Abstract
Zearalenone (ZEN) and deoxynivalenol (DON) are mycotoxins produced by various species of Fusarium fungi. They contaminate agricultural products and negatively influence human and animal health, thus representing a serious problem of the agricultural industry. Earlier we showed that compactin, a secondary metabolite of Penicillium citrinum, is able to completely suppress the aflatoxin B1 biosynthesis by Aspergillus flavus. Using the F. culmorum strain FC-19 able to produce DON and ZEN, we demonstrated that compactin also significantly suppressed both DON (99.3%) and ZEN (100%) biosynthesis. The possible mechanisms of this suppression were elucidated by qPCR-based analysis of expression levels of 48 biosynthetic and regulatory genes. Expression of eight of 13 TRI genes, including TRI4, TRI5, and TRI101, was completely suppressed. A significant down-regulation was revealed for the TRI10, TRI9, and TRI14 genes. TRI15 was the only up-regulated gene from the TRI cluster. In the case of the ZEN cluster, almost complete suppression was observed for PKS4, PKS13, and ZEB1 genes, and the balance between two ZEB2 isoforms was altered. Among regulatory genes, an increased expression of GPA1 and GPA2 genes encoding α- and β-subunits of a G-protein was shown, whereas eight genes were down-regulated. The obtained results suggest that the main pathway for a compactin-related inhibition of the DON and ZEN biosynthesis affects the transcription of genes involved in the G-protein-cAMP-PKA signaling pathway. The revealed gene expression data may provide a better understanding of genetic mechanisms underlying mycotoxin production and its regulation.
1. Introduction
Fusarium fungi are harmful pathogens infesting cereal crops in the temperate climatic zones and causing diseases such as Fusarium ear rot, Fusarium head blight, and various foot and root rots [1,2]. Some fungi of this genus may produce various mycotoxins, including deoxynivalenol (DON) and zearalenone (ZEN), which are among the most important and regulated mycotoxins at the global scale [3,4].
DON is considered to be the most common mycotoxin contaminating cereals worldwide [5,6,7]. The main mechanism of the trichothecene action is the inhibition of a protein biosynthesis in eukaryotic cells via binding to ribosomal peptidyltransferase [8,9]. DON and its derivatives induce apoptosis, affect intestinal and immune functions, cause reproductive disorders [10,11], and act as virulence factors in plants [12,13]. Another important fusariotoxin is zearalenone (ZEN), a xenoestrogen synthesized via a polyketide pathway [14]. ZEN is capable of binding to estrogen receptors in mammals, thus leading to reproductive disorders [15,16]. According to the International Agency for Research on Cancer, it is classified as a Group 3 carcinogen [17]. Several reports demonstrated that ZEN possesses neurotoxic, immunotoxic, and hepatotoxic properties [18,19,20].
Genes responsible for the DON and ZEN biosynthesis are organized in clusters. Each cluster consists of genes encoding enzymes, transcription factors, and transport proteins [21,22]. This type of organization significantly facilitates coordinated regulation of a cluster gene’s expression and its control. Genes involved in these clusters are under the control of specific regulators, in addition to global regulation factors mediating signals from different environmental stimuli.
In the case of the main DON producers, F. graminearum and F. culmorum, the core TRI cluster includes 12 genes, among which two (TRI6 and TRI10) encode transcription factors and one (TRI12) encodes an efflux pump, involved in self-defense against the mycotoxin; the functions of TRI9 and TRI14 genes are still unknown [23]. The remaining seven genes encode enzymes responsible for different stages of a DON biosynthesis. In addition to the core cluster, there are at least two loci (TRI1-TRI16 and TRI101) located in different parts of the genome, but also involved in the biosynthesis of this mycotoxin [24]. Some researchers considered the TRI15 gene, which encodes a regulatory Cys2His2 zinc finger and is located outside the core cluster, to be another locus related to trichothecene production [25]. Genes crucial for ZEN biosynthesis include PKS4, PKS13, ZEB1, and ZEB2, which encode reducing and non-reducing polyketide synthases, isoamyl alcohol oxidase, and transcription factor, respectively. These neighboring genes form a cluster, coordinately regulated at the transcriptional level [26,27,28].
Traditional management of fungal diseases and mycotoxin contamination of food and feed is based on complex approaches, which include crop rotation, use of resistant cultivars, and application of fungicides. At the same time, the first two approaches are unable to completely prevent mycotoxin accumulation, while fungicidal treatments, especially in inadequate doses, may result in a chemical stress in attacked fungi, causing even more active synthesis of mycotoxins [29]. In addition, fungicides may have negative effects on human and animal health, and may lead to the emergence of resistant pathogen lines. From this point of view, natural compounds of microbial or plant origin able to inhibit mycotoxin production without suppression of fungal growth represent a promising alternative to synthetic fungicides, primarily in terms of safety and prevention of the development of resistance [30,31,32].
Earlier we showed that 6-demethylmevinolin (6-DMM or compactin), produced by Penicillium citrinum and known as an inhibitor of HMG-CoA reductase regulating cholesterol biosynthesis, was able to efficiently block the biosynthesis of a polyketide mycotoxin aflatoxin B1 (AFB1), and prevent melanin production and spore formation in Aspergillus flavus [33]. Interestingly, no other known inhibitor of mycotoxin biosynthesis provides such a triple effect. Since both AFB1 and melanin are synthesized in A. flavus via the polyketide pathway, we suggested that the revealed effect of compactin may be determined by its interaction with enzymes or regulating proteins of this biosynthetic pathway and, therefore, may be also applied to other polyketide mycotoxins. A preliminary study confirmed the inhibiting activity of compactin towards ZEN biosynthesis [34]. The purpose of this study was elucidation of the mechanisms of this phenomenon via the analysis of the effect of compactin on the levels of expression of various genes involved in ZEN production. Since the toxigenic strain of F. culmorum used in this study was also able to produce DON, a possible compactin effect on the DON production and expression of genes from the corresponding biosynthetic and regulatory clusters was additionally investigated. The results of the study demonstrated that the inhibitor acts at the transcriptional level, down- and up-regulating biosynthetic and regulatory genes involved in both ZEN and DON production. The obtained data are important for the development of new plant protection strategies, and for the better understanding of genetic mechanisms underlying mycotoxin production and its regulation.
2. Materials and Methods
2.1. Fungal Strains and Culture Conditions
A toxigenic Fusarium culmorum strain FC-19 was provided by the State Collection of Plant Pathogenic Microorganisms of the All-Russian Research Institute of Phytopathology (Bolshie Vyazemy, Russia). A stock culture of the pathogen maintained on potato dextrose agar (PDA) slants was resumed by culturing for two weeks at 25 °C in Petri plates with the same medium to obtain actively growing and spore-producing colonies. Conidia were collected from a colony surface by flooding the mycelium with sterilized water and gently rubbing with a glass rod. The obtained spore suspension was filtered through sterile cotton wool to remove mycelium debris and adjusted to 1 × 105–1 × 106 conidia/mL to be used as the inoculum.
Submerged cultures of FC-19 used in the experiments were grown in 250 mL flasks with 50 mL of a liquid Myro medium of the following composition (g/L): soybean flour, 2.5; sucrose, 40; glycerol, 10; (NH4)2HPO4, 1; KH2PO4, 3; MgSO4, 2; NaCl, 5 (pH 5.9). Compactin (Sigma-Aldrich, St.-Louis, MO, USA) diluted in a minimal volume of ethanol was added into the autoclaved medium prior to inoculation up to a final concentration of 25 μg/mL, which, according to the preliminary experiments, had no inhibitory effect on the fungus growth, but completely suppressed ZEN production; for DON variants, the tested compactin concentrations were 10, 20, and 50 μg/mL. The equivalent amount of ethanol was added as the control.
After inoculation with 2 mL of conidial suspension, flasks were incubated on an Infors HT RT-TK rotary shaker (Informs AG, Bottmingen, Switzerland) at 25 °C and 250 rpm for 4 (gene expression analysis) or 8 (mycotoxin analysis) days. All experimental and control variants were arranged in three replications.
2.2. Fungal Biomass and Mycotoxin Production Determination
To confirm the inhibitory effect of compactin, the content of ZEN and DON in control and compactin-treated FC-19 cultures was determined by HPLC using a Waters 1525 Breeze HPLC system equipped with a WAT 045905 detector (Waters Corp, Milford, MA, USA). At the end of the 8-day cultivation, each flask with a fungal culture was supplemented with 50 mL of dichloromethane and incubated for 1 h on a shaker under the same conditions. Then, the mycelium was separated by filtration, dried to a constant weight at 60 °C, and weighed to determine its dry weight. The filtered extract was passed through a layer of anhydrous sodium sulfate and evaporated at 40 °C on a Rotavapor R110 rotary evaporator (BÜCHI Labortechnik AG, Essen, Germany). The obtained residue was dissolved in a minimum volume of the mobile phase (see below), and a 10 μL aliquot of the prepared solution was applied to a temperature-controlled (27 °C) Symmetry C18 column (5 µm; 4.6 × 150 mm; Waters Corp., Milford, MA, USA). ZEN and DON elution from the column was performed using an acetonitrile:methanol:water mixture at the ratio of 1:1:0.75 (ZEN) or 1:1:4 (DON) as the mobile phase and a flow rate of 0.8 mL/min. The detection was performed at 254 nm. The mycotoxin content was quantified using commercial ZEN and DON preparations (Sigma-Aldrich, St.-Louis, MO, USA) as the standards.
2.3. RNA Isolation and Reverse Transcriptase-Polymerase Chain Reaction
A total RNA was isolated from 4-day FC-19 cultures grown in the presence or absence of compactin (10 or 25 μg/mL for DON and ZEN gene clusters, respectively) using an RNeasy® Plant MiniKit (QIAGEN, Hilden, Germany) in accordance with the manufacturer’s recommendations. A preliminary homogenization of the mycelium was carried out using liquid nitrogen. During isolation, samples were treated with an RNase-free DNAse set (QIAGEN, Hilden, Germany) according to the manufacturer’s instruction. The quality of the isolated RNA was evaluated spectrophotometrically using a Qubit 4 fluorometer (Thermo Fischer Scientific (Waltham, MA, USA) and by electrophoresis in 1% agarose gel. The reverse transcription reaction was performed using a QuantiTect® Reverse Transcription Kit (QIAGEN, Hilden, Germany). The possible presence of DNA impurities was additionally assessed via amplification of cDNA using a pair of primers complementary to two exons of the TEF1α gene followed by electrophoresis. The presence of genomic DNA was determined by the size of the specific product (~600 or ~250 bp in the presence or absence of introns, respectively). Samples suspected to contain DNA contamination were not used in the further study.
2.4. Gene Expression Analysis by qPCR
The design of primer sets and the corresponding hydrolysis (TaqMan® [35]) probes were conducted based on the genomic data of Fusarium graminearum strain PH-1 (GenBank accession numbers CP087871-74) and the search for potential homologues of the corresponding genes in the genome of Fusarium culmorum strain Class2-1B (GenBank accession numbers CP064747-50). Primers and probes were universal for both of these species. Oligonucleotides for the detection of transcripts of PKS4, PKS13, TRI5, and TRI6 genes were described earlier [36]. Primer and probe sequences are listed in Table S1.
The qPCR reactions were carried out using a DT-96 thermocycler (DNA-Technology, Moscow, Russia) and the following universal amplification conditions: 94 °C for 1 min followed by 45 cycles at 94 °C for 10 s, 64 °C for 30 s, 72 °C for 5 s, and, finally, 72 °C for 5 min. The reaction mix was described in [37]. The total volume of a cDNA sample added to the reaction mix was 2 µL.
Two independent experiments were carried out, and each included three biological replicates.
2.5. qPCR Data Analysis
Amplification efficiency was calculated using the Cq slope method (see Table S1). The relative expression of target genes was estimated according to the mathematical model proposed by Pfaffl [38] using a QGene software package [39]. Results are represented as fold changes in treated samples calculated with respect to the same gene in the control sample (considered as 1.0).
2.6. Statistical Data Treatment
The statistical analysis of the obtained data was carried out using the Statistica 6.0 software package (StatSoft Inc., Tulsa, OK, USA). The significance of differences (p < 0.05) in the means between the experimental and control values were determined using a t-test for independent variables.
3. Results
3.1. Effect of Compactin on the ZEN and DON Production by F. culmorum FC-19
The results of the evaluation of the compactin effect on the ZEN and DON production by FC-19 are shown in Table 1. Like in the preliminary study [34], compactin demonstrated a significant inhibiting activity towards ZEN production. Addition of 25 μg/mL of compactin completely suppressed ZEN production by FC-19, since the residual ZEN content was below the limit of detection (2.0 ng/mL); at the same time, its negative effect on the mycelium growth was rather insignificant (~8% of the control).

Table 1.
Effect of compactin on the zearalenone (ZEN) and deoxynivalenol (DON) production by F. culmorum FC-19 1.
No DON production was detected in the presence of 20 and 50 μg/mL of compactin. A very low DON content (<1% of the control) was observed for the lowest studied compactin concentration (10 μg/mL), while no significant effect on the fungal growth was revealed. Based on these results, this concentration (10 μg/mL) was used in the further gene expression experiments.
3.2. Effect of Compactin on the Expression of Genes Responsible for the ZEN Biosynthesis and Regulation in F. culmorum FC-19
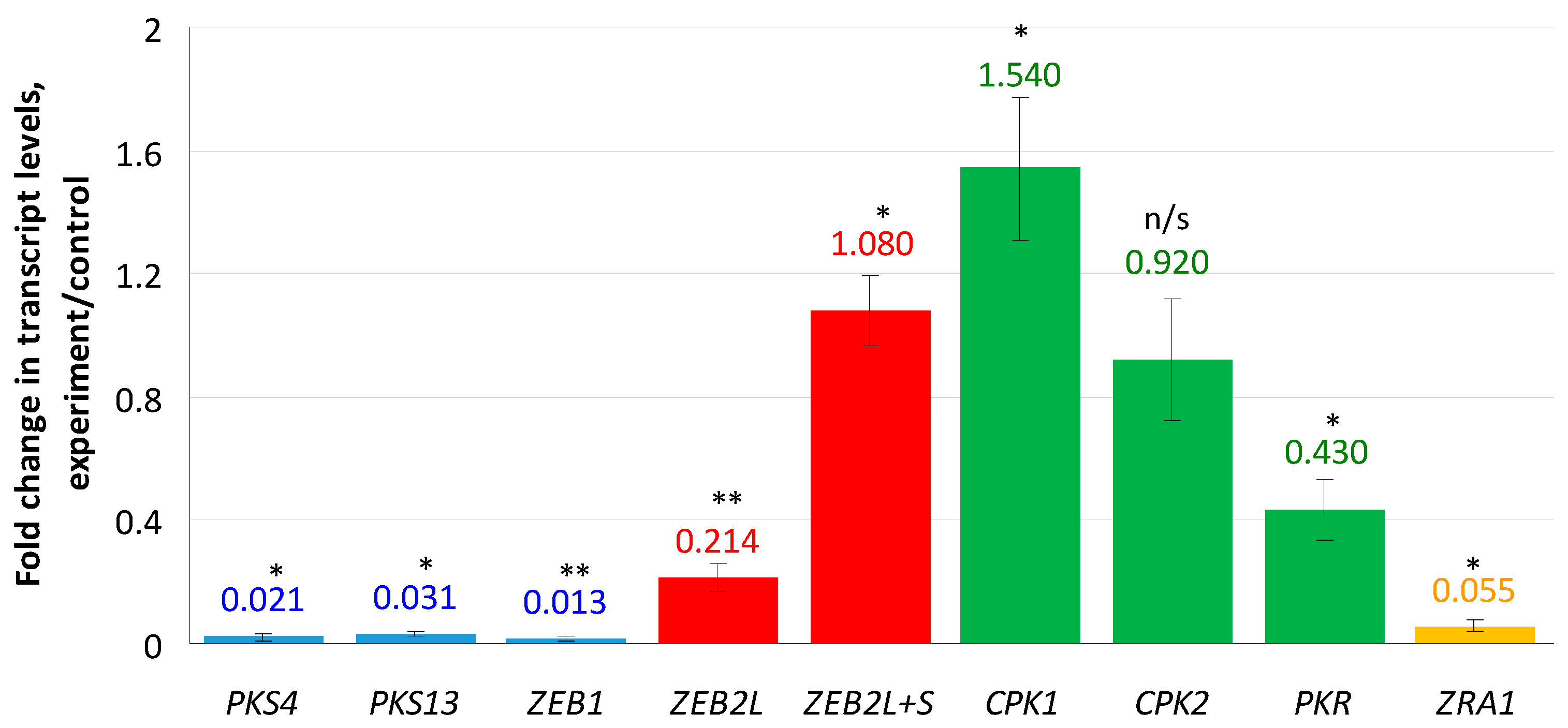
Genes encoding enzymes responsible for the ZEN biosynthesis were shown to be almost completely down-regulated (Figure 1): 0.02 fold change (f.c.) for PKS4, 0.03 f.c. for PKS13, and 0.013 f.c. for ZEB1. The most interesting results were obtained for the transcription factor ZEB2 encoded by the ZEB2 gene and regulating expression of ZEN biosynthetic genes. This factor is represented by two isoforms: ZEB2L (long) and ZEB2S (short). Since it was impossible to design a pair of primers specific to the ZEB2S isoform, we used two primer pairs: the first pair was specific for the “long” isoform, and the second pair was able to detect both “long” and “short” isoforms. As a result, we found a 0.214-fold decrease in the ZEB2L isoform expression. In contrast, when tested with the second primer pair, the expression level was increased (1.08 f.c.). This result can be explained by enhanced transcription of the ZEB2S isoform and suppressed transcription of the ZEB2L isoform under the compactin treatment conditions. In addition, expression of a CPK1 gene encoding the catalytic subunit 1 of cAMP-dependent protein kinase (PKA), which regulates ZEN production, was increased 1.54-fold, whereas the change in the expression of the CPK2 gene was insignificant (0.92 f.c., p > 0.05). The PKR and ZRA1 genes encoding the PKA regulatory subunit and the ABC transporter required for ZEN production, respectively, were down-regulated (0.43- and 0.05-fold, respectively).
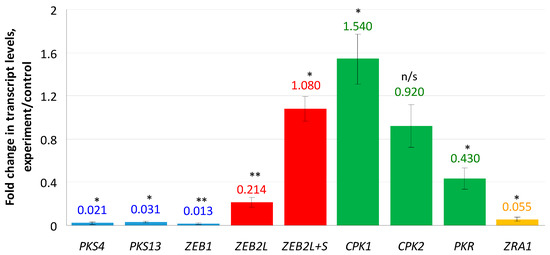
Figure 1.
Effect of compactin on the relative expression of genes responsible for ZEN biosynthesis and regulation. The expression level (EL) for the untreated control was considered as 1.0; EL above or below 1.0 means up- or down-regulation, respectively. Blue bars indicate genes encoding biosynthetic enzymes; red bars indicate transcripts of a regulatory ZEB2 gene; yellow bar indicates a ZRA1 gene encoding the transport protein; green bars indicate genes encoding cAMP-dependent protein kinase subunits. Each bar is shown as M ± SD; n/s, no significant changes; * p < 0.05; ** p < 0.01.
3.3. Effect of Compactin on the Expression of Genes Responsible for the DON Biosynthesis and Regulation in F. culmorum FC-19
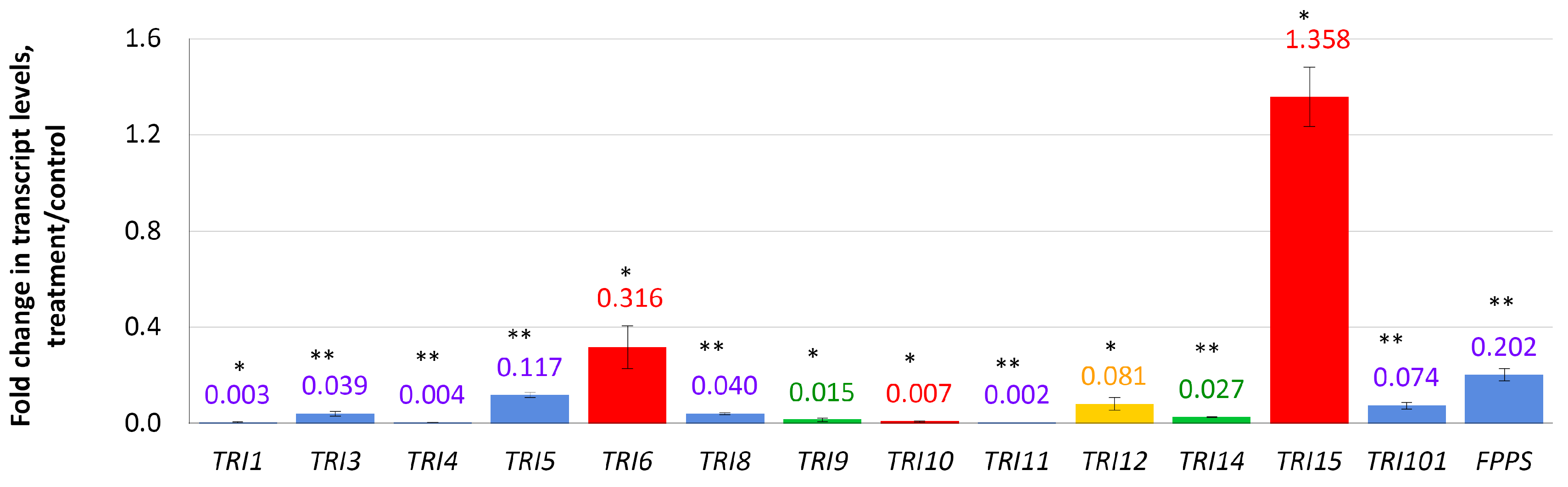
In this experiment, relative expression levels of 13 TRI genes related to the DON biosynthesis, and a FPPS gene encoding farnesyl pyrophosphate synthase, were analyzed. Four days after the addition of compactin to the cultivation medium, the expression of eight TRI genes was almost completely suppressed (Figure 2). The most inhibited gene was TRI11 (0.002 f.c.) followed by TRI1, TRI4 (0.003 f.c. each), and TRI10 (0.007 f.c.) genes. The TRI5 gene encoding trichodiene synthase (the first enzyme of the trichothecene biosynthetic pathway) was 0.12-fold down-regulated. A global regulator-encoding TRI6 gene was the least down-regulated gene of the TRI cluster (0.316 f.c.). Two genes with unknown functions (TRI9 and TRI14) were down-regulated (0.014- and 0.026-fold, respectively). The only up-regulated gene among those analyzed was TRI15 (1.36 f.c.).
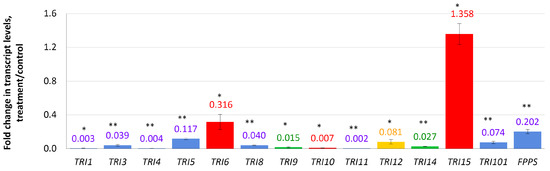
Figure 2.
Effect of compactin on the relative expression of genes responsible for DON biosynthesis and regulation. The expression level for the untreated control was considered as 1.0. EL above or below 1.0 means up- or down-regulation, respectively. Blue bars indicate genes encoding biosynthetic enzymes; red bars indicate genes encoding regulation factors; yellow bar indicates the TRI12 gene encoding transport protein (TRI efflux pump); green bars indicate genes with unknown functions. Each bar is shown as M ± SD; * p < 0.05; ** p < 0.01.
3.4. Effect of Compaction on the Expression of Key Regulatory Genes in F. culmorum FC-19
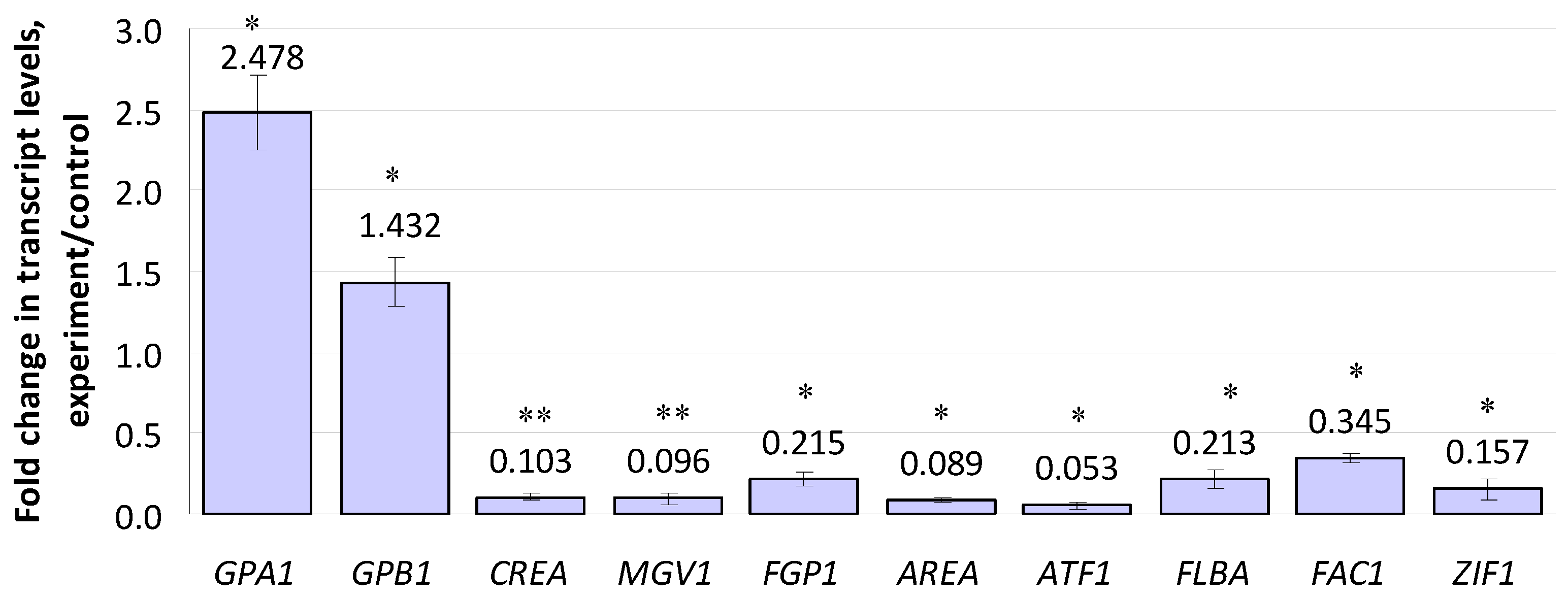
Ten of the regulatory genes included in the study were up- or down-regulated by compactin (Figure 3). Among these, the expression of genes encoding α- and β-subunits (GPA1 and GPB1) of a heteromeric G-protein showed a 2.47- and 1.43-fold increase, respectively. Note that the changes in the expression levels of genes encoding other Gα subunits (GPA2 and GPA3) were insignificant (Table S2). The most down-regulated regulatory gene was ATF1 (0.053 f.c.), which encoded a transcription factor; it was followed by AREA (0.089 f.c.), MGV1 (0.096 f.c.), and CREA (0.103 f.c.). The ZIF1 gene encoding a b-ZIP transcription factor demonstrated a 0.16-fold down-regulation. The expression levels of FLBA (encoding a regulator of G-protein signaling) and P1 (encoding a Wor1-like protein) genes showed a 0.21- and 0.22-fold decrease, respectively. The least-inhibited gene (0.345 f.c.) was FAC1 encoding adenylate cyclase. Fifteen other regulatory genes, including those of the velvet regulatory complex (VE1, VELB, LAEA), were insignificantly affected by compactin (Table S2).
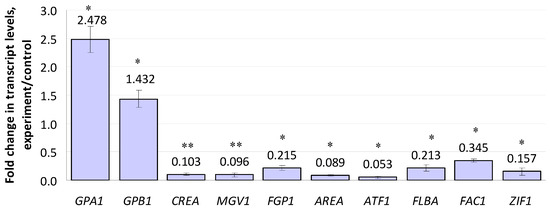
Figure 3.
Effect of compactin on the relative expression of genes encoding regulatory factors. The expression level for the untreated control was considered as 1.0. EL above or below 1.0 means up- or down-regulation, respectively. Each bar is shown as M ± SD; * p < 0.05; ** p < 0.01.
4. Discussion
An appropriate mycotoxin control in food and feed products is a very important problem in modern agriculture. From this point of view, prevention of its production and accumulation represents a good practical approach. Since the existing pre-harvest technologies for the control of toxigenic fungi are unable to completely prevent mycotoxin contamination of agricultural products, the development of efficient and safe post-harvest approaches, including suppression of mycotoxin production or biodegradation of already accumulated mycotoxins, is of great interest. Furthermore, the majority of studies in this area are focused on biodegradation aspects, whereas the search for potential efficient inhibitors of mycotoxin production among natural compounds of plant or microbial origin seems to be underestimated. To date, the number of such studies is relatively low, and these are mainly focused on the AFB1 biosynthesis inhibitors (see, for example, [40,41]). Moreover, some compounds reported to suppress mycotoxin biosynthesis also possess antifungal activity; thus, it often remains unclear if they reduce mycotoxin content via suppression of its biosynthesis, or simply by reducing the mycelial biomass producing them.
Biosynthesis of mycotoxins and its regulation is a complex process involved in global regulatory networks and controlled by different factors [42]. To elucidate the possible mechanism of inhibition of DON and ZEN biosynthesis by compactin, a qPCR-based system for the estimation of relative expression of 48 genes was developed. The analyzed genes included those located in TRI and ZEN biosynthetic clusters, and genes encoding global regulators. Surprisingly, our study showed compactin to be able to suppress production of DON belonging to another group of mycotoxins, whose biosynthetic pathways differ from those of polyketide mycotoxins (AFB1 and ZEN). The obtained results demonstrated that the expression of eight of 13 TRI genes (TRI7 and TRI13 genes are non-functional in DON-producing strains [43] and, therefore, were excluded from the analysis) was almost completely repressed. The most inhibited genes included those responsible for the early stages of the trichothecene biosynthesis (TRI4, TRI5, TRI101). These data correlate well with the data of the earlier studies demonstrating the down-regulation of these genes in F. graminearum [44] and F. culmorum [45] by phenolic acids possessing inhibiting activity towards mycotoxin biosynthesis. However, the authors of the mentioned studies analyzed only eight and seven genes, respectively. In our study, we also examined other genes involved in DON biosynthesis. Among the genes encoding specific regulators of the TRI biosynthetic pathway, TRI10 was significantly more suppressed compared to TRI6. This fact probably indicates that TRI10 or one of its regulators represents the main target of a compactin-related inhibition mechanism. This suggestion is supported by a report on the strong down-regulation of genes coordinated by TRI10, such as TRI3, TRI11, and TRI12 [46]. Another interesting result is the almost complete inhibition of transcription of TRI9 and TRI14 genes, whose functions are unknown. Dyer et al. 2005 [47] demonstrated that TRI14 is required for DON biosynthesis and pathogen virulence in planta, but not in vitro. At the same time, expression of TRI9 and especially TRI14 in F. graminearum was significantly increased by agmatine, a compound promoting DON biosynthesis [48]. These genes probably encode unique Fusarium-specific regulation factors, whose exact functions are yet to be elucidated. Among the studied genes, TRI15 was the only upregulated gene from the TRI cluster. This gene is predicted to encode a Cys2His2 zinc finger protein, but its role in a trichothecene biosynthesis is also unclear. Disruption of TRI15 in F. sporotrichioides did not affect the synthesis of a T-2 toxin [25], but Ponts et al. (2007) suggested it can negatively regulate DON biosynthesis in F. graminearum [49]. The up-regulation of the TRI10-controlled TRI15 gene can be explained by the presence of an unknown alternative regulation mechanism.
A qPCR-based approach helped to decipher a possible transcriptional mechanism of a compactin-related inhibition of a ZEN biosynthesis. In the case of F. graminearum, expression of PKS4, PKS13, and ZEB1 genes is controlled by a ZEB2 transcription factor [50]. ZEB2 can be translated in two isoforms, ZEB2L (long) and ZEB2S (short). MRNAs encoding these isoforms are transcribed via an alternative promoter. Earlier it was shown that ZEB2L acts as an activator of genes from the ZEN biosynthetic cluster, whereas ZEB2S inhibits them. Genes responsible for the ZEN biosynthesis are transcribed only in the presence of a ZEB2L isoform alone. Under ZEN-inducing conditions, ZEB2L transcript was detected on the 3rd day of incubation, reaching the peak value by the 6th day. ZEB2S transcript appeared after 5 days of incubation and was present up to the 10th day. In our study, ZEB2L expression measured on the 4th day of the FC-19 incubation in the presence of compactin was significantly decreased (0.214 f.c.). At the same time, a transcript detected using primers specific for both isoforms demonstrated a slight up-regulation (1.08 f.c.). These data indicate that, in the presence of compactin, the ratio between L and S isoforms changes, and the S isoform inhibits ZEN biosynthesis via suppression of transcription of PKS4, PKS13, and ZEB1 genes. Another ZEB2-controlled down-regulated gene was ZRA1, which encodes the ABC transporter that is also important for the ZEN biosynthesis [51]. In F. graminearum, ZEN production is controlled via the G-protein-cAMP-PKA signaling pathway [52]. In our study, the CPK1 gene, encoding the subunit 1 of cAMP-dependent protein kinase, was up-regulated, whereas expression of the CPK2 gene was not significantly changed, and a regulatory subunit-encoding PKR gene was down-regulated. These results are in accordance with the data obtained by Park et al. (2016), who demonstrate that CPK1 and PKR are negative and positive regulators of the ZEN biosynthesis, respectively [53].
An increased expression of α- and β-subunits of a heterotrimeric G-protein reaffirms the principal role of a compactin-related effect of G-protein-cAMP-PKA pathway on the inhibition of a mycotoxin biosynthesis in F. culmorum. According to Yu et al. (2008), GzGPA1 and GzGPB1 genes negatively regulate mycotoxin production in F. graminearum [54]. Genes encoding some regulators of a G-protein signaling pathway, such as RGS protein (FLBA) or adenylate cyclase (FAC1), were also shown to be down-regulated. At the same time, expression of the RAS1 gene, which encodes Ras GTPase and is suggested to play an important role in the cAMP signaling pathway [55], was not significantly changed in the compactin-treated sample. Among other results of our study, attention should be paid to a significant down-regulation of the AREA and CREA genes encoding transcription factors related to the metabolism of nutrients [21,42]. This fact indicates that the mechanism of compactin-related inhibition of the mycotoxin biosynthesis in F. culmorum is more complex and involves various factors and metabolic networks. Interestingly, genes encoding proteins of an important velvet regulatory complex (VE1, LAEA, VELB) were not affected by the compactin treatment.
To date, the landscape of studies describing efficient inhibitors of ZEN and DON is relatively sparse. Reported compounds possessing such activity include some phenolic and flavonoid compounds of plant origin, antibiotics, and other microbial metabolites (see, for example [31,56,57,58,59]). The vast majority of such studies was carried out under in vitro conditions, although some phenolic compounds were also tested on wheat spikes [32]. As far as the authors are aware, there are no reports revealing any compounds able to efficiently inhibit different types of mycotoxins. From this point of view, the results obtained in our study are novel and of great interest. The revealed property of compactin, namely, the complete suppression of the biosynthesis of polyketide (AFB1 [33] and ZEN) and trichothecene (DON) mycotoxins at concentrations not influencing fungal growth, was confirmed for both in vitro conditions (compactin-containing nutrient media) and on artificially infected grain; thus, compactin may have good potential for practical application. The performed gene expression analysis showed compactin had a significant influence, not only on genes from DON and ZEN biosynthetic clusters, but also on the higher-order genes regulating general cell activities. As far as the authors are aware, no such studies have been carried out for other mycotoxin-inhibiting compounds. Thus, these data may be also considered to be novel. In general, the results of this study are interesting, since they demonstrate the existence of a kind of “multi-purpose” inhibitor that is able to suppress the biosynthesis of different mycotoxins without suppressing the growth of their producers. Such compounds may have some benefits for practical application because agricultural products are often contaminated with several mycotoxin-producing fungi or fungi able to produce several mycotoxins.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms10071347/s1, Table S1: Target genes and corresponding oligonucleotides, Table S2: Relative expression changes for genes insignificantly affected by the compactin treatment.
Author Contributions
Conceptualization, A.A.S.; methodology, A.A.S., D.V.E. and O.D.M.; formal analysis, A.A.S. and N.V.S.; investigation, A.A.S., D.V.E., E.A.M. and O.D.M.; writing—original draft preparation, A.A.S., D.V.E. and N.V.S.; writing—review and editing, A.A.S. and N.V.S.; project administration, A.A.S.; funding acquisition, A.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, project number 19-76-10031.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are shown in this manuscript and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wachowska, U.; Packa, D.; Wiwart, M. Microbial inhibition of Fusarium pathogens and biological modification of trichothecenes in cereal grains. Toxins 2017, 9, 408. [Google Scholar] [CrossRef] [PubMed]
- Mielniczuk, E.; Skwaryło-Bednarz, B. Fusarium head blight, mycotoxins and strategies for their reduction. Agronomy 2020, 10, 509. [Google Scholar] [CrossRef]
- BIOMIN Mycotoxin Survey Q3 2021 Results. Available online: https://www.biomin.net/science-hub/biomin-mycotoxin-survey-q3-2021-results/ (accessed on 22 May 2022).
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Tabuc, C.; Nicolau, A.; Aprodu, I.; Puel, O.; et al. Current situation of mycotoxin contamination and co-occurrence in animal feed—Focus on Europe. Toxins 2012, 4, 788–809. [Google Scholar] [CrossRef] [PubMed]
- Foroud, N.A.; Baines, D.; Gagkaeva, T.Y.; Thakor, N.; Badea, A.; Steiner, B.; Bürstmayr, M.; Bürstmayr, H. Trichothecenes in cereal grains—An update. Toxins 2019, 11, 634. [Google Scholar] [CrossRef]
- Guo, H.; Ji, J.; Wang, J.S.; Sun, X.L. Deoxynivalenol: Masked forms, fate during food processing, and potential biological remedies. Compr. Rev. Food Sci. Food Saf. 2020, 19, 895–926. [Google Scholar] [CrossRef] [PubMed]
- Chtioui, W.; Balmas, V.; Delogu, G.; Migheli, Q.; Oufensou, S. Bioprospecting phenols as inhibitors of trichothecene-producing Fusarium: Sustainable approaches to the management of wheat pathogens. Toxins 2022, 14, 72. [Google Scholar] [CrossRef] [PubMed]
- Cundliffe, E.; Cannon, M.; Davies, J. Mechanism of inhibition of eukaryotic protein synthesis by trichothecene fungal toxins. Proc. Nat. Acad. Sci. USA 1974, 71, 30–34. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, Y.; Abraham, N.; Li, X.-Z.; Kimber, M.; Zhou, T. The ribosome-binding mode of trichothecene mycotoxins rationalizes their structure-activity relationships. Int. J. Mol. Sci. 2021, 22, 1604. [Google Scholar] [CrossRef]
- Rocha, O.; Ansari, K.; Doohan, F.M. Effects of trichothecene mycotoxins on eukaryotic cells: A review. Food Addit. Contam. 2005, 22, 369–378. [Google Scholar] [CrossRef]
- Arunachalam, C.; Doohan, F.M. Trichothecene toxicity in eukaryotes: Cellular and molecular mechanisms in plants and animals. Toxicol. Lett. 2013, 217, 149–158. [Google Scholar] [CrossRef]
- Abbas, H.K.; Yoshizawa, T.; Shier, W.T. Cytotoxicity and phytotoxicity of trichothecene mycotoxins produced by Fusarium spp. Toxicon 2013, 74, 68–75. [Google Scholar] [CrossRef]
- Scherm, B.; Orrù, M.; Balmas, V.; Spanu, F.; Azara, E.; Delogu, G.; Hammond, T.M.; Keller, N.P.; Migheli, Q. Altered trichothecene biosynthesis in TRI6-silenced transformants of Fusarium culmorum influences the severity of crown and foot rot on durum wheat seedlings. Mol. Plant Pathol. 2011, 12, 759–771. [Google Scholar] [CrossRef]
- Zinedine, A.; Soriano, J.M.; Molto, J.C.; Manes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Tiemann, U.; Dänicke, S. In vivo and in vitro effects of the mycotoxins zearalenone and deoxynivalenol on different non-reproductive and reproductive organs in female pigs: A review. Food Addit. Contam. 2007, 24, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Minervini, F.; Dell’Aquila, M.E. Zearalenone and reproductive function in farm animals. Int. J. Mol. Sci. 2008, 9, 2570–2584. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Das, M.; Tripathi, A. Occurrence and toxicity of a fusarium mycotoxin, zearalenone. Crit. Rev. Food Sci. Nutr. 2019, 60, 2710–2729. [Google Scholar] [CrossRef]
- Agahi, F.; Juan, C.; Font, G.; Juan-García, A. Neurotoxicity of zearalenone’s metabolites and beauvericin mycotoxins via apoptosis and cell cycle disruption. Toxicology 2021, 456, 152784. [Google Scholar] [CrossRef]
- Hueza, I.M.; Raspantini, P.C.F.; Raspantini, L.E.R.; Latorre, A.O.; Górniak, S.L. Zearalenone, an estrogenic mycotoxin, is an immunotoxic compound. Toxins 2014, 6, 1080–1095. [Google Scholar] [CrossRef]
- Gao, X.; Xiao, Z.-H.; Liu, M.; Zhang, N.-Y.; Khalil, M.M.; Gu, C.-Q.; Qi, D.-S.; Sun, L.-H. Dietary silymarin supplementation alleviates zearalenone-induced hepatotoxicity and reproductive toxicity in rats. J. Nutr. 2018, 148, 1209–1216. [Google Scholar] [CrossRef]
- Woloshuk, C.P.; Shim, W.-B. Aflatoxins, fumonisins, and trichothecenes: A convergence of knowledge. FEMS Microbiol. Rev. 2013, 37, 94–109. [Google Scholar] [CrossRef]
- Kim, J.E.; Son, H.; Lee, Y.-W. Biosynthetic mechanism and regulation of zearalenone in Fusarium graminearum. JSM Mycotoxins 2018, 68, 1–6. [Google Scholar] [CrossRef]
- Villafana, R.T.; Ramdass, A.C.; Rampersad, S.N. Selection of Fusarium trichothecene toxin genes for molecular detection depends on TRI gene cluster organization and gene function. Toxins 2019, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Alexander, N.J.; Proctor, R.H.; McCormick, S.P. Genes, gene clusters, and biosynthesis of trichothecenes and fumonisins in Fusarium. Toxin Rev. 2009, 28, 198–215. [Google Scholar] [CrossRef]
- Alexander, N.J.; McCormick, S.P.; Larson, T.M.; Jurgenson, J.E. Expression of Tri15 in Fusarium sporotrichioides. Curr. Genet. 2004, 45, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Gaffoor, I.; Trail, F. Characterization of two polyketide synthase genes involved in zearalenone biosynthesis in Gibberella zeae. Appl. Environ. Microbiol. 2006, 72, 1793–1799. [Google Scholar] [CrossRef]
- Huffman, J.; Gerber, R.; Du, L. Recent advancements in the biosynthetic mechanisms for polyketide-derived mycotoxins. Biopolymers 2010, 93, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Mahato, D.K.; Devi, S.; Pandhi, S.; Sharma, B.; Maurya, K.K.; Mishra, S.; Dhawan, K.; Selvakumar, R.; Kamle, M.; Mishra, A.K.; et al. Occurrence, impact on agriculture, human health, and management strategies of zearalenone in food and feed: A review. Toxins 2021, 13, 92. [Google Scholar] [CrossRef]
- Hope, R.; Aldred, D.; Magan, N. Comparison of environmental profiles for growth and deoxynivalenol production by Fusarium culmorum and F. graminearum on wheat grain. Lett. Appl. Microbiol. 2005, 40, 295–300. [Google Scholar] [CrossRef]
- Picot, A.; Atanasova-Penichon, V.; Pons, S.; Marchegay, G.; Barreau, C.; Pinson Gadais, L.; Roucolle, J.; Daveau, F.; Caron, D.; Richard-Forget, F. Maize kernel antioxidants and their potential involvement in Fusarium ear rot resistance. J. Agric. Food Chem. 2013, 61, 3389–3395. [Google Scholar] [CrossRef]
- Pani, G.; Scherm, B.; Azara, E.; Balmas, V.; Jahansiri, Z.; Carta, P.; Fabbri, D.; Dettori, M.A.; Fadda, A.; Dessì, A.; et al. Natural and natural-like phenolic inhibitors of type B trichothecene in vitro production by the wheat (Triticum sp.) pathogen Fusarium culmorum. J. Agric. Food Chem. 2014, 62, 4969–4978. [Google Scholar] [CrossRef]
- Malbrán, I.; Mourelos, C.A.; Pardi, M.; Oufensou, S.; Balmas, V.; Delogu, G.; Migheli, Q.; Lori, G.A.; Patricia Juárez, M.; Girotti, J.R. Commercially available natural inhibitors of trichothecene production in Fusarium graminearum: A strategy to manage Fusarium head blight of wheat. Crop Protect. 2020, 138, 105313. [Google Scholar] [CrossRef]
- Dzhavakhiya, V.G.; Voinova, T.M.; Popletaeva, S.B.; Statsyuk, N.V.; Limantseva, L.A.; Shcherbakova, L.A. Effect of various compounds blocking the colony pigmentation on the aflatoxin B1 production by Aspergillus flavus. Toxins 2016, 8, 313. [Google Scholar] [CrossRef] [PubMed]
- Mikityuk, O.D.; Voinova, T.M.; Statsyuk, N.V.; Dzhavakhiya, V.G. Suppression of sporulation, pigmentation, and zearalenone production in Fusarium culmorum by 6-demethylmevinolin, an inhibitor of the aflatoxin B1 biosynthesis. AIP Conf. Proc. 2022, 2390, 030058. [Google Scholar] [CrossRef]
- Holland, P.M.; Abramson, R.D.; Watson, R.; Gelfand, D.H. Detection of specific polymerase chain reaction product by utilizing the 5’ → 3’ exonuclease activity of Thermus aquaticus DNA polymerase. Proc. Natl. Acad. Sci. USA 1991, 88, 7276–7280. [Google Scholar] [CrossRef] [PubMed]
- Shcherbakova, L.; Mikityuk, O.; Arslanova, L.; Stakheev, A.; Erokhin, D.; Zavriev, S.; Dzhavakhiya, V. Studying the ability of thymol to improve fungicidal effects of tebuconazole and difenoconazole against some plant pathogenic fungi in seed or foliar treatments. Front. Microbiol. 2021, 12, 629429. [Google Scholar] [CrossRef]
- Stakheev, A.A.; Khairulina, D.R.; Zavriev, S.K. Four-locus phylogeny of Fusarium avenaceum and related species and their species-specific identification based on partial phosphate permease gene sequences. Int. J. Food Microbiol. 2016, 225, 27–37. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucl. Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Simon, P. Q-Gene: Processing quantitative real-time PCR data. Bioinformatics 2003, 19, 1439–1440. [Google Scholar] [CrossRef]
- Sakuda, S.; Yoshinari, T.; Furukawa, T.; Jermnak, U.; Takagi, K.; Iimura, K.; Yamamoto, T.; Suzuki, M.; Nagasawa, H. Search for aflatoxin and trichothecene production inhibitors and analysis of their modes of action. Biosci. Biotechnol. Biochem. 2016, 80, 43–54. [Google Scholar] [CrossRef]
- Inoguchi, H.; Furukawa, T.; Yoshinari, T.; Sakuda, S. Inhibition of aflatoxin production by protein tyrosine phosphatase inhibitors, blasticidin A and dephostatin. JSM Mycotoxins 2019, 69, 71–79. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.; Kistler, H.C.; Ma, Z. Fusarium graminearum trichothecene mycotoxins: Biosynthesis, regulation, and management. Annu. Rev. Phytopathol. 2019, 57, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Han, Y.K.; Kim, K.H.; Yun, S.-H.; Lee, Y.-W. Tri13 and Tri7 determine deoxynivalenol- and nivalenol-producing chemotypes of Gibberella zeae. Appl. Environ. Microbiol. 2002, 68, 2148–2154. [Google Scholar] [CrossRef] [PubMed]
- Boutigny, A.-L.; Barreau, C.; Atanasova-Penichon, V.; Verdal-Bonnin, M.-N.; Pinson-Gadais, L.; Richard-Forget, F. Ferulic acid, an efficient inhibitor of type B trichothecene biosynthesis and Tri gene expression in Fusarium liquid cultures. Mycol. Res. 2009, 113, 746–753. [Google Scholar] [CrossRef]
- Boutigny, A.-L.; Atanasova-Penichon, V.; Benet, M.; Barreau, C.; Richard-Forget, F. Natural phenolic acids from wheat bran inhibit Fusarium culmorum trichothecene biosynthesis in vitro by repressing Tri gene expression. Eur. J. Plant Pathol. 2010, 127, 275–286. [Google Scholar] [CrossRef]
- Peplow, A.W.; Tag, A.G.; Garifulina, G.F.; Beremand, M.N. Identification of new genes positively regulated by Tri10 and a regulatory network for trichothecene mycotoxin production. Appl. Environ. Microbiol. 2003, 69, 2731–2736. [Google Scholar] [CrossRef]
- Dyer, R.B.; Plattner, R.D.; Kendra, D.F.; Brown, D.W. Fusarium graminearum TRI14 is required for high virulence and DON production on wheat but not for DON biosynthesis in vitro. J. Agric. Food Chem. 2005, 53, 9281–9287. [Google Scholar] [CrossRef]
- Gardiner, D.M.; Kazan, K.; Manners, J.M. Novel genes of Fusarium graminearum that negatively regulate deoxynivalenol production and virulence. Mol. Plant Microbe Interact. 2009, 22, 1588–1600. [Google Scholar] [CrossRef]
- Ponts, N.; Pinson-Gadais, L.; Barreau, C.; Richard-Forget, F.; Ouellet, T. Exogenous H2O2 and catalase treatments interfere with Tri genes expression in liquid cultures of Fusarium graminearum. FEBS Lett. 2007, 581, 443–447. [Google Scholar] [CrossRef]
- Park, A.R.; Son, H.; Min, K.; Park, J.; Goo, J.H.; Rhee, S.; Chae, S.-K.; Lee, Y.-W. Autoregulation of ZEB2 expression for zearalenone production in Fusarium graminearum. Mol. Microbiol. 2015, 97, 942–956. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Son, H.; Lee, J.; Lee, Y.-R.; Lee, Y.W. A putative ABC transporter gene, ZRA1, is required for zearalenone production in Gibberella zeae. Curr. Genet. 2011, 57, 343–351. [Google Scholar] [CrossRef]
- Hu, S.; Zhou, X.; Gu, X.; Cao, S.; Wang, C.; Xu, J.-R. The cAMP-PKA pathway regulates growth, sexual and asexual differentiation, and pathogenesis in Fusarium graminearum. Mol. Plant Microbe Interact. 2014, 27, 557. [Google Scholar] [CrossRef] [PubMed]
- Park, A.R.; Fu, M.; Shin, J.Y.; Son, H.; Lee, Y.-W. The protein kinase A pathway regulates zearalenone production by modulating alternative ZEB2 transcription. J. Microbiol. Biotechnol. 2016, 26, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.Y.; Seo, J.-A.; Kim, J.-E.; Han, K.-H.; Shim, W.-B.; Yun, S.-H.; Lee, Y.-W. Functional analyses of heterotrimeric G protein Gα and Gβ subunits in Gibberella zeae. Microbiology 2008, 154, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Bluhm, B.H.; Zhao, X.; Flaherty, J.E.; Xu, J.R.; Dunkle, L.D. RAS2 regulates growth and pathogenesis in Fusarium graminearum. Mol. Plant-Microbe Interact. 2007, 20, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Bollina, V.; Kushalappa, A.C. In Vitro inhibition of trichothecene biosynthesis in Fusarium graminearum by resistance-related endogenous metabolites identified in barley. Mycology 2011, 2, 291–296. [Google Scholar] [CrossRef]
- Oufensou, S.; Balmas, V.; Azara, E.; Fabbri, D.; Dettori, M.A.; Schüller, C.; Zehetbauer, F.; Strauss, J.; Delogu, G.; Migheli, Q. Naturally occurring phenols modulate vegetative growth and deoxynivalenol biosynthesis in Fusarium graminearum. ACS Omega 2020, 5, 29407–29415. [Google Scholar] [CrossRef]
- Li, J.; Duan, Y.; Bian, C.; Pan, X.; Yao, C.; Wang, J.; Zhou, M. Effects of validamycin in controlling Fusarium head blight caused by Fusarium graminearum: Inhibition of DON biosynthesis and induction of host resistance. Pestic. Biochem. Physiol. 2019, 153, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Nakajima, Y.; Maeda, K.; Matsuyama, M.; Kanamaru, K.; Kobayashi, T.; Ohsato, S.; Kimura, M. Inhibition of Fusarium trichothecene biosynthesis by yeast extract components extractable with ethyl acetate. Int. J. Food Microbiol. 2019, 289, 24–29. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).