Effects of Volatile Organic Compounds on Biofilms and Swimming Motility of Agrobacterium tumefaciens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacteria and Conditions of Growth
2.2. Detection of the Effect of VOCs on the Formation of Colony Biofilms
2.3. Detection of the Effect of VOCs on Bacterial Cell Survival in Mature Biofilms
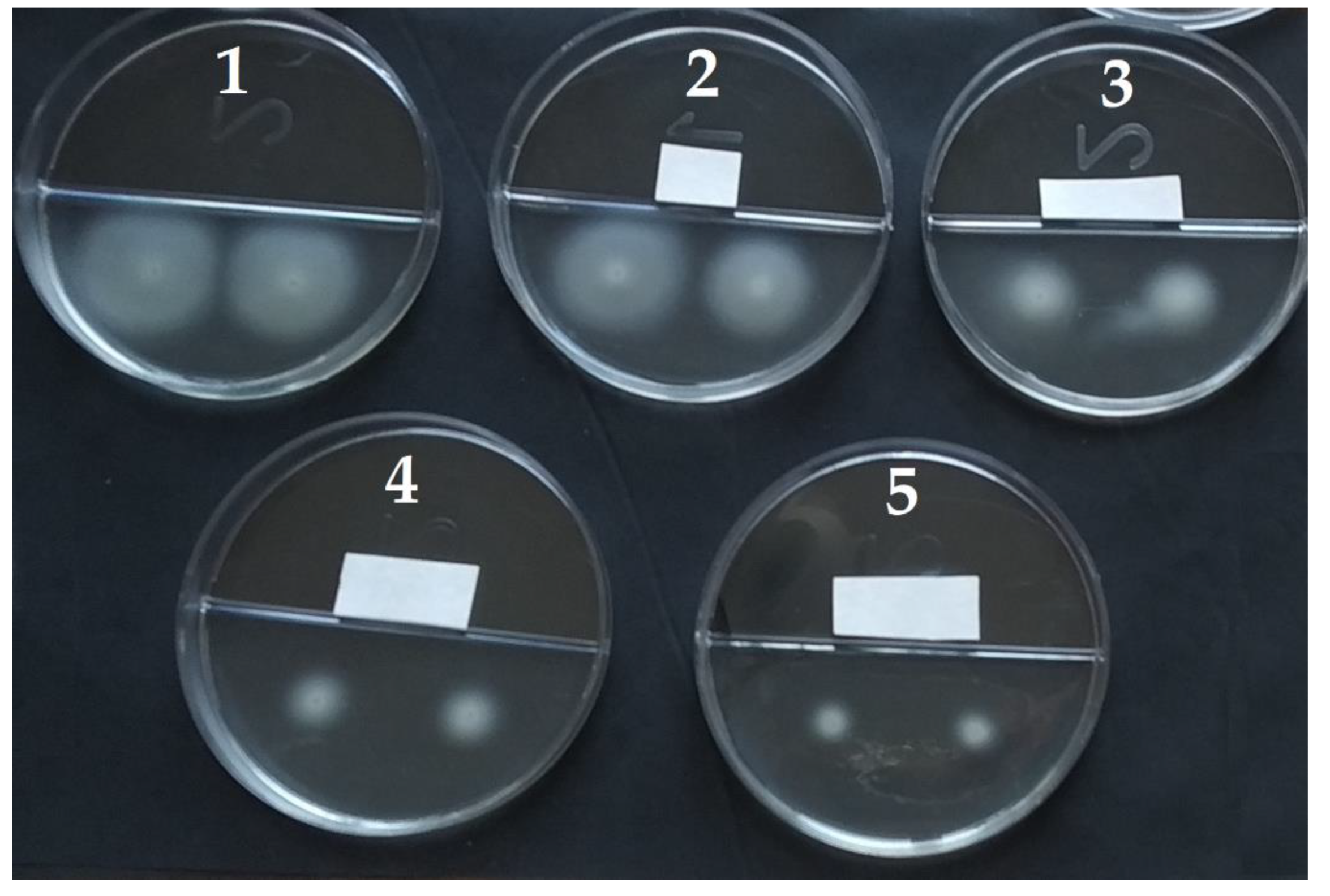
2.4. Influence of VOCs on the Motility of A. tumefaciens Strains
2.5. Statistical Analysis
3. Results
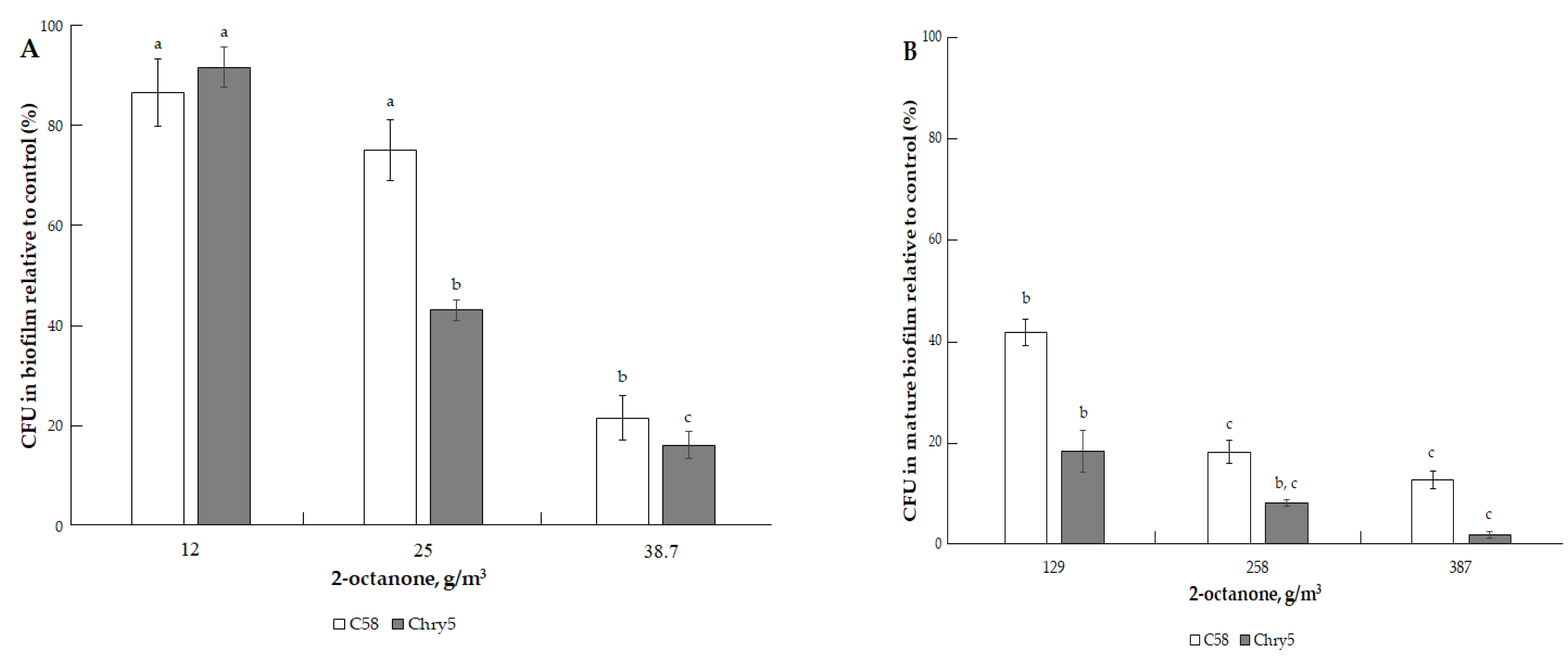
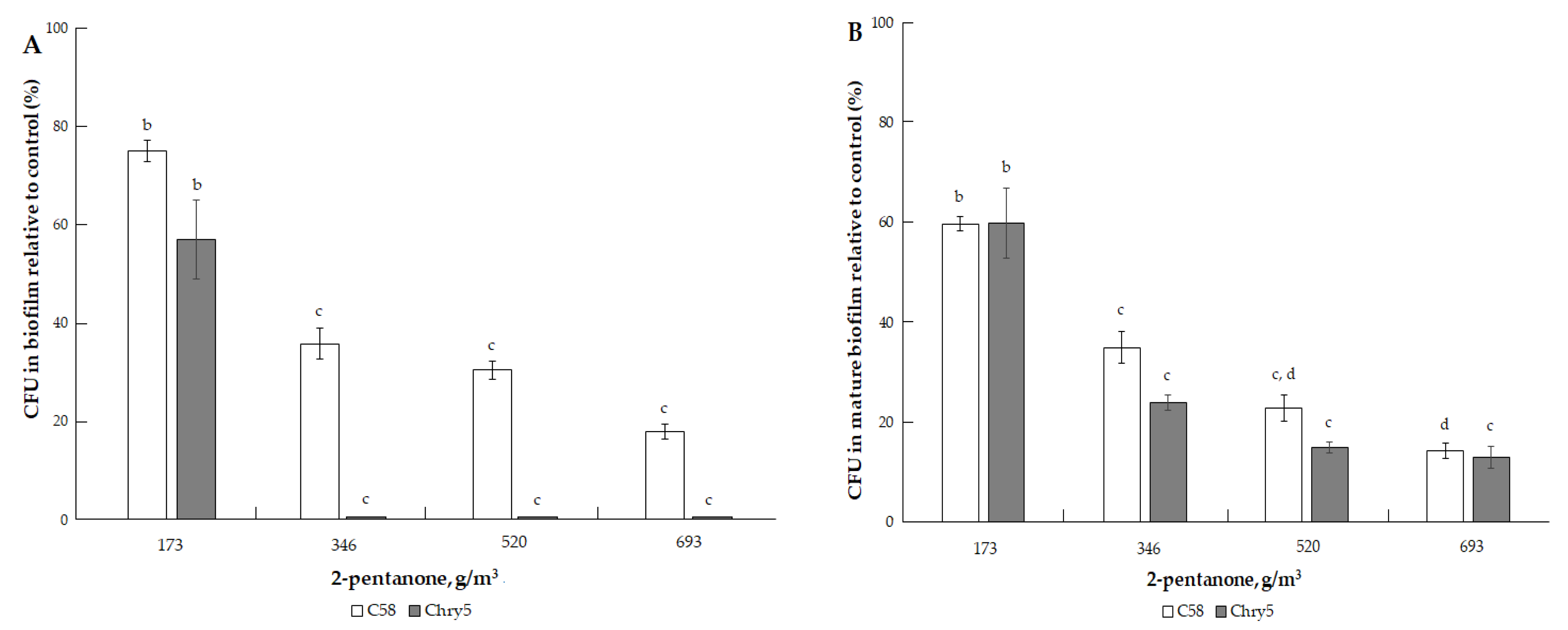
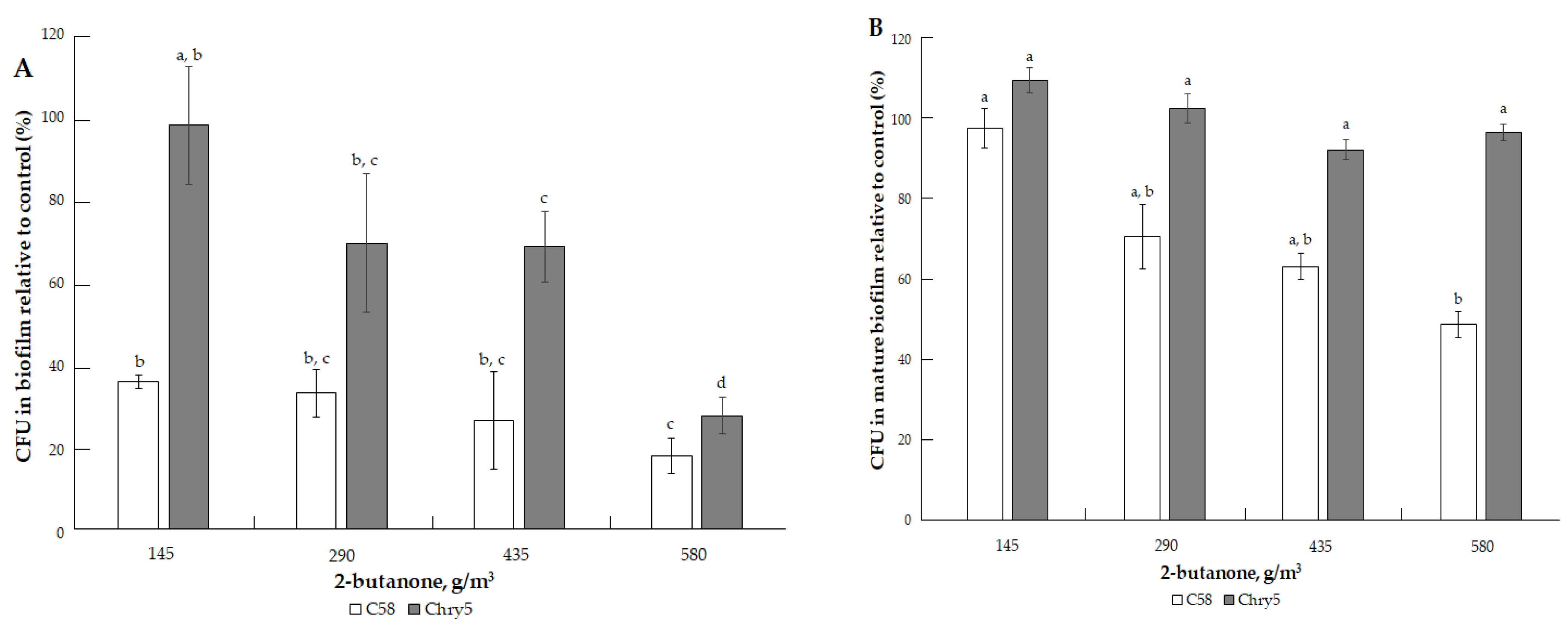
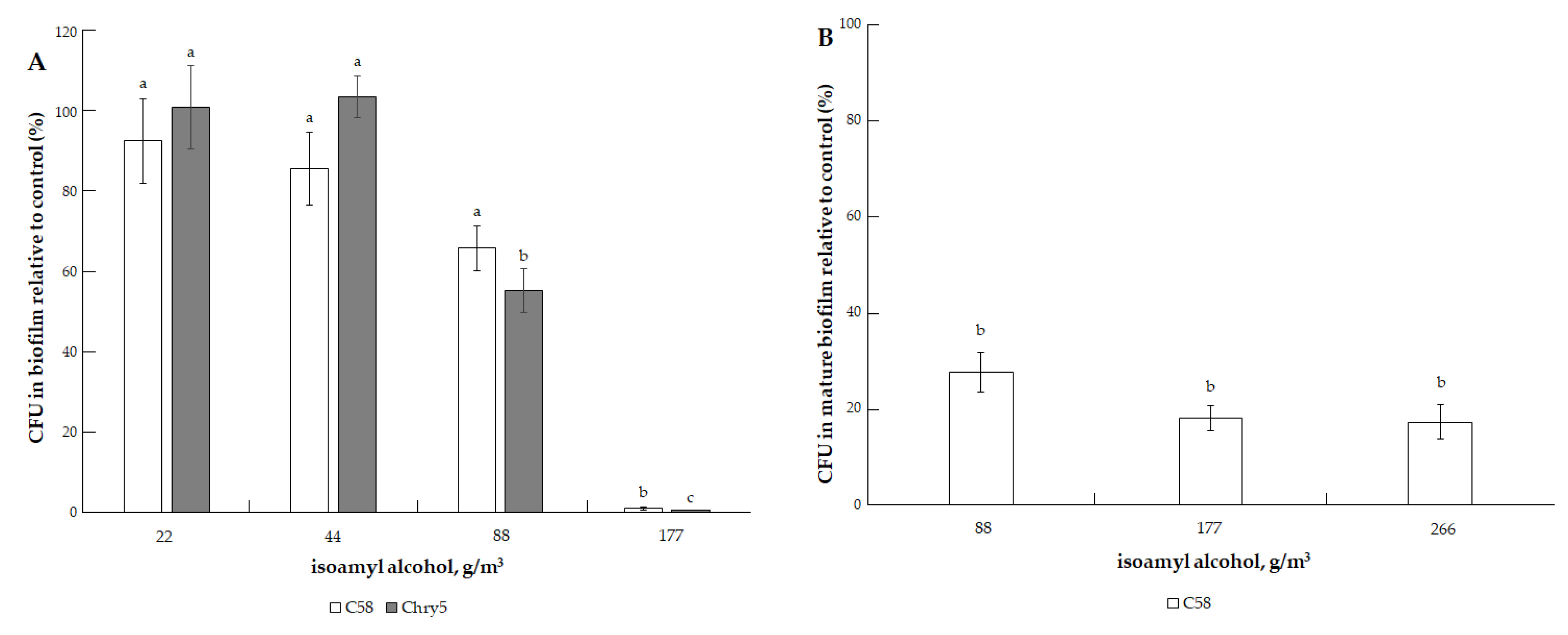
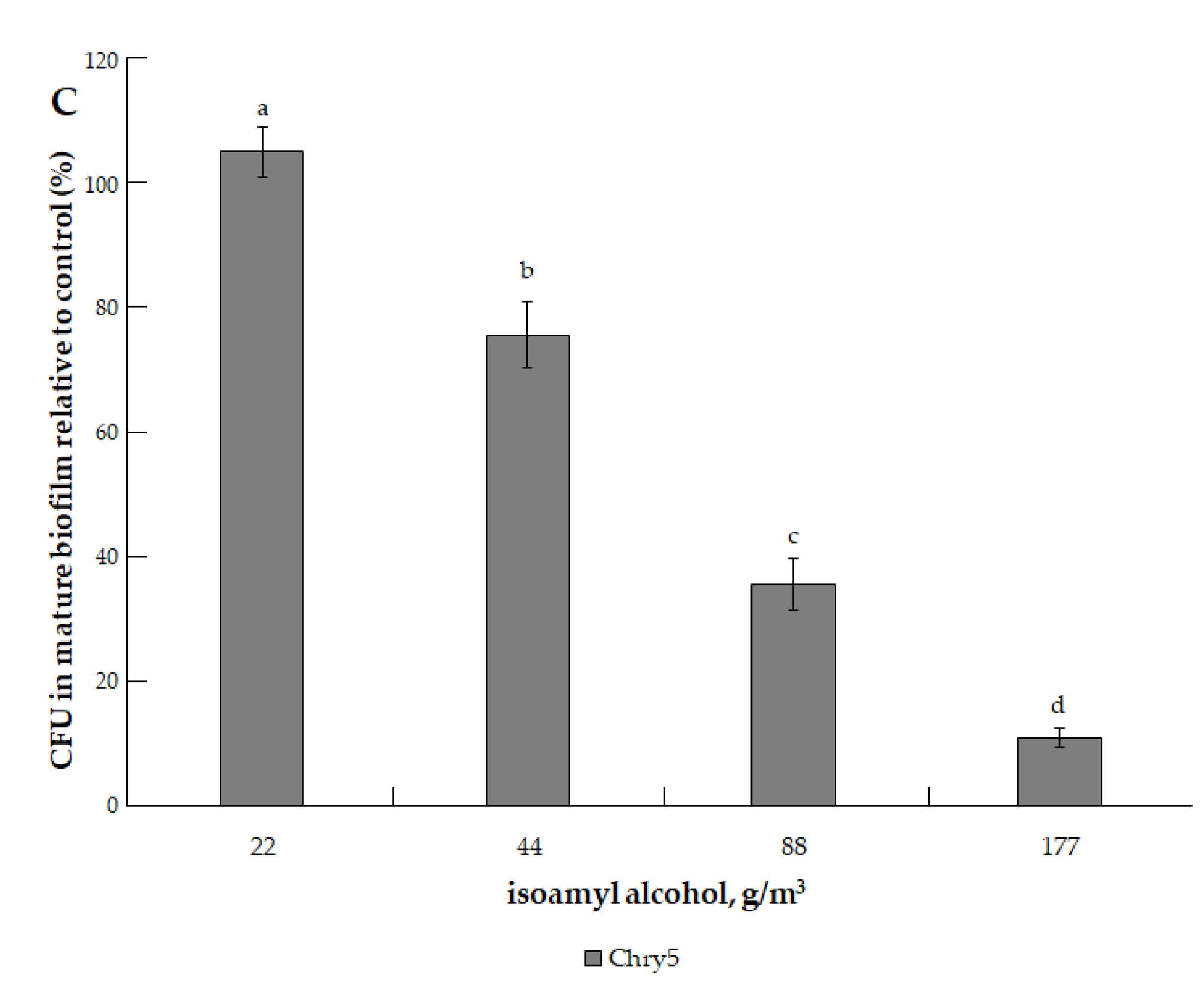
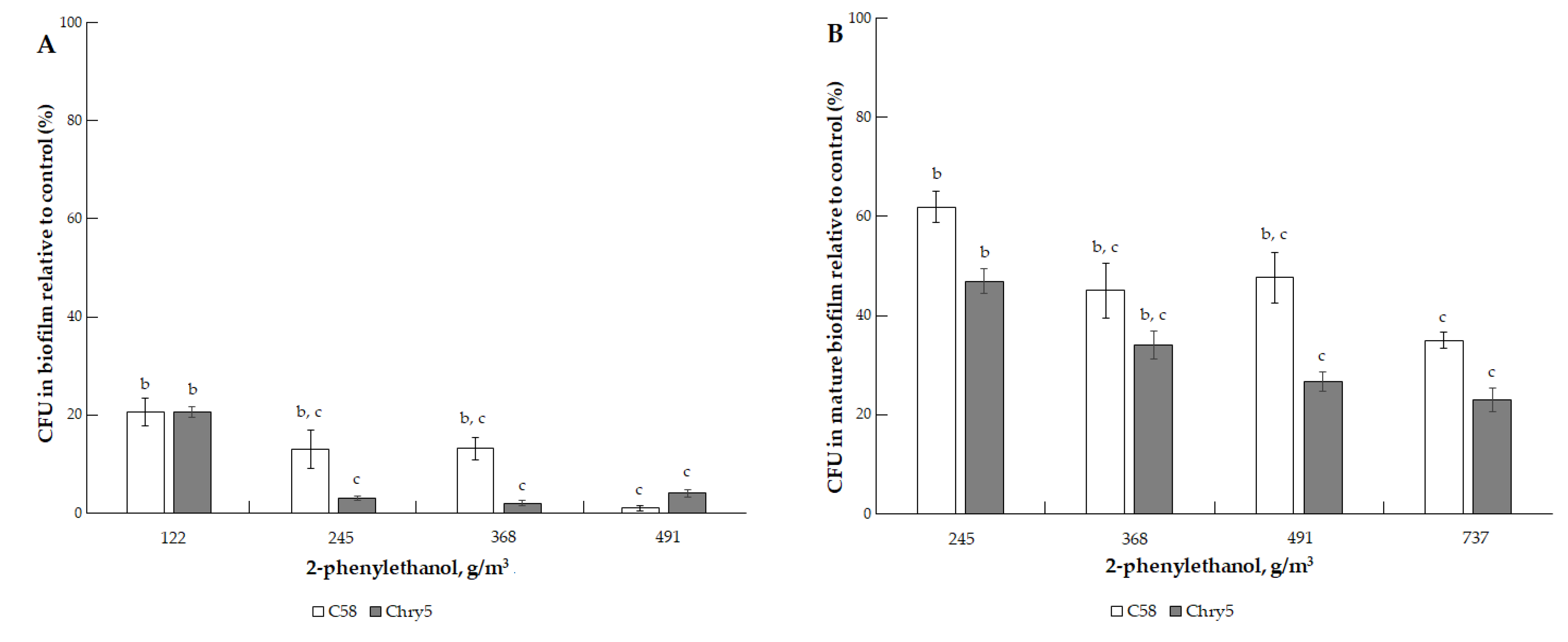
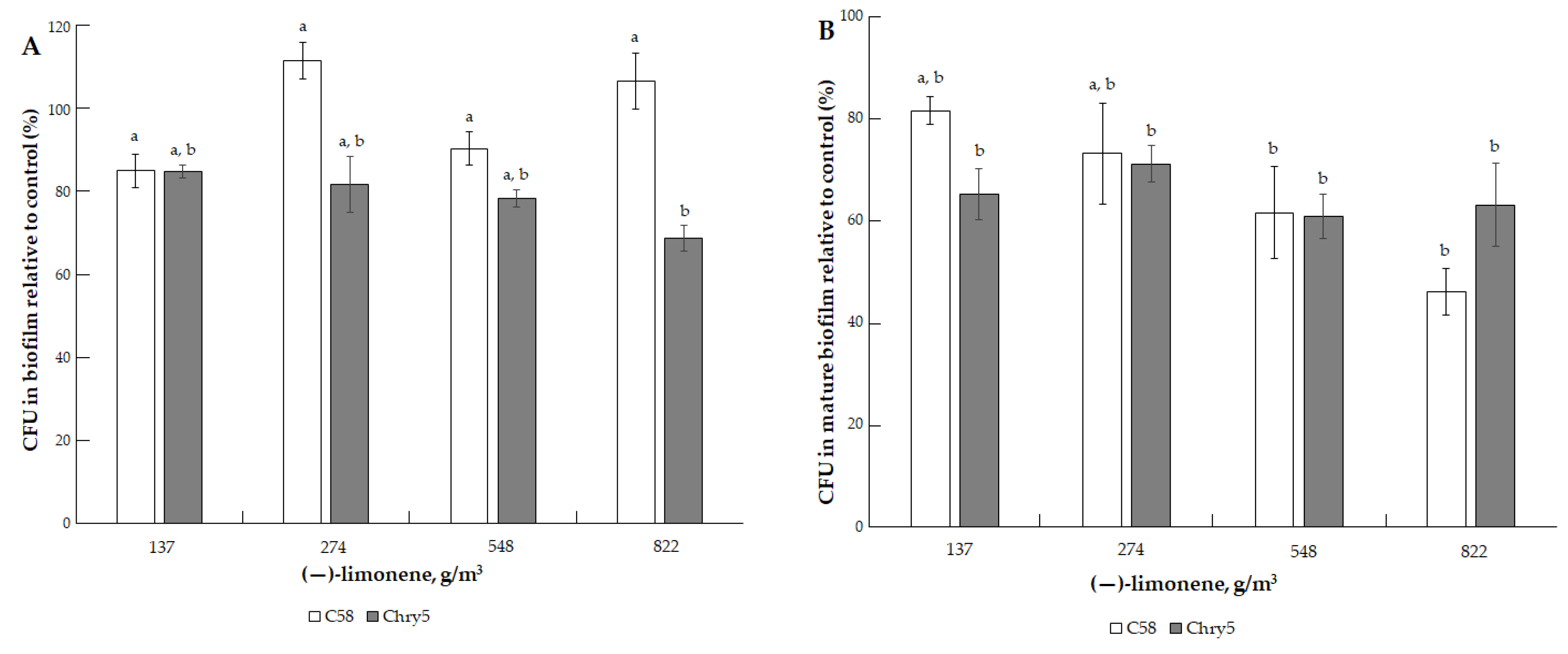
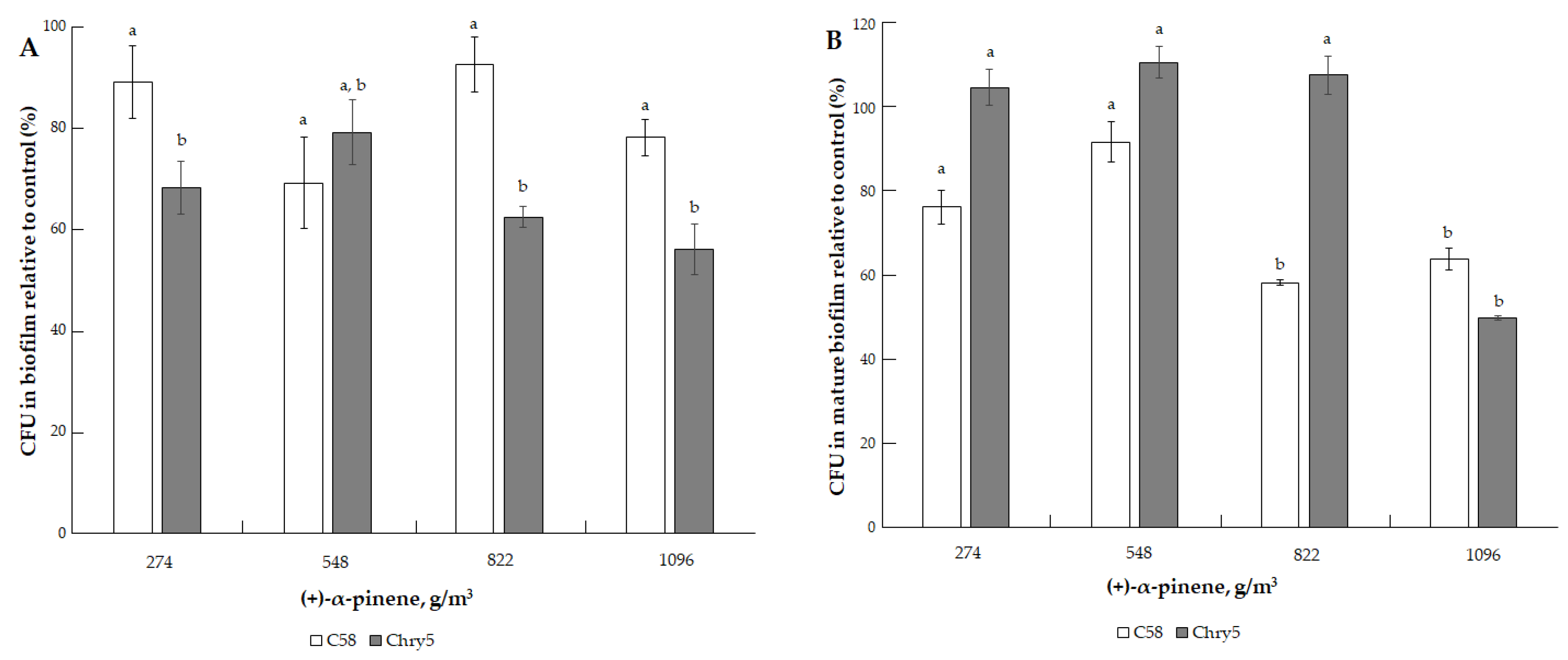
3.1. The Effects of VOCs on A. tumefaciens Colony Biofilms
3.2. The Action of VOCs on the Swimming Motility of A. tumefaciens
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kai, M.; Haustein, M.; Molina, F.; Petri, A.; Scholz, B.; Piechulla, B. Bacterial Volatiles and Their Action Potential. Appl. Microbiol. Biotechnol. 2009, 81, 1001–1012. [Google Scholar] [CrossRef]
- Effmert, U.; Kalderás, J.; Warnke, R.; Piechulla, B. Volatile Mediated Interactions Between Bacteria and Fungi in the Soil. J. Chem. Ecol. 2012, 38, 665–703. [Google Scholar] [CrossRef] [PubMed]
- Audrain, B.; Farag, M.A.; Ryu, C.M.; Ghigo, J.M. Role of Bacterial Volatile Compounds in Bacterial Biology. FEMS Microbiol. Rev. 2015, 39, 222–233. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, R.; Cordovez, V.; De Boer, W.; Raaijmakers, J.; Garbeva, P. Volatile Affairs in Microbial Interactions. ISME J. 2015, 9, 2329–2335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyc, O.; Song, C.; Dickschat, J.S.; Vos, M.; Garbeva, P. The Ecological Role of Volatile and Soluble Secondary Metabolites Produced by Soil Bacteria. Trends Microbiol. 2017, 25, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Piechulla, B.; Lemfack, M.C.; Magnus, N. Chapter 2. Bioactive Bacterial Volatiles: An Overview and Critical Comments. In Bacterial Volatile Compounds as Mediators of Airborne Interactions; Ryu, C.-M., Weisskopf, L., Piechulla, B., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2020; pp. 39–92. [Google Scholar] [CrossRef]
- Veselova, M.A.; Plyuta, V.A.; Khmel, I.A. Volatile Compounds of Bacterial Origin: Structure, Biosynthesis, and Biological Activity. Microbiology 2019, 88, 261–274. [Google Scholar] [CrossRef]
- Netzker, T.; Shepherdson, E.M.F.; Zambri, M.P.; Elliot, M.A. Bacterial Volatile Compounds: Functions in Communication, Cooperation, and Competition. Annu. Rev. Microbiol. 2020, 74, 409–430. [Google Scholar] [CrossRef]
- Lemfack, M.C.; Gohlke, B.O.; Toguem, S.M.T.; Preissner, S.; Piechulla, B.; Preissner, R. MVOC 2.0: A Database of Microbial Volatiles. Nucleic Acids Res. 2018, 46, D1261–D1265. [Google Scholar] [CrossRef] [Green Version]
- Ryu, C.M.; Farag, M.A.; Hu, C.H.; Reddy, M.S.; Kloepper, J.W.; Paré, P.W. Bacterial Volatiles Induce Systemic Resistance in Arabidopsis. Plant Physiol. 2004, 134, 1017–1026. [Google Scholar] [CrossRef] [Green Version]
- Piechulla, B.; Degenhardt, J. The Emerging Importance of Microbial Volatile Organic Compounds. Plant Cell Environ. 2014, 37, 811–812. [Google Scholar] [CrossRef] [Green Version]
- Schulz-Bohm, K.; Martín-Sánchez, L.; Garbeva, P. Microbial Volatiles: Small Molecules with an Important Role in Intra- and Inter-Kingdom Interactions. Front. Microbiol. 2017, 8, 2484. [Google Scholar] [CrossRef] [PubMed]
- Avalos, M.; van Wezel, G.P.; Raaijmakers, J.M.; Garbeva, P. Healthy Scents: Microbial Volatiles as New Frontier in Antibiotic Research? Curr. Opin. Microbiol. 2018, 45, 84–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fincheira, P.; Quiroz, A. Microbial Volatiles as Plant Growth Inducers. Microbiol. Res. 2018, 208, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Weisskopf, L.; Schulz, S.; Garbeva, P. Microbial Volatile Organic Compounds in Intra-Kingdom and Inter-Kingdom Interactions. Nat. Rev. Microbiol. 2021, 19, 391–404. [Google Scholar] [CrossRef]
- Popova, A.A.; Koksharova, O.A.; Lipasova, V.A.; Zaitseva, J.V.; Katkova-Zhukotskaya, O.A.; Eremina, S.I.; Mironov, A.S.; Chernin, L.S.; Khmel, I.A. Inhibitory and Toxic Effects of Volatiles Emitted by Strains of Pseudomonas and Serratia on Growth and Survival of Selected Microorganisms, Caenorhabditis Elegans, and Drosophila Melanogaster. Biomed Res. Int. 2014, 2014, 125704. [Google Scholar] [CrossRef] [Green Version]
- Plyuta, V.A.; Chernikova, A.S.; Sidorova, D.E.; Kupriyanova, E.V.; Koksharova, O.A.; Chernin, L.S.; Khmel, I.A. Modulation of Arabidopsis thaliana Growth by Volatile Substances Emitted by Pseudomonas and Serratia Strains. World J. Microbiol. Biotechnol. 2021, 37, 82. [Google Scholar] [CrossRef]
- Sidorova, D.E.; Plyuta, V.A.; Padiy, D.A.; Kupriyanova, E.V.; Roshina, N.V.; Koksharova, O.A.; Khmel, I.A. The Effect of Volatile Organic Compounds on Different Organisms: Agrobacteria, Plants and Insects. Microorganisms 2022, 10, 69. [Google Scholar] [CrossRef]
- Davies, D.G.; Parsek, M.R.; Pearson, J.P.; Iglewski, B.H.; Costerton, J.W.; Greenberg, E.P. The Involvement of Cell-to-Cell Signals in the Development of a Bacterial Biofilm. Science 1998, 280, 295–298. [Google Scholar] [CrossRef] [Green Version]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [Green Version]
- Morris, C.E.; Monier, J.M. The Ecological Significance of Biofilm Formation by Plant-Associated Bacteria. Annu. Rev. Phytopathol. 2003, 41, 429–453. [Google Scholar] [CrossRef]
- Danhorn, T.; Fuqua, C. Biofilm Formation by Plant-Associated Bacteria. Annu. Rev. Microbiol. 2007, 61, 401–422. [Google Scholar] [CrossRef] [PubMed]
- Rudrappa, T.; Biedrzycki, M.L.; Bais, H.P. Causes and Consequences of Plant-Associated Biofilms. FEMS Microbiol. Ecol. 2008, 64, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Plyuta, V.; Lipasova, V.; Popova, A.; Koksharova, O.; Kuznetsov, A.; Szegedi, E.; Chernin, L.; Khmel, I. Influence of Volatile Organic Compounds Emitted by Pseudomonas and Serratia Strains on Agrobacterium tumefaciens Biofilms. Apmis 2016, 124, 586–594. [Google Scholar] [CrossRef]
- Sciaky, D.; Montoya, A.L.; Chilton, M.D. Fingerprints of Agrobacterium Ti Plasmids. Plasmid 1978, 1, 238–253. [Google Scholar] [CrossRef]
- Bush, A.L.; Pueppke, S.G. Characterization of an Unusual New Agrobacterium tumefaciens Strain from Chrysanthemum Morifolium Ram. Appl. Environ. Microbiol. 1991, 57, 2468–2472. [Google Scholar] [CrossRef] [Green Version]
- Merritt, J.H.; Kadouri, D.E.; O’Toole, G.A. Growing and Analytical Statistic Biofilms. Curr. Protoc. Microbiol. 2005, 1, Unit 1B.1. [Google Scholar] [CrossRef] [Green Version]
- Peterson, S.B.; Irie, Y.; Borlee, B.R.; Murakami, K.; Harrison, J.J.; Colvin, K.M.; Parsek, M.R. Chapter 15. Different Methods for Culturing Biofilms In Vitro. In Biofilm Infections; Bjarnsholt, T., Jensen, P., Moser, C., Høiby, N., Eds.; Springer: New York, NY, USA, 2011; pp. 251–266. [Google Scholar] [CrossRef]
- Déziel, E.; Comeau, Y.; Villemur, R. Initiation of Biofilm Formation by Pseudomonas aeruginosa 57RP Correlates with Emergence of Hyperpiliated and Highly Adherent Phenotypic Variants Deficient in Swimming, Swarming, and Twitching Motilities. J. Bacteriol. 2001, 183, 1195–1204. [Google Scholar] [CrossRef] [Green Version]
- Givskov, M.; de Nys, R.; Manefield, M.; Gram, L.; Maximilien, R.; Eberl, L.; Molin, S.; Steinberg, P.D.; Kjelleberg, S. Eukaryotic interference with homoserine lactone-mediated prokaryotic signalling. J. Bacteriol. 1996, 178, 6618–6622. [Google Scholar] [CrossRef] [Green Version]
- Daubert, T.E.; Danner, R.P. Physical and Thermodynamic Properties of Pure Chemicals: Data Compilation; Hemisphere Publishing Corporation: New York, NY, USA, 1989; ISBN 0891169482. [Google Scholar]
- Lide, D.R. (Ed.) CRC Handbook of Chemistry and Physics, Internet Version 2006, 86th ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2006; ISBN 0849304865. [Google Scholar]
- Riddick, J.A.; Bunger, W.B.; Sakano, T.K. Techniques of Chemistry, Organic Solvents, 4th ed.; John Wiley and Sons: New York, NY, USA, 1986; Volume II, ISBN 0471084670. [Google Scholar]
- Korpi, A.; Järnberg, J.; Pasanen, A.L. Microbial volatile organic compounds. Crit. Rev. Toxicol. 2009, 39, 139–193. [Google Scholar] [CrossRef]
- Alarie, Y.; Nielsen, G.D.; Andonian-Haftvan, J.; Abraham, M.H. Physicochemical properties of nonreactive volatile organic chemicals to estimate RD50: Alternatives to animal studies. Toxicol. Appl. Pharmacol. 1995, 134, 92–99. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency DSSTox. DTXSID5021916. Available online: https://comptox.epa.gov/dashboard/DTXSID5021916 (accessed on 23 July 2022).
- Fichan, I.; Larroche, C.; Gros, J.B. Water Solubility, Vapor Pressure, and Activity Coefficients of Terpenes and Terpenoids. J. Chem. Eng. Data 1998, 44, 56–62. [Google Scholar] [CrossRef]
- Sell, C.S. Terpenoids. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons: New York, NY, USA, 2006; Volume 24. [Google Scholar] [CrossRef]
- OECD. SIDS Initial Assessment Report for SIAM 20, beta-Ionone (CAS 79-77-6), April 2004, UNEP Publications. 13 July 2015. Available online: https://hpvchemicals.oecd.org/ui/handler.axd?id=27DDCF61-620E-4C8B-A7ED-606148694B0B (accessed on 23 July 2022).
- U.S. Environmental Protection Agency DSSTox. DTXSID6047078. Available online: https://comptox.epa.gov/dashboard/DTXSID6047078 (accessed on 23 July 2022).
- Nadais, M.H.; Bernardo-Gil, M.G. Vapour—liquid equilibria of α-pinene + limonene at reduced pressures. Fluid Phase Equilibria 1993, 91, 321–330. [Google Scholar] [CrossRef]
- Nikunen, E.; Leinonen, R.; Kemilainen, B.; Kultamaa, A. Environmental Properties of Chemicals; Finnish Environment Institute, Edita: Helsinki, Finland, 2000; Volume 1, ISBN 952-11-0670-0. Available online: https://helda.helsinki.fi/handle/10138/228880 (accessed on 30 May 2022).
- Merritt, P.M.; Danhorn, T.; Fuqua, C. Motility and chemotaxis in Agrobacterium tumefaciens surface attachment and biofilm formation. J. Bacteriol. 2007, 189, 8005–8014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunesson, A.-L. Volatile Metabolites from Microorganisms in Indoor Environments—Sampling, Analysis and Identification. Ph.D. Thesis, Umea University and National Institute for Working Life, Umea, Sweden, 1995; pp. 1–88. [Google Scholar]
- Saha, S.; Pal, D. Chapter 14. log P. In Encyclopedia of Physical Organic Chemistry; Wang, Z., Wille, U., Juaristi, E., Eds.; Wiley InterScience: New York, NY, USA, 2017; pp. 629–650. [Google Scholar] [CrossRef]
- Kim, K.S.; Lee, S.; Ryu, C.M. Interspecific Bacterial Sensing through Airborne Signals Modulates Locomotion and Drug Resistance. Nat. Commun. 2013, 4, 1809–1812. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| VOC | M, g/mol | Vapor Pressure of VOC, mm Hg (Pa) at 25 °C | Boiling Point of VOC, °C (K) at 760 mm Hg (101.3 kPa) | Octanol: Water Partition Coefficient, log P (log Kow) | Amount of VOC, µmol | Concentration of VOC *, g/m3 |
|---|---|---|---|---|---|---|
| alcohols | ||||||
| 2-phenylethanol (phenethyl alcohol, C8H10O) | 122.16 | 0.0868 mm Hg (11.57 Pa) [31]. | 218.2 °C (491.35 K) [32]. | 1.36 (exp) [32]. | 25, 50, 100, 200, 300, 400, and 600 µmol | 30.74, 61.47, 122.94, 245.89, 368.83, 491.78, and 737.67 g/m3 |
| isoamyl alcohol (3-methyl-1-butanol, C5H12O) | 88.15 | 2.37 mm Hg (315.97 Pa) [33]. | 131.1 °C (404.25 K) [32]. | 1.16 (exp) [34]. | 25, 50, 75, 100, 200, 300, and 400 µmol | 22.18, 44.36, 66.54, 88.71, 177.43, 266.14, and 354.86 g/m3 |
| ketones | ||||||
| 2-butanone (C4H8O) | 72.11 | 90.6 mm Hg (12079 Pa) [35]. | 79.59 °C (352.74 K) [32]. | 0.26; 0.29 (exp) [34]. | 200, 400, 600, and 800 µmol | 145.15, 290.29, 435.44, and 580.58 g/m3 |
| 2-pentanone (C5H10O) | 86.13 | 35.4 mm Hg (4719.6 Pa) [33]. | 102.26 °C (375.41 K) [32]. | 0.84; 0.91 (exp) [32,34]. | 50, 100, 200, 300, 400, 600 and 800 µmol | 43.34, 86.68, 173.37, 260.05, 346.73, 520.10, and 693.46 g/m3 |
| 2-heptanone ** (C7H14O) | 114.19 | 3.85 mm Hg (513.29 Pa) [36] | 151.05 °C (424.2K) [32]. | 1.98 [32]. | 25, 50, 75, 100, 200, 300, and 400 * µmol | 28.73, 57.46, 86.19, 114.92, 229.84, 344.76, and 459.68 * g/m3 |
| 2-octanone (C8H16O) | 128.22 | 1.35 mm Hg (179.99 Pa) [33] | 172.5 °C (445.65 K) [32]. | 2.37 (cal) [34]. | 10, 20, 30, 50, 100,200, and 300 µmol | 12.90, 25.81, 38.71, 64.52, 129.04, 258.09, and 387.13 g/m3 |
| 2-nonanone ** (C9H18O) | 142.24 | 0.645 mm Hg (85 Pa) [34]. | 195.3 °C (468.45 K) [32]. | 3.16 (cal) [32]. | 5, 10, 15, 20, 25, 35, and 50 * µmol | 7.16, 14.32, 21.47, 28.63, 35.79, 50.10, and 71.58 * g/m3 |
| 2-undecanone ** (C11H22O) | 170.30 | 0.0975 mm Hg (13 Pa) [34]. | 231.5 °C (504.65 K) [32]. | 4.09 (cal) [32]. | 25, 50, 100, 200, 300, 400, and 600 * µmol | 42.85, 85.70, 171.39, 342.79, 514.18, 685.57, and 1028.36 * g/m3 |
| β-ionone (unsaturated ketone, C13H20O) | 192.30 | 0.054 mm Hg (7.2 Pa) [37]. | 271 °C (544.15 K) [38]. | 3.995 (exp) [39]. | 25, 50, 100, 200, 400, 600, and 800 µmol | 48.38, 96.77, 193.53, 387.07, 774.14, 1161.21, and 1548.28 g/m3 |
| terpenes | ||||||
| (–)-limonene (C10H16) | 136.24 | 1.55 mm Hg (206.65 Pa) [40]. | 177.5 °C (450.65 K) [41]. | 4.57 (cal) [34]. | 100, 200, 400, 500, and 600 µmol | 137.11, 274.23, 548.46, 685.57, and 822.69 g/m3 |
| (+)-α-pinene (C10H16) | 136.23 | 4.75 mm Hg (633.28 Pa) [31]. | 155.6 °C (428.75 K) [41]. | 4.83 (cal) [34]. | 50, 100, 200, 400, 600, and 800 µmol | 68.55, 137.10, 274.21, 548.42, 822.63, and 1096.84 g/m3 |
| sulfur compound | ||||||
| Dimethyl disulfide ** (DMDS, C2H6S2) | 94.19 | 28.7 mm Hg (3830 Pa) [34]. | 109.74 °C (382.89 K) [32]. | 1.77 (exp) [34]. | 50, 100, 150, 200, 300, 400, and 600 * µmol | 47.40, 94.79, 142.19, 189.59, 284.38, 379.18 and 568.77 * g/m3 |
| Concentration of VOCs, g/m3 (μmol) | Radius of A. tumefaciens Swimming Zones Relative to Control (%) | |
|---|---|---|
| C58 | Chry5 | |
| 2-butanone | ||
| 145.15 (200) | (78.5 ± 2.5) b | (97.7 ± 5.3) a |
| 290.29 (400) | (52.9 ± 2.5) c | (60.9 ± 2.0) b |
| 435.44 (600) | (38.6 ± 4.3) d | (29.9 ± 2.0) c |
| 580.58 (800) | (24.3 ± 6.6) e | (25.3 ± 4.0) c |
| 2-pentanone | ||
| 43.34 (50) | (82.9 ± 5.0) a | (93.2 ± 2.0) a |
| 86.68 (100) | (57.1 ± 2.5) b | (77.3 ± 5.2) b |
| 173.37 (200) | (42.9 ± 4.3) b,c | (38.6 ± 2.0) c |
| 260.05 (300) | (32.9 ± 13.8) c | (21.6 ± 7.1) d |
| 2-octanone | ||
| 12.90 (10) | (85.3 ± 4.6) a,b | (79.6 ± 10.4) b |
| 25.81 (20) | (81.3 ± 8.3) b | (71.6 ± 3.4) b |
| 38.71 (30) | (61.3 ± 4.6) c | (52.3 ± 3.9) c |
| 64.52 (50) | (41.3 ± 10.1) d | (18.2 ± 7.9) d |
| β-ionone | ||
| 48.38 (25) | (98.3 ± 6.1) a | (91.4 ± 4.6) a |
| 96.77 (50) | (89.5 ± 9.1) a | (87.1 ± 8.9) a |
| 193.53 (100) | (91.2 ± 6.1) a | (84.3 ± 6.6) a |
| 387.07 (200) | (94.7 ± 10.5) a | (84.3 ± 13.8) a |
| isoamyl alcohol | ||
| 22.18 (25) | (75.7 ± 2.5) b | (58.0 ± 9.0) b |
| 44.36 (50) | (78.6 ± 10.8) b | (47.7 ± 3.4) b |
| 66.54 (75) | (68.6 ± 11.3) b | (21.6 ± 2.0) c |
| 88.71 (100) | (68.6 ± 7.4) b | (19.3 ± 5.2) c |
| 2-phenylethanol | ||
| 30.74 (25) | (95.5 ± 6.8) a | (69.1 ± 5.5) b |
| 61.47 (50) | (73.1 ± 2.6) b | (56.0 ± 2.1) c |
| 122.94 (100) | (55.2 ± 9.3) c | (33.3 ± 7.4) d |
| 245.89 (200) | (53.7 ± 7.8) c | (32.1 ± 3.6) d |
| (−)-limonene | ||
| 274.23 (200) | (91.8 ± 11.4) a | (94.3 ± 8.6) a |
| 548.46 (400) | (100.0 ± 7.5) a | (88.6 ± 9.9) a |
| 685.57 (500) | (85.2 ± 5.7) a | (87.1 ± 8.9) a |
| 822.69 (600) | (101.6 ± 20.5) a | (87.1 ± 17.3) a |
| (+)-α-pinene | ||
| 68.55 (50) | (93.8 ± 9.9) a | (91.2 ± 6.9) a |
| 137.10 (100) | (87.5 ± 5.7) a | (98.9 ± 11.9) a |
| 274.21 (200) | (90.0 ± 6.5) a | (93.4 ± 19.9) a |
| 548.42 (400) | (80.0 ± 15.2) a | (96.7 ± 10.1) a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidorova, D.E.; Skripka, M.I.; Khmel, I.A.; Koksharova, O.A.; Plyuta, V.A. Effects of Volatile Organic Compounds on Biofilms and Swimming Motility of Agrobacterium tumefaciens. Microorganisms 2022, 10, 1512. https://doi.org/10.3390/microorganisms10081512
Sidorova DE, Skripka MI, Khmel IA, Koksharova OA, Plyuta VA. Effects of Volatile Organic Compounds on Biofilms and Swimming Motility of Agrobacterium tumefaciens. Microorganisms. 2022; 10(8):1512. https://doi.org/10.3390/microorganisms10081512
Chicago/Turabian StyleSidorova, Daria E., Mariia I. Skripka, Inessa A. Khmel, Olga A. Koksharova, and Vladimir A. Plyuta. 2022. "Effects of Volatile Organic Compounds on Biofilms and Swimming Motility of Agrobacterium tumefaciens" Microorganisms 10, no. 8: 1512. https://doi.org/10.3390/microorganisms10081512
APA StyleSidorova, D. E., Skripka, M. I., Khmel, I. A., Koksharova, O. A., & Plyuta, V. A. (2022). Effects of Volatile Organic Compounds on Biofilms and Swimming Motility of Agrobacterium tumefaciens. Microorganisms, 10(8), 1512. https://doi.org/10.3390/microorganisms10081512







