Abstract
Antimicrobial resistance (AMR) has become a global public health issue and antibiotic agents have lagged behind the rise in bacterial resistance. We are searching for a new method to combat AMR and phages are viruses that can effectively fight bacterial infections, which have renewed interest as antibiotic alternatives with their specificity. Large phage products have been produced in recent years to fight AMR. Using the “one health” approach, this review summarizes the phage products used in plant, food, animal, and human health. In addition, the advantages and disadvantages and future perspectives for the development of phage therapy as an antibiotic alternative to combat AMR are also discussed in this review.
1. Introduction
Antimicrobial resistance is a naturally evolving phenomenon that emerged soon after the discovery of penicillin in 1940 [1]. Antibiotics are highly efficient against bacterial infections, saving millions of lives and drastically reducing mortality rates. However, multidrug-resistant bacteria (MDR), extensively drug-resistant bacteria (XDR), and even pan-resistant bacteria (PDR) have evolved as a result of antibiotic overuse, abuse, and misuse. In particular, ESKAPE bacteria seriously threaten human health worldwide. According to the latest estimates, approximately 700,000 people worldwide die directly from AMR bacteria each year, with that number possibly rising to 1 billion by 2050 [2]. AMR is one of the top ten global public health threats facing humans, according to the WHO. As a result, FAO, WOAH, and WHO attach great importance to this and jointly launched the “one health” approach to combat AMR [3]. The interdependent relationship between the food chain and the environment makes resistant bacteria widespread in plants, animals, food, and humans, and the “one health” approach trinity model is ideally suited to address AMR [4,5]. Phages are currently one of the antibiotic alternatives with the most potential because of their ability to effectively combat bacterial infections. Phages are a new alternative therapy under the “one health” approach that can be used to control bacteria in plants, animals, food, and humans [6]. Currently, phage therapy is emerging globally, and in this review, we summarize the application of phage products for plants, animals, food, and human health from the perspective of “one health” from the two databases of phage companies and bacteriophage news [7,8]. Furthermore, the advantages and disadvantages of phages as antibiotic alternatives to combat AMR and their future development prospects are also discussed in detail.
2. Phage Biology
As early as 1896, Ernest Hankin discovered antibacterial substances against Vibrio cholerae from water extracted from the Ganges and Jumna rivers in India, laying the foundation for the subsequent discovery of phages [9,10]. The term “phage” was introduced by Félix d’Hérelle after he discovered the “anti-microbe” Shigella in 1917 [11]. Phages are abundant entities on the planet, with a population of 1031, which is 10–100 times that of their obligatory parasitic host bacterium [12]. The genome of phages is composed of single-stranded (ss) or double-stranded (ds) DNA or RNA, which is encapsulated by a wide variety of protein capsids. The universal viral taxonomy established by the International Committee on Taxonomy of Viruses (ICTV) divides phages into polyhedral, filamentous, pleomorphic, and tailed according to capsid morphology [13]. Phages can be classified into temperate and virulent based on their life cycle and reproductive characteristics. However, the process of bacterial infection is different between temperate and virulent phages.
Virulent phages enter the lytic cycle, which usually consists of five stages. The tail filament first adsorbs to a specific receptor on the surface of the host bacteria. These receptors can be located on cell walls, capsular polysaccharides, outer membrane proteins, efflux pumps, or appendages, such as pili and flagella [11] (Figure 1A). Second, the phage-derived enzymes (such as endolysins) lyse the peptidoglycan of the cell wall and the tail pipe penetrates through the cell membrane to inject its DNA into the host bacteria [14]. Third, phages perform biosynthesis, such as nucleic acid replication, RNA transcription, and protein translation in dormitory cells. Fourth, phages assemble into progeny phages. Finally, when the number of progeny phages reaches a certain threshold, bacteria lyse and release progeny phages [15] (Figure 1B). For Gram-negative bacteria, lysis is achieved by three different functional proteins, holins, endolysins, and spanins, which act on the inner membrane, peptidoglycan, and outer membranes of the cell envelope, respectively [16]. Temperate phages differ from virulent phages in a number of important ways. For example, temperate phages integrate their genomes into the chromosomes of host bacteria, which do not lyse but enter the lysogenic cycle. Phages grow and multiply with host bacteria [17]. Under certain conditions, temperate phages can also enter the lytic cycle, depending mainly on phage-encoded repressors and regulators, as well as the control of phage enzymes [18]. For example, under stress responses and light, temperate phages can initiate the expression of lytic genes. Temperate phages can regulate the gene expression and behavior of bacteria through different mechanisms and enhance phage-host fitness [19]. In addition, both virulent and temperate phages have a pseudo-lysogenic nature, which means that viral DNA is present in the host bacteria in a form similar to a plasmid and the host at this moment is only the vector of the phage [14,18].
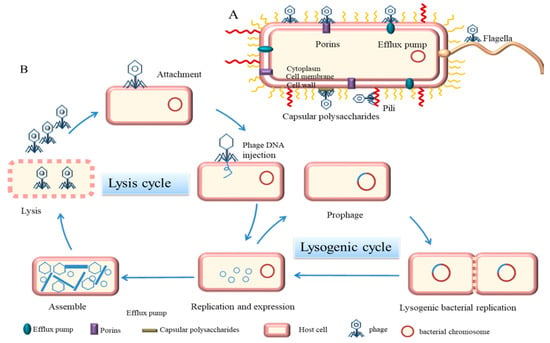
Figure 1.
Mechanism of phage infestation of bacteria. (A) Receptors for phage adsorption on bacteria (purple represents porins, blue represents efflux pumps, yellow represents flagella, red represents pili, and gray represents capsular polysaccharide) (B) Described five phases of a phage lytic cycle and a lysogenic cycle.
The key to killing bacteria by phages depends on lysing the bacterial cell wall and virulent phages are generally selected for treatment. It has commonly been assumed that icosahedral DS DNA phages containing tails can effectively treat human and animal infections [20]. Bacteria may acquire resistance genes or genes with pathogenic potential after the lysogenic transformation of temperate phages and they are generally not recommended for therapeutic purposes [11]. However, with advances in synthetic biology, temperate phages can be designed to interfere with bacterial intracellular processes and cause bacterial cell death. Alternatively, genomes of temperate phages were engineered to eliminate known virulence genes involved in the lysogenic cycle [21]. The current crisis of AMR makes phage therapy re-emerge globally and the cases of phage therapy in preclinical research are also gradually increasing [22,23,24].
3. Phages Products in Plant Health
More than 200 plant bacteria have been reported to cause significant crop losses during preharvest, storage, and transport [25]. Antibiotics have also been used against plant pathogens since World War II, and AMR has been widespread in some plants and crops due to the dissemination of resistance genes in the environment. For example, antibiotic resistance genes (strAB) have emerged in Pseudomonas syringae, Xanthomonas campestris, and Erwinia amylovora, triggering resistance to streptomycin [26]. The first experimental evidence that phages may be associated with plant pathogenic bacteria was the discovery that filtrates obtained from cabbage were able to inhibit cabbage decay caused by Xanthomonas campestris pv. [27]. Subsequently, in 1925 Kotila and Coons used phages to prevent soft rot caused by Pectobacterium atrosepticum and Pectobacterium carotovorum subsp on potato tuber and carrot slices, respectively. In 1935, Thomas used phage against the phytopathogen Pantoea stewartii to significantly reduce the incidence [28]. Modern studies have shown the effectiveness of phages for plant health and can target drug-resistant plant-bacteria with extremely high efficiency. For example, the isolation of a novel phage Xoo-sp2 infected with Xanthomonas oryzae from soil can effectively control bacterial blight in rice [29]. The engineered phage Y2 can effectively control and rapidly detect Erwinia amylovora, a fire blight pathogen [30]. Recently, three phage cocktails (φEa2345-6, φEa1337-26, and Eh21-5) are effective against fire blight in apples and pears. Four phage cocktails (Eram2, Eram26, Eram24, and Eram45) are effective against fire in blighted pears [31]. Xylella fastidiosa (Xf) is a novel plant pathogen with a wide range of plant hosts and a spectrum of insect species that are now causing significant damage to world agriculture. Phage is a novel therapy to control diseases caused by Xf [32,33]. Given the current invasion of Xf into agroecosystems, these phages can be implemented as biological agents and are excellent candidates for development into phage cocktails.
As phage studies advance, the number of phage products targeting plant pathogens in the market is also increasing. At present, the United States Environmental Protection Agency (USEPA) has approved several phage products to fight plant pathogens, and commercial phage products are summarized in Table 1. Only six phage products for plant health have been commercialized, mostly for soft rot Enterobacteriacea, Clavibacter michiganensis, Xanthomonas citri, and Erwinia amylovora, which are all prevalent plant pathogens. OmniLytics (Kuala Lumpur, MY, USA) was the earliest registered phage-based biopesticide product and its AgriPhage™ product line has been approved by the USEPA for the control of bacterial diseases in citrus, tomato, apple, and pear. In addition, the product also obtains an Organic Materials Review Institute (OMRI) listing for commercial organic growers. Enviroinvest Erwiphage PLUS (Hungary) is the second company to obtain a biopesticide registration and its product Erwiphage can fight fire blight caused by Rosaceae plants. The Biolyse® BP product, developed by APS Biocontrol (Dundee, UK), is a phage-based potato tuber wash for the prevention of soft rot during storage. Fixed-Phage (Glasgow, UK) also has a product for a variety of bacteria (agriPHIX™) that plays a significant role in improving the storage of a range of crops. In conclusion, there are a few phage products for plants that need to be further developed.

Table 1.
Commercial phage products for plant health.
4. Phage Products in Animal Health
The indiscriminate and extensive use of antibiotics in animals has been one of the principal reasons for the rapid spread of AMR. The new EU law prohibiting the prophylactic use of antibiotics in farmed animals was implemented in 2022 and the use of antibiotics is also strictly regulated in the United States and Canada [39]. The recent resurgence of phage therapy has also prompted the extensive application of phages in veterinary medicine. The first known therapeutic use of phages in veterinary medicine was associated with Felix d’Herelle, who used phages in 1919 to prevent and treat Salmonella infections in chickens and effectively reduce mortality in chickens [40]. However, when Pyle used phage therapy in 1926 to treat Salmonella Enteritidis infection in chickens, the results were less than encouraging [41]. Until the 1980s, William Smith reconsidered the use of phage therapy in animals and experimented with chickens, cattle, and pigs [42]. The early British team conducted a small clinical trial of a phage cocktail for canine otitis media caused by P. aeruginosa in 2010 and the results were greatly encouraging [43]. Subsequently, the application of phages in animals has been increasing, mainly for treating E. coli and Salmonella infections in poultry and pigs, as well as mastitis in cattle caused by S. aureus [44]. In aquaculture, phages are also effective against Vibrio, Pseudomonas, and Aeromonas, reducing fish mortality [45].
The preharvest application of phages can effectively reduce the infection and colonization of live poultry and minimize the risk of pathogens entering the food chain, thereby reducing the infection of zoonotic bacteria [46]. At present, phage products in animals mainly focus on the preharvest application of livestock, poultry, and the clinical application of pets. The detailed commercial phage products used in animals are shown in Table 2. A total of 9 of the 38 veterinary phage products counted have been approved by the FDA and 3 by the European EFSA. The products developed by Intralytix (Baltimore, AR, USA) mainly focus on pet food safety and preharvest intervention. The product line of PhagePharm (Qingdao, China) focuses on common pathogens in the environment and is used as an environmental improver, and the product line of Fixed-Phage (UK) is mostly phage cocktails. Figure 2A reveals that 20 mono-component phage products in the market almost target E. coli, Salmonella, and C. perfringens in poultry. A variety of phage cocktail products also target the infection of these bacteria. Most veterinary phage products are mainly in the form of food additives in animal feed or drinking water to prevent and control bacterial diseases. Only a few phage products are made into gel formulations for topical epidermic medication, such as Staphage Lysate (SPL)®, a phage product from Delmont Laboratories (Swarthmore, PA, USA), which is also the only staphylococcal product approved for use in Staphylococcus canis skin infections [47]. In contrast, phage products targeting companion animals remain to be further investigated, especially the study of bacterial dermatology, which may have significance in the future.

Table 2.
Commercial phage products for animal health.
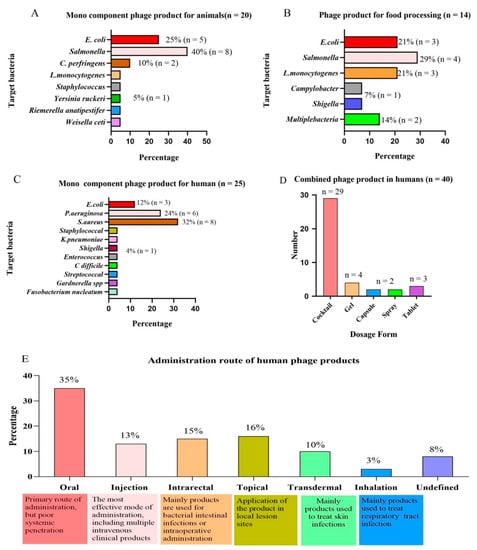
Figure 2.
Analysis of phage products from the perspective of the “one health” approach. (A) Targeted bacteria for mono-component phage products in animals. (B) Targeted bacteria for phage products in food processing. (C) Targeted bacteria for mono-component phage products in humans. (D) Main dosage forms of phage products in human therapy. (E) Main routes of administration for human phage products.
5. Phages Products in Food Health
The use of antibiotics aggravates AMR in livestock and poultry products and the high morbidity and mortality caused by foodborne pathogens has been a global burden [68]. Contamination caused by foodborne pathogens can be transmitted from production lines to humans, ultimately threatening human health. Phages are desirable for the biological control of foodborne pathogens as an effective natural and ecological alternative [69]. There are also increasing studies on the effectiveness of phages against foodborne pathogens. For example, Mengzhe demonstrated that phage STP4-A with a wide host range is effective against Salmonella as a food additive [70]. Vikram demonstrated that phage preparation can effectively reduce the level and prevalence of E. coli O157:H7 in food [71]. As early as 1958, the U.S. Food and Drug Administration (FDA) recognized phages and their derivatives as GRAS (generally recognized as safe) through the Food Additives Amendment to the Federal Food, Drug, and Cosmetic Act [72]. Phages are primarily used in three departments: primary production, biological preservation, and biological harmlessness in the food industry to ensure food safety [53]. Phages used in primary food production can prevent foodborne pathogens from entering the human body through the food chain, which is an excellent pre-harvest strategy. Livestock and poultry products are contaminated with pathogens in the production, processing, distribution, and consumption links. The application of phage products in postharvest can effectively reduce the presence of pathogens on carcasses, packaging, and RTE poultry products [68]. The benefit of phages for postharvest poultry processing is that they do not affect the quality senses and nutritional value of food [48].
Phage products are currently used with high safety to eliminate pathogens in animal food (meat products and dairy products) and plant food (fruits and vegetables). The FDA has granted phage products GRAS approval, allowing them to be used in livestock and poultry products. The use of phage products in food is also approved by health agencies in Israel, Canada, China, Switzerland, Australia, New Zealand, and the European Union [53]. Since the FDA approved the first phage product, ListShield™, as a food preservative in 2006, a significant number of phage products have emerged worldwide to combat foodborne pathogens [34,73]. As of November 2021, 14 phage products have been used in food processing, of which 11 have been approved by the FDA, including Intralytix (USA) and Micreos (Utrecht, Netherlands). Table 3 lists the commercial phage products used to combat foodborne pathogens in detail. The statistics of approved commercial phage products against foodborne pathogens revealed that Intralytix (USA) has made remarkable contributions to the field, with five products for marketing, and has gained Jewish cleansing and halal certification. Figure 2B reveals that commercial phage products primarily compete with E. coli, Salmonella, and L. monocytogenes, which seriously threaten human health. It is worth noting that Campylobacter is the most commonly reported foodborne pathogen, but there are no commercial Campylobacter phage products. A recent project (C-SNiper) directed by the Spanish Technology Center (AZTI) developed a prototype phage product for Campylobacter that is expected to be globally commercialized in 2022 [74].

Table 3.
Commercial phage products fighting foodborne pathogens in food.
6. Phage Products in Human Health
The application of phages to treat human diseases dates back to the successful injection of phage preparations in France in 1921 to treat five children with dysentery caused by the Shigella infection [24]. Belgian researchers published the first paper in the same year on the successful use of phages to treat furuncles and carbuncles of human skin [9]. Initially, the French company L’Oréal sold five phage preparations for the treatment of bacterial infections, Antipiol (Deutsch, Germany) produced Enterofagos, and EliLily (Indianapolis, IN, USA) first sold “Staphylofel” phage preparations for the treatment of streptococci and E. coli [86]. D’Herelle and Eliava first used phages to control cholera in India in 1931 and found no side effects following treatment [87]. During World War II, phages were also applied by both Soviet and German armies to treat wound infections, with the German army using Shigella phage preparation “Polyfagin” by Behringwerke Leverkusen to treat and prevent dysentery in soldiers [88]. In the late 1930s, however, the Committee on Pharmacy and Chemistry of the American Medical Association stated that the efficacy of phage therapy was unclear and further research was needed. Together with the discovery of penicillin, which led to the successful introduction and widespread use of antibiotics, interest in phage therapy has diminished, with only the Soviet Union and some countries in Eastern Europe still investigating it [14].
Common infections or minor injuries may be fatal with the increasing threat of AMR to humans. Researchers found great potential for phage therapy and phage therapies are increasingly being used for human bacterial diseases. In 2000, clinical human trials using phage therapy as a potential antibiotic alternative officially began in the United States, and phase I clinical data was first published in 2009. Clinical trials have revealed that phage cocktails against E. coli, S. aureus, and P. aeruginosa are safe for the treatment of wounds [89]. In 2013, the European Commission supported the large multinational phage therapy multicenter clinical research program “Phagoburn”, which treated 27 patients infected with P. aeruginosa burn wounds with phage therapy in France, Belgium, and Switzerland [90]. Despite the intended purpose not being achieved, this is the first time that three national regulatory agencies reached a consensus about phage cocktails for human therapy. At present, there are five phage therapy institutions worldwide, which are: Eliava Phage Therapy Center (Tbilisi, Georgia), Phage Therapy Center (Tbilisi, Georgia), Center for Innovative Phage Applications and Therapeutics (West Philadelphia, PA, USA), Phage International (San Ramon, CA, USA), and Phage Therapy Unit (Wrocław, Poland). Eliava Phage Therapy, founded in 1923, was the first institution to focus on phage therapy and has marketed phage cocktail products targeting specific pathogenic bacteria to treat human bacterial infections [91].
It has been confirmed that phage therapy has a lethal impact on a range of bacteria, which has contributed to an increase in phage therapy research and development for human diseases by multiple institutions around the world. However, no phage products have been approved for human use in the European Union or the United States. The FDA has merely opened up the regulatory pathway for phages to provide a green channel for phage products for clinical use in emergencies. Phage therapies are approved for use in emergency treatment plans in the European Union, Australia, France, and Belgium [92]. Detailed information on phage products currently approved and in preclinical studies worldwide is provided in Table 4. Figure 2C reveals that phages in preclinical products are almost exclusively targeted at MDR bacteria, especially “ESKPAEE” pathogens, including E. faecium, S. aureus, K. pneumoniae, E. coli, and others. It can treat the infections caused by these bacteria at different sites, including bone and joint infections (IOA), diabetic foot ulcers (UPD), and MARS caused by S. aureus [93,94]. It can treat the fibrosis and burn infections caused by P. aeruginosa [95]. It can treat urinary tract infections and IBD caused by E.coli and K. pneumonia [96,97]. Among the preclinical phage products, the cocktail products composed of 4–8 phage mixtures account for 60% of the total.

Table 4.
Phage products for human health.
Phage cocktails can increase the host range and avoid targeting a specific pathogen. In addition, rapid identification of bacterial pathogens is a time-consuming and laborious process before individualized treatment with phages [121]. Notably, phage cocktails are still targets for treating bacterial diseases caused by “ESKPAEE” pathogens. Figure 2D reveals that phage cocktail products that have been marketed in Russia and Georgia are also basically liquid phage cocktails, with only a few gels, capsules, and tablets available. Due to the effective identification of phages in the reticuloendothelial system, the half-life of phages in humans is usually relatively short [122]. Figure 2E reveals that the route of administration has a significant impact on the efficacy of phage absorption into the human body. Currently, the administration routes of phage products in preclinical studies mainly include oral, topical, transdermal, inhalation, and intrarectal administration. There are various routes of administration for phage products, with oral administration accounting for 35% of the total and remaining the most prevalent, followed by topical and intrarectal administration. Oral administration is effective in delivering phages to the gastrointestinal tract but it is the least effective route for systemic penetration. The most effective mode of delivery is an injection, which may deliver phages to practically all organs and tissues in minutes. Therefore, the efficacy of phage therapy is determined by the route of administration.
Endolysin and virosome-associated lysozyme (VAL), which are phage-derived peptidoglycan-degrading enzymes, are also bactericidal. Endolysins are enzymes used by phages to lyse the bacterial cell wall at the end of the replication cycle, while the VAL is responsible for the injection of genetic material into infected cells for peptidoglycan degradation [18,123]. Many studies on the antibacterial effect of endolysin are currently being implemented in human medicine clinics. Endolysin is also one of the alternatives to antibiotics. It has the advantages of killing the host quickly, host specificity, preservation of the normal microbial community, reduction of AMR risk, and efficiency against multidrug-resistant bacteria and biofilms when compared to antibiotics [124]. The benefits of endolysin therapy have attracted the attention of researchers and pharmaceutical companies to its commercial potential and several commercial products based on endolysin have now been developed. The first human endolysin product developed by Micreos, Staphefekt SA. 100, specifically for the treatment of chronic S. aureus associated skin diseases, has been marketed. All three clinical patients had a positive therapeutic effect and did not develop resistance [125]. Artilysin has developed an Artilysin® product line(Lysando AG, Regensburg, Germany) that is effective against resistant P. aeruginosa and A. baumannii in various forms including spray, nebulizer, solution, lyophilization, gel, and coating [126]. Rephasin® SAL200 (Intron Biotechnology, Seongnam, Korea) is now in phase II of human clinical trials [105,127]. ContraFect has developed a novel direct lysing agent called Amurin peptide, which is effective against numerous Gram-negative pathogens. The other is a lysin-based direct lytic agent, containing Exebacase CF301, which is effective against S. aureus, including MARS, and is the first phage lytic enzyme to enter human clinical trials in the United States [128,129]. Criteria used for the preclinical analysis of small molecule antibiotics may be more readily translated into the preclinical assessment of phage lytic enzymes than phages, so clinical evaluation of phage lytic enzymes is progressing significantly faster [130].
7. Advantages and Disadvantages of Phage Therapy
Compared to antibiotics, phages are characterized by host specificity, which means only lysing the host bacterial cell wall without destroying the microbiota [62]. There is a process of adaptation versus counter-adaptation in the coevolution of phages and bacterial hosts and the risk of developing resistance is low [40]. For example, coevolutionary phage training can delay the evolution of phage resistance. Researchers conducted coevolution experiments using E. coli and untrained or trained phages to assess the potential of phage-training treatments and found that trained phages were able to inhibit host bacteria for a longer period of time [131]. The coevolution of phages with host bacteria has also driven bacteria to evolve a variety of highly specific phage defense mechanisms. For example, mutations in phage receptors, the R-M system, the DISARM system, the superinfection exclusions (SIEs) system, the abortive infection (Abi) system, and the adaptive immune system CRISPR-Cas all make phages resistant [132,133]. Hussain studied the evolutionary trajectory of resistance in wild-type phages, which showed that the rapid evolution of mobile phage defense elements (PDEs) drove bacterial resistance to phages [134]. Studies have shown an evolutionary trade-off between phage and antibiotic resistance, with bacteria sometimes showing increased susceptibility to antibiotics when phage resistance evolves. Barber studies have demonstrated that efflux pumps play a dual role in antibiotic resistance and phage sensitivity, and when phage resistance leads to the loss of bacterial capsules, they will subsequently become sensitive to antibiotics [135]. However, when Burmeister studied E. coli phages, it was found that bacterial interaction with phages may depend on efflux pump protein TolC and structural barrier molecule lipopolysaccharide (LPS), and when these two mutants were constructed, some phage resistance mutations conferred an increase in antibiotic resistance [136]. Therefore, there are not only synergistic effects but also antagonistic effects between phages and antibiotics, and their intrinsic mechanisms of action remain to be further studied. In addition, phages replicate only in the target bacteria at the site of infection and treatment causes fewer adverse effects and is safer. Oral phage preparations are generally harmless and researchers have found the presence of adverse effects associated with phage therapy when assessing animal and clinical phage therapy safety and toxicity, but with a small probability of events [137]. Finally, phages are widespread in the environment and provide an inexhaustible resource. It only takes days to weeks to produce a new natural phage preparation, and if a phage develops resistance, phages that use other new receptors can also be quickly found. Screening for a new natural phage preparation takes a few days, and phages using other new receptors can also be quickly found if the phage develops resistance.
Despite the favorable results of various studies on phage applications, phage therapy still has certain shortcomings and unknowns. First, there are still some potential risks associated with the application of phage therapy, which are largely observational with existing phage therapies or performed in small non-randomized trials, where side effects may be underestimated. Second, the route of administration, frequency of administration, dose, phage resistance, pharmacokinetic and pharmacodynamic characteristics of phages, and the mechanism of phage entry into eukaryotic cells and the immune system need to continue to be studied in depth [40,138]. Third, legal regulation is a significant obstacle to the implementation of phage therapy and regulatory authorities classify phages as biological substances, which differs from the approval and production of antibiotics, making it difficult to use phage therapy. European legislators have been advocating for a regulatory framework specifically targeting individualized phage preparations but they have been strongly resisted [139]. Fourth, considering phages are natural entities, they entrap pharmaceutical companies in intellectual property issues [121]. Fifth, animal prophylactic phage products do not remove phages immediately after use and may lead to phage mutation and the cultivation of phage mutants. This problem also needs to be solved by using the regular rotation of phages and continuous detection, such as antibiotics [35]. Sixth, phages can transfer bacterial resistance genes and even contain toxic genes, implying that, as much as possible, the selection of lytic phages ensures that therapeutic phage products must be deeply purified and must remove endotoxins during processing [140]. Seventh, when the scope of phage application expands, including antibiotic substitutes, carrier delivery drugs, vaccines, and phage display technology, the demand for large-scale production of phage increases. The Phage on Tap (PoT) protocol has been studied for the rapid formulation of high titer phage formulations and a systematic procedure has also been developed for the isolation, up-culture, concentration, and purification of phages for pharmaceutical use [141]. The procedure can combine modified classical techniques, modern membrane filtration processes, and no organic solvents in 16 to 21 days, producing an average of 23 mL of 1011 PFU/mL phage [142]. Despite the enormous efforts of researchers for phage technology, there remain challenges for the production and expansion of wild-type phages for biological control. Finally, doctors and the public at large are unaware of the use of phages to treat diseases and the public believes that viruses are exclusively harmful to the human body, not realizing that they may also be beneficial [143].
8. Conclusions and Prospects
Antibiotic resistance poses a threat to global health. Russia approved the addition of phages to the official pharmacopoeia in 2016. The European Pharmacopoeia included “phage therapeutic active ingredients and pharmaceutical products for human and veterinary use” in 2021. To ensure its safety and effectiveness, pharmaceutical authorities such as the FDA and EMA require that any modern phage therapy product meet GMP standards, which poses challenges [144]. According to the statistics, Figure 3C reveals that 20 countries began to develop phages and had phage products approved for use. According to the analysis of 123 products in 20 nations, Figure 3A reveals that 53% of the products were used for human health, and Russia, Georgia, and the United States have rich experience in phage therapy for human diseases. Figure 3B reveals that the FDA has approved twenty phage products for animals and food, but only seven investigational new drugs (INDs) for humans. Except for Russia and Georgia, which have focused on phage therapy for human diseases and have sold many phage products, phage product research and development in other nations remains to be further developed. Overall, phage products in the United States are rapidly developing and the FDA has also approved several products. In the future, as a novel alternative therapy under the “one health” approach, phage research and development will continue to focus on making products that are environmentally friendly, safe, and successful in combating AMR.
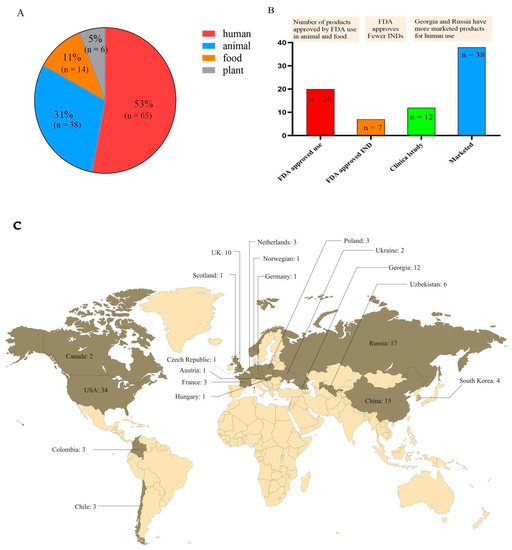
Figure 3.
Total phage products are distributed and approved. (A) The proportion of phage products in plants, animals, food, and humans. (B) The number of phage products that have been approved by the FDA and are in clinical research and marketed for human use. (C) Worldwide distribution of the number of phage products.
Author Contributions
Y.H. collected data and wrote the first draft. W.W. and Z.Z. contributed to the data collection of the manuscript. Y.G., A.H. and J.W. made suggestions for revision of the manuscript. H.H. provides supervision, guidance and financial support. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by grants from National Key Research and Development Program (2021YFD1800600), National Natural Science Foundation of China (32172914), Fundamental Research Funds for the Central Universities (2662022DKYJC005).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank the editors and peer reviewers for reading this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lobanovska, M.; Pilla, G. Penicillin’s Discovery and Antibiotic Resistance: Lessons for the Future? Yale J. Biol. Med. 2017, 90, 135–145. [Google Scholar]
- Oechslin, F. Resistance Development to Bacteriophages Occurring during Bacteriophage Therapy. Viruses 2018, 10, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aslam, B.; Khurshid, M.; Arshad, M.I.; Muzammil, S.; Rasool, M.; Yasmeen, N.; Shah, T.; Chaudhry, T.H.; Rasool, M.H.; Shahid, A.; et al. Antibiotic Resistance: One Health One World Outlook. Front. Cell Infect. Microbiol. 2021, 11, 771510. [Google Scholar] [CrossRef]
- McEwen, S.A.; Collignon, P.J. Antimicrobial Resistance: A One Health Perspective. Microbiol. Spectr. 2018, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.W.; Cha, C.J. Antibiotic resistome from the One-Health perspective: Understanding and controlling antimicrobial resistance transmission. Exp. Mol. Med. 2021, 53, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.P.; Costa, A.R.; Pinto, G.; Meneses, L.; Azeredo, J. Current challenges and future opportunities of phage therapy. FEMS Microbiol. Rev. 2020, 44, 684–700. [Google Scholar] [CrossRef]
- Group The Bacteriophage Ecology. Phage Companies. Available online: http://companies.phage.org/ (accessed on 29 April 2022).
- Bacteriophage.news. Bacteriophage Products. Available online: https://www.bacteriophage.news/phage-products/ (accessed on 29 April 2022).
- Squires, R.A. Bacteriophage therapy for challenging bacterial infections: Achievements, limitations and prospects for future clinical use by veterinary dermatologists. Vet. Dermatol. 2021, 32, 587-e158. [Google Scholar] [CrossRef] [PubMed]
- Abd-Allah, I.M.; El-Housseiny, G.S.; Yahia, I.S.; Aboshanab, K.M.; Hassouna, N.A. Rekindling of a Masterful Precedent; Bacteriophage: Reappraisal and Future Pursuits. Front. Cell Infect. Microbiol. 2021, 11, 635597. [Google Scholar] [CrossRef]
- Gordillo Altamirano, F.L.; Barr, J.J. Phage Therapy in the Postantibiotic Era. Clin. Microbiol. Rev. 2019, 32, e00066-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moelling, K.; Broecker, F.; Willy, C. A Wake-Up Call: We Need Phage Therapy Now. Viruses 2018, 10, 688. [Google Scholar] [CrossRef] [Green Version]
- Dion, M.B.; Oechslin, F.; Moineau, S. Phage diversity, genomics and phylogeny. Nat. Rev. Microbiol. 2020, 18, 125–138. [Google Scholar] [CrossRef]
- Salmond, G.P.; Fineran, P.C. A century of the phage: Past, present and future. Nat. Rev. Microbiol. 2015, 13, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Olszak, T.; Latka, A.; Roszniowski, B.; Valvano, M.A.; Drulis-Kawa, Z. Phage Life Cycles Behind Bacterial Biodiversity. Curr. Med. Chem. 2017, 24, 3987–4001. [Google Scholar] [CrossRef] [Green Version]
- Cahill, J.; Young, R. Phage Lysis: Multiple Genes for Multiple Barriers. Adv. Virus Res. 2019, 103, 33–70. [Google Scholar]
- Sunderland, K.S.; Yang, M.; Mao, C. Phage-Enabled Nanomedicine: From Probes to Therapeutics in Precision Medicine. Angew. Chem. Int. Ed. Engl. 2017, 56, 1964–1992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maciejewska, B.; Olszak, T.; Drulis-Kawa, Z. Applications of bacteriophages versus phage enzymes to combat and cure bacterial infections: An ambitious and also a realistic application? Appl. Microbiol. Biotechnol. 2018, 102, 2563–2581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argov, T.; Azulay, G.; Pasechnek, A.; Stadnyuk, O.; Ran-Sapir, S.; Borovok, I.; Sigal, N.; Herskovits, A.A. Temperate bacteriophages as regulators of host behavior. Curr. Opin. Microbiol. 2017, 38, 81–87. [Google Scholar] [CrossRef]
- Górski, A.; Międzybrodzki, R.; Węgrzyn, G.; Jończyk-Matysiak, E.; Borysowski, J.; Weber-Dąbrowska, B. Phage therapy: Current status and perspectives. Med. Res. Rev. 2020, 40, 459–463. [Google Scholar] [CrossRef]
- Monteiro, R.; Pires, D.P.; Costa, A.R.; Azeredo, J. Phage Therapy: Going Temperate? Trends Microbiol. 2019, 27, 368–378. [Google Scholar] [CrossRef] [Green Version]
- Bhargava, K.; Nath, G.; Bhargava, A.; Aseri, G.K.; Jain, N. Phage therapeutics: From promises to practices and prospectives. Appl. Microbiol. Biotechnol. 2021, 105, 9047–9067. [Google Scholar] [CrossRef]
- Principi, N.; Silvestri, E.; Esposito, S. Advantages and Limitations of Bacteriophages for the Treatment of Bacterial Infections. Front. Pharmacol. 2019, 10, 513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luong, T.; Salabarria, A.C.; Roach, D.R. Phage Therapy in the Resistance Era: Where Do We Stand and Where Are We Going? Clin. Ther. 2020, 42, 1659–1680. [Google Scholar] [CrossRef] [PubMed]
- Farooq, T.; Hussain, M.D.; Shakeel, M.T.; Tariqjaveed, M.; Aslam, M.N.; Naqvi SA, H.; Amjad, R.; Tang, Y.; She, X.; He, Z. Deploying Viruses against Phytobacteria: Potential Use of Phage Cocktails as a Multifaceted Approach to Combat Resistant Bacterial Plant Pathogens. Viruses 2022, 14, 171. [Google Scholar] [CrossRef] [PubMed]
- Sundin, G.W.; Wang, N. Antibiotic Resistance in Plant-Pathogenic Bacteria. Annu. Rev. Phytopathol. 2018, 56, 161–180. [Google Scholar] [CrossRef]
- Svircev, A.; Roach, D.; Castle, A. Framing the Future with Bacteriophages in Agriculture. Viruses 2018, 10, 218. [Google Scholar] [CrossRef] [Green Version]
- Buttimer, C.; McAuliffe, O.; Ross, R.P.; Hill, C.; O’Mahony, J.; Coffey, A. Bacteriophages and Bacterial Plant Diseases. Front. Microbiol. 2017, 8, 34. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.; Xing, S.; Liu, J.; Tang, X.; Ruan, L.; Sun, M.; Tong, Y.; Peng, D. Isolation and characterization of a novel phage Xoo-sp2 that infects Xanthomonas oryzae pv. oryzae. J. Gen. Virol. 2018, 99, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Born, Y.; Fieseler, L.; Thöny, V.; Leimer, N.; Duffy, B.; Loessner, M.J. Engineering of Bacteriophages Y2::dpoL1-C and Y2::luxAB for Efficient Control and Rapid Detection of the Fire Blight Pathogen, Erwinia amylovora. Appl. Environ. Microbiol. 2017, 83, e00341-17. [Google Scholar] [CrossRef] [Green Version]
- Kering, K.K.; Kibii, B.J.; Wei, H. Biocontrol of phytobacteria with bacteriophage cocktails. Pest. Manag. Sci. 2019, 75, 1775–1781. [Google Scholar] [CrossRef]
- Clavijo-Coppens, F.; Ginet, N.; Cesbron, S.; Briand, M.; Jacques, M.A.; Ansaldi, M. Novel Virulent Bacteriophages Infecting Mediterranean Isolates of the Plant Pest Xylella fastidiosa and Xanthomonas albilineans. Viruses 2021, 13, 725. [Google Scholar] [CrossRef] [PubMed]
- Ahern, S.J.; Das, M.; Bhowmick, T.S.; Young, R.; Gonzalez, C.F. Characterization of novel virulent broad-host-range phages of Xylella fastidiosa and Xanthomonas. J. Bacteriol. 2014, 196, 459–471. [Google Scholar] [CrossRef] [PubMed]
- de Melo, A.G.; Levesque, S.; Moineau, S. Phages as friends and enemies in food processing. Curr. Opin. Biotechnol. 2018, 49, 185–190. [Google Scholar] [CrossRef]
- Sommer, J.; Trautner, C.; Witte, A.K.; Fister, S.; Schoder, D.; Rossmanith, P.; Mester, P.J. Don’t Shut the Stable Door after the Phage Has Bolted-The Importance of Bacteriophage Inactivation in Food Environments. Viruses 2019, 11, 468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Połaska, M.; Sokołowska, B. Bacteriophages-a new hope or a huge problem in the food industry. AIMS Microbiol. 2019, 5, 324–346. [Google Scholar] [CrossRef] [PubMed]
- PLUS Erwiphage. Erwiphage PLUS. 2022. Available online: https://www.erwiphage.com/ (accessed on 29 April 2022).
- Phage Fixed. Applications. 2022. Available online: https://www.fixed-phage.com/ (accessed on 29 April 2022).
- Protection World Animal. EU Bans the Routine Use of Antibiotics in Farmed Animals. 2022. Available online: https://www.worldanimalprotection.org/european-union-bans-antibiotic-overuse-farmed-animals-animal-welfare (accessed on 29 April 2022).
- Kortright, K.E.; Chan, B.K.; Koff, J.L.; Turner, P.E. Phage Therapy: A Renewed Approach to Combat Antibiotic-Resistant Bacteria. Cell Host Microbe 2019, 25, 219–232. [Google Scholar] [CrossRef] [Green Version]
- Gigante, A.; Atterbury, R.J. Veterinary use of bacteriophage therapy in intensively-reared livestock. Virol. J. 2019, 16, 155. [Google Scholar] [CrossRef] [Green Version]
- Loponte, R.; Pagnini, U.; Iovane, G.; Pisanelli, G. Phage Therapy in Veterinary Medicine. Antibiotics 2021, 10, 421. [Google Scholar] [CrossRef]
- Hawkins, C.; Harper, D.; Burch, D.; Anggård, E.; Soothill, J. Topical treatment of Pseudomonas aeruginosa otitis of dogs with a bacteriophage mixture: A before/after clinical trial. Vet. Microbiol. 2010, 146, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Dec, M.; Wernicki, A.; Urban-Chmiel, R. Efficacy of experimental phage therapies in livestock. Anim. Health Res. Rev. 2020, 21, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Han, G.; Li, Z.; Cun, S.; Hao, B.; Zhang, J.; Liu, X. Bacteriophage therapy in aquaculture: Current status and future challenges. Folia Microbiol. 2022, 1–18. [Google Scholar] [CrossRef]
- Islam, M.R.; Martinez-Soto, C.E.; Lin, J.T.; Khursigara, C.M.; Barbut, S.; Anany, H. A systematic review from basics to omics on bacteriophage applications in poultry production and processing. Crit. Rev. Food Sci. Nutr. 2021, 1–33. [Google Scholar] [CrossRef]
- Laboratories Delmont. What is Staphage Lysate (SPL)? 2022. Available online: https://delmontlabs.com/ (accessed on 29 April 2022).
- Moye, Z.D.; Woolston, J.; Sulakvelidze, A. Bacteriophage Applications for Food Production and Processing. Viruses 2018, 10, 205. [Google Scholar] [CrossRef] [Green Version]
- Pharmaceuticals Proteon. Products. 2022. Available online: https://www.proteonpharma.com/ (accessed on 29 April 2022).
- Phagelab. Development. 2022. Available online: https://phage-lab.com/development/ (accessed on 29 April 2022).
- Heyse, S.; Hanna, L.F.; Woolston, J.; Sulakvelidze, A.; Charbonneau, D. Bacteriophage cocktail for biocontrol of Salmonella in dried pet food. J. Food Prot. 2015, 78, 97–103. [Google Scholar] [CrossRef]
- Soffer, N.; Abuladze, T.; Woolston, J.; Li, M.; Hanna, L.F.; Heyse, S.; Charbonneau, D.; Sulakvelidze, A. Bacteriophages safely reduce Salmonella contamination in pet food and raw pet food ingredients. Bacteriophage 2016, 6, e1220347. [Google Scholar] [CrossRef] [Green Version]
- Alomari, M.M.M.; Dec, M.; Urban-Chmiel, R. Bacteriophages as an Alternative Method for Control of Zoonotic and Foodborne Pathogens. Viruses 2021, 13, 2348. [Google Scholar] [CrossRef]
- Wójcik, E.A.; Stańczyk, M.; Wojtasik, A.; Kowalska, J.D.; Nowakowska, M.; Łukasiak, M.; Bartnicka, M.; Kazimierczak, J.; Dastych, J. Comprehensive Evaluation of the Safety and Efficacy of BAFASAL(®) Bacteriophage Preparation for the Reduction of Salmonella in the Food Chain. Viruses 2020, 12, 742. [Google Scholar] [CrossRef]
- UniFAHS. Products. 2022. Available online: https://www.unifahs.com/# (accessed on 29 April 2022).
- Żbikowska, K.; Michalczuk, M.; Dolka, B. The Use of Bacteriophages in the Poultry Industry. Animals 2020, 10, 872. [Google Scholar] [CrossRef]
- Clavijo, V.; Baquero, D.; Hernandez, S.; Farfan, J.C.; Arias, J.; Arévalo, A.; Donado-Godoy, P.; Vives-Flores, M. Phage cocktail SalmoFREE® reduces Salmonella on a commercial broiler farm. Poult. Sci. 2019, 98, 5054–5063. [Google Scholar] [CrossRef]
- Phageseeker. Products. 2022. Available online: http://www.phageseeker.com/ (accessed on 29 April 2022).
- Miller, R.W.; Skinner, E.J.; Sulakvelidze, A.; Mathis, G.F.; Hofacre, C.L. Bacteriophage therapy for control of necrotic enteritis of broiler chickens experimentally infected with Clostridium perfringens. Avian Dis. 2010, 54, 33–40. [Google Scholar] [CrossRef]
- Pharma ACD. CUSTUS®YRS: Bacteriophages against Yersiniosis. 2022. Available online: https://acdpharma.com/custusyrs-eng/ (accessed on 29 April 2022).
- SciPhage. Products. 2022. Available online: http://sciphage.com/products/ (accessed on 29 April 2022).
- Schulz, P.; Pajdak-Czaus, J.; Robak, S.; Dastych, J.; Siwicki, A.K. Bacteriophage-based cocktail modulates selected immunological parameters and post-challenge survival of rainbow trout (Oncorhynchus mykiss). J. Fish Dis. 2019, 42, 1151–1160. [Google Scholar] [CrossRef]
- Runda Biotechnology Pharmaceutical. News. 2022. Available online: http://www.rundabio.cn/news/1946.html (accessed on 29 April 2022).
- Cytophage. BACTERIOPHAGE. 2022. Available online: https://cytophage.com/ (accessed on 29 April 2022).
- Intermediates Pathway. Products. 2022. Available online: http://www.pathway-intermediates.com/ (accessed on 29 April 2022).
- Bacteriophage. Phage-Products. 2022. Available online: https://www.bacteriophage.news/phage-products/ (accessed on 1 May 2022).
- MicroMir. Micromir Drugs. 2022. Available online: https://micromir.bio/ (accessed on 29 April 2022).
- Gambino, M.; Brøndsted, L. Looking into the future of phage-based control of zoonotic pathogens in food and animal production. Curr. Opin. Biotechnol. 2021, 68, 96–103. [Google Scholar] [CrossRef]
- López-Cuevas, O.; Medrano-Félix, J.A.; Castro-Del Campo, N.; Chaidez, C. Bacteriophage applications for fresh produce food safety. Int. J. Environ. Health Res. 2021, 31, 687–702. [Google Scholar] [CrossRef]
- Li, M.; Lin, H.; Jing, Y.; Wang, J. Broad-host-range Salmonella bacteriophage STP4-a and its potential application evaluation in poultry industry. Poult. Sci. 2020, 99, 3643–3654. [Google Scholar] [CrossRef]
- Vikram, A.; Tokman, J.I.; Woolston, J.; Sulakvelidze, A. Phage Biocontrol Improves Food Safety by Significantly Reducing the Level and Prevalence of Escherichia coli O157:H7 in Various Foods. J. Food Prot. 2020, 83, 668–676. [Google Scholar] [CrossRef]
- Ushanov, L.; Lasareishvili, B.; Janashia, I.; Zautner, A.E. Application of Campylobacter jejuni Phages: Challenges and Perspectives. Animals 2020, 10, 279. [Google Scholar] [CrossRef] [Green Version]
- Rehman, S.; Ali, Z.; Khan, M.; Bostan, N.; Naseem, S. The dawn of phage therapy. Rev. Med. Virol. 2019, 29, e2041. [Google Scholar] [CrossRef]
- (FSN) Food Safety News. EU Project Uses Phages to Tackle Campylobacter in Poultry. 2020. Available online: https://www.foodsafetynews.com/2020/11/eu-project-uses-phages-to-tackle-campylobacter-in-poultry/ (accessed on 1 May 2022).
- Dissanayake, U.; Ukhanova, M.; Moye, Z.D.; Sulakvelidze, A.; Mai, V. Bacteriophages Reduce Pathogenic Escherichia coli Counts in Mice Without Distorting Gut Microbiota. Front. Microbiol. 2019, 10, 1984. [Google Scholar] [CrossRef]
- Phageguard. Phage Technology Which Food Pathogen Can We Help You Fight? 2022. Available online: https://phageguard.com/ (accessed on 29 April 2022).
- Zhang, X.; Niu, Y.D.; Nan, Y.; Stanford, K.; Holley, R.; McAllister, T.; Narváez-Bravo, C. SalmoFresh™ effectiveness in controlling Salmonella on romaine lettuce, mung bean sprouts and seeds. Int. J. Food Microbiol. 2019, 305, 108250. [Google Scholar] [CrossRef]
- Grant, A.; Parveen, S.; Schwarz, J.; Hashem, F.; Vimini, B. Reduction of Salmonella in ground chicken using a bacteriophage. Poult. Sci. 2017, 96, 2845–2852. [Google Scholar] [CrossRef]
- Production Arm & Hammer Animal and Food. Phage Technology Reduces Salmonella in Ground Turkey. 2020. Available online: https://ahfoodchain.com/en/segments/food-production/products/finalyse-sal (accessed on 29 April 2022).
- Diana, G.; Lorena, R.-R.; Lucía, F.; Beatriz, M.; Ana, R.; Pilar, G. Applicability of commercial phage-based products against Listeria monocytogenes for improvement of food safety in Spanish dry-cured ham and food contact surfaces. Food Control 2017, 73, 1474–1482. [Google Scholar]
- Chibeu, A.; Agius, L.; Gao, A.; Sabour, P.M.; Kropinski, A.M.; Balamurugan, S. Efficacy of bacteriophage LISTEX™P100 combined with chemical antimicrobials in reducing Listeria monocytogenes in cooked turkey and roast beef. Int. J. Food Microbiol. 2013, 167, 208–214. [Google Scholar] [CrossRef]
- Truchado, P.; Elsser-Gravesen, A.; Gil, M.I.; Allende, A. Post-process treatments are effective strategies to reduce Listeria monocytogenes on the surface of leafy greens: A pilot study. Int. J. Food Microbiol. 2020, 313, 108390. [Google Scholar] [CrossRef]
- Pennone, V.; Sanz-Gaitero, M.; O’Connor, P.; Coffey, A.; Jordan, K.; van Raaij, M.J.; McAuliffe, O. Inhibition of L. monocytogenes Biofilm Formation by the Amidase Domain of the Phage vB_LmoS_293 Endolysin. Viruses 2019, 11, 722. [Google Scholar] [CrossRef] [Green Version]
- Intralytix. Products. 2022. Available online: http://intralytix.com/index.php?page=prod (accessed on 29 April 2022).
- Soffer, N.; Woolston, J.; Li, M.; Das, C.; Sulakvelidze, A. Bacteriophage preparation lytic for Shigella significantly reduces Shigella sonnei contamination in various foods. PLoS ONE 2017, 12, e0175256. [Google Scholar] [CrossRef] [Green Version]
- Suh, G.A.; Lodise, T.P.; Tamma, P.D.; Knisely, J.M.; Alexander, J.; Aslam, S.; Barton, K.D.; Bizzell, E.; Totten KM, C.; Campbell, J.L.; et al. Considerations for the Use of Phage Therapy in Clinical Practice. Antimicrob. Agents Chemother. 2022, 66, e0207121. [Google Scholar] [CrossRef]
- Nikolich, M.P.; Filippov, A.A. Bacteriophage Therapy: Developments and Directions. Antibiotics 2020, 9, 135. [Google Scholar] [CrossRef] [Green Version]
- Reuter, M.; Kruger, D.H. Approaches to optimize therapeutic bacteriophage and bacteriophage-derived products to combat bacterial infections. Virus Genes 2020, 56, 136–149. [Google Scholar] [CrossRef]
- Rhoads, D.D.; Wolcott, R.D.; Kuskowski, M.A.; Wolcott, B.M.; Ward, L.S.; Sulakvelidze, A. Bacteriophage therapy of venous leg ulcers in humans: Results of a phase I safety trial. J. Wound Care 2009, 18, 237–238, 240–243. [Google Scholar] [CrossRef]
- Jault, P.; Leclerc, T.; Jennes, S.; Pirnay, J.P.; Que, Y.A.; Resch, G.; Rousseau, A.F.; Ravat, F.; Carsin, H.; Le Floch, R.; et al. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): A randomised, controlled, double-blind phase 1/2 trial. Lancet Infect. Dis. 2019, 19, 35–45. [Google Scholar] [CrossRef]
- Kakasis, A.; Panitsa, G. Bacteriophage therapy as an alternative treatment for human infections. A comprehensive review. Int. J. Antimicrob. Agents 2019, 53, 16–21. [Google Scholar] [CrossRef]
- Górski, A.; Międzybrodzki, R.; Łobocka, M.; Głowacka-Rutkowska, A.; Bednarek, A.; Borysowski, J.; Jończyk-Matysiak, E.; Łusiak-Szelachowska, M.; Weber-Dąbrowska, B.; Bagińska, N.; et al. Phage Therapy: What Have We Learned? Viruses 2018, 10, 288. [Google Scholar] [CrossRef] [Green Version]
- Petrovic Fabijan, A.; Lin RC, Y.; Ho, J.; Maddocks, S.; Ben Zakour, N.L.; Iredell, J.R. Safety of bacteriophage therapy in severe Staphylococcus aureus infection. Nat. Microbiol. 2020, 5, 465–472. [Google Scholar] [CrossRef]
- Kifelew, L.G.; Warner, M.S.; Morales, S.; Vaughan, L.; Woodman, R.; Fitridge, R.; Mitchell, J.G.; Speck, P. Efficacy of phage cocktail AB-SA01 therapy in diabetic mouse wound infections caused by multidrug-resistant Staphylococcus aureus. BMC Microbiol. 2020, 20, 204. [Google Scholar] [CrossRef]
- Law, N.; Logan, C.; Yung, G.; Furr, C.L.; Lehman, S.M.; Morales, S.; Rosas, F.; Gaidamaka, A.; Bilinsky, I.; Grint, P.; et al. Successful adjunctive use of bacteriophage therapy for treatment of multidrug-resistant Pseudomonas aeruginosa infection in a cystic fibrosis patient. Infection 2019, 47, 665–668. [Google Scholar] [CrossRef]
- Terwilliger, A.; Clark, J.; Karris, M.; Hernandez-Santos, H.; Green, S.; Aslam, S.; Maresso, A. Phage Therapy Related Microbial Succession Associated with Successful Clinical Outcome for a Recurrent Urinary Tract Infection. Viruses 2021, 13, 2049. [Google Scholar] [CrossRef]
- Abedon, S.T.; Danis-Wlodarczyk, K.M.; Alves, D.R. Phage Therapy in the 21st Century: Is There Modern, Clinical Evidence of Phage-Mediated Efficacy? Pharmaceuticals 2021, 14, 1157. [Google Scholar] [CrossRef]
- Galtier, M.; De Sordi, L.; Sivignon, A.; de Vallée, A.; Maura, D.; Neut, C.; Rahmouni, O.; Wannerberger, K.; Darfeuille-Michaud, A.; Desreumaux, P.; et al. Bacteriophages Targeting Adherent Invasive Escherichia coli Strains as a Promising New Treatment for Crohn’s Disease. J. Crohns. Colitis 2017, 11, 840–847. [Google Scholar] [CrossRef] [Green Version]
- Pharma Pherecydes. Candidats. 2022. Available online: https://www.pherecydes-pharma.com/produits-candidats/ (accessed on 29 April 2022).
- Therapeutics Phico. Products. 2022. Available online: https://www.phicotx.co.uk/#products (accessed on 29 April 2022).
- Microgen. Products. 2022. Available online: https://www.microgen.ru/en/products/bakteriofagi/ (accessed on 29 April 2022).
- Biomx. Pipeline. 2022. Available online: https://www.biomx.com/ (accessed on 29 April 2022).
- Phagelux. Leading Products. 2022. Available online: http://www.phagelux.com/ (accessed on 29 April 2022).
- Czaplewski, L.; Bax, R.; Clokie, M.; Dawson, M.; Fairhead, H.; Fischetti, V.A.; Foster, S.; Gilmore, B.F.; Hancock, R.E.; Harper, D.; et al. Alternatives to antibiotics-a pipeline portfolio review. Lancet Infect. Dis. 2016, 16, 239–251. [Google Scholar] [CrossRef] [Green Version]
- Kim, N.H.; Park, W.B.; Cho, J.E.; Choi, Y.J.; Choi, S.J.; Jun, S.Y.; Kang, C.K.; Song, K.H.; Choe, P.G.; Bang, J.H.; et al. Effects of Phage Endolysin SAL200 Combined with Antibiotics on Staphylococcus aureus Infection. Antimicrob. Agents Chemother. 2018, 62, e00731-18. [Google Scholar] [CrossRef] [Green Version]
- Jun, S.Y.; Jang, I.J.; Yoon, S.; Jang, K.; Yu, K.S.; Cho, J.Y.; Seong, M.W.; Jung, G.M.; Yoon, S.J.; Kang, S.H. Pharmacokinetics and Tolerance of the Phage Endolysin-Based Candidate Drug SAL200 after a Single Intravenous Administration among Healthy Volunteers. Antimicrob. Agents Chemother. 2017, 61, e02629-16. [Google Scholar] [CrossRef] [Green Version]
- Cass, J.; Barnard, A.; Fairhead, H. Engineered Bacteriophage as a Delivery Vehicle for Antibacterial Protein, SASP. Pharmaceuticals 2021, 14, 1038. [Google Scholar] [CrossRef] [PubMed]
- BioPreparation Eliava. Products. 2022. Available online: https://eliava-institute.org/?lang=en (accessed on 29 April 2022).
- Mai, V.; Ukhanova, M.; Reinhard, M.K.; Li, M.; Sulakvelidze, A. Bacteriophage administration significantly reduces Shigella colonization and shedding by Shigella-challenged mice without deleterious side effects and distortions in the gut microbiota. Bacteriophage 2015, 5, e1088124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R&D BioNTech. Synthetic lysins. 2022. Available online: https://www.phagomed.com/overview (accessed on 29 April 2022).
- Zheng, D.W.; Dong, X.; Pan, P.; Chen, K.W.; Fan, J.X.; Cheng, S.X.; Zhang, X.Z. Phage-guided modulation of the gut microbiota of mouse models of colorectal cancer augments their responses to chemotherapy. Nat. Biomed. Eng. 2019, 3, 717–728. [Google Scholar] [CrossRef]
- Biochimpharm. Products. 2022. Available online: https://biochimpharm.ge/en/ (accessed on 29 April 2022).
- Ghosh, C.; Sarkar, P.; Issa, R.; Haldar, J. Alternatives to Conventional Antibiotics in the Era of Antimicrobial Resistance. Trends Microbiol. 2019, 27, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Immunopreparat Aziya. Production. 2022. Available online: https://www.aziyaimmunopreparat.uz/eng/ (accessed on 29 April 2022).
- Pharma, M.B. Products. 2022. Available online: https://www.mbph.cz/produkty (accessed on 29 April 2022).
- Phagex. Phage Products. 2022. Available online: https://bacteriophages.info/en/bacteriophage/ (accessed on 29 April 2022).
- Therapeutics Adaptive Phage. Development Pipeline. 2022. Available online: https://www.aphage.com/science/pipeline/ (accessed on 29 April 2022).
- Selle, K.; Fletcher, J.R.; Tuson, H.; Schmitt, D.S.; McMillan, L.; Vridhambal, G.S.; Rivera, A.J.; Montgomery, S.A.; Fortier, L.C.; Barrangou, R.; et al. In Vivo Targeting of Clostridioides difficile Using Phage-Delivered CRISPR-Cas3 Antimicrobials. mBio 2020, 11, e00019-20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Science Ellis Day Skin. Serums. 2022. Available online: https://www.ellisdayskinscience.com/products/balancing-phage-serum (accessed on 29 April 2022).
- Phyla. Our Technology: Bacteriophages. 2022. Available online: https://phylabiotics.com/ (accessed on 29 April 2022).
- Domingo-Calap, P.; Delgado-Martínez, J. Bacteriophages: Protagonists of a Post-Antibiotic Era. Antibiotics 2018, 7, 66. [Google Scholar] [CrossRef] [Green Version]
- Pirnay, J.P.; Merabishvili, M.; Van Raemdonck, H.; De Vos, D.; Verbeken, G. Bacteriophage Production in Compliance with Regulatory Requirements. Methods Mol. Biol. 2018, 1693, 233–252. [Google Scholar]
- Oliveira, H.; São-José, C.; Azeredo, J. Phage-Derived Peptidoglycan Degrading Enzymes: Challenges and Future Prospects for In Vivo Therapy. Viruses 2018, 10, 292. [Google Scholar] [CrossRef] [Green Version]
- Gondil, V.S.; Harjai, K.; Chhibber, S. Endolysins as emerging alternative therapeutic agents to counter drug-resistant infections. Int. J. Antimicrob. Agents 2020, 55, 105844. [Google Scholar] [CrossRef]
- Totté, J.E.E.; van Doorn, M.B.; Sgma, P. Successful Treatment of Chronic Staphylococcus aureus-Related Dermatoses with the Topical Endolysin Staphefekt SA.100: A Report of 3 Cases. Case Rep. Dermatol. 2017, 9, 19–25. [Google Scholar] [CrossRef]
- Gerstmans, H.; Rodríguez-Rubio, L.; Lavigne, R.; Briers, Y. From endolysins to Artilysin®s: Novel enzyme-based approaches to kill drug-resistant bacteria. Biochem. Soc. Trans. 2016, 44, 123–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, J.Y.; Jun, K.I.; Kang, C.K.; Song, K.H.; Choe, P.G.; Bang, J.H.; Kim, E.S.; Park, S.W.; Kim, H.B.; Kim, N.J.; et al. Efficacy of Intranasal Administration of the Recombinant Endolysin SAL200 in a Lethal Murine Staphylococcus aureus Pneumonia Model. Antimicrob. Agents Chemother. 2019, 63, e02009-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fowler, V.G., Jr.; Das, A.F.; Lipka-Diamond, J.; Schuch, R.; Pomerantz, R.; Jáuregui-Peredo, L.; Bressler, A.; Evans, D.; Moran, G.J.; Rupp, M.E.; et al. Exebacase for patients with Staphylococcus aureus bloodstream infection and endocarditis. J. Clin. Investig. 2020, 130, 3750–3760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moorthy, G.S.; Greenberg, R.G.; Hornik, C.D.; Cassino, C.; Ghahramani, P.; Kumar, K.R.; Fowler, V.G.; Cohen-Wolkowiez, M. Safety and Pharmacokinetics of Exebacase in an Infant with Disseminated Staphylococcus aureus Infection. Clin. Infect. Dis. 2021, 1–12. [Google Scholar] [CrossRef]
- Abdelkader, K.; Gerstmans, H.; Saafan, A.; Dishisha, T.; Briers, Y. The Preclinical and Clinical Progress of Bacteriophages and Their Lytic Enzymes: The Parts are Easier than the Whole. Viruses 2019, 11, 96. [Google Scholar] [CrossRef] [Green Version]
- Borin, J.M.; Avrani, S.; Barrick, J.E.; Petrie, K.L.; Meyer, J.R. Coevolutionary phage training leads to greater bacterial suppression and delays the evolution of phage resistance. Proc. Natl. Acad. Sci. USA 2021, 118, e2104592118. [Google Scholar] [CrossRef]
- Hampton, H.G.; Watson, B.N.J.; Fineran, P.C. The arms race between bacteria and their phage foes. Nature 2020, 577, 327–336. [Google Scholar] [CrossRef]
- Barrangou, R.; Horvath, P. A decade of discovery: CRISPR functions and applications. Nat. Microbiol. 2017, 2, 17092. [Google Scholar] [CrossRef]
- Hussain, F.A.; Dubert, J.; Elsherbini, J.; Murphy, M.; VanInsberghe, D.; Arevalo, P.; Kauffman, K.; Rodino-Janeiro, B.K.; Gavin, H.; Gomez, A.; et al. Rapid evolutionary turnover of mobile genetic elements drives bacterial resistance to phages. Science 2021, 374, 488–492. [Google Scholar] [CrossRef]
- Barber, O.W.; Miramontes, I.M.; Jain, M.; Ozer, E.A.; Hartmann, E.M. The Future of Bacteriophage Therapy Will Promote Antimicrobial Susceptibility. mSystems 2021, 6, e0021821. [Google Scholar] [CrossRef]
- Burmeister, A.R.; Fortier, A.; Roush, C.; Lessing, A.J.; Bender, R.G.; Barahman, R.; Turner, P.E. Pleiotropy complicates a trade-off between phage resistance and antibiotic resistance. Proc. Natl. Acad. Sci. USA 2020, 117, 11207–11216. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Van Belleghem, J.D.; de Vries, C.R.; Burgener, E.; Chen, Q.; Manasherob, R.; Aronson, J.R.; Amanatullah, D.F.; Tamma, P.D.; Suh, G.A. The Safety and Toxicity of Phage Therapy: A Review of Animal and Clinical Studies. Viruses 2021, 13, 1268. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Silk route to the acceptance and re-implementation of bacteriophage therapy. Biotechnol. J. 2016, 11, 595–600. [Google Scholar]
- Fauconnier, A. Phage Therapy Regulation: From Night to Dawn. Viruses 2019, 11, 352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres-Barceló, C. The disparate effects of bacteriophages on antibiotic-resistant bacteria. Emerg. Microbes Infect. 2018, 7, 168. [Google Scholar] [CrossRef]
- Bonilla, N.; Barr, J.J. Phage on Tap: A Quick and Efficient Protocol for the Preparation of Bacteriophage Laboratory Stocks. Methods Mol. Biol. 2018, 1838, 37–46. [Google Scholar]
- Luong, T.; Salabarria, A.C.; Edwards, R.A.; Roach, D.R. Standardized bacteriophage purification for personalized phage therapy. Nat. Protoc. 2020, 15, 2867–2890. [Google Scholar] [CrossRef]
- Sybesma, W.; Rohde, C.; Bardy, P.; Pirnay, J.P.; Cooper, I.; Caplin, J.; Chanishvili, N.; Coffey, A.; De Vos, D.; Scholz, A.H.; et al. Silk Route to the Acceptance and Re-Implementation of Bacteriophage Therapy-Part II. Antibiotics 2018, 7, 35. [Google Scholar]
- Bretaudeau, L.; Tremblais, K.; Aubrit, F.; Meichenin, M.; Arnaud, I. Good Manufacturing Practice (GMP) Compliance for Phage Therapy Medicinal Products. Front. Microbiol. 2020, 11, 1161. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).