Katz, S.; Zer, A.; Benouaich-Amiel, A.; Ben-Zvi, H.; Moskovits, N.; Brenner, B.; et al. Evaluation of Seropositivity Following BNT162b2 Messenger RNA Vaccination for SARS-CoV-2 in Patients Undergoing Treatment for Cancer. JAMA Oncol. 2021, 7, 1133–1140. [Google Scholar] [CrossRef]
- Tenforde, M.W.; Patel, M.M.; Gaglani, M.; Ginde, A.A.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; Mohr, N.M.; Zepeski, A.; McNeal, T.; et al. Effectiveness of a Third Dose of Pfizer-BioNTech and Moderna Vaccines in Preventing COVID-19 Hospitalization Among Immunocompetent and Immunocompromised Adults—United States, August–December. MMWR. Morb. Mortal. Wkly. Rep. 2022, 71, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Tenforde, M.W.; Self, W.H.; Adams, K.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; et al. Association Between mRNA Vaccination and COVID-19 Hospitalization and Disease Severity. JAMA 2021, 326, 2043–2054. [Google Scholar] [CrossRef] [PubMed]
- Janetzki, S.; Price, L.; Schroeder, H.; Britten, C.M.; Welters, M.J.; Hoos, A. Guidelines for the automated evaluation of Elispot assays. Nat. Protoc. 2015, 10, 1098–1115. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.H.; Tenforde, M.W.; Gaglani, M.; Talbot, H.K.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Casey, J.D.; Mohr, N.M.; et al. mRNA Vaccine Effectiveness Against COVID-19 Hospitalization Among Solid Organ Transplant Recipients. J. Infect. Dis. 2022, jiac118. [Google Scholar] [CrossRef] [PubMed]
- Hall, V.G.; Ferreira, V.H.; Ku, T.; Ierullo, M.; Majchrzak-Kita, B.; Chaparro, C.; Selzner, N.; Schiff, J.; McDonald, M.; Tomlinson, G.; et al. Randomized Trial of a Third Dose of mRNA-1273 Vaccine in Transplant Recipients. N. Engl. J. Med. 2021, 385, 1244–1246. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef]
- Al Jurdi, A.; Gassen, R.B.; Borges, T.J.; Lape, I.T.; Morena, L.; Efe, O.; Solhjou, Z.; El Fekih, R.; Deban, C.; Bohan, B.; et al. Suboptimal antibody response against SARS-CoV-2 Omicron variant after third dose of mRNA vaccine in kidney transplant recipients. Kidney Int. 2022, 101, 1282–1286. [Google Scholar] [CrossRef]
- Kumar, D.; Hu, Q.; Samson, R.; Ferreira, V.H.; Hall, V.G.; Ierullo, M.; Majchrzak-Kita, B.; Hardy, W.; Gingras, A.C.; Humar, A. Neutralization against Omicron variant in transplant recipients after three doses of mRNA vaccine. Am. J. Transplant. 2022. [Google Scholar] [CrossRef]
- Wang, P. Significance of IgA antibody testing for early detection of SARS-CoV-2. J. Med. Virol. 2021, 93, 1888–1889. [Google Scholar] [CrossRef] [PubMed]
- Infantino, M.; Manfredi, M.; Grossi, V.; Lari, B.; Fabbri, S.; Benucci, M.; Fortini, A.; Damiani, A.; Mobilia, E.M.; Panciroli, M.; et al. Closing the serological gap in the diagnostic testing for COVID-19: The value of anti-SARS-CoV-2 IgA antibodies. J. Med. Virol. 2021, 93, 1436–1442. [Google Scholar] [CrossRef]
- Yu, H.Q.; Sun, B.Q.; Fang, Z.F.; Zhao, J.C.; Liu, X.Y.; Li, Y.M.; Sun, X.Z.; Liang, H.F.; Zhong, B.; Huang, Z.F.; et al. Distinct features of SARS-CoV-2-specific IgA response in COVID-19 patients. Eur. Respir. J. 2020, 56, 2001526. [Google Scholar] [CrossRef] [PubMed]
- Siracusano, G.; Brombin, C.; Pastori, C.; Cugnata, F.; Noviello, M.; Tassi, E.; Princi, D.; Cantoni, D.; Malnati, M.S.; Maugeri, N.; et al. Profiling Antibody Response Patterns in COVID-19: Spike S1-Reactive IgA Signature in the Evolution of SARS-CoV-2 Infection. Front. Immunol. 2021, 12, 772239. [Google Scholar] [CrossRef] [PubMed]
- Busa, R.; Sorrentino, M.C.; Russelli, G.; Amico, G.; Miceli, V.; Miele, M.; Di Bella, M.; Timoneri, F.; Gallo, A.; Zito, G.; et al. Specific Anti-SARS-CoV-2 Humoral and Cellular Immune Responses After Booster Dose of BNT162b2 Pfizer-BioNTech mRNA-Based Vaccine: Integrated Study of Adaptive Immune System Components. Front. Immunol. 2022, 13, 856657. [Google Scholar] [CrossRef] [PubMed]

| Variable | SOTRs (n = 43) |
|---|---|
| Age, mean yr (SD) | 54.2 (12.7) |
| Gender, M (%) | 26 (60.4) |
| Type of transplant, n (%) | |
| kidney | 11 (25.6) |
| lung | 14 (32.6) |
| liver | 10 (23.2) |
| heart | 7 (16.3) |
| liver-kidney | 1 (2.3) |
| Time from transplant, median yr (range) | 6 (1–27) |
| Immunosuppressive treatment, n (%), | |
| Calcineurin inhibitors 1 (3.27 to 12.43 ng/mL) mean ng/mL (SD) | 40 (93), 7.2 (2.0) |
| mTOR inhibitors 2 (2.43 to 5.47 ng/mL) mean ng/mL (SD) | 3 (7), 3.6 (1.60) |
| Mycophenolate-mofetil (MMF) (180 to 2000 mg)mean mg (SD) | 30 (69.7), 968.5 (487.6) |
| Steroids (1.25 to 12.18 mg) mean mg (SD) | 23 (53.5), 5.4 (2.4) |
| Timespan between T1/T2, mean days (range) | 187.7 (114–244) |
| Timespan between T1/sampling, mean days (range) | 36.51 (15–136) 3 |
| Timespan between T2/sampling, mean days (range) | 40.3 (11–132) 3 |
| Comorbidities, n (%) | |
| Diabetes | 9 (20.93) |
| Obesity | 7 (16.27) |
| Hypertension | 17 (39.53) |
| Dyslipidaemia | 8 (18.60) |
| Active or previous smoke | 14 (32.56) |
| Cardiovascular disease | 12 (27.90) |
| Kidney disease | 6 (13.95) |
| Pulmonary disease | 6 (13.95) |
| Gastrointestinal disease | 12 (30.23) |
| Endocrinal disease | 7 (16.27) |
| Hepatopancreatic disease | 3 (6.97) |
| History of malignancy | 16 (37.20) |
| Basal Characteristics | Immunosuppressive Therapy | Immune Response after 3rd Dose (T2) | Clinical Presentation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient (n = 14) | Age | Tx | Years since Tx | Calcineurin INHIBITORS (ng/mL) | MMF (mg/die) | Steroid (mg/die) | Anti-Spike IgG (BAU/mL) | Anti-Spike IgA (Ratio) | T Cell Response (SFC/million PBMC) | Symptoms | Treatment | Outcome |
| TxVAC1 | 65 | liver | 27 | -- | 1000 | 21.5 | 0.6 | 311 | severe | aVT, steroid | Hospitalized, discharged | |
| TxVAC8 | 53 | lung | 8 | 12.4 | 2000 | 5 | 220.9 | 0.9 | 320 | moderate | Nonhospitalized, recovered | |
| TxVAC9 | 69 | lung | 1 | 10.2 | 1000 | 10 | 73.3 | 0.44 | 0 | severe | mAb | Hospitalized, discharged |
| TxVAC11 | 41 | kidney | 3 | 6.8 | 720 | 5 | 6.8 | 0 | 0 | severe/ critical | mAb, ECMO | Hospitalized, died |
| TxVAC19 | 70 | kidney | 1 | 6.9 | 1080 | -- | 18.1 | 0 | 0 | severe/ critical | aVT, mAb | Hospitalized, died |
| TxVAC25 | 41 | lung | 5 | 10.9 | 2000 | 5 | 20.9 | 0.9 | 0 | moderate | aVT | Nonhospitalized, recovered |
| TxVAC26 | 20 | lung | 6 | 10.2 | 1000 | 5 | 21.6 | 1 | 198 | moderate | - | Nonhospitalized, recovered |
| TxVAC28 | 58 | kidney | 18 | 6.4 | 1000 | - | 170.8 | 1.03 | 312 | moderate | - | Nonhospitalized, recovered |
| TxVAC48 | 50 | lung | 3 | 7.2 | 500 | 5 | 82.9 | 0.56 | 9 | severe | mAb | Hospitalized, discharged |
| TxVAC66 | 49 | liver | 3 | 5 | 250 | 5 | 166.4 | 0.18 | 839 | moderate | - | Nonhospitalized, recovered |
| TxVAC68 | 53 | liver | 11 | 4.2 | 1000 | - | 111 | 0.46 | 42 | mid | - | Nonhospitalized, recovered |
| TxVAC74 | 59 | heart | 12 | 6.3 | 1000 | - | 938.6 | 2.9 | 210 | mild | - | Nonhospitalized, recovered |
| TxVAC83 | 40 | heart | 12 | 6.4 | 1000 | - | >1040 | 5.6 | 365 | moderate | - | Nonhospitalized, recovered |
| TxVAC85 | 51 | lung | 25 | 8.8 | 1000 | 5 | >1040 | 0.8 | 94 | moderate | - | Nonhospitalized, recovered |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miele, M.; Busà, R.; Russelli, G.; Sorrentino, M.C.; Di Bella, M.; Timoneri, F.; Vitale, G.; Calzolari, E.; Vitulo, P.; Mularoni, A.; et al. Analysis of the Specific Immune Response after the Third Dose of mRNA COVID-19 Vaccines in Organ Transplant Recipients: Possible Spike-S1 Reactive IgA Signature in Protection from SARS-CoV-2 Infection. Microorganisms 2022, 10, 1563. https://doi.org/10.3390/microorganisms10081563
Miele M, Busà R, Russelli G, Sorrentino MC, Di Bella M, Timoneri F, Vitale G, Calzolari E, Vitulo P, Mularoni A, et al. Analysis of the Specific Immune Response after the Third Dose of mRNA COVID-19 Vaccines in Organ Transplant Recipients: Possible Spike-S1 Reactive IgA Signature in Protection from SARS-CoV-2 Infection. Microorganisms. 2022; 10(8):1563. https://doi.org/10.3390/microorganisms10081563
Chicago/Turabian StyleMiele, Monica, Rosalia Busà, Giovanna Russelli, Maria Concetta Sorrentino, Mariangela Di Bella, Francesca Timoneri, Giampiero Vitale, Elisa Calzolari, Patrizio Vitulo, Alessandra Mularoni, and et al. 2022. "Analysis of the Specific Immune Response after the Third Dose of mRNA COVID-19 Vaccines in Organ Transplant Recipients: Possible Spike-S1 Reactive IgA Signature in Protection from SARS-CoV-2 Infection" Microorganisms 10, no. 8: 1563. https://doi.org/10.3390/microorganisms10081563
APA StyleMiele, M., Busà, R., Russelli, G., Sorrentino, M. C., Di Bella, M., Timoneri, F., Vitale, G., Calzolari, E., Vitulo, P., Mularoni, A., Conaldi, P. G., & Bulati, M. (2022). Analysis of the Specific Immune Response after the Third Dose of mRNA COVID-19 Vaccines in Organ Transplant Recipients: Possible Spike-S1 Reactive IgA Signature in Protection from SARS-CoV-2 Infection. Microorganisms, 10(8), 1563. https://doi.org/10.3390/microorganisms10081563






