Urinary Tract Infections Caused by Uropathogenic Escherichia coli Strains—New Strategies for an Old Pathogen
Abstract
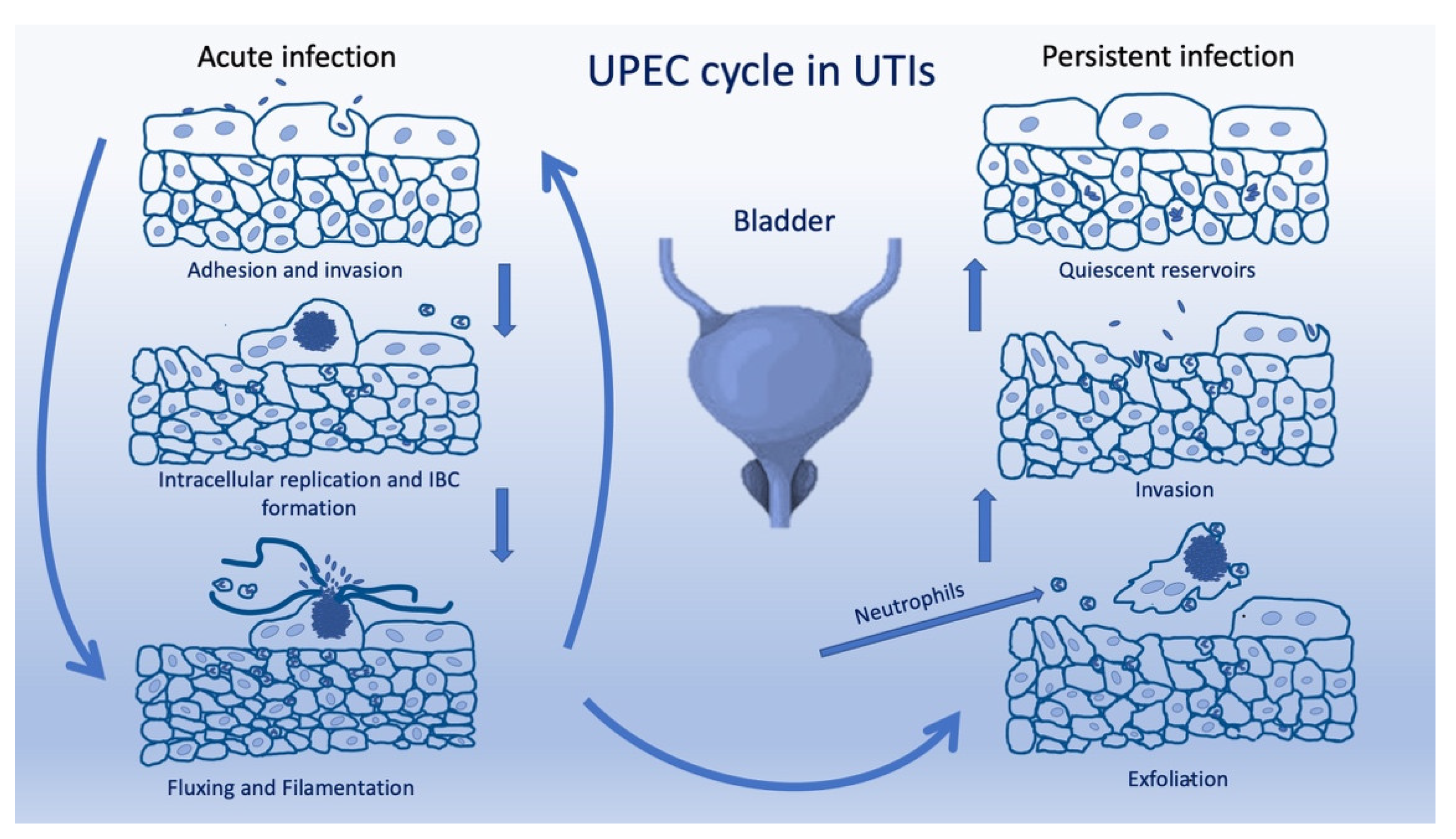
:1. Introduction
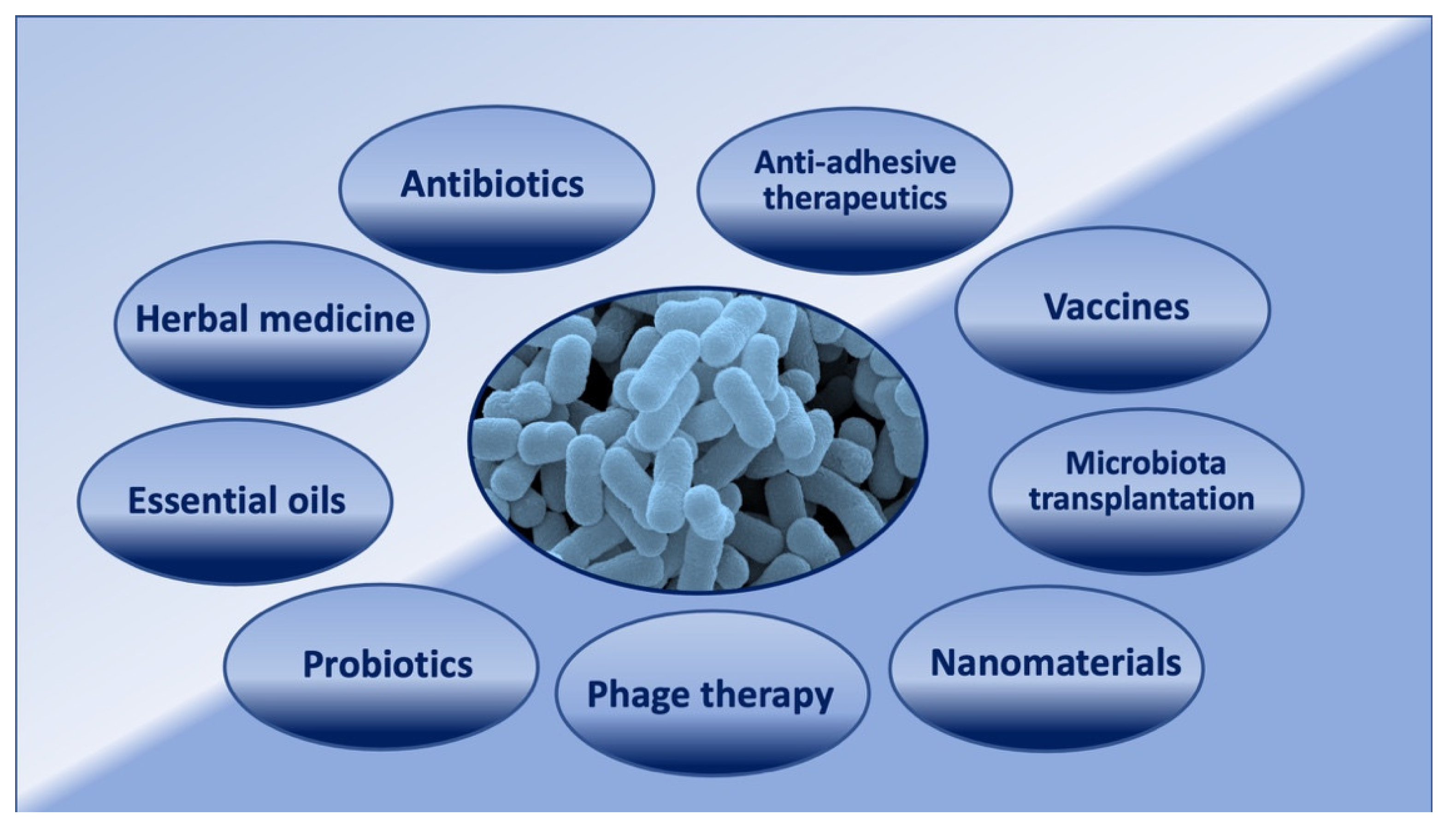
2. Antibiotics and UPECs
3. Natural Products Used in the UTI Treatment
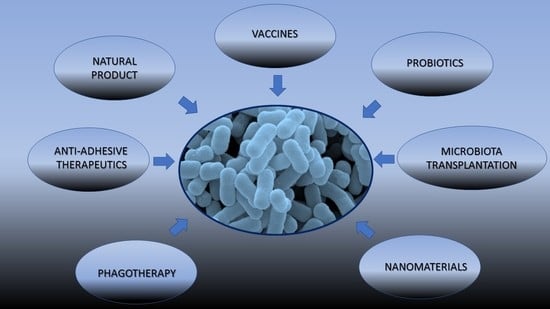
4. Novel Strategies in the Prevention and Treatment of UTIs
4.1. Vaccination
4.2. Probiotics
4.3. Anti-Adhesive Therapeutics
4.4. Phage Therapy
4.5. Microbiota Transplantation
4.6. Nanomaterials
5. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Bruxvoort, K.J.; Bider-Canfield, Z.; Casey, J.A.; Qian, L.; Pressman, A.; Liang, A.S.; Robinson, S.; Jacobsen, S.J.; Tartof, S.Y. Outpatient Urinary Tract Infections in an Era of Virtual Healthcare: Trends From 2008 to 2017. Clin. Infect. Dis. 2020, 71, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Device-associated Module UTI. Urinary Tract Infection (Catheter-Associated Urinary Tract Infection [CAUTI] and Non-Catheter-Associated Urinary Tract Infection [UTI]) Events. 2022. Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/7psccauticurrent.pdf (accessed on 7 July 2022).
- Klein, R.D.; Hultgren, S.J. Urinary tract infections: Microbial pathogenesis, host-pathogen interactions and new treatment strategies. Nat. Rev. Microbiol. 2020, 18, 211–226. [Google Scholar] [PubMed]
- Murray, B.O.; Flores, C.; Williams, C.; Flusberg, D.A.; Marr, E.E.; Kwiatkowska, K.M.; Charest, J.L.; Isenberg, B.C.; Rohn, J.R. Recurrent Urinary Tract Infection: A Mystery in Search of Better Model Systems. Front. Cell. Infect. Microbiol. 2021, 11, 691210. [Google Scholar] [CrossRef]
- Foxman, B. The epidemiology of urinary tract infection. Nat. Rev. Urol. 2010, 7, 653–660. [Google Scholar] [CrossRef]
- Subashchandrabose, S.; Mobley, H.L. Virulence and Fitness Determinants of Uropathogenic Escherichia coli. Microbiol. Spectr. 2015, 3, 101128. [Google Scholar] [CrossRef] [Green Version]
- Lipsky, B.A.; Byren, I.; Hoey, C.T. Treatment of bacterial prostatitis. Clin. Infect. Dis. 2010, 50, 1641–1652. [Google Scholar]
- Terlizzi, M.E.; Gribaudo, G.; Maffei, M.E. UroPathogenic Escherichia coli (UPEC) infections: Virulence factors, bladder responses, antibiotic, and non-antibiotic antimicrobial strategies. Front. Microbiol. 2017, 8, 1566. [Google Scholar] [CrossRef]
- Marrs, C.F.; Zhang, L.; Foxman, B. Escherichia coli mediated urinary tract infections: Are there distinct uropathogenic E. coli (UPEC) pathotypes? FEMS Microbiol. Lett. 2005, 252, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Mulvey, M.A.; Schilling, J.D.; Martinez, J.J.; Hultgren, S.J. Bad bugs and beleaguered bladders: Interplay between uropathogenic Escherichia coli and innate host defenses. Proc. Natl. Acad. Sci. USA 2000, 97, 8829–8835. [Google Scholar]
- Schwab, S.; Jobin, K.; Kurts, C. Urinary tract infection: Recent insight into the evolutionary arms race between uropathogenic Escherichia coli and our immune system. Nephrol. Dial. Transplant. 2017, 32, 1977–1983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spaulding, C.N.; Hultgren, S.J. Adhesive pili in UTI pathogenesis and drug development. Pathogens 2016, 5, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreiber, H.L., IV; Spaulding, C.N.; Dodson, K.W.; Livny, J.; Hultgren, S.J. One size doesn’t fit all: Unraveling the diversity of factors and interactions that drive E. coli urovirulence. Ann. Transl. Med. 2017, 5, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neugent, M.L.; Hulyalkar, N.V.; Nguyen, V.H.; Zimmern, P.E.; De Nisco, N.J. Advances in understanding the human urinary microbiome and its potential role in urinary tract infection. mBio 2020, 11, 218–220. [Google Scholar] [CrossRef]
- Conover, M.S.; Hadjifrangiskou, M.; Palermo, J.J.; Hibbing, M.E.; Dodson, K.W.; Hultgren, S.J. Metabolic requirements of Escherichia coli in intracellular bacterial communities during urinary tract infection pathogenesis. mBio 2016, 7, e00104-16. [Google Scholar] [CrossRef] [Green Version]
- Tamadonfar, K.O.; Omattage, N.S.; Spaulding, C.N.; Hultgren, S.J. Reaching the end of the line: Urinary tract infections. Microbiol. Spectr. 2019, 7, 101128. [Google Scholar] [CrossRef]
- Lane, M.C.; Alteri, C.J.; Smith, S.N.; Mobley, H.L. Expression of flagella is coincident with uropathogenic Escherichia coli ascension to the upper urinary tract. Proc. Natl. Acad. Sci. USA 2007, 104, 16669–16674. [Google Scholar] [CrossRef] [Green Version]
- Hannan, T.J.; Totsika, M.; Mansfield, K.J.; Moore, K.H.; Schembri, M.A.; Hultgren, S.J. Host–pathogen checkpoints and population bottlenecks in persistent and intracellular uropathogenic Escherichia coli bladder infection. FEMS Microbiol. Rev. 2012, 36, 616–648. [Google Scholar]
- Sarkar, S.; Ulett, G.C.; Totsika, M.; Phan, M.D.; Schembri, M.A. Role of capsule and O antigen in the virulence of uropathogenic Escherichia coli. PLoS ONE 2014, 9, 94786. [Google Scholar] [CrossRef] [Green Version]
- Asadi Karam, M.R.; Habibi, M.; Bouzari, S. Urinary tract infection: Pathogenicity, antibiotic resistance and development of effective vaccines against Uropathogenic Escherichia coli. Mol. Immunol. 2019, 108, 56–67. [Google Scholar] [CrossRef]
- Firoozeh, F.; Zibaei, M.; Badmasti, F.; Khaledi, A. Virulence factors, antimicrobial resistance and the relationship between these characteristics in uropathogenic Escherichia coli. Gene Rep. 2022, 27, 101622. [Google Scholar] [CrossRef]
- Soto, S.M. Importance of Biofilms in Urinary Tract Infections: New Therapeutic Approaches. Adv. Biol. 2014, 2014, 543974. [Google Scholar] [CrossRef]
- Eberly, A.R.; Floyd, K.A.; Beebout, C.J.; Colling, S.J.; Fitzgerald, M.J.; Stratton, C.W.; Schmitz, J.E.; Hadjifrangiskou, M. Biofilm Formation by Uropathogenic Escherichia coli Is Favored under Oxygen Conditions That Mimic the Bladder Environment. Int. J. Mol. Sci. 2017, 18, 2077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartoletti, R.; Cai, T.; Wagenlehner, F.M.; Naber, K.; Bjerklund Johansen, T.E. Treatment of Urinary Tract Infections and Antibiotic Stewardship. Eur. Urol. 2016, 15, 81–87. [Google Scholar] [CrossRef]
- Bonkat, G.; Bartoletti, R.; Bruyère, R.; Cai, T.; Geerlings, S.E.; Köves, B.; Schubert, S.; Pilatz, A.; Veeratteraoillay, R.; Wagenlehner, F. EAU Guidelines on Urological Infections; European Association of Urology: Arnhem, The Netherlands, 2022; Available online: https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-Guidelines-on-Urological-Infections-2022.pdf (accessed on 7 July 2022).
- Ong, L.T. Antibiotics for complicated urinary tract infection and acute pyelonephritis: A systematic review. World J. Clin. Infect. Dis. 2020, 10, 33–41. [Google Scholar] [CrossRef]
- Cai, T.; Palagin, I.; Brunelli, R.; Cipelli, R.; Pellini, E.; Truzzi, J.C.; Van Bruwaene, S. Office-based approach to urinary tract infections in 50 000 patients: Results from the REWIND study. Int. J. Antimicrob. Agents 2020, 56, 105966. [Google Scholar] [CrossRef]
- Gonzalez, M.J.; Da Cunda, P.; Notejane, M.; Zunino, P.; Scavone, P.; Robino, L. Fosfomycin tromethamine activity on biofilm and intracellular bacterial communities produced by uropathogenic Escherichia coli isolated from patients with urinary tract infection. Pathog. Dis. 2019, 77, ftz022. [Google Scholar]
- Gonzalez, M.J.; Robino, L.; Iribarnegaray, V.; Zunino, P.; Scavone, P. Effect of different antibiotics on biofilm produced by uropathogenic Escherichia coli isolated from children with urinary tract infection. Pathog. Dis. 2017, 75, ftx053. [Google Scholar] [CrossRef] [Green Version]
- Blango, M.G.; Mulvey, M.A. Persistence of uropathogenic Escherichia coli in the face of multiple antibiotics. Antimicrob. Agents Chemother. 2010, 54, 1855–1863. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.C.; Han, X.M.; Shi, M.; Pang, Z.L. Persistence of uropathogenic Escherichia coli in the bladders of female patients with sterile urine after antibiotic therapies. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2016, 36, 710–715. [Google Scholar]
- Sharma, K.; Dhar, N.; Thacker, V.V.; Simonet, T.M.; Signorino-Gelo, F.; Knott, G.W.; McKinney, J.D. Dynamic persistence of UPEC intracellular bacterial communities in a human bladder-chip model of urinary tract infection. Elife 2021, 10, e66481. [Google Scholar] [CrossRef] [PubMed]
- Huen, K.H.; Nik-Ahd, F.; Chen, L.; Lerman, S.; Singer, J. Neomycin-polymyxin or gentamicin bladder instillations decrease symptomatic urinary tract infections in neurogenic bladder patients on clean intermittent catheterization. J. Pediatr. Urol. 2019, 15, 178.e1–178.e7. [Google Scholar] [CrossRef] [PubMed]
- Harding, C.; Mossop, H.; Homer, T.; Chadwick, T.; King, W.; Carnell, S.; Lecouturier, J.; Abouhajar, A.; Vale, L.; Watson, G.; et al. Alternative to prophylactic antibiotics for the treatment of recurrent urinary tract infections in women: Multicentre, open label, randomised, non-inferiority trial. BMJ 2022, 376, e068229. [Google Scholar] [CrossRef]
- Arakawa, S.; Kawahara, K.; Kawahara, M.; Yasuda, M.; Fujimoto, G.; Sato, A.; Yokokawa, R.; Yoshinari, T.; Rhee, E.G.; Aoyama, N. The efficacy and safety of tazobactam/ceftolozane in Japanese patients with uncomplicated pyelonephritis and complicated urinary tract infection. J. Infect. Chemother. 2019, 25, 104–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzariol, A.; Bazaj, A.; Cornaglia, G. Multi-drug-resistant Gram-negative bacteria causing urinary tract infections: A review. J. Chemother. 2017, 29, 2–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kot, B. Antibiotic Resistance Among Uropathogenic Escherichia coli. Pol. J. Microbiol. 2019, 68, 403–415. [Google Scholar] [CrossRef] [Green Version]
- Longhi, C.; Conte, M.P.; Marazzato, M.; Iebba, V.; Totino, V.; Santangelo, F.; Gallinelli, C.; Pallecchi, L.; Riccobono, E.; Schippa, S.; et al. Plasmid-mediated fluoroquinolone resistance determinants in Escherichia coli from community uncomplicated urinary tract infection in an area of high prevalence of quinolone resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1917–1921. [Google Scholar] [CrossRef]
- Stapleton, A.E.; Wagenlehner, F.M.E.; Mulgirigama, A.; Twynholm, M. Escherichia coli Resistance to Fluoroquinolones in Community Acquired Uncomplicated Urinary Tract Infection in Women: A Systematic Review. Antimicrob. Agents Chemother. 2020, 64, e00862-20. [Google Scholar] [CrossRef]
- Abduzaimovic, A.; Aljicevic, M.; Rebic, V.; Vranic, S.; Abduzaimovic, K.; Sestic, S. Antibiotic resistance in urinary isolates of Escherichia coli. Mater. Sociomed. 2016, 28, 416–419. [Google Scholar] [CrossRef] [Green Version]
- Kresken, M.; Körber-Irrgang, B.; Biedenbach, D.J.; Batista, N.; Besard, V.; Cantón, R.; García-Castillo, M.; Kalka-Moll, W.; Pascual, A.; Schwarz, R.; et al. Comparative in vitro activity of oral antimicrobial agents against Enterobacteriaceae from patients with community acquired urinary tract infections in three European countries. Clin. Microbiol. Infect. 2016, 22, 63.e1–63.e5. [Google Scholar]
- Lavigne, J.P.; Thibault, M.; Costa, P.; Combescure, C.; Sotto, A.; Cariou, G.; Ronco, E.; Lanotte, P.; Bruyère, F.; Coloby, P.; et al. Resistance and virulence potential of uropathogenic Escherichia coli strains isolated from patients hospitalized in urology departments: A French prospective multicentre study. J. Med. Microbiol. 2016, 65, 530–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hrbacek, J.; Cermak, P.; Zachoval, R. Current Antibiotic Resistance Trends of Uropathogens in Central Europe: Survey from a Tertiary Hospital Urology Department 2011–2019. Antibiotics 2020, 9, 630–640. [Google Scholar]
- Bandyopadhyay, D.; Mukherjee, M. Combination of bactericidal antibiotics and inhibitors of Universal stress protein A (UspA): A potential therapeutic alternative against multidrug resistant Escherichia coli in urinary tract infections. J. Antibiotics 2022, 75, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Das, S. Natural therapeutics for urinary tract infections-a review. Futur J. Pharm. Sci. 2020, 6, 64–76. [Google Scholar] [PubMed]
- Jamshidi-Kia, F.; Lorigooini, Z.; Amini-Khoei, H. Medicinal plants: Past history and future perspective. J. Herbmed. Pharmacol. 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Head, K.A. Natural approaches to prevention and treatment of infections of the lower urinary tract. Altern. Med. Rev. 2008, 13, 227–244. [Google Scholar]
- Petronio, G.P.; Cutuli, M.A.; Magnifico, I.; Venditti, N.; Pietrangelo, L.; Vergalito, F.; Pane, A.; Scapagnini, G.; Di Marco, R. In Vitro and In Vivo Biological Activity of Berberine Chloride against Uropathogenic E. coli Strains Using Galleria mellonella as a Host Model. Molecules 2020, 25, 5010. [Google Scholar] [CrossRef]
- González de Llano, D.; Moreno-Arribas, M.V.; Bartolomé, B. Cranberry Polyphenols and Prevention against Urinary Tract Infections: Relevant Considerations. Molecules 2020, 25, 3523. [Google Scholar] [CrossRef]
- Scharf, B.; Schmidt, T.J.; Rabbani, S.; Stork, C.; Dobrindt, U.; Sendker, J.; Ernst, B.; Hensel, A. Antiadhesive natural products against uropathogenic E. coli: What can we learn from cranberry extract? J. Ethnopharmacol. 2020, 15, 112889. [Google Scholar]
- EFSA (European Food Safety Authority). Scientific Opinion on the substantiation of health claims related to proanthocyanidins from cranberry (Vaccinium macrocarpon Aiton) fruit and defence against bacterial pathogens in the lower urinary tract (ID 1841, 2153, 2770, 3328), “powerful protectors of our gums” (ID 1365), and “heart health” (ID 2499) pursuant to Article 13(1) of Regulation (EC) No 1924/20061. EFSA J. 2011, 9, 2215. [Google Scholar]
- FDA (Food and Drug Administration). Health Claim Response Cranberry UTI. 2018. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwiCwIGIqfr3AhV6SPEDHVXkDr8QFnoECAkQAQ&url=https%3A%2F%2Fwww.fda.gov%2Fmedia%2F140304%2Fdownload&usg=AOvVaw3j5x-tIPZvPcHLnprqDk_j (accessed on 4 July 2022).
- Yarnell, E. Botanical medicines for the urinary tract. World J. Urol. 2002, 20, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Flower, A.; Harman, K.; Lewith, G.; Flower, A.; Harman, K.; Lewith, G.; Moore, M.; Bishop, F.L.; Stuart, B.; Lampertet, N. Standardised Chinese herbal treatment delivered by GPs compared with individualised treatment administered by practitioners of Chinese herbal medicine for women with recurrent urinary tract infections (RUTI): Study protocol for a randomised controlled trial. Trials 2016, 17, 358–367. [Google Scholar] [PubMed] [Green Version]
- Yang, W.; Liu, P.; Chen, Y.; Lv, Q.; Wang, Z.; Huang, W.; Jiang, H.; Zheng, Y.; Jiang, Y.; Sun, L. Dictamnine Inhibits the Adhesion to and Invasion of Uropathogenic Escherichia coli (UPEC) to Urothelial Cells. Molecules 2022, 27, 272. [Google Scholar] [CrossRef] [PubMed]
- Lagha, R.; Ben Abdallah, F.; Al-Sarhan, B.O.; Al-Sodany, Y. Antibacterial and Biofilm Inhibitory Activity of Medicinal Plant Essential Oils Against Escherichia coli Isolated from UTI Patients. Molecules 2019, 24, 1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitanza, L.; Maccelli, A.; Marazzato, M.; Scazzocchio, F.; Comanducci, A.; Fornarini, S.; Crestoni, M.E.; Filippi, A.; CFraschetti, C.; Rinaldi, F.; et al. Satureja montana L. essential oil and its antimicrobial activity alone or in combination with gentamicin. Microb. Pathog. 2019, 126, 323–331. [Google Scholar] [CrossRef]
- Scazzocchio, F.; Mondì, L.; Ammendolia, M.G.; Goldoni, P.; Comanducci, A.; Marazzato, M.; Conte, M.P.; Rinaldi, F.; Crestoni, M.E.; Fraschetti, C.; et al. Coriander (Coriandrum sativum) Essential Oil: Effect on Multidrug Resistant Uropathogenic Escherichia coli. Nat. Prod. Commun. 2017, 12, 623–626. [Google Scholar]
- Tache, A.M.; Dinu, L.D.; Vamanu, E. Novel Insights on Plant Extracts to Prevent and Treat Recurrent Urinary Tract Infections. Appl. Sci. 2022, 12, 2635–2651. [Google Scholar]
- Bartlett, J.G.; Gilbert, D.N.; Spellberg, B. Seven ways to preserve the miracle of antibiotics. Clin. Infect. Dis. 2013, 56, 1445–1450. [Google Scholar]
- Loubet, P.; Ranfaing, J.; Dinh, A.; Dunyach-Remy, C.; Bernard, L.; Bruyère, F.; Lavigne, J.F.; Sotto, A. Alternative Therapeutic Options to Antibiotics for the Treatment of Urinary Tract Infections. Front. Microbiol. 2020, 11, 1509–1526. [Google Scholar]
- Langermann, S.; Möllby, R.; Burlein, J.E.; Palaszynski, S.R.; Auguste, C.G.; De Fusco, A.; Strouse, R.; Schenerman, M.A.; Hultgren, S.J.; Pinkner, J.S.; et al. Vaccination with FimH adhesion protects cynomolgus monkeys from colonization and infection by uropathogenic Escherichia coli. J. Infect. Dis. 2000, 181, 774–778. [Google Scholar] [CrossRef] [Green Version]
- Savar, N.S.; Jahanian-Najafabadi, A.; Mohammad, M.M.; Shokrgozard, A.; Jafari, A.; Bouzariet, S. In silico and in vivo studies of truncated forms of flagellin (FliC) of enteroaggregative Escherichia coli fused to FimH from uropathogenic Escherichia coli as a vaccine candidate against urinary tract infections. J. Biotechnol. 2014, 175, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Mobley, H.L.; Alteri, C.J. Development of a vaccine against Escherichia coli urinary tract infections. Pathogens 2016, 5, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunden, F.; Hakansson, L.; Ljunggren, E.; Wullt, B. Escherichia coli bacteriuria protects against recurrent lower urinary tract infections in patients with incomplete bladder emptying. J. Urol. 2010, 184, 179–185. [Google Scholar] [CrossRef] [Green Version]
- Darouiche, R.O.; Green, B.G.; Donovan, W.H.; Chen, D.; Schwartz, M.; Merritt, J.; Mendez, M.; Hull, R.A. Multicenter randomized controlled trial of bacterial interference for prevention of urinary tract infection in patients with neurogenic bladder. Urology 2011, 78, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Stork, C.; Kovács, B.; Rózsai, B.; Putze, J.; Kiel, M.; Dorn, Á.; Kovács, J.; Melegh, S.; Leimbach, A.; Kovács, T.; et al. Characterization of Asymptomatic Bacteriuria Escherichia coli Isolates in Search of Alternative Strains for Efficient Bacterial Interference against Uropathogens. Front. Microbiol. 2018, 9, 214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akgül, T.; Karakan, T. The role of probiotics in women with recurrent urinary tract infections. Turk. J. Urol. 2018, 44, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.L.; Brown, J.; Wyman, J.F.; Berry, A.; Newman, D.K.; Stapleton, A.E. Treatment and prevention of recurrent lower urinary tract infections in women: A rapid review with practice recommendations. J. Urol. 2018, 200, 1174–1191. [Google Scholar] [CrossRef]
- Aggarwal, N.; Breedon, A.M.E.; Davis, C.M.; Hwang, I.Y.; Chang, M.W. Engineering probiotics for therapeutic applications: Recent examples and translational outlook. Curr. Opinion Biotechnol. 2020, 65, 171–179. [Google Scholar]
- Trivedi, D.; Kumar, J.P.; Seshadri, S. Colicin E2 Expression in Lactobacillus brevis DT24, A Vaginal Probiotic Isolate, against Uropathogenic Escherichia coli. ISRN Urol. 2014, 869610, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Spaulding, C.N.; Klein, R.D.; Ruer, S.; Kau, A.L.; Schreiber, H.L., IV; Cusumano, Z.T.; Dodson, K.W.; Pinkner, J.S.; Fremont, D.H.; Janetka, J.W.; et al. Selective depletion of uropathogenic E. coli from the gut by a FimH antagonist. Nature 2017, 546, 528–532. [Google Scholar] [CrossRef] [Green Version]
- Kalograiaki, I.; Abellán-Flos, M.; Fernández, L.Á.; Menéndez, M.; Vincent, S.P.; Solís, D. Direct evaluation of live uropathogenic Escherichia coli adhesion and efficiency of antiadhesive compounds using a simple microarray approach. Anal. Chem. 2018, 90, 12314–12321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berne, C.; Ducret, A.; Hardy, G.G.; Brun, Y.V. Adhesins involved in attachment to abiotic surfaces by Gram-negative bacteria. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behzadi, P.; Behzadi, E.; Pawlak-Adamska, E.A. Urinary tract infections (UTIs) or genital tract infections (GTIs)? It’s the diagnostics that count. GMS Hyg. Infect. Control. 2019, 14, 2196–5226. [Google Scholar]
- Wurpel, D.J.; Beatson, S.A.; Totsika, M.; Petty, N.K.; Schembri, M.A. Chaperone-Usher fimbriae of Escherichia coli. PLoS ONE 2013, 8, e52835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarshar, M.; Behzadi, P.; Ambrosi, C.; Zagaglia, C.; Palamara, A.T.; Scribano, D. FimH and Anti-Adhesive Therapeutics: A Disarming Strategy Against Uropathogens. Antibiotics 2020, 10, 397. [Google Scholar] [CrossRef]
- Scribano, D.; Sarshar, M.; Prezioso, C.; Lucarelli, M.; Angeloni, A.; Zagaglia, C.; Palamara, A.T.; Ambrosi, C. d-Mannose Treatment neither Affects Uropathogenic Escherichia coli Properties nor Induces Stable FimH Modifications. Molecules 2020, 13, 316. [Google Scholar] [CrossRef] [Green Version]
- Malik, S.; Sidhu, P.K.; Rana, J.S.; Nehra, K. Managing urinary tract infections through phage therapy: A novel approach. Folia Microbiol. 2019, 65, 316. [Google Scholar] [CrossRef]
- Nishikawa, H.; Yasuda, M.; Uchiyama, J.; Rashel, M.; Maeda, Y.; Takemura, I.; Sugihara, S.; Ujihara, T.; Shimizu, Y.; Shuin, T.; et al. T-even-related bacteriophages as candidates for treatment of Escherichia coli urinary tract infections. Arch. Virol. 2008, 153, 507–515. [Google Scholar] [CrossRef]
- Ujmajuridze, A.; Chanishvili, N.; Goderdzishvili, M.; Leitner, L.; Mehnert, U.; Chkhotua, A.; Kessler, T.M.; Sybesma, W. Adapted bacteriophages for treating urinary tract infections. Front. Microbiol. 2018, 9, 1832–1838. [Google Scholar] [CrossRef] [Green Version]
- Zalewska-Piatek, B.; Piatek, R. Phage Therapy as a Novel Strategy in the Treatment of Urinary Tract Infections Caused by E. coli. Antibiotics 2020, 9, 304–324. [Google Scholar]
- Hatfull, G.F.; Dedrick, R.M.; Schooley, R.T. Phage Therapy for Antibiotic-Resistant Bacterial Infections. Annu. Rev. Med. 2022, 73, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Ghani, R.; Mullish, B.H.; Roberts, L.A.; Davies, F.J.; Marchesi, J.R. The potential utility of fecal (or intestinal) microbiota transplantation in controlling infectious diseases. Gut Microbes 2022, 14, e2038856. [Google Scholar]
- Culligan, E.P.; Sleator, R.D. Advances in the Microbiome: Applications to Clostridium difficile Infection. J. Clinic. Med. 2016, 5, 83–102. [Google Scholar]
- Kenneally, C.; Murphy, C.P.; Sleator, R.D.; Culligan, E.P. The urinary microbiome and biological therapeutics: Novel therapies for urinary tract infections. Microbiol. Res. 2022, 259, 127010. [Google Scholar] [PubMed]
- Pardi, D.S.; Tosh, P.K.; Walker, R.C.; Razonable, R.R.; Khanna, S. Fecal microbiota transplantation for recurrent clostridium difficile infection reduces recurrent urinary tract infection frequency. Clin. Infect. Dis. 2017, 65, 1745–1747. [Google Scholar]
- Hocquart, M.; Pham, T.; Kuete, E.; Tomei, E.; Lagier, J.C.; Raoult, D. Successful fecal microbiota transplantation in a patient suffering from irritable bowel syndrome and recurrent urinary tract infections. Open Forum Infect. Dis. 2019, 6, ofz398. [Google Scholar] [CrossRef] [Green Version]
- Qindeel, M.; Barani, M.; Rahdar, A.; Arshad, R.; Cucchiarini, M. Nanomaterials for the Diagnosis and Treatment of Urinary Tract Infections. Nanomaterials 2021, 11, 546. [Google Scholar] [CrossRef]
- Wang, L.; Yang, J.; Yang, X.; Hou, Q.; Liu, S.; Zheng, W.; Long, Y.; Jiang, X. Mercaptophenylboronic acid-activated gold nanoparticles as nanoantibiotics against multidrug-resistant bacteria. ACS Appl. Mater. Interfaces 2020, 12, 51148–51159. [Google Scholar] [CrossRef]
- Abdal Dayem, A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef] [Green Version]
- Aderibigbe, B.A. Metal-based nanoparticles for the treatment of infectious diseases. Molecules 2017, 22, 1370. [Google Scholar] [CrossRef]
- Rodríguez-Serrano, C.; Guzmán-Moreno, J.; Ángeles-Chávez, C.; Rodríguez-González, V.; Ortega-Sigala, J.J.; Ramírez-Santoyo, R.M.; Vidales-Rodríguez, L.E. Biosynthesis of silver nanoparticles by Fusarium scirpi and its potential as antimicrobial agent against uropathogenic Escherichia coli biofilms. PLoS ONE 2020, 15, e0230275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agnihotri, S.; Dhiman, N.K. Development of nano-antimicrobial biomaterials for biomedical applications. Adv. Biomater. Biomed. Appl. 2017, 66, 479–545. [Google Scholar]
- Dutta, R.; Nenavathu, B.P.; Gangishetty, M.K.; Reddy, A. Antibacterial effect of chronic exposure of low concentration ZnO nanoparticles on E. coli. J. Environ. Sci. Health A 2013, 48, 871–878. [Google Scholar] [CrossRef]
- Dayyoub, E.; Frant, M.; Pinnapireddy, S.R.; Liefeith, K.; Bakowsky, U. Antibacterial and anti-encrustation biodegradable polymer coating for urinary catheter. Int. J. Pharm. 2017, 531, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Brauner, B.; Semmler, J.; Rauch, D.; Nokaj, M.; Haiss, P.; Schwarz, P.; Wirth, M.; Gabor, F. Trimethoprim-loaded PLGA nanoparticles grafted with WGA as potential intravesical therapy of urinary tract infections—Studies on adhesion to SV-HUCs under varying time, pH, and drug-loading conditions. ACS Omega 2020, 5, 17377–17384. [Google Scholar] [CrossRef]
- Cano, A.; Ettcheto, M.; Espina, M.; López-Machado, A.; Cajal, Y.; Rabanal, F.; Sánchez-López, E.; Camins, A.; García, M.L.; Souto, E.B. State-of-the-art polymeric nanoparticles as promising therapeutic tools against human bacterial infections. J. Nanobiotechnol. 2020, 18, 156. [Google Scholar] [CrossRef]
- Gao, L.; Wang, Y.; Li, Y.; Xu, M.; Sun, G.; Zou, T.; Wang, F.; Xu, S.; Da, J.; Wang, L. Biomimetic biodegradable Ag@ Au nanoparticle embedded ureteral stent with a constantly renewable contact-killing antimicrobial surface and antibiofilm and extraction-free properties. Acta Biomater. 2020, 114, 117–132. [Google Scholar]
- Ashmore, D.A.; Chaudhari, A.; Barlow, B.; Barlow, B.; Harper, T.; Vig, K.; Miller, M.; Singh, S.; Nelson, E.; Pillai, S. Evaluation of E. coli inhibition by plain and polymer-coated silver nanoparticles. Rev. Do Inst. Med. Trop. São Paulo 2018, 6, e18. [Google Scholar] [CrossRef] [Green Version]
- Lu, D.; Tao, R.; Wang, Z. Carbon-based materials for photodynamic therapy: A mini-review. Front. Chem. Sci. Eng. 2019, 13, 310–323. [Google Scholar] [CrossRef]
- Dybowska-Sarapuk, Ł.; Kotela, A.; Krzemiński, J.; Wróblewska, M.; Marchel, H.; Romaniec, M.; Łęgosz, P.; Jakubowska, M. Graphene nanolayers as a new method for bacterial biofilm prevention: Preliminary results. J. AOAC Int. 2017, 100, 900–904. [Google Scholar] [CrossRef]
- Rouhani, P.; Singh, R.N. Polyethyleneimine-functionalized magnetic Fe3O4 and nanodiamond particles as a platform foramoxicillin delivery. J. Nanosci. Nanotechnol. 2020, 20, 3957–3970. [Google Scholar] [PubMed]
- Iyer, J.K.; Dickey, A.; Rouhani, P.; Kaul, A.; Govindaraju, N.; Singh, R.N.; Kaul, R. Nanodiamonds facilitate killing of intracellular uropathogenic E. coli in an in vitro model of urinary tract infection pathogenesis. PLoS ONE 2018, 13, e0191020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfei, S.; Schito, A.M. From nanobiotechnology, positively charged biomimetic dendrimers as novel antibacterial agents: A review. Nanomaterials 2020, 10, 2022. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Yu, F.; Chen, H.; Wang, J.; Lopez, A.I.; Chen, Q.; Li, S.; Long, Y.; Darouiche, R.O.; Hull, R.A. Coating of silicone with mannoside-PAMAM dendrimers to enhance formation of non-pathogenic Escherichia coli biofilms against colonization of uropathogens. Acta Biomater. 2017, 64, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Gupta, S.; Tyagi, A.; Sharma, R.K.; Ali, J.; Gabrani, R.; Dang, S. Development of nanoemulsion based gel loaded with phytoconstituents for the treatment of urinary tract infection and in vivo biodistribution studies. Adv. Pharm. Bull. 2017, 7, 611–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zagaglia, C.; Ammendolia, M.G.; Maurizi, L.; Nicoletti, M.; Longhi, C. Urinary Tract Infections Caused by Uropathogenic Escherichia coli Strains—New Strategies for an Old Pathogen. Microorganisms 2022, 10, 1425. https://doi.org/10.3390/microorganisms10071425
Zagaglia C, Ammendolia MG, Maurizi L, Nicoletti M, Longhi C. Urinary Tract Infections Caused by Uropathogenic Escherichia coli Strains—New Strategies for an Old Pathogen. Microorganisms. 2022; 10(7):1425. https://doi.org/10.3390/microorganisms10071425
Chicago/Turabian StyleZagaglia, Carlo, Maria Grazia Ammendolia, Linda Maurizi, Mauro Nicoletti, and Catia Longhi. 2022. "Urinary Tract Infections Caused by Uropathogenic Escherichia coli Strains—New Strategies for an Old Pathogen" Microorganisms 10, no. 7: 1425. https://doi.org/10.3390/microorganisms10071425
APA StyleZagaglia, C., Ammendolia, M. G., Maurizi, L., Nicoletti, M., & Longhi, C. (2022). Urinary Tract Infections Caused by Uropathogenic Escherichia coli Strains—New Strategies for an Old Pathogen. Microorganisms, 10(7), 1425. https://doi.org/10.3390/microorganisms10071425