New Variants of Pseudomonas aeruginosa High-Risk Clone ST233 Associated with an Outbreak in a Mexican Paediatric Hospital
Abstract
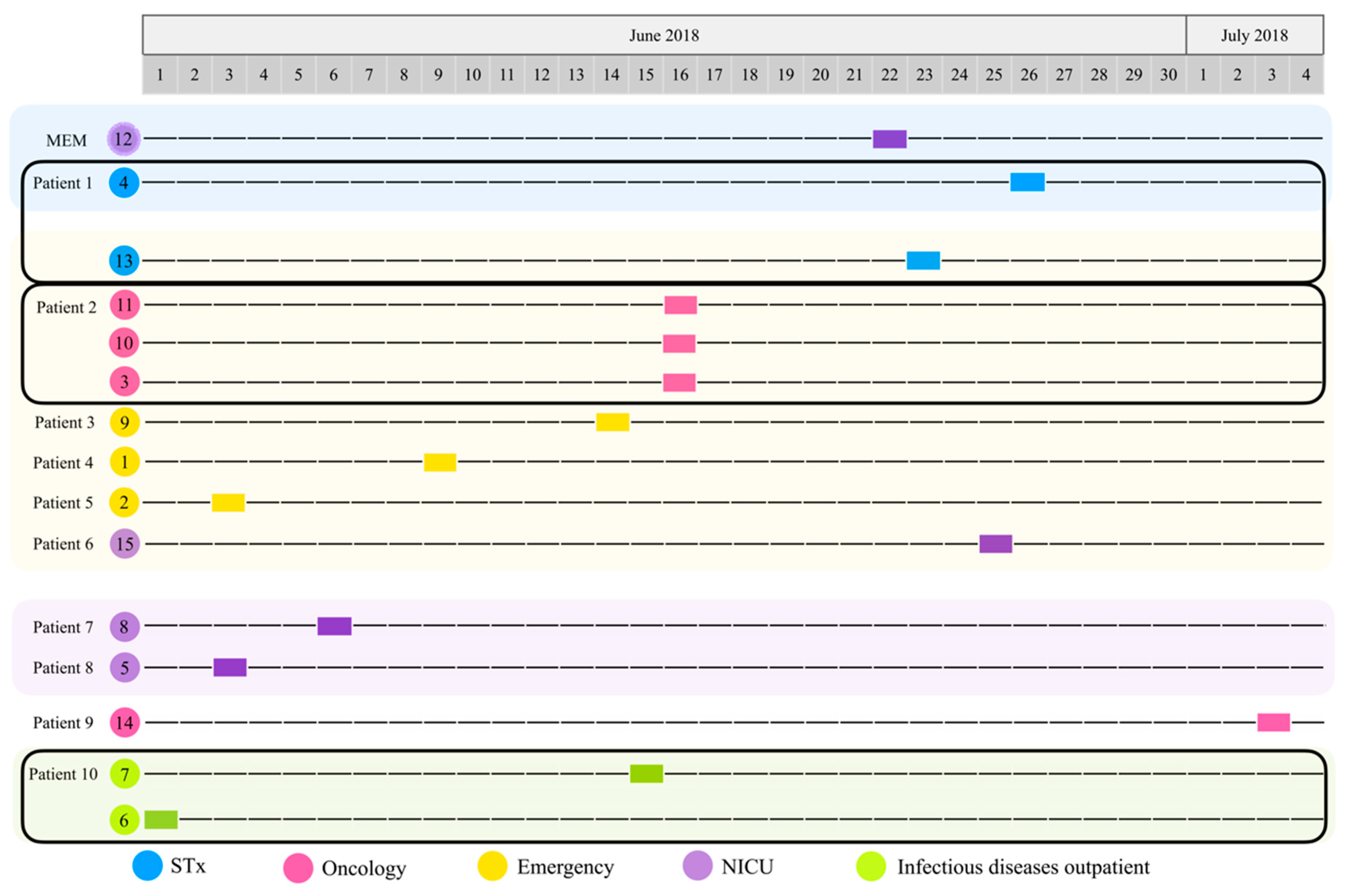
1. Introduction
2. Materials and Methods
2.1. Biological Samples
2.2. Susceptibility Profile
2.3. Phenotypic Screening and Detection of Carbapenemases
2.4. Phenotypic Production of Pyocyanin and Pyoverdine
2.5. Phenotypic Production of Biofilm
2.6. Pulsed-Field Gel Electrophoresis (PFGE)
2.7. Multi-Locus Sequence Typing (MLST)
2.8. MexAB-OprM Efflux Pump Repressor Genes’ Characterization
2.9. Phylogenetic Analysis
3. Results
3.1. Isolation, Identification, and Characterization of Pseudomonas aeruginosa from Biological Samples
3.2. P. aeruginosa Strains’ Susceptibility Profile
3.3. Carbapenemases’ Production
3.4. Phenotypic Production of Pyocyanin and Pyoverdine
3.5. Phenotypic Production of Biofilm
3.6. Pulsed-Field Gel Electrophoresis (PFGE)
3.7. Multi-Locus Sequence Typing (MLST)
3.8. Characterization of the MexAB-OprM Efflux Pump Repressor Genes (mexR, nalC, nalD)
3.9. Phylogenetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, P.R.; Rosenthal, K.S.; Pfaller, M.A. Microbiología Médica, 7th ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Ryan, K.J.; Ray, C.G.; Sherris. Microbiología Médica, 5th ed.; McGraw-Hill Interamericana: Mexico City, Mexico, 2011. [Google Scholar]
- NOM-045-SSA2-2005; Norma Oficial Mexicana: Para la Vigilancia Epidemiológica, Prevención y Control de las Infecciones Nosocomiales. Estados Unidos Mexicanos-Secretaria de Salud: Mexico City, Mexico, 2005.
- Gutiérrez, M.J.; Morayta, R.C.A.; Martínez, B.M.E.; Coria, L.J.J.; Armenta, G.L.; Ayala, F.J.R.; Bernal, G.S.M.; Flores, Z.F.J.; García, P.F.E.; Monjardín, R.J.A.; et al. Estudio multicéntrico de resistencias bacterianas nosocomiales en México. Rev. Latinoam. Infectología Pediátrica 2017, 30, 68–75. [Google Scholar]
- Organización Mundial de la Salud (OMS). Prevención de las Infecciones Nosocomiales: Guía Práctica [Internet]. Ducel, G., Fabray, J., Nicolle, L., Eds.; 2nd ed 2002. Available online: https://apps.who.int/iris/handle/10665/67877 (accessed on 22 July 2022).
- S Sundermann, A.J.; Chen, J.; Miller, J.K.; Saul, M.I.; Shutt, K.A.; Griffith, M.P.; Mustapha, M.M.; Ezeonwuka, C.; Waggle, K.; Srinivasa, V.; et al. Outbreak of Pseudomonas aeruginosa infections from a contaminated gastroscope detected by whole genome sequencing surveillance. Clin. Infect. Dis. 2021, 73, e638–e642. [Google Scholar] [CrossRef]
- Horcajada, J.P.; Padilla, B. Endemia y epidemia: Investigación de un brote epidémico nosocomial. Enferm. Infecc. Microbiol. Clínica 2013, 31, 181–186. [Google Scholar] [CrossRef]
- Parcell, B.J.; Oravcova, K.; Pinheiro, M.; Holden, M.; Phillips, G.; Turton, J.F.; Gillespie, S.H. Pseudomonas aeruginosa intensive care unit outbreak: Winnowing of transmissions with molecular and genomic typing. J. Hosp. Infect. 2018, 98, 282–288. [Google Scholar] [CrossRef]
- Martak, D.; Meunier, A.; Sauget, M.; Cholley, P.; Thouverez, M.; Bertrand, X.; Valot, B.; Hocquet, D. Comparison of pulsed-field gel electrophoresis and whole-genome-sequencing-based typing confirms the accuracy of pulsed-field gel electrophoresis for the investigation of local Pseudomonas aeruginosa outbreaks. J. Hosp. Infect. 2020, 105, 643–647. [Google Scholar] [CrossRef]
- Mulet, X.; Cabot, G.; Ocampo-Sosa, A.A.; Domínguez, M.A.; Zamorano, L.; Juan, C.; Tubau, F.; Rodríguez, C.; Moyà, B.; Peña, C.; et al. Biological markers of Pseudomonas aeruginosa epidemic high-risk clones. Spanish Network for Research in Infectious Diseases (REIPI). Antimicrob. Agents Chemother. 2013, 57, 5527–5535. [Google Scholar] [CrossRef]
- Correa, A.; del Campo, R.; Perenguez, M.; Blanco, V.M.; Rodríguez-Baños, M.; Perez, F.; Maya, J.J.; Rojas, L.; Cantón, R.; Arias, C.A.; et al. Dissemination of high-risk clones of extensively drug-resistant Pseudomonas aeruginosa in Colombia. Antimicrob. Agents Chemother. 2015, 59, 2421–2425. [Google Scholar] [CrossRef]
- Oliver, A. Epidemiología y mecanismos de resistencia a carbapenemas en Pseudomonas aeruginosa: Papel de los clones de alto riesgo en la multirresistencia. Enferm. Infecc. Microbiol. Clínica 2017, 35, 137–138. [Google Scholar] [CrossRef]
- Hu, Y.; Peng, W.; Wu, Y.; Li, H.; Wang, Q.; Yi, H.; Zhang, R.; Shao, B.; Zhu, K. A potential high-risk clone of Pseudomonas aeruginosa ST463. Front. Microbiol. 2021, 12, 670202. [Google Scholar] [CrossRef]
- Del Barrio-Tpfiño, E.; López-Causapé, C.; Oliver, A. Pseudomonas aeruginosa epidemic high-risk clones and their association with horizontally-acquired β-lactamases: 2020 update. Int. J. Antimicrob. Agents 2020, 56, 106196. [Google Scholar] [CrossRef]
- Maatallah, M.; Cheriaa, J.; Backhrouf, A.; Iversen, A.; Grundmann, H.; Do, T.; Lanotte, P.; Mastouri, M.; Elghmati, M.S.; Rojo, F.; et al. Population structure of Pseudomonas aeruginosa from five Mediterranean countries: Evidence for frequent recombination and epidemic occurrence of CC235. PLoS ONE 2011, 6, e25617. [Google Scholar] [CrossRef] [PubMed]
- López-Causapé, C.; Rojo-Molinero, E.; Mulet, X.; Cabot, G.; Moyà, B.; Figuerola, J.; Togores, B.; Pérez, J.L.; Oliver, A. Clonal dissemination, emergence of mutator lineages and antibiotic resistance evolution in Pseudomonas aeruginosa cystic fibrosis chronic lung infection. PLoS ONE 2013, 8, e71001. [Google Scholar] [CrossRef]
- Oliver, A.; Mulet, X.; López-Causapé, C.; Juan, C. The increasing threat of Pseudomonas aeruginosa high-risk clones. Drug Resist. Updates 2015, 21, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Rodea, P.; Zúñiga, G.; Rodríguez-Espino, B.A.; Olivares Cervantes, A.L.; Gamiño Arroyo, A.E.; Moreno-Espinosa, S.; de la Rosa Zamboni, D.; López Martínez, B.; Castellanos-Cruz, M.D.; Parra-Ortega, I.; et al. Identification of extensive drug resistant Pseudomonas aeruginosa strains: New clone ST1725 and high-risk clone ST233. PLoS ONE 2017, 12, e0172882. [Google Scholar] [CrossRef]
- Sánchez, A.; Salso, S.; Culebras, E.; Picazo, J.J. Carbapenem resistance determined by metalloenzymes in clinical isolates of Pseudomonas aeruginosa. Rev. Española Quimioter. 2004, 17, 336–340. [Google Scholar]
- Aguilar-Rodea, P.; Zúñiga, G.; Cerritos, R.; Rodríguez-Espino, B.A.; Gomez-Ramirez, U.; Nolasco-Romero, C.G.; López-Marceliano, B.; Rodea, G.E.; Mendoza-Elizalde, S.; Reyes-López, A.; et al. Nucleotide substitutions in the mexR, nalC and nalD regulator genes of the MexAB-OprM efflux pump are maintained in Pseudomonas aeruginosa genetic lineages. PLoS ONE 2022, 17, e0266742. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 31st ed.; Wayne, P.A., Ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2021. [Google Scholar]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R; R Studio, Inc.: Boston, MA, USA, 2015. [Google Scholar]
- Wongsaroj, L.; Saninjuk, K.; Romsang, A.; Duang-Nkern, J.; Trinachartvanit, W.; Vattanaviboon, P.; Mongkolsuk, S. Pseudomonas aeruginosa glutathione biosynthesis genes play multiple roles in stress protection, bacterial virulence and biofilm formation. PLoS ONE 2018, 13, e0205815. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Rudden, M.; Smyth, T.J.; Dooley, J.; Marchant, R.; Banat, I.M. Natural quorum sensing inhibitors effectively downregulate gene expression of Pseudomonas aeruginosa virulence factors. Appl. Microbiol. Biotechnol. 2019, 103, 3521–3535. [Google Scholar] [CrossRef]
- O’Toole, G.A. Microtiter dish biofilm formation assay. J. Vis. Exp. 2011, 30, 2437. [Google Scholar] [CrossRef]
- Tenover, F.C.; Arbeit, R.D.; Goering, R.V.; Mickelsen, P.A.; Murray, B.E.; Persing, D.H.; Swaminathan, B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: Criteria for bacterial strain typing. J. Clin. Microbiol. 1995, 33, 2233–2239. [Google Scholar] [CrossRef]
- Curran, B.; Jonas, D.; Grundmann, H.; Pitt, T.; Dowson, C.G. Development of a multi locus sequence typing scheme for the opportunistic pathogen Pseudomonas aeruginosa. J. Clin. Microbiol. 2004, 42, 5644–5649. [Google Scholar] [CrossRef]
- FinchTV 1. 4.0: A Brilliant Trace Viewer; Geospiza, Inc.: Seattle, WA, USA, 2006; Available online: http://www.geospiza.com (accessed on 22 July 2022).
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R. ClustalW and ClustalX version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Gouy, M.; Guindon, S.; Gascuel, O. SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 2010, 27, 221–224. [Google Scholar] [CrossRef]
- Ribeiro-Gonçalves, B.; Francisco, A.P.; Vaz, C.; Ramirez, M.; Carriço, J.A. PHYLOViZ Online: Web-based tool for visualization, phylogenetic inference, analysis and sharing of minimum spanning trees. Nucleic Acids Res. 2016, 44, W246–W251. Available online: https://online.phyloviz.net/ (accessed on 22 July 2022). [CrossRef]
- Quale, J.; Bratu, S.; Gupta, J.; Landman, D. Interplay of efflux system, ampC, and oprD expression in carbapenem resistance of Pseudomonas aeruginosa clinical isolates. Antimicrob. Agents Chemother. 2006, 50, 1633–1641. [Google Scholar] [CrossRef]
- Suresh, M.; Nithya, N.; Jayasree, P.R.; Vimal, K.P.; Manish Kumar, P.R. Mutational analyses of regulatory genes, mexR, nalC, nalD and mexZ of mexAB-oprM and mexXY operons, in efflux pump hyperexpressing multidrug-resistant clinical isolates of Pseudomonas aeruginosa. World J. Microbiol. Biotechnol. 2018, 34, 83. [Google Scholar] [CrossRef]
- Pankuch, G.A.; Lin, G.; Seifert, H.; Appelbaum, P.C. Activity of meropenem with and without ciprofloxacin and colistin against Pseudomonas aeruginosa and Acinetobacter baumannii. Antimicrob. Agents Chemother. 2008, 52, 333–336. [Google Scholar] [CrossRef][Green Version]
- Ochoa, S.A.; Cruz, A.C.; Rodea, G.E.; Cázares-Domínguez, V.; Escalona, G.; Arellano-Galindo, J.; Hernández-Castro, R.; Reyes-López, A.; Xicohtencatl-Cortes, J. Phenotypic characterization of multidrug-resistant Pseudomonas aeruginosa strains isolated from pediatric patients associated to biofilm formation. Microbiol. Res. 2015, 172, 68–78. [Google Scholar] [CrossRef]
- Almangour, T.A.; Aljabri, A.; Musawa, M.; Almohaizeie, A.; Almuhisen, S.; Damfu, N.; Alfozan, A.; Alraddadi, B.M.; Alattas, M.; Qutub, M.; et al. Ceftolozane-tazobactam vs. colistin for the treatment of infections due to multidrug-resistant Pseudomonas aeruginosa: A multicentre cohort study. J. Glob. Antimicrob. Resist. 2022, 28, 288–294. [Google Scholar] [CrossRef]
- Biswas, S.; Brunel, J.M.; Dubus, J.C.; Reynaud-Gaubert, M.; Rolain, J.M. Colistin: An update on the antibiotic of the 21st century. Expert Rev. Anti-Infect. Ther. 2012, 10, 917–934. [Google Scholar] [CrossRef]
- Angles-Yanqui, E.; Chumbes-Pérez, J.; Huaringa-Marcelo, J. Colistina en el tratamiento de infecciones por Pseudomonas aeruginosa y Acinetobacter baumanii extensamente resistentes (XDR) en un hospital de tercer nivel. Rev. Infect. 2020, 24, 201–207. [Google Scholar] [CrossRef]
- Ribeiro, Á.; Crozatti, M.; Silva, A.; Macedo, R.S.; Machado, A.; Silva, A. Pseudomonas aeruginosa in the ICU: Prevalence, resistance profile, and antimicrobial consumption. Rev. Soc. Bras. Med. Trop. 2019, 53, 1–6. [Google Scholar] [CrossRef]
- Biswal, I.; Arora, B.S.; Kasana, D.; Neetushree. Incidence of multidrug resistant Pseudomonas aeruginosa isolated from burn patients and environment of teaching institution. J. Clin. Diagn. Res. 2014, 8, DC26–DC29. [Google Scholar] [CrossRef]
- Schalk, I.J.; Guillon, L. Pyoverdine biosynthesis and secretion in Pseudomonas aeruginosa: Implications for metal homeostasis. Environ. Microbiol. 2013, 15, 161–173. [Google Scholar] [CrossRef]
- Hansen, S.; Stamm-Balderjahn, S.; Zuschneid, I.; Behnke, M.; Rüden, H.; Vonberg, R.P.; Gastmeier, P. Closure of medical departments during nosocomial outbreaks: Data from a systematic analysis of the literature. J. Hosp. Infect. 2007, 65, 348–353. [Google Scholar] [CrossRef]
- Seyman, D.; Inan, D.; Ozen, N.S.; Ogunc, E. Un brote epidémico de endocarditis por Pseudomonas aeruginosa secundario a angiografía coronaria. Rev. Chil. Infectología 2014, 31, 261–267. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Deplano, A.; Denis, O.; Poirel, L.; Hocquet, D.; Nonhoff, C.; Byl, B.; Nordmann, P.; Vincent, J.L.; Struelens, M.J. Molecular characterization of an epidemic clone of panantibiotic-resistant Pseudomonas aeruginosa. J. Clin. Microbiol. 2005, 43, 1198–1204. [Google Scholar] [CrossRef] [PubMed]
- Baquero, F.; Coque, T.M.; de la Cruz, F. Ecology and evolution as targets: The need for novel eco-evo drugs and strategies to fight antibiotic resistance. Antimicrob. Agents Chemother. 2011, 55, 3649–3660. [Google Scholar] [CrossRef] [PubMed]
- Micek, S.T.; Wunderink, R.G.; Kollef, M.H.; Chen, C.; Rello, J.; Chastre, J.; Antonelli, M.; Welte, T.; Clair, B.; Ostermann, H.; et al. An international multicenter retrospective study of Pseudomonas aeruginosa nosocomial pneumonia: Impact of multidrug resistance. Crit. Care 2015, 19, 219. [Google Scholar] [CrossRef]
- González-Olvera, E.M.; Pérez-Morales, R.; González, A.; Castro-Escarpulli, G.; Palma-Martínez, I.; Alba-Romero, J.J. Antibiotic resistance, virulence factors and genotyping of Pseudomonas aeruginosa in public hospitals of northeastern Mexico. J. Infect. Dev. Ctries. 2019, 13, 374–383. [Google Scholar] [CrossRef]
- Uc-Cachón, A.H.; Gracida-Osorno, C.; Luna-Chi, I.G.; Jiménez-Guillermo, J.G.; Molina-Salinas, G.M. High prevalence of antimicrobial resistance among Gram-negative isolated bacilli in intensive care units at a tertiary-care hospital in Yucatán Mexico. Medicina 2019, 55, 588. [Google Scholar] [CrossRef]
- Javed, M.; Jentzsch, B.; Heinrich, M.; Ueltzhoeffer, V.; Peter, S.; Schoppmeier, U.; Angelov, A.; Schwarz, S.; Willmann, M. Transcriptomic basis of serum resistance and virulence related traits in XDR P. aeruginosa evolved under antibiotic pressure in a morbidostat device. Front. Microbiol. 2021, 11, 619542. [Google Scholar] [CrossRef]
- Rodulfo, H.; Arcia, A.; Hernández, A.; Michelli, E.; Martinez, D.; Guzman, M.; Sharma, A.; Donato, M. Virulence factors and integrons are associated with MDR and XDR phenotypes in nosocomial strains of Pseudomonas aeruginosa in a Venezuelan university hospital. Rev. Inst. Med. Trop. Sao Paulo 2019, 61, e20. [Google Scholar] [CrossRef]
- Gajdács, M.; Baráth, Z.; Kárpáti, K.; Szabó, D.; Usai, D.; Zanetti, S.; Donadu, M.G. No correlation between biofilm formation, virulence factors, and antibiotic resistance in Pseudomonas aeruginosa: Results from a laboratory-based in vitro study. Antibiotics 2021, 10, 1134. [Google Scholar] [CrossRef]
- Horcajada, J.P.; Montero, M.; Oliver, A.; Sorlí, L.; Luque, S.; Gómez-Zorrilla, S.; Benito, N.; Grau, S. Epidemiology and treatment of multidrug-resistant and extensively drug-resistant Pseudomonas aeruginosa infections. Clin. Microbiol. Rev. 2019, 32, e00031-e19. [Google Scholar] [CrossRef]
- Pesingi, P.V.; Singh, B.R.; Pesingi, P.K.; Bhardwaj, M.; Singh, S.V.; Kumawat, M.; Sinha, D.K.; Gandham, R.K. MexAB-OprM efflux pump of Pseudomonas aeruginosa offers resistance to carvacrol: A herbal antimicrobial agent. Front. Microbiol. 2019, 10, 2664. [Google Scholar] [CrossRef]
- Hwnag, W.; Yoon, S.S. Virulence characteristics and an action mode of antibiotic resistance in multidrug-resistant Pseudomonas aeruginosa. Sci. Rep. 2019, 9, 487. [Google Scholar] [CrossRef]
- Daigle, D.M.; Cao, L.; Fraud, S.; Wilke, M.S.; Pacey, A.; Klinoski, R.; Strynadka, N.C.; Dean, C.R.; Poole, K. Protein modulator of multidrug efflux gene expression in Pseudomonas aeruginosa. J. Bacteriol. 2007, 189, 5441–5451. [Google Scholar] [CrossRef]
- Ghosh, S.; Cremers, C.M.; Jakob, U.; Love, N.G. Chlorinated phenols control the expression of the multidrug resistance efflux pump MexAB-OprM in Pseudomonas aeruginosa by interacting with NalC. Mol. Microbiol. 2011, 79, 1547–1556. [Google Scholar] [CrossRef]
- Chen, W.; Wang, D.; Zhou, W.; Sang, H.; Liu, X.; Ge, Z.; Zhang, J.; Lan, L.; Yang, C.G.; Chen, H.; et al. Novobiocin binding to NalD induces the expression of the MexAB-OprM pump in Pseudomonas aeruginosa. Mol. Microbiol. 2016, 100, 749–758. [Google Scholar] [CrossRef]
- Miyoshi-Akiyama, T.; Tada, T.; Ohmagari, N.; Viet Hung, N.; Tharavichitkul, P.; Pokhrel, B.M.; Gniadkowski, M.; Shimojima, M.; Kirikae, T. Emergence and spread of epidemic multidrug-resistant Pseudomonas aeruginosa. Genome Biol. Evol. 2017, 9, 3238–3245. [Google Scholar] [CrossRef]
- Woodford, N.; Turton, J.F.; Livermore, D.M. Multiresistant gram-negative bacteria: The role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol. Rev. 2011, 35, 736–755. [Google Scholar] [CrossRef]
- García-Castillo, M.; del Campo, R.; Morosini, M.I.; Riera, E.; Cabot, G.; Willems, R.; van Mansfeld, R.; Oliver, A.; Cantón, R. Wide dispersion of ST175 clone despite high genetic diversity of carbapenem-nonsusceptible Pseudomonas aeruginosa clinical strains in 16 Spanish hospitals. J. Clin. Microbiol. 2011, 49, 2905–2910. [Google Scholar] [CrossRef]
- Pragasam, A.; Veeraraghavan, B.; Anandan, S.; Narasiman, V.; Sistla, S.; Kapil, A.; Mathur, P.; Ray, P.; Wattal, C.; Bhattacharya, S.; et al. Dominance of international high-risk clones in carbapenemase producing Pseudomonas aeruginosa: Multicentric molecular epidemiology report from India. Indian J. Med. Microbiol. 2018, 36, 344–351. [Google Scholar] [CrossRef]


| Strain | Patient | ID | Source | H. Ward | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Suscept. | Carb | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GEN | TOB | AK | IMI | MEM | CAZ | CPM | CIP | LEV | CB * | P/T | AZT | FOS * | CS | |||||||
| 12 | MMV | HIM12/18 | MMV | NICU | 1024 | 128 | 256 | 256 | 128 | 32 | 256 | 32 | 32 | >1024 | 512 | 4 | 128 | 2 | MDR | M |
| 4 | P1 | HIM4/18 | B | STx | 512 | 128 | 256 | 256 | 64 | 64 | 128 | 32 | 32 | >1024 | 256 | 8 | 256 | 1 | XDR | M |
| 13 | HIM13/18 | B | STx | 1024 | 128 | 256 | 256 | 128 | 64 | 256 | 32 | 32 | >1024 | 512 | 4 | 256 | 2 | XDR | M | |
| 11 | P2 | HIM11/18 | B | O | 1024 | 128 | 256 | 256 | 128 | 64 | 256 | 4 | 32 | >1024 | 512 | 4 | 256 | 1 | XDR | M |
| 10 | HIM10/18 | B | O | 1024 | 128 | 256 | 256 | 128 | 32 | 256 | 32 | 32 | >1024 | 256 | 4 | 256 | 2 | XDR | M | |
| 3 | HIM3B/18 | B | O | 1024 | 128 | 256 | 256 | 64 | 64 | 256 | 32 | 32 | >1024 | 512 | 8 | 256 | 1 | XDR | M | |
| 9 | P3 | HIM9/18 | B | E | 1024 | 128 | 256 | 256 | 128 | 32 | 256 | 32 | 32 | >1024 | 256 | 8 | 256 | 2 | XDR | M |
| 1 | P4 | HIM1/18 | B | E | 1024 | 128 | 512 | 256 | 128 | 32 | 256 | 32 | 16 | >1024 | 256 | 8 | 128 | 2 | MDR | M |
| 2 | P5 | HIM2/18 | B | E | 1024 | 128 | 512 | 256 | 64 | 32 | 256 | 32 | 32 | >1024 | 512 | 8 | 256 | 1 | XDR | M |
| 15 | P6 | HIM15/18 | U | NICU | 2 | 8 | 8 | 2 | 0.25 | 1 | 4 | 0.125 | 0.5 | 64 | 8 | 4 | 128 | 2 | S | - |
| 8 | P7 | HIM8/18 | U | NICU | 0.125 | 16 | 16 | 128 | 128 | 32 | 32 | 16 | 16 | >1024 | 64 | 16 | 8 | 2 | MDR | M |
| 5 | P8 | HIM5/18 | B | NICU | 0.125 | 32 | 16 | 128 | 64 | 32 | 32 | 16 | 16 | >1024 | 64 | 8 | 4 | 1 | MDR | M |
| 14 | P9 | HIM14/18 | B | O | 1024 | 128 | 512 | 256 | 128 | 32 | 256 | 32 | 32 | >1024 | 256 | 4 | 128 | 2 | MDR | M |
| 7 | P10 | HIM7/18 | U | I | 256 | 128 | 256 | 16 | 128 | >1024 | 512 | 32 | 16 | >1024 | 128 | 64 | >1024 | 1 | XDR | S |
| 6 | HIM6/18 | U | I | 256 | 64 | 128 | 32 | 256 | >1024 | 1024 | 16 | 8 | >1024 | 512 | 256 | >1024 | 1 | XDR | S | |
| Strain | acsA | aroE | guaA | mutL | nuoD | ppsA | trpE | ST |
|---|---|---|---|---|---|---|---|---|
| 1 | 83 | 5 | 30 | 218 | 4 | 31 | 41 | 3237 |
| 2 | 83 | 5 | 30 | 11 | 45 | 31 | 41 | 3749 |
| 3 | 16 | 5 | 30 | 11 | 45 | 31 | 41 | 3238 |
| 4 | 16 | 5 | 30 | 11 | 45 | 31 | 41 | 3238 |
| 5 | 63 | 5 | 5 | 4 | 45 | 4 | 3 | 3750 |
| 6 | 82 | 91 | 3 | 13 | 1 | 2 | 4 | 3239 |
| 7 | 38 | 11 | 3 | 13 | 1 | 2 | 4 | 235 * |
| 8 | 17 | 5 | 5 | 4 | 45 | 4 | 3 | 3240 |
| 9 | 16 | 5 | 30 | 218 | 45 | 31 | 41 | 3241 |
| 10 | 16 | 5 | 30 | 218 | 45 | 31 | 41 | 3241 |
| 11 | 16 | 5 | 30 | 218 | 45 | 31 | 41 | 3241 |
| 12 | 16 | 5 | 30 | 140 | 45 | 31 | 41 | 3751 |
| 13 | 16 | 5 | 30 | 218 | 45 | 31 | 41 | 3241 |
| 14 | 16 | 5 | 30 | 216 | 45 | 31 | 41 | 3752 |
| 15 | 7 | 5 | 7 | 7 | 45 | 12 | 7 | 3242 |
| Repressor Gene | Genetic Variation | Total n = 15 | Nucleotide Variations | Amino Acid Variation |
|---|---|---|---|---|
| mexR | No substitution | 11 | - | - |
| Synonymous substitution (n = 5) | 4 | 60G→A, 264C→T, 327G→A, 384G→A, 411G→A | V20V, S88S, E109E, Q128Q, Q137Q | |
| Nonsynonymous substitution (n = 2) | 4 | 377T→A, 392T→A | V126E, L131Q | |
| nalC | No substitution | 0 | - | - |
| Synonymous substitution (n = 15) | 3 | 12T→G, 15T→C, 69T→C, 123A→T, 147G→A, 177G→A, 354C→T, 358C→A, 369G→A, 411T→C, 415C→T, 420C→G, 426G→A, 435C→A, 447T→C | A4A, S5S, A23A, I41I, G49G, E59E, S118S, R120R, A123A, Y137Y, L139L, E142E, A145A, A145A, P149P | |
| Nonsynonymous substitution (n = 15) | 15 | 194T→G, 212G→A, 223G→T, 283G→T, 402G→C, 422G→T, 428G→A, 433G→A, 440T→C, 457G→C, 459G→T, 486G→C, 517C→A, 556G→A, 625A→C | V65G, G71E, D75Y, G95C, Q134H, S141I, R143Q, A145T, V147A, E153Q, E153D, Q162H, L173I, A186T, S209R | |
| nalD | No substitution | 11 | - | - |
| Synonymous substitution (n = 5) | 4 | 169C→T, 276C→T, 295T→C, 333C→T, 540C→T | L57L, C92C, L99L, I111I, D180D | |
| Nonsynonymous substitution (n = 0) | 0 | - | - |
| Strain | ST | Haplotype | mexR | nalC | nalD | Total Mutations |
|---|---|---|---|---|---|---|
| 1 | 3237 | 12 | G71E, E153D, A186T | 3 | ||
| 2 | 3749 | |||||
| 3, 4 | 3238 | |||||
| 10, 11, 13 | 3241 | |||||
| 12 | 3751 | |||||
| 14 | 3752 | |||||
| 5 | 3750 | 28 | S88S, E109E, Q128Q, Q137Q, V126E | G71E | L57L | 7 |
| 8 | 3240 | |||||
| 6 | 3239 | 29 | V20V, E109E, Q128Q, Q137Q, V126E | A4A, S5S, A23A, I41I, G49G, E59E, S118S, R120R, A123A, Y137Y, A145A, P149P, G71E, E153Q, S209R | C92C, L99L, I111I, D180D | 24 |
| 7 | 235 | 30 | V20V, E109E, Q128Q, Q137Q, V126E, L131Q | A4A, S5S, A23A, I41I, G49G, E59E, S118S, R120R, A123A, Y137Y, A145A, P149P, G71E, E153Q, S209R | C92C, L99L, I111I, D180D | 25 |
| 9 | 3241 | 31 | L139L, E142E, A145A, V65G, G71E, D75Y, G95C, Q134H, S141I, R143Q, A145T, V147A, Q162H, L173I | 14 | ||
| 15 | 3242 | 32 | G71E | 1 | ||
| Number of haplotypes | 3 | 4 | 3 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar-Rodea, P.; Estrada-Javier, E.L.; Jiménez-Rojas, V.; Gomez-Ramirez, U.; Nolasco-Romero, C.G.; Rodea, G.E.; Rodríguez-Espino, B.A.; Mendoza-Elizalde, S.; Arellano, C.; López-Marcelino, B.; et al. New Variants of Pseudomonas aeruginosa High-Risk Clone ST233 Associated with an Outbreak in a Mexican Paediatric Hospital. Microorganisms 2022, 10, 1533. https://doi.org/10.3390/microorganisms10081533
Aguilar-Rodea P, Estrada-Javier EL, Jiménez-Rojas V, Gomez-Ramirez U, Nolasco-Romero CG, Rodea GE, Rodríguez-Espino BA, Mendoza-Elizalde S, Arellano C, López-Marcelino B, et al. New Variants of Pseudomonas aeruginosa High-Risk Clone ST233 Associated with an Outbreak in a Mexican Paediatric Hospital. Microorganisms. 2022; 10(8):1533. https://doi.org/10.3390/microorganisms10081533
Chicago/Turabian StyleAguilar-Rodea, Pamela, Elia L. Estrada-Javier, Verónica Jiménez-Rojas, Uriel Gomez-Ramirez, Carolina G. Nolasco-Romero, Gerardo E. Rodea, Benjamín Antonio Rodríguez-Espino, Sandra Mendoza-Elizalde, Cesar Arellano, Beatriz López-Marcelino, and et al. 2022. "New Variants of Pseudomonas aeruginosa High-Risk Clone ST233 Associated with an Outbreak in a Mexican Paediatric Hospital" Microorganisms 10, no. 8: 1533. https://doi.org/10.3390/microorganisms10081533
APA StyleAguilar-Rodea, P., Estrada-Javier, E. L., Jiménez-Rojas, V., Gomez-Ramirez, U., Nolasco-Romero, C. G., Rodea, G. E., Rodríguez-Espino, B. A., Mendoza-Elizalde, S., Arellano, C., López-Marcelino, B., de la Rosa Zamboni, D., Gamiño-Arroyo, A. E., Mora-Suárez, R., Torres García, M., Franco Hernández, I., Parra-Ortega, I., Campos-Valdez, G., Velázquez-Guadarrama, N., & Rosas-Pérez, I. (2022). New Variants of Pseudomonas aeruginosa High-Risk Clone ST233 Associated with an Outbreak in a Mexican Paediatric Hospital. Microorganisms, 10(8), 1533. https://doi.org/10.3390/microorganisms10081533









