Phascinating Phages
Abstract
:1. Introduction
2. Phage Biology
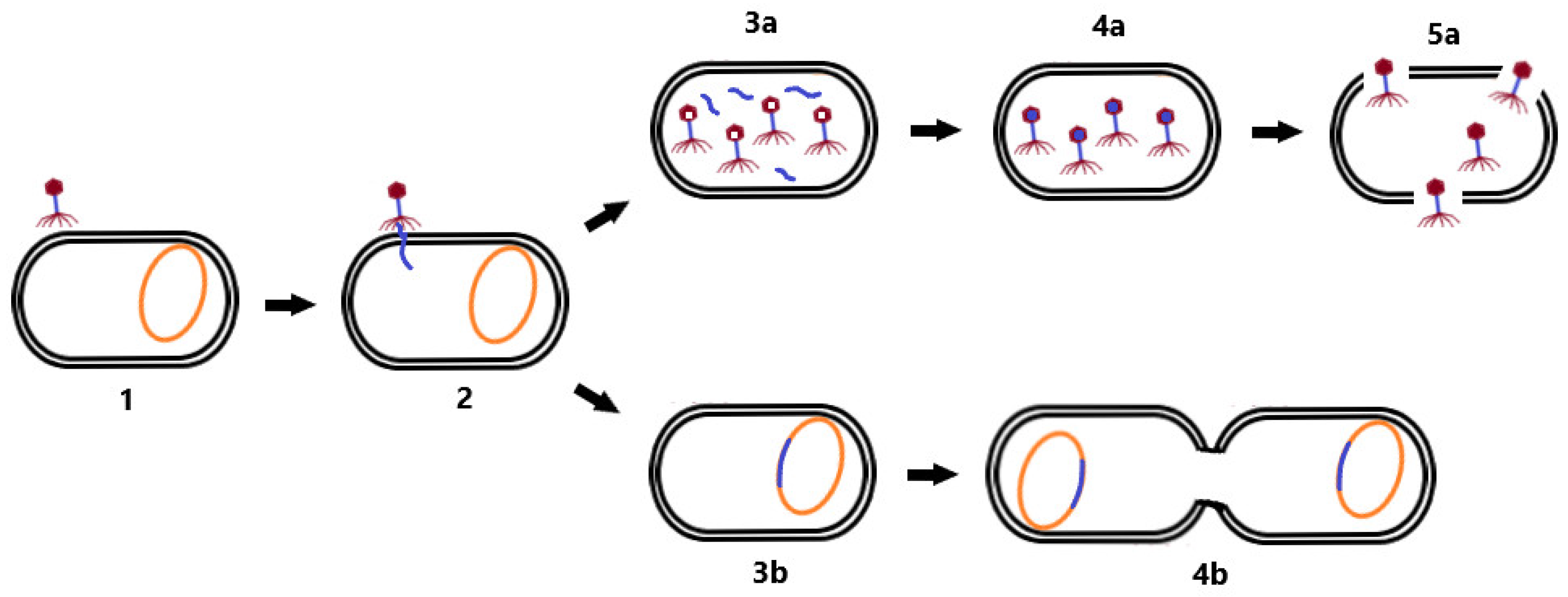
2.1. Phage Life Cycle
2.2. Phages versus Bacteria
3. Properties of Phage Therapy
3.1. Antibiotic versus Phage Therapy
3.2. Phage Administration
4. Phage Therapy at Present
4.1. Phage Laboratory Studies
4.2. Phage Case Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Serapide, F.; Quirino, A.; Scaglione, V.; Morrone, H.L.; Longhini, F.; Bruni, A.; Garofalo, E.; Matera, G.; Marascio, N.; Scarlata, G.G.M.; et al. Is the Pendulum of Antimicrobial Drug Resistance Swinging Back after COVID-19? Microorganisms 2022, 10, 957. [Google Scholar] [CrossRef] [PubMed]
- Jennes, S.; Merabishvili, M.; Soentjens, P.; Pang, K.W.; Rose, T.; Keersebilck, E.; Soete, O.; François, P.M.; Teodorescu, S.; Verween, G.; et al. Use of bacteriophages in the treatment of colistin-only-sensitive Pseudomonas aeruginosa septicaemia in a patient with acute kidney injury-a case report. Crit. Care 2017, 21, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, A.; Hawkins, C.H.; Anggård, E.E.; Harper, D.R. A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy. Clin. Otolaryngol. 2009, 34, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Koulenti, D.; Xu, E.; Mok, I.Y.S.; Song, A.; Karageorgopoulos, D.E.; Armaganidis, A.; Lipman, J.; Tsiodras, S. Novel Antibiotics for Multidrug-Resistant Gram-Positive Microorganisms. Microorganisms 2019, 7, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koreň, J.; Hubenáková, Z.; Maliar, T.; Krčméry, V.; Wawruch, M. In vitro efficacy of novel and current antimicrobial agents against carbapenem-resistant/carbapenemase-producing Enterobacterales/Enterobacteriaceae (CRE, CPE) strains isolated from hospitalized patients. Lek. Obz. 2020, 69, 404–408. [Google Scholar]
- Bittner Fialová, S.; Rendeková, K.; Mucaji, P.; Slobodníková, L. Plant Natural Agents: Polyphenols, Alkaloids and Essential Oils as Perspective Solution of Microbial Resistance. Curr. Org. Chem. 2017, 21, 1875–1884. [Google Scholar] [CrossRef]
- Zemanová, M.; Slobodníková, L.; Čambal, M.; Labaš, P. Excellent antibacterial activity of Slovak honeys on bacteria mostly infecting chronic wounds. Bratisl. Med. J. 2021, 122, 519–525. [Google Scholar] [CrossRef]
- Sánchez, M.C.; Ribeiro-Vidal, H.; Esteban-Fernández, A.; Bartolomé, B.; Figuero, E.; Moreno-Arribas, M.V.; Sanz, M.; Herrera, D. Antimicrobial activity of red wine and oenological extracts against periodontal pathogens in a validated oral biofilm model. BMC Complement. Altern. Med. 2019, 19, 145. [Google Scholar] [CrossRef]
- López-López, N.; Muñoz Resta, I.; de Llanos, R.; Miravet, J.F.; Mikhaylov, M.; Sokolov, M.N.; Ballesta, S.; García-Luque, I.; Galindo, F. Photodynamic Inactivation of Staphylococcus aureus Biofilms Using a Hexanuclear Molybdenum Complex Embedded in Transparent polyHEMA Hydrogels. ACS Biomater. Sci. Eng. 2020, 6, 6995–7003. [Google Scholar] [CrossRef]
- Ivanová, K.; Fernandes, M.M.; Franchesko, A.; Mendoza, E.; Guezguez, J.; Burnet, M.; Tzanov, T. Quorum-Quenching and Matrix-Degrading Enzymes in Multilayer Coatings Synergistically Prevent Bacterial Biofilm Formation on Urinary Catheters. ACS Appl. Mater. Interfaces 2015, 7, 27066–27077. [Google Scholar] [CrossRef]
- Grein, K.; Jungbäck, C.; Kubiak, V. Autogenous vaccines: Quality of production and movement in a common market. Biologicals 2022, 76, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Romero-Calle, D.; Guimarães Benevides, R.; Góes-Neto, A.; Billington, C. Bacteriophages as Alternatives to Antibiotics in Clinical Care. Antibiotics 2019, 8, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirnay, J.P. Phage Therapy in the Year 2035. Front. Microbiol. 2020, 11, 1171. [Google Scholar] [CrossRef] [PubMed]
- Kakasis, A.; Panitsa, G. Bacteriophage therapy as an alternative treatment for human infections. A comprehensive review. Int. J. Antimicrob. Agents 2019, 53, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Weber-Dąbrowska, B.; Jończyk-Matysiak, E.; Żaczek, M.; Łobocka, M.; Łusiak-Szelachowska, M.; Górski, A. Bacteriophage Procurement for Therapeutic Purposes. Front. Microbiol. 2016, 7, 1177. [Google Scholar] [CrossRef] [Green Version]
- Reyes, A.; Semenkovich, N.P.; Whiteson, K.; Rohwer, F.; Gordon, J.I. Going viral: Next-generation sequencing applied to phage populations in the human gut. Nat. Rev. Microbiol. 2012, 10, 607–617. [Google Scholar] [CrossRef]
- Gregory, A.C.; Zablocki, O.; Howell, A.; Bolduc, B.; Sullivan, M.B. The human gut virome database. BioRxiv 2019. Preprint. [Google Scholar] [CrossRef]
- Sutton, T.D.S.; Hill, C. Gut Bacteriophage: Current Understanding and Challenges. Front. Endocrinol. 2019, 29, 784. [Google Scholar] [CrossRef]
- Koskella, B.; Meaden, S. Understanding bacteriophage specificity in natural microbial communities. Viruses 2013, 11, 806–823. [Google Scholar] [CrossRef] [Green Version]
- Howard-Varona, C.; Hargreaves, K.R.; Abedon, S.T.; Sullivan, M.B. Lysogeny in nature: Mechanisms, impact and ecology of temperate phages. ISME J. 2017, 11, 1511–1520. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y. Bacteriophage-Derived Endolysins Applied as Potent Biocontrol Agents to Enhance Food Safety. Microorganisms 2020, 8, 724. [Google Scholar] [CrossRef] [PubMed]
- Liptáková, A.; Krčméry, V.; Slobodníková, L.; Buc, M.; Predný, J.; Koreň, J. Lekárska Mikrobiológia, 1st ed.; Herba: Bratislava, Slovakia, 2019; p. 920. [Google Scholar]
- Boakes, E.; Kearns, A.M.; Ganner, M.; Perry, C.; Hill, R.L.; Ellington, M.J. Distinct bacteriophages encoding Panton-Valentine leukocidin (PVL) among international methicillin-resistant Staphylococcus aureus clones harboring PVL. J. Clin. Microbiol. 2011, 49, 684–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholl, D.; Adhya, S.; Merril, C. Escherichia coli K1’s capsule is a barrier to bacteriophage T7. Appl. Environ. Microbiol. 2005, 71, 4872–4874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef] [Green Version]
- Cumby, N.; Reimer, K.; Mengin-Lecreulx, D.; Davidson, A.R.; Maxwell, K.L. The phage tail tape measure protein, an inner membrane protein and a periplasmic chaperone play connected roles in the genome injection process of E. coli phage HK97. Mol. Microbiol. 2015, 96, 437–447. [Google Scholar] [CrossRef]
- Tock, M.R.; Dryden, D.T. The biology of restriction and anti-restriction. Curr. Opin. Microbiol. 2005, 8, 466–472. [Google Scholar] [CrossRef]
- Rostøl, J.T.; Marraffini, L. (Ph)ighting Phages: How Bacteria Resist Their Parasites. Cell Host Microbe 2019, 13, 184–194. [Google Scholar] [CrossRef] [Green Version]
- Kronheim, S.; Daniel-Ivad, M.; Duan, Z.; Hwang, S.; Wong, A.I.; Mantel, I.; Nodwell, J.R.; Maxwell, K.L. A chemical defence against phage infection. Nature 2018, 564, 283–286. [Google Scholar] [CrossRef]
- Ram, G.; Chen, J.; Kumar, K.; Ross, H.F.; Ubeda, C.; Damle, P.K.; Lane, K.D.; Penadés, J.R.; Christie, G.E.; Novick, R.P. Staphylococcal pathogenicity island interference with helper phage reproduction is a paradigm of molecular parasitism. Proc. Natl. Acad. Sci. USA 2012, 109, 16300–16305. [Google Scholar] [CrossRef] [Green Version]
- Dy, R.L.; Przybilski, R.; Semeijn, K.; Salmond, G.P.; Fineran, P.C. A widespread bacteriophage abortive infection system functions through a Type IV toxin-antitoxin mechanism. Nucleic Acids Res. 2014, 42, 4590–4605. [Google Scholar] [CrossRef]
- Van Houte, S.; Buckling, A. Westra, E.R. Evolutionary Ecology of Prokaryotic Immune Mechanisms. Microbiol. Mol. Biol. Rev. 2016, 80, 745–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leiman, P.G.; Battisti, A.J.; Bowman, V.D.; Stummeyer, K.; Mühlenhoff, M.; Gerardy-Schahn, R.; Scholl, D.; Molineux, I.J. The structures of bacteriophages K1E and K1-5 explain processive degradation of polysaccharide capsules and evolution of new host specificities. J. Mol. Biol. 2007, 371, 836–849. [Google Scholar] [CrossRef] [PubMed]
- Warwick-Dugdale, J.; Solonenko, N.; Moore, K.; Chittick, L.; Gregory, A.C.; Allen, M.J.; Sullivan, M.B.; Temperton, B. Long-read viral metagenomics captures abundant and microdiverse viral populations and their niche-defining genomic islands. PeerJ 2019, 7, e6800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bondy-Denomy, J.; Pawluk, A.; Maxwell, K.L.; Davidson, A.R. Bacteriophage genes that inactivate the CRISPR/Cas bacterial immune system. Nature 2013, 493, 429–432. [Google Scholar] [CrossRef] [Green Version]
- Bair, C.L.; Black, L.W. A type IV modification dependent restriction nuclease that targets glucosylated hydroxymethyl cytosine modified DNAs. J. Mol. Biol. 2007, 366, 768–778. [Google Scholar] [CrossRef] [Green Version]
- Seed, K.D.; Lazinski, D.W.; Calderwood, S.B.; Camilli, A. A bacteriophage encodes its own CRISPR/Cas adaptive response to evade host innate immunity. Nature 2013, 494, 489–491. [Google Scholar] [CrossRef]
- Lv, X.; Zhang, J.; Jiang, M.; Liu, Y.; Ren, W.; Fang, Z. Clostridium difficile-associated diarrhea following the therapy with antibiotic and proton pump inhibitors in a 77-year-old man with several comorbidities: A case report. Medicine 2019, 98, e15004. [Google Scholar] [CrossRef]
- Loc-Carrillo, C.; Abedon, S.T. Pros and cons of phage therapy. Bacteriophage 2011, 1, 111–114. [Google Scholar] [CrossRef] [Green Version]
- Dvořáčková, M.; Růžička, F.; Benešík, M.; Pantůček, R.; Dvořáková-Heroldová, M. Antimicrobial effect of commercial phage preparation Stafal® on biofilm and planktonic forms of methicillin-resistant Staphylococcus aureus. Folia Microbiol. 2019, 64, 121–126. [Google Scholar] [CrossRef]
- Ferriol-González, C.; Domingo-Calap, P. Phages for Biofilm Removal. Antibiotics 2020, 9, 268. [Google Scholar] [CrossRef]
- Krut, O.; Bekeredjian-Ding, I. Contribution of the Immune Response to Phage Therapy. J. Immunol. 2018, 200, 3037–3044. [Google Scholar] [CrossRef] [PubMed]
- Morozova, V.V.; Vlassov, V.V.; Tikunova, N.V. Applications of Bacteriophages in the Treatment of Localized Infections in Humans. Front. Microbiol. 2018, 9, 1696. [Google Scholar] [CrossRef] [Green Version]
- Jault, P.; Leclerc, T.; Jennes, S.; Pirnay, J.P.; Que, Y.A.; Resch, G.; Rousseau, A.F.; Ravat, F.; Carsin, H.; Le Floch, R.; et al. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): A randomised, controlled, double-blind phase 1/2 trial. Lancet Infect. Dis. 2019, 19, 35–45. [Google Scholar] [CrossRef]
- Dedrick, R.M.; Guerrero-Bustamante, C.A.; Garlena, R.A.; Russell, D.A.; Ford, K.; Harris, K.; Gilmour, K.C.; Soothill, J.; Jacobs-Sera, D.; Schooley, R.T.; et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat. Med. 2019, 25, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Dedrick, R.; Jacobs-Sera, D.; Bustamante, C.; Garlena, R.A.; Mavrich, T.N.; Pope, W.H.; Reyes, J.C.C.; Russell, D.A.; Adair, T.; Alvey, R.; et al. Prophage-mediated defence against viral attack and viral counter-defence. Nat. Microbiol. 2017, 2, 16251. [Google Scholar] [CrossRef] [PubMed]
- Duplessis, C.; Stockelman, M.G.; Hamilton, T.; Merril, G.L.; Brownstein, M.J.; Bishop-Lilly, K.A.; Schooley, R.T.; Henry, M.S.; Horne, B.; Sisson, B.M.; et al. A Case Series of Emergency Investigational New Drug Applications for Bacteriophages Treating Recalcitrant Multi-drug Resistant Bacterial Infections: Confirmed Safety and a Signal of Efficacy. J. Intensive Crit. Care 2019, 5, 11. [Google Scholar]
- Schooley, R.T.; Biswas, B.; Gill, J.J.; Hernandez-Morales, A.; Lancaster, J.; Lessor, L.; Barr, J.J.; Reed, S.L.; Rohwer, F.; Bneler, S.; et al. Development and Use of Personalized Bacteriophage-Based Therapeutic Cocktails to Treat a Patient with a Disseminated Resistant Acinetobacter baumannii Infection. Antimicrob. Agents Chemother. 2017, 61, e00954-17. [Google Scholar] [CrossRef] [Green Version]
- Nale, J.Y.; Spencer, J.; Hargreaves, K.R.; Buckley, A.M.; Trzepiński, P.; Douce, G.R.; Clokie, M.R. Bacteriophage Combinations Significantly Reduce Clostridium difficile Growth In Vitro and Proliferation In Vivo. Antimicrob. Agents Chemother. 2015, 60, 968–981. [Google Scholar] [CrossRef] [Green Version]
- Malik, D.J.; Sokolov, I.J.; Vinner, G.K.; Mancuso, F.; Cinquerrui, S.; Vladisavljevic, G.T.; Clokie, M.R.J.; Garton, N.J.; Stapley, A.G.F.; Kirpichnikova, A. Formulation, stabilisation and encapsulation of bacteriophage for phage therapy. Adv. Colloid Interface Sci. 2017, 249, 100–133. [Google Scholar] [CrossRef] [Green Version]
- Eliava Institute. Available online: http://eliava-institute.org (accessed on 11 June 2022).
- FSUE NPO Microgen. Available online: https://www.microgen.ru/en (accessed on 10 June 2022).
- Bakteriofag Stafylokokovyy. Available online: https://www.bacteriofag.ru/products/bakteriofag-stafilokokkovyy-20-ml (accessed on 10 June 2022).
- Sextaphag®polyvalent Pyobacteriophage. Available online: https://www.microgen.ru/en/products/bakteriofagi/sekstafag-piobakteriofag-polivalentnyy (accessed on 10 June 2022).
- Staphylococcal Bacteriophage—ELIAVA. Available online: http://phage.ge/products/staphylococcal-bacteriophage (accessed on 11 June 2022).
- Fersisi-Bacteriophage—ELIAVA. Available online: https://phage.ge/products/fersisi-bacteriophage (accessed on 11 June 2022).
- Ses-Bacteriophage—ELIAVA. Available online: https://phage.ge/products/ses-bacteriophage (accessed on 11 June 2022).
- Intesti-Bacteriophage—ELIAVA. Available online: https://phage.ge/products/intesti-bacteriophage/?lang=en (accessed on 11 June 2022).
- Enko-Bacteriophage—ELIAVA. Available online: https://phage.ge/products/enko-bacteriophage (accessed on 11 June 2022).
- Pyo-Bacteriophage—ELIAVA. Available online: http://phage.ge/products/pyo-bacteriophage (accessed on 11 June 2022).
- Instytut Imunologii i Terapii Doświadczalnej im. Ludwika Hirszfelda. Available online: https://www.iitd.pan.wroc.pl (accessed on 11 June 2022).
- STAFAL®. Available online: https://www.bohemiapharm.eu/en/products/products/stafal (accessed on 11 June 2022).
- Fauconnier, A. Regulating phage therapy: The biological master file concept could help to overcome the regulatory challenge of personalized medicines. EMBO Rep. 2017, 18, 198–200. [Google Scholar] [CrossRef]
- Phagoburn. Available online: http://www.phagoburn.eu (accessed on 11 June 2022).
- Tkhilaishvili, T.; Wang, L.; Tavanti, A.; Trampuz, A.; Di Luca, M. Antibacterial Efficacy of Two Commercially Available Bacteriophage Formulations, Staphylococcal Bacteriophage and PYO Bacteriophage, Against Methicillin-Resistant Staphylococcus aureus: Prevention and Eradication of Biofilm Formation and Control of a Systemic Infection of Galleria mellonella Larvae. Front. Microbiol. 2020, 11, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verstappen, K.M.; Tulinski, P.; Duim, B.; Fluit, A.C.; Carney, J.; van Nes, A.; Wagenaar, J.A. The Effectiveness of Bacteriophages against Methicillin-Resistant Staphylococcus aureus ST398 Nasal Colonization in Pigs. PLoS ONE 2016, 11, e0160242. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.; Zou, W.; Zhang, M.; Xu, L.; Liu, F.; Li, X.; Wang, L.; Xu, Y. Evaluation of phage therapy in the treatment of Staphylococcus aureus-induced mastitis in mice. Folia Microbiol. 2020, 65, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Gundogdu, A.; Bolkvadze, D.; Kilic, H. In vitro Effectiveness of Commercial Bacteriophage Cocktails on Diverse Extended-Spectrum Beta-Lactamase Producing Escherichia coli Strains. Front. Microbiol. 2016, 7, 1761. [Google Scholar] [CrossRef] [PubMed]
- Slobodníková, L.; Markusková, B.; Kajsík, M.; Andrezál, M.; Straka, M.; Liptáková, A.; Drahovská, H. Characterization of Anti-Bacterial Effect of the Two New Phages against Uropathogenic Escherichia coli. Viruses 2021, 13, 1348. [Google Scholar] [CrossRef]
- Namonyo, S.; Carvalho, G.; Guo, J.; Weynberg, K.D. Novel Bacteriophages Show Activity against Selected Australian Clinical Strains of Pseudomonas aeruginosa. Microorganisms 2022, 10, 210. [Google Scholar] [CrossRef]
- Camens, S.; Liu, S.; Hon, K.; Bouras, G.S.; Psaltis, A.J.; Wormald, P.-J.; Vreugde, S. Preclinical Development of a Bacteriophage Cocktail for Treating Multidrug Resistant Pseudomonas aeruginosa Infections. Microorganisms 2021, 9, 2001. [Google Scholar] [CrossRef]
- Fong, S.A.; Drilling, A.; Morales, S.; Cornet, M.E.; Woodworth, B.A.; Fokkens, W.J.; Psaltis, A.J.; Vreugde, S.; Wormald, P.J. Activity of Bacteriophages in Removing Biofilms of Pseudomonas aeruginosa Isolates from Chronic Rhinosinusitis Patients. Front. Cell. Infect. Microbiol. 2017, 7, 418. [Google Scholar] [CrossRef] [Green Version]
- Shield, C.G.; Swift, B.M.C.; McHugh, T.D.; Dedrick, R.M.; Hatfull, G.F.; Satta, G. Application of Bacteriophages for Mycobacterial Infections, from Diagnosis to Treatment. Microorganisms 2021, 9, 2366. [Google Scholar] [CrossRef]
- Kutateladze, M.; Adamia, R. Phage therapy experience at the Eliava Institute. Med. Mal. Infect. 2008, 38, 426–430. [Google Scholar] [CrossRef]
- Cano, E.J.; Caflisch, K.M.; Bollyky, P.L.; Van Belleghem, J.D.; Patel, R.; Fackler, J.; Brownstein, M.J.; Horne, B.; Biswas, B.; Henry, M.; et al. Phage Therapy for Limb-threatening Prosthetic Knee Klebsiella pneumoniae Infection: Case Report and In Vitro Characterization of Anti-biofilm Activity. Clin. Infect. Dis. 2021, 73, e144–e151. [Google Scholar] [CrossRef] [PubMed]
- Ujmajuridze, A.; Chanishvili, N.; Goderdzishvili, M.; Leitner, L.; Mehnert, U.; Chkhotua, A.; Kessler, T.M.; Sybesma, W. Adapted Bacteriophages for Treating Urinary Tract Infections. Front. Microbiol. 2018, 9, 1832. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Property | Phage Therapy | Antibiotic Therapy |
|---|---|---|
| Pharmacokinetics | Phages replicate at the site of infection; after elimination of the bacterial host, spontaneous disappearance occurs | Antibiotics are metabolized and eliminated by human body |
| Spectrum of action | Phages attack bacteria based on their host specificity | Antibiotics usually have a much broader spectrum of action |
| Resistance | Phages are suitable for the treatment of infections caused also by antibiotic-resistant bacteria; phage-resistant bacterial strains have lower fitness | The number of antibiotic-resistant bacterial strains is increasing |
| Manufacture | Simple isolation of phages and their modification for therapeutic use | Challenging development of new antibiotics with high financial costs |
| Effect on biofilm | Production of depolymerization enzymes for biofilm elimination | Reduced effect on the bacteria in the biofilm |
| Clinical validation | Few clinical studies | Many clinical studies |
| Combined therapy | Efficacy is higher with combined therapy | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Straka, M.; Dubinová, M.; Liptáková, A. Phascinating Phages. Microorganisms 2022, 10, 1365. https://doi.org/10.3390/microorganisms10071365
Straka M, Dubinová M, Liptáková A. Phascinating Phages. Microorganisms. 2022; 10(7):1365. https://doi.org/10.3390/microorganisms10071365
Chicago/Turabian StyleStraka, Marek, Martina Dubinová, and Adriána Liptáková. 2022. "Phascinating Phages" Microorganisms 10, no. 7: 1365. https://doi.org/10.3390/microorganisms10071365
APA StyleStraka, M., Dubinová, M., & Liptáková, A. (2022). Phascinating Phages. Microorganisms, 10(7), 1365. https://doi.org/10.3390/microorganisms10071365







