Lactobacillus paracasei CCFM1223 Protects against Lipopolysaccharide-Induced Acute Liver Injury in Mice by Regulating the “Gut–Liver” Axis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bacterial Strain Cultures
2.3. Animal Experiment
2.4. Blood, Liver, and Colon Analysis
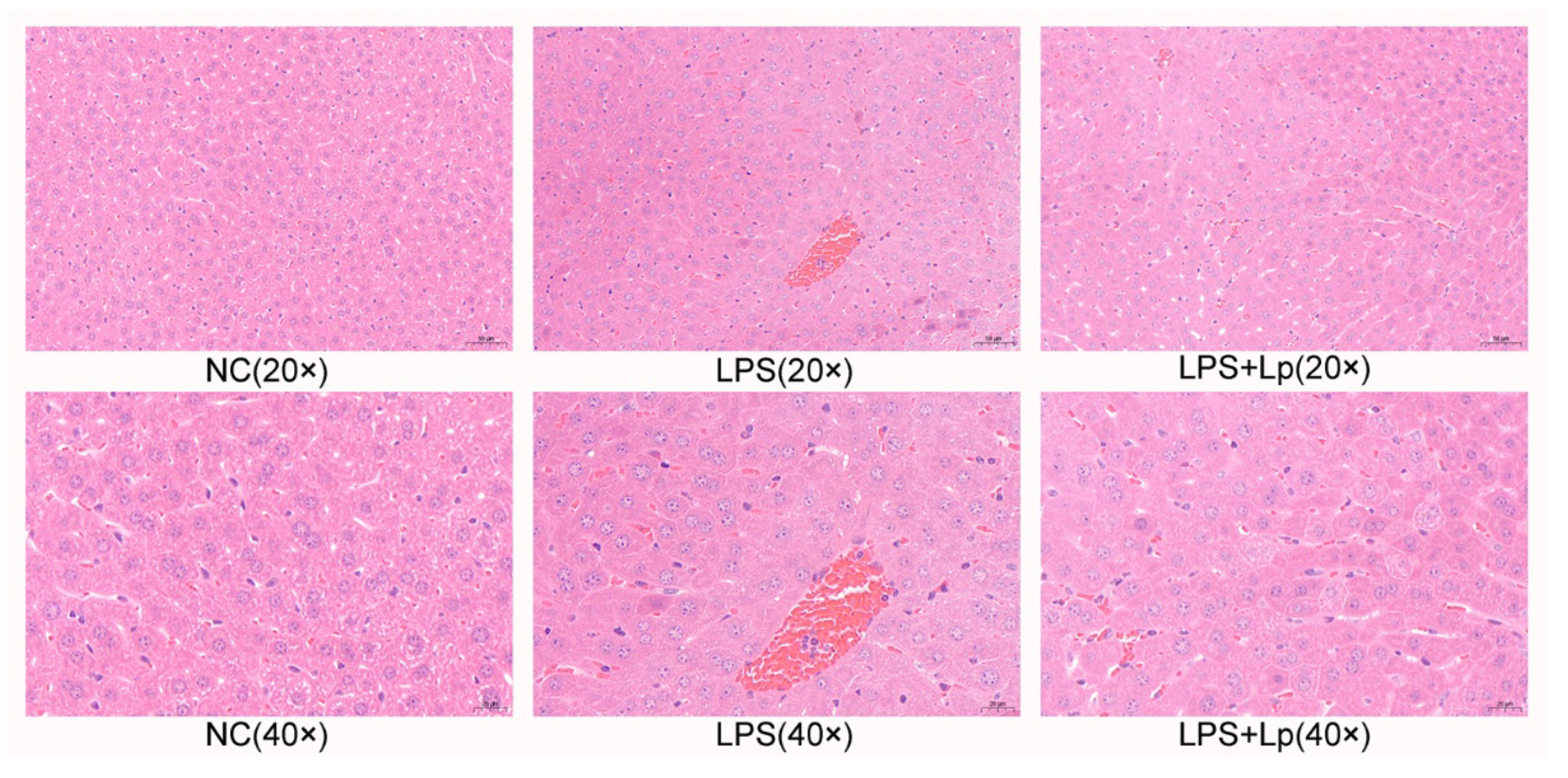
2.5. Hematoxylin and Eosin (H&E) Staining
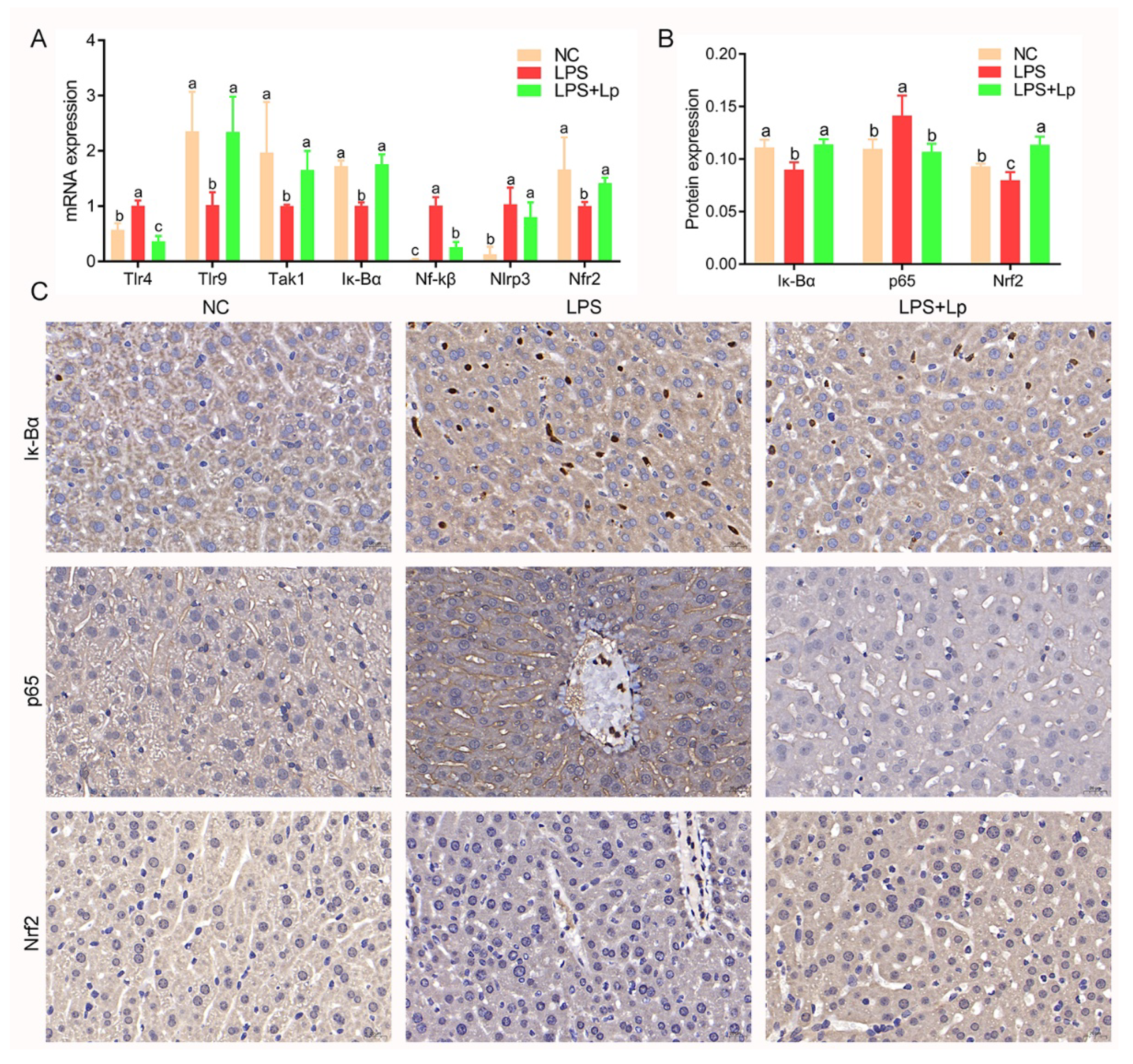
2.6. Immunohistochemistry
2.7. Cecal Short-Chain Fatty Acids Analysis
2.8. Real-Time Quantitative PCR Analysis
2.9. Intestinal Microbiota Analysis
2.10. Statistical Analysis
3. Results
3.1. Effect of L. paracasei CCFM1223 on the Body Weight, Serum Biochemical Index in ALI Mice
3.2. L. paracasei CCFM1223 Pretreatment Stimulated the IL-22 Production in ALI Mice
3.3. L. paracasei CCFM1223 Pretreatment Inhibited the Inflammation and Oxidation Stress in ALI Mice
3.4. L. paracasei CCFM1223 Pretreatment Shifted the SCFAs in ALI Mice
3.5. L. paracasei CCFM1223 Pretreatment Reshaped the Intestinal Microbiota in ALI Mice
3.6. Correlation of Key Intestinal Microbiota with ALI Indicators
3.7. L. paracasei CCFM1223 Alters the Expression of Genes in ALI Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, Y.; Ma, N.; Liu, X.; Yi, J.; Cai, S. Preventive effects of Chinese sumac fruits against acetaminophen-induced liver injury in mice via regulating oxidative stress, inflammation and apoptosis. J. Funct. Foods 2021, 87, 104830. [Google Scholar] [CrossRef]
- Xu, Q.; Xu, J.; Zhang, K.; Zhong, M.; Cao, H.; Wei, R.; Jin, L.; Gao, Y. Study on the protective effect and mechanism of Dicliptera chinensis (L.) Juss (Acanthaceae) polysaccharide on immune liver injury induced by LPS. Biomed. Pharmacother. 2021, 134, 111159. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, L.; Dong, R.; Huang, K.; Wang, C.; Gu, J.; Luo, H.; Liu, K.; Wu, J.; Sun, H.; et al. Luteolin ameliorates LPS-induced acute liver injury by inhibiting TXNIP-NLRP3 inflammasome in mice. Phytomedicine 2021, 87, 153586. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, Z.; Hu, Y.; Yang, S.; Cheng, F.; Rao, J.; Wang, X. Hyperglycemia-triggered ATF6-CHOP pathway aggravates acute inflammatory liver injury by β-catenin signaling. Cell Death Discov. 2022, 8, 115. [Google Scholar] [CrossRef]
- Ding, Q.; Cao, F.; Lai, S.; Zhuge, H.; Chang, K.; Valencak, T.G.; Liu, J.; Li, S.; Ren, D. Lactobacillus plantarum ZY08 relieves chronic alcohol-induced hepatic steatosis and liver injury in mice via restoring intestinal flora homeostasis. Food Res. Int. 2022, 157, 111259. [Google Scholar] [CrossRef]
- Lv, X.C.; Chen, M.; Huang, Z.R.; Guo, W.L.; Ai, L.Z.; Bai, W.D.; Yu, X.D.; Liu, Y.L.; Rao, P.F.; Ni, L. Potential mechanisms underlying the ameliorative effect of Lactobacillus paracasei FZU103 on the lipid metabolism in hyperlipidemic mice fed a high-fat diet. Food Res. Int. 2021, 139, 109956. [Google Scholar] [CrossRef]
- Dong, J.; Ping, L.; Xie, Q.; Liu, D.; Zhao, L.; Evivie, S.E.; Wang, Z.; Li, B.; Huo, G. Lactobacillus plantarum KLDS1.0386 with antioxidant capacity ameliorates the lipopolysaccharide-induced acute liver injury in mice by NF-κB and Nrf2 pathway. Food Biosci. 2022, 47, 101589. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, H.; Zhang, J.; Mu, J.; Zalan, Z.; Hegyi, F.; Takács, K.; Zhao, X.; Du, M. Protective effect of Lactobacillus fermentum CQPC04 on dextran sulfate sodium–induced colitis in mice is associated with modulation of the nuclear factor-κB signaling pathway. J. Dairy Sci. 2019, 102, 9570–9585. [Google Scholar] [CrossRef]
- Duan, C.; Zhao, Y.; Huang, C.; Zhao, Z.; Gao, L.; Niu, C.; Wang, C.; Liu, X.; Zhang, C.; Li, S. Hepatoprotective effects of Lactobacillus plantarum C88 on LPS/D-GalN–induced acute liver injury in mice. J. Funct. Foods 2018, 43, 146–153. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, H.; Tan, F.; Yi, R.; Li, W.; Long, X.; Mu, J.; Zhao, X. Lactobacillus plantarum KFY02 enhances the prevention of CCl4-induced liver injury by transforming geniposide into genipin to increase the antioxidant capacity of mice. J. Funct. Foods 2020, 73, 104128. [Google Scholar] [CrossRef]
- Zhou, Q.; Wu, F.; Chen, S.; Cen, P.; Yang, Q.; Guan, J.; Cen, L.; Zhang, T.; Zhu, H.; Chen, Z. Lactobacillus reuteri improves function of the intestinal barrier in rats with acute liver failure through Nrf-2/HO-1 pathway. Nutrition 2022, 99–100, 111673. [Google Scholar] [CrossRef] [PubMed]
- Sonnenberg, G.F.; Fouser, L.A.; Artis, D. Border patrol: Regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat. Immunol. 2011, 12, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, N.; Amiya, T.; Aoki, R.; Taniki, N.; Koda, Y.; Miyamoto, K.; Teratani, T.; Suzuki, T.; Chiba, S.; Chu, P.S.; et al. Commensal Lactobacillus controls immune tolerance during acute liver injury in mice. Cell Rep. 2017, 21, 1215–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, W.; Mao, B.; Cui, S.; Tang, X.; Zhang, Q.; Zhao, J.; Zhang, H. Protective effects of a novel probiotic Bifidobacterium pseudolongum on the intestinal barrier of colitis mice via modulating the PPARγ/STAT3 pathway and intestinal microbiota. Foods 2022, 11, 1551. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wang, C.; Mai, C.; Chen, J.; Lai, X.; He, L.; Huang, S.; Zhang, X. Flavonoids from Livistona chinensis fruit ameliorates LPS/D-GalN-induced acute liver injury by inhibiting oxidative stress and inflammation. J. Funct. Foods 2019, 61, 103460. [Google Scholar] [CrossRef]
- Wang, H.; Wei, X.; Wei, X.; Sun, X.; Huang, X.; Liang, Y.; Xu, W.; Zhu, X.; Lin, X.; Lin, J. 4-hydroxybenzo[d]oxazol-2(3H)-one ameliorates LPS/D-GalN-induced acute liver injury by inhibiting TLR4/NF-κB and MAPK signaling pathways in mice. Int. Immunopharmacol. 2020, 83, 106445. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, B.; Hu, J.; Nie, S.; Xiong, T.; Xie, M. Intervention of five strains of Lactobacillus on obesity in mice induced by high-fat diet. J. Funct. Foods 2020, 72, 104078. [Google Scholar] [CrossRef]
- Guo, W.L.; Pan, Y.Y.; Li, L.; Li, T.T.; Liu, B.; Lv, X.C. Ethanol extract of Ganoderma lucidum ameliorates lipid metabolic disorders and modulates the gut microbiota composition in high-fat diet fed rats. Food Funct. 2018, 9, 3419–3431. [Google Scholar] [CrossRef]
- Sharma, N.; Navik, U.; Tikoo, K. Unveiling the presence of epigenetic mark by Lactobacillus supplementation in high-fat diet-induced metabolic disorder in Sprague-Dawley rats. J. Nutr. Biochem. 2020, 84, 108442. [Google Scholar] [CrossRef]
- Guo, W.; Xiang, Q.; Mao, B.; Tang, X.; Cui, S.; Li, X.; Zhao, J.; Zhang, H.; Chen, W. Protective effects of microbiome-derived inosine on lipopolysaccharide-induced acute liver damage and inflammation in mice via mediating the TLR4/NF-κB pathway. J. Agric. Food Chem. 2021, 69, 7619–7628. [Google Scholar] [CrossRef]
- Yu, Y.; Li, X.; Hu, W.; Cu, S.; Dai, J.; Gao, Y.; Zhang, Y.; Bai, X.; Shi, D. Self-developed NF-κB inhibitor 270 protects against LPS-induced acute kidney injury and lung injury through improving inflammation. Biomed. Pharmacother. 2022, 147, 112615. [Google Scholar] [CrossRef]
- Bal, S.S.; Leishangthem, G.D.; Sethi, R.S.; Singh, A. P-coumaric acid ameliorates fipronil induced liver injury in mice through attenuation of structural changes, oxidative stress and inflammation. Pestic. Biochem. Physiol. 2022, 180, 104997. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.L.; Cao, Y.J.; You, S.Z.; Wu, Q.; Zhang, F.; Han, J.Z.; Lv, X.C.; Rao, P.F.; Ai, L.Z.; Ni, L. Ganoderic acids-rich ethanol extract from Ganoderma lucidum protects against alcoholic liver injury and modulates intestinal microbiota in mice with excessive alcohol intake. Curr. Res. Food Sci. 2022, 5, 515–530. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.C.; Wu, Q.; Cao, Y.J.; Lin, Y.C.; Guo, W.L.; Rao, P.; Zhang, Y.Y.; Chen, Y.T.; Ai, L.Z.; Ni, L. Ganoderic acid A from Ganoderma lucidum protects against alcoholic liver injury through ameliorating lipid metabolism and modulating intestinal microbial composition. Food Funct. 2022, 13, 5820–5837. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Tao, J.; Xiao, S.; Jiang, S.; Shang, E.; Zhu, Z.; Qian, D.; Duan, J. Xiexin Tang improves the symptom of type 2 diabetic rats by modulation of the gut microbiota. Sci. Rep. 2018, 8, 3685. [Google Scholar] [CrossRef]
- Mao, Z.; Ren, Y.; Zhang, Q.; Dong, S.; Han, K.; Feng, G.; Wu, H.; Zhao, Y. Glycated fish protein supplementation modulated gut microbiota composition and reduced inflammation but increased accumulation of advanced glycation end products in high-fat diet fed rats. Food Funct. 2019, 10, 3439–3451. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, S.; Cheng, Y.; Liu, Q.; Su, L.; Yang, Y.; Zhang, X.; Wu, M.; Choi, J.I.; Tong, H. Sargassum fusiforme alginate relieves hyperglycemia and modulates intestinal microbiota and metabolites in type 2 diabetic mice. Nutrients 2021, 13, 2887. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Song, S.; Shu, A.; Liu, L.; Jiang, J.; Jiang, M.; Wu, Q.; Xu, H.; Sun, J. The herb pair radix rehmanniae and cornus officinalis attenuated testicular damage in mice with diabetes mellitus through butyric acid/glucagon-like peptide-1/glucagon-like peptide-1 receptor pathway mediated by gut microbiota. Front. Microbiol. 2022, 13, 831881. [Google Scholar]
- Youssef, O.; Lahti, L.; Kokkola, A.; Karla, T.; Tikkanen, M.; Ehsan, H.; Carpelan-Holmström, M.; Koskensalo, S.; Böhling, T.; Rautelin, H.; et al. Stool microbiota composition differs in patients with stomach, colon, and rectal neoplasms. Dig. Dis. Sci. 2018, 63, 2950–2958. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Wang, Y.; Hao, X.; Duan, Y.; Meng, Z.; An, X.; Qi, J. Dietary fermented soybean meal replacement alleviates diarrhea in weaned piglets challenged with enterotoxigenic Escherichia coli K88 by modulating inflammatory cytokine levels and cecal microbiota composition. BMC Vet. Res. 2020, 16, 245. [Google Scholar] [CrossRef]
- Zhang, X.; Monnoye, M.; Mariadassou, M.; Beguet-Crespel, F.; Lapaque, N.; Heberden, C.; Douard, V. Glucose but not fructose alters the intestinal paracellular permeability in association with gut inflammation and dysbiosis in mice. Front. Immunol. 2021, 12, 742584. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yu, T.; Huang, X.; Bilotta, A.J.; Xu, L.; Lu, Y.; Sun, J.; Pan, F.; Zhou, J.; Zhang, W.; et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat. Commun. 2020, 11, 4457. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Han, B.; Yao, Q.; Zhang, Y.; Liu, H.; Zhang, S. Curcumin attenuates Concanavalin A-induced liver injury in mice by inhibition of Toll-like receptor (TLR) 2, TLR4 and TLR9 expression. Int. Immunopharm. 2012, 12, 151–157. [Google Scholar] [CrossRef]
- Arshad, T.; Mansur, F.; Palek, R.; Manzoor, S.; Liska, V. A double edged sword role of interleukin-22 in wound healing and tissue regeneration. Front. Immunol. 2020, 11, 2148. [Google Scholar] [CrossRef]
- Nolte, M.; Margadant, C. Controlling immunity and inflammation through integrin-dependent regulation of TGF-β. Trends Cell Biol. 2020, 30, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Marciano Deborah, L.; Leeman Susan, E.; Amar, S. LPS induces the interaction of a transcription factor, LPS-induced TNF-α factor, and STAT6(B) with effects on multiple cytokines. Proc. Natl. Acad. Sci. USA 2005, 102, 5132–5137. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Yu, J.; Jia, G.; Li, Z.; Xiong, H. Crocin attenuates NF-κB-mediated inflammation and proliferation in breast cancer cells by down-regulating PRKCQ. Cytokine 2022, 154, 155888. [Google Scholar] [CrossRef]
- Kimura, A.; Naka, T.; Muta, T.; Takeuchi, O.; Akira, S.; Kawase, I.; Kishimoto, T. Suppressor of cytokine signaling-1 selectively inhibits LPS-induced IL-6 production by regulating JAK–STAT. Proc. Natl. Acad. Sci. USA 2005, 102, 17089–17094. [Google Scholar] [CrossRef] [Green Version]
- Flores-Costa, R.; Duran-Güell, M.; Casulleras, M.; López-Vicario, C.; Alcaraz-Quiles, J.; Diaz, A.; Lozano, J.J.; Titos, E.; Hall, K.; Sarno, R.; et al. Stimulation of soluble guanylate cyclase exerts antiinflammatory actions in the liver through a VASP/NF-κB/NLRP3 inflammasome circuit. Proc. Natl. Acad. Sci. USA 2020, 117, 28263–28274. [Google Scholar] [CrossRef]





| NC | LPS | LPS+Lp | |
|---|---|---|---|
| TNF-α (pg/mg prot.) | 33.82 ± 13.52 c | 59.71 ± 20.71 a | 41.42 ± 6.38 b |
| IL-1β (pg/mg prot.) | 18.12 ± 7.40 c | 50.23 ± 12.02 a | 28.36 ± 5.38 b |
| IL-6 (pg/mg prot.) | 7.85 ± 2.45 a | 10.54 ± 3.64 a | 8.19 ± 1.04 a |
| IL-17 (pg/mg prot.) | 72.16 ± 18.54 b | 122.76 ± 41.84 a | 109.33 ± 17.04 a |
| IL-10 (pg/mg prot.) | 295.09 ± 72.99 a | 196.71 ± 83.54 b | 328.35 ± 43.48 a |
| TGF-β (pg/mg prot.) | 10.48 ± 0.16 a | 4.76 ± 2.05 b | 13.42 ± 3.52 a |
| LPS (EU/mg prot.) | 1.57 ± 0.46 b | 3.13 ± 0.49 a | 2.15 ± 0.54 b |
| MDA (nmol/mg prot.) | 1.03 ± 0.33 b | 1.82 ± 0.65 a | 0.79 ± 0.30 b |
| SOD (U/mg prot.) | 3.14 ± 1.2 a | 0.90 ± 0.84 b | 2.61 ± 2.13 ab |
| GSH-Px (U/mg prot.) | 23.36 ± 8.94 ab | 16.71 ± 5.96 b | 25.87 ± 7.18 a |
| CAT (U/mg prot.) | 2.74 ± 0.92 a | 1.10 ± 0.13 b | 2.72 ± 0.26 a |
| T-AOC (U/mg prot.) | 23.36 ± 8.93 a | 16.71 ± 5.96 c | 25.87 ± 7.18 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, W.; Mao, B.; Tang, X.; Zhang, Q.; Zhao, J.; Cui, S.; Zhang, H. Lactobacillus paracasei CCFM1223 Protects against Lipopolysaccharide-Induced Acute Liver Injury in Mice by Regulating the “Gut–Liver” Axis. Microorganisms 2022, 10, 1321. https://doi.org/10.3390/microorganisms10071321
Guo W, Mao B, Tang X, Zhang Q, Zhao J, Cui S, Zhang H. Lactobacillus paracasei CCFM1223 Protects against Lipopolysaccharide-Induced Acute Liver Injury in Mice by Regulating the “Gut–Liver” Axis. Microorganisms. 2022; 10(7):1321. https://doi.org/10.3390/microorganisms10071321
Chicago/Turabian StyleGuo, Weiling, Bingyong Mao, Xin Tang, Qiuxiang Zhang, Jianxin Zhao, Shumao Cui, and Hao Zhang. 2022. "Lactobacillus paracasei CCFM1223 Protects against Lipopolysaccharide-Induced Acute Liver Injury in Mice by Regulating the “Gut–Liver” Axis" Microorganisms 10, no. 7: 1321. https://doi.org/10.3390/microorganisms10071321
APA StyleGuo, W., Mao, B., Tang, X., Zhang, Q., Zhao, J., Cui, S., & Zhang, H. (2022). Lactobacillus paracasei CCFM1223 Protects against Lipopolysaccharide-Induced Acute Liver Injury in Mice by Regulating the “Gut–Liver” Axis. Microorganisms, 10(7), 1321. https://doi.org/10.3390/microorganisms10071321






