Fungal Enzymes Involved in Plastics Biodegradation
Abstract
1. Introduction
1.1. Plastic Pollution
1.2. Plastic Biodegradation
2. Fungal Enzymes Involved in Plastic Biodegradation
2.1. Laccases (EC 1.10.3.2)
2.2. Peroxidases (EC 1.11.1)
2.3. Cutinases (E.C. 3.1.1.74)
2.4. Lipases (EC 3.1.1.3)
3. Types of Plastics and Their Biodegradation by Fungi
3.1. Polyethylene (PE)
3.2. Fungal Enzymes Involved in PE Biodegradation
3.3. Polyethylene Terephthalate (PET)
3.4. Fungal Enzymes Involved in PET Biodegradation
3.4.1. Cutinases Involved in PET Biodegradation
3.4.2. Lipases Involved in PET Biodegradation
3.4.3. Polyesterases Involved in PET Biodegradation
3.4.4. Synergic Action of Cutinase HiC and Lipase CALB
3.4.5. PET Hydrophobicity Modification after Fungal Enzymes Action
3.5. Polyurethane (PUR)
3.6. Fungal Enzymes Involved in PUR Biodegradation
3.6.1. Esterases Involved in Polyester-PUR Biodegradation
3.6.2. Lipases Involved in Polyester-PUR Biodegradation
3.6.3. Cutinase Involved in Polyester-PUR Biodegradation
3.6.4. Fungal Hydrolyses Involved in Polyether-PUR Biodegradation
3.7. Polyvinyl Chloride (PVC)
3.8. Fungal Enzymes Involved in PVC Biodegradation
3.9. Polypropylene (PP)
3.10. Fungal Enzymes Involved in PP Biodegradation
3.11. Polystyrene (PS)
3.12. Fungal Enzymes Involved in PS Biodegradation
4. Conclusions and Perspectives for Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fried, J.R. Polymer Science and Technology; Prentice Hall: Upper Saddle River, NJ, USA, 1995; pp. 4–9. [Google Scholar]
- Millet, H.; Vangheluwe, P.; Block, C.; Sevenster, A.; Garcia, L.; Antonopoulos, R. The nature of plastics and their societal usage. In Plastic and the Environment; Hester, R.E., Ed.; Royal Society of Chemistry’s: London, UK, 2018; pp. 1–20. [Google Scholar]
- Matjašič, T.; Simčič, T.; Medvešček, N.; Bajt, O.; Dreo, T.; Mori, N. Critical evaluation of biodegradation studies on synthetic plastics through a systematic literature review. Sci. Total Environ. 2021, 752, 141959. [Google Scholar] [CrossRef] [PubMed]
- Plastic Europe. Plastics–The Facts 2021: An Analysis of European Plastics Production, Demand and Waste Data. 2021. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2021/ (accessed on 19 January 2021).
- Bahl, S.; Dolma, J.; Singh, J.J.; Sehgal, S. Biodegradation of plastics: A state of the art review. Mater. Today Proc. 2021, 39, 31–34. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Devi, R.S.; Kannan, V.R.; Natarajan, K.; Nivas, D.; Kannan, K.; Chandru, S.; Antony, A.R. The role of microbes in plastic degradation. Environ. Waste Manag. 2016, 341, 341–370. [Google Scholar]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Shen, M.; Huang, W.; Chen, M.; Song, B.; Zeng, G.; Zhang, Y. (Micro) plastic crisis: Un-ignorable contribution to global greenhouse gas emissions and climate change. J. Clean Prod. 2020, 254, 120138. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Suh, S. Degradation rates of plastics in the environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Kumar, M.; Chen, H.; Sarsaiya, S.; Qin, S.; Liu, H.; Awasthi, M.K.; Taherzadeh, M.J. Current research trends on micro-and nano-plastics as an emerging threat to global environment: A review. J. Hazard. Mater. 2021, 409, 124967. [Google Scholar] [CrossRef]
- Chen, Q.; Allgeier, A.; Yin, D.; Hollert, H. Leaching of endocrine disrupting chemicals from marine microplastics and mesoplastics under common life stress conditions. Environ. Int. 2019, 130, 104938. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, H.; Allgeier, A.; Zhou, Q.; Ouellet, J.D.; Crawford, S.E.; Hollert, H. Marine microplastics bound dioxin-like chemicals: Model explanation and risk assessment. J. Hazard. Mater. 2019, 364, 82–90. [Google Scholar] [CrossRef]
- Turner, A.; Filella, M. Hazardous metal additives in plastics and their environmental impacts. Environ. Int. 2021, 156, 106622. [Google Scholar] [CrossRef]
- Mohsen, M.; Lin, C.; Tu, C.; Zhang, C.; Xu, S.; Yang, H. Association of heavy metals with plastics used in aquaculture. Mar. Pollut. Bull. 2022, 174, 113312. [Google Scholar] [CrossRef]
- Kumar, P. Role of plastics on human health. Indian J. Pediatr. 2018, 85, 384–389. [Google Scholar] [CrossRef]
- Yang, D.; Shi, H.; Li, L.; Li, J.; Jabeen, K.; Kolandhasamy, P. Microplastic pollution in table salts from China. Environ. Sci. Technol. 2015, 49, 13622–13627. [Google Scholar] [CrossRef]
- Istrate, I.R.; Medina-Martos, E.; Galvez-Martos, J.L.; Dufour, J. Assessment of the energy recovery potential of municipal solid waste under future scenarios. Appl. Energy 2021, 293, 116915. [Google Scholar] [CrossRef]
- Kunwar, B.; Cheng, H.N.; Chandrashekaran, S.R.; Sharma, B.K. Plastics to fuel: A review. Renew. Sust. Energ. Rev. 2016, 54, 421–428. [Google Scholar] [CrossRef]
- Verma, R.; Vinoda, K.S.; Papireddy, M.; Gowda, A.N.S. Toxic pollutants from plastic waste—A review. Procedia Environ. Sci. 2016, 35, 701–708. [Google Scholar] [CrossRef]
- Aguado, J.; Serrano, D.P.; Escola, J.M. Fuels from waste plastics by thermal and catalytic processes: A review. Ind. Eng. Chem. Res. 2008, 47, 7982–7992. [Google Scholar] [CrossRef]
- Thiounn, T.; Smith, R.C. Advances and approaches for chemical recycling of plastic waste. J. Polym. Sci. 2020, 58, 1347–1364. [Google Scholar] [CrossRef]
- Chiellini, E.; Solaro, R. Biodegradable polymeric materials. Adv. Mater. 1996, 8, 305–313. [Google Scholar] [CrossRef]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef] [PubMed]
- Amobonye, A.; Bhagwat, P.; Singh, S.; Pillai, S. Plastic biodegradation: Frontline microbes and their enzymes. Sci. Total Environ. 2021, 759, 143536. [Google Scholar] [CrossRef] [PubMed]
- Harms, H.; Schlosser, D.; Wick, L.Y. Untapped potential: Exploiting fungi in bioremediation of hazardous chemicals. Nat. Rev. Microbiol. 2011, 9, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Bhandari, G.; Bhatt, K.; Maithani, D.; Mishra, S.; Gangola, S.; Bhatt, R.; Huang, Y.; Chen, S. Plasmid-mediated catabolism for the removal of xenobiotics from the environment. J. Hazard. Mater. 2021, 420, 126618. [Google Scholar] [CrossRef]
- Lu, H.; Diaz, D.J.; Czarnecki, N.J.; Zhu, C.; Kim, W.; Shroff, R.; Alper, H.S. Machine learning-aided engineering of hydrolases for PET depolymerization. Nature 2022, 604, 662–667. [Google Scholar] [CrossRef]
- Bhatt, P.; Pandey, S.C.; Joshi, S.; Chaudhary, P.; Pathak, V.M.; Huang, Y.; Chen, S. Nanobioremediation: A sustainable approach for the removal of toxic pollutants from the environment. J. Hazard. Mater. 2022, 427, 128033. [Google Scholar] [CrossRef]
- Zara, Z.; Mishra, D.; Pandey, S.K.; Csefalvay, E.; Fadaei, F.; Minofar, B.; Řeha, D. Surface Interaction of Ionic Liquids: Stabilization of Polyethylene Terephthalate-Degrading Enzymes in Solution. Molecules 2022, 27, 119. [Google Scholar] [CrossRef]
- Wallace, N.E.; Adams, M.C.; Chafin, A.C.; Jones, D.D.; Tsui, C.L.; Gruber, T.D. The highly crystalline PET found in plastic water bottles does not support the growth of the PETase-producing bacterium Ideonella sakaiensis. Environ. Microbiol. Rep. 2020, 12, 578–582. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P.; Ugwu, C.U.; Aiba, S. Biodegradability of plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef]
- Taghavi, N.; Zhuang, W.Q.; Baroutian, S. Enhanced biodegradation of non-biodegradable plastics by UV radiation: Part 1. J. Environ. Chem. Eng. 2021, 9, 106464. [Google Scholar] [CrossRef]
- Vedrtnam, A.; Kumar, S.; Chaturvedi, S. Experimental study on mechanical behavior, biodegradability, and resistance to natural weathering and ultraviolet radiation of wood-plastic composites. Compos. B. Eng. 2019, 176, 107282. [Google Scholar] [CrossRef]
- Arutchelvi, J.; Sudhakar, M.; Arkatkar, A.; Doble, M.; Bhaduri, S.; Uppara, P.V. Biodegradation of polyethylene and polypropylene. IJBT 2008, 7, 9–22. [Google Scholar]
- Jacquin, J.; Cheng, J.; Odobel, C.; Pandin, C.; Conan, P.; Pujo-Pay, M.; Ghiglione, J.F. Microbial ecotoxicology of marine plastic debris: A review on colonization and biodegradation by the “Plastisphere”. Front. Microbiol. 2019, 10, 865. [Google Scholar] [CrossRef]
- Dussud, C.; Ghiglione, J.F. Bacterial degradation of synthetic plastics. In Marine Litter in the Mediterranean and Black Seas; Briand, F., Ed.; CIESM: Paris, France, 2014; Volume 46, pp. 49–54. [Google Scholar]
- Zhang, J.; Gao, D.; Li, Q.; Zhao, Y.; Li, L.; Lin, H.; Zhao, Y. Biodegradation of polyethylene microplastic particles by the fungus Aspergillus flavus from the guts of wax moth Galleria mellonella. Sci. Total Environ. 2020, 704, 135931. [Google Scholar] [CrossRef]
- Wei, R.; Zimmermann, W. Microbial enzymes for the recycling of recalcitrant petroleum-based plastics: How far are we? Microb. Biotechnol. 2017, 10, 1308–1322. [Google Scholar] [CrossRef]
- Daccò, C.; Girometta, C.; Asemoloye, M.D.; Carpani, G.; Picco, A.M.; Tosi, S. Key fungal degradation patterns, enzymes and their applications for the removal of aliphatic hydrocarbons in polluted soils: A review. Int. Biodeterior. Biodegrad. 2020, 147, 104866. [Google Scholar] [CrossRef]
- Sánchez, C. Fungal potential for the degradation of petroleum-based polymers: An overview of macro-and microplastics biodegradation. Biotechnol. Adv. 2020, 40, 107501. [Google Scholar] [CrossRef]
- Da Luz, J.M.R.; da Silva, M.D.C.S.; dos Santos, L.F.; Kasuya, M.C.M. Plastics polymers degradation by fungi. In Microorganisms; Blumenberg, M., Ed.; IntechOpen: London, UK, 2019; pp. 261–270. [Google Scholar]
- Vertommen, M.A.M.E.; Nierstrasz, V.A.; van der Veer, M.; Warmoeskerken, M.M.C.G. Enzymatic surface modification of poly (ethylene terephthalate). J. Biotechnol. 2005, 120, 376–386. [Google Scholar] [CrossRef]
- Krueger, M.C.; Hofmann, U.; Moeder, M.; Schlosser, D. Potential of wood-rotting fungi to attack polystyrene sulfonate and its depolymerisation by Gloeophyllum trabeum via hydroquinone-driven Fenton chemistry. PLoS ONE 2015, 10, e0131773. [Google Scholar] [CrossRef]
- Webb, H.; Arnott, J.; Crawford, R.; Ivanova, E. Plastic degradation and its environmental implicationswith special reference to poly(ethylene terephthalate). Polymers 2013, 5, 1. [Google Scholar] [CrossRef]
- Cregut, M.; Bedas, M.; Durand, M.J.; Thouand, G. New insights into polyurethane biodegradation andrealistic prospects for the development of a sustainablewaste recycling process. Biotechnol. Adv. 2013, 31, 1634–1647. [Google Scholar] [CrossRef]
- Álvarez-Barragán, J.; Domínguez-Malfavón, L.; Vargas-Suárez, M.; González-Hernández, R.; Aguilar-Osorio, G.; Loza-Tavera, H. Biodegradative activities of selected environmental fungi on a polyester polyurethane varnish and polyether polyurethane foams. Appl. Environ. Microbiol. 2016, 82, 5225–5235. [Google Scholar] [CrossRef]
- Gómez-Méndez, L.D.; Moreno-Bayona, D.A.; Poutou-Piñales, R.A.; Salcedo-Reyes, J.C.; Pedroza-Rodríguez, A.M.; Vargas, A.; Bogoya, J.M. Biodeterioration of plasma pretreated LDPE sheets by Pleurotus ostreatus. PLoS ONE 2018, 13, e0203786. [Google Scholar] [CrossRef]
- Restrepo-Florez, J.M.; Bassi, A.; Thompson, M.R. Microbial degradation and deterioration of polyethylene—A review. Int. Biodeterior. Biodegrad. 2014, 88, 83–90. [Google Scholar] [CrossRef]
- Daly, P.; Cai, F.; Kubicek, C.P.; Jiang, S.; Grujic, M.; Rahimi, M.J.; Druzhinina, I.S. From lignocellulose to plastics: Knowledge transfer on the degradation approaches by fungi. Biotechnol. Adv. 2021, 50, 107770. [Google Scholar] [CrossRef]
- Arya, G.C.; Cohen, H. The Multifaceted Roles of Fungal Cutinases during Infection. J. Fungi 2022, 8, 199. [Google Scholar] [CrossRef]
- Mehta, A.; Bodh, U.; Gupta, R. Fungal lipases: A review. J. Biotech Res. 2017, 8, 58–77. [Google Scholar]
- Da Silva Santiago, S.R.S.; Santiago, P.A.L.; de Oliveira, M.R.; de Souza Rodrigues, R.; Barbosa, A.N.; da Silva, G.F.; de Souza, A.Q.L. Evaluation of enzymatic production of hydrolases and oxyredutases by Fusarium pseudocircinatum and Corynespora torulosa isolated from caesarweed (Urena lobata L., 1753). Res. Soc. Dev. 2022, 11, e13211225325. [Google Scholar] [CrossRef]
- Kabir, S.M.M.; Koh, J. Sustainable textile processing by enzyme applications. In Biodegradation Technology of Organic and Inorganic Pollutants; Mendes, K.F., de Sousa, R.O., Mielke, K.C., Eds.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Drozłowska, E. The use of enzymatic fungal activity in the food industry-review. World Sci. News 2019, 116, 222–229. [Google Scholar]
- Behbudi, G.; Yousefi, K.; Sadeghipour, Y. Microbial Enzymes Based Technologies for Bioremediation of Pollutions. J. Environ. Treat. Tech. 2021, 9, 463–469. [Google Scholar]
- Siddiqui, N.M.; Dahiya, P. Enzyme-based biodegradation of toxic environmental pollutants. In Development in Wastewater Treatment Research and Processes; Rodriguez-Couto, S., Shah, M.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 311–333. [Google Scholar]
- Bilal, M.; Rasheed, T.; Nabeel, F.; Iqbal, H.M.; Zhao, Y. Hazardous contaminants in the environment and their laccase-assisted degradation—A review. J. Environ. Manag. 2019, 234, 253–264. [Google Scholar] [CrossRef]
- Demarche, P.; Junghanns, C.; Nair, R.R.; Agathos, S.N. Harnessing the power of enzymes for environmental stewardship. Biotechnol. Adv. 2012, 30, 933–953. [Google Scholar] [CrossRef]
- Surwase, S.V.; Patil, S.A.; Srinivas, S.; Jadhav, J.P. Interaction of small molecules with fungal laccase: A surface plasmon resonance based study. Enzym. Microb. Technol. 2016, 82, 110–114. [Google Scholar] [CrossRef]
- Desai, S.S.; Nityanand, C. Microbial laccases and their applications: A review. Asian J. Biotechnol. 2011, 3, 98–124. [Google Scholar] [CrossRef]
- Giardina, P.; Faraco, V.; Pezzella, C.; Piscitelli, A.; Vanhulle, S.; Sannia, G. Laccases: A never-ending story. Cell Mol. Life Sci. 2010, 67, 369–385. [Google Scholar] [CrossRef]
- Madhavi, V.; Lele, S.S. Laccase: Properties and applications. BioResources 2009, 4, 1694–1717. [Google Scholar]
- Kunamneni, A.; Ballesteros, A.; Plou, F.J.; Alcalde, M. Fungal laccase—A versatile enzyme for biotechnological applications. In Communicating Current Research and Educational Topics and Trends in Applied Microbiology; Méndez-Vilas, A., Ed.; Formatex: Guadalajara, Mexico, 2007; pp. 233–245. [Google Scholar]
- Rivera-Hoyos, C.M.; Morales-Álvarez, E.D.; Poutou-Pinales, R.A.; Pedroza-Rodríguez, A.M.; Rodriguez-Vazquez, R.; Delgado-Boada, J.M. Fungal laccases. Fungal. Biol. Rev. 2013, 27, 67–82. [Google Scholar] [CrossRef]
- Thurston, C.F. The structure and function of fungal laccases. Microbiology 1994, 140, 19–26. [Google Scholar] [CrossRef]
- Henson, J.M.; Butler, M.J.; Day, A.W. The dark side of the mycelium: Melanins of phytopathogenic fungi. Annu. Rev. Phytopathol. 1999, 37, 447–471. [Google Scholar] [CrossRef]
- Ikehata, K.; Buchanan, I.D.; Smith, D.W. Recent developments in the production of extracellular fungal peroxidases and laccases for waste treatment. J. Environ. Eng. Sci. 2004, 3, 1–19. [Google Scholar] [CrossRef]
- Welinder, K.G. Structure and evolution of peroxidase. In Plant Peroxidases, Biochemistry and Physiology, 1st ed.; Welinder, K.G., Rasmussen, S.K., Penel, C., Greppin, H., Eds.; University of Geneva: Geneva, Switzerland, 1993; pp. 35–42. [Google Scholar]
- Conesa, A.; Punt, P.J.; van den Hondel, C.A. Fungal peroxidases: Molecular aspects and applications. J. Biotechnol. 2002, 93, 143–158. [Google Scholar] [CrossRef]
- Ayuso-Fernández, I.; Martínez, A.T.; Ruiz-Dueñas, F.J. Experimental recreation of the evolution of lignin-degrading enzymes from the Jurassic to date. Biotechnol. Biofuels 2017, 10, 67. [Google Scholar] [CrossRef]
- Tien, M.; Kirk, T.K. Lignin-degrading enzyme from the hymenomycete Phanerochaete chrysosporium Burds. Science 1983, 221, 661–663. [Google Scholar] [CrossRef]
- Tien, M.; Kirk, T.K. Lignin-degrading enzyme from Phanerochaete chrysosporium: Purification, characterization, and catalytic properties of a unique H2O2-requiring oxygenase. Proc. Natl. Acad. Sci. USA 1984, 81, 2280–2284. [Google Scholar] [CrossRef]
- Huynh, V.B.; Crawford, R.L. Novel extracellular enzymes (ligninases) of Phanerochaete chrysosporium. FEMS Microbiol. Lett. 1985, 28, 119–123. [Google Scholar] [CrossRef][Green Version]
- Paszczynski, A.; Huynh, V.B.; Crawford, R. Enzymatic activities of an extracellular, manganese-dependent peroxidase from Phanerochaete chrysosporium. FEMS Microbiol. Lett. 1985, 29, 37–41. [Google Scholar] [CrossRef]
- Sundaramoorthy, M.; Gold, M.H.; Poulos, T.L. Ultrahigh (0.93Å) resolution structure of manganese peroxidase from Phanerochaete chrysosporium: Implications for the catalytic mechanism. J. Inorg. Biochem. 2010, 104, 683–690. [Google Scholar] [CrossRef]
- Maciel, M.J.M.; Ribeiro, H.C.T. Industrial and biotechnological applications of ligninolytic enzymes of the basidiomycota: A review. Electron. J. Biotechnol. 2010, 13, 14–15. [Google Scholar] [CrossRef]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic transformation of lignin for the production of chemicals and fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef]
- Sung, H.J.; Khan, M.F.; Kim, Y.H. Recombinant lignin peroxidase-catalyzed decolorization of melanin using in-situ generated H2O2 for application in whitening cosmetics. Int. J. Biol. Macromol. 2019, 136, 20–26. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Zhang, X.; Geng, A. Purification and characterization of a novel manganese peroxidase from white-rot fungus Cerrena unicolor BBP6 and its application in dye decolorization and denim bleaching. Process Biochem. 2018, 66, 222–229. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, L.; Jia, R.; Wang, N. Cloning and expression of a new manganese peroxidase from Irpex lacteus F17 and its application in decolorization of reactive black 5. Process Biochem. 2015, 50, 1748–1759. [Google Scholar] [CrossRef]
- Lee, H.; Jang, Y.; Choi, Y.S.; Kim, M.J.; Lee, J.; Lee, H.; Hong, J.-H.; Lee, Y.M.; Kim, G.H.; Kim, J.J. Biotechnological procedures to select white rot fungi for the degradation of PAHs. J. Microbiol. Methods 2014, 97, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Torres-Farradá, G.; Manzano León, A.M.; Rineau, F.; Ledo Alonso, L.L.; Sánchez-López, M.I.; Thijs, S.; Colpaert, J.; Ramos-Leal, M.; Guerra, G.; Vangronsveld, J. Diversity of ligninolytic enzymes and their genes in strains of the genus Ganoderma: Applicable for biodegradation of xenobiotic compounds? Front. Microbiol. 2017, 8, 898. [Google Scholar] [CrossRef] [PubMed]
- Pozdnyakova, N.N. Involvement of the ligninolytic system of white-rot and litter-decomposing fungi in the degradation of polycyclic aromatic hydrocarbons. Biotechnol. Res. Int. 2012, 2012, 243217. [Google Scholar] [CrossRef]
- Qayyum, H.; Maroof, H.; Yasha, K. Remediation and treatment of organopollutants mediated by peroxidases: A review. Crit. Rev. Biotechnol. 2009, 29, 94–119. [Google Scholar] [CrossRef]
- Skamnioti, P.; Furlong, R.F.; Gurr, S.J. Evolutionary history of the ancient cutinase family in five filamentous Ascomycetes reveals differential gene duplications and losses and in Magnaporthe grisea shows evidence of sub- and neo-functionalization. New Phytol. 2008, 180, 711–721. [Google Scholar] [CrossRef]
- Chen, S.; Tong, X.; Woodard, R.W.; Du, G.; Wu, J.; Chen, J. Identification and characterization of bacterial cutinase. J. Biol. Chem. 2008, 283, 25854–25862. [Google Scholar] [CrossRef]
- Longhi, S.; Czjzek, M.; Nicolas, A.; Cambillau, C. Atomic resolution (1.0 Å) crystalstructure of Fusarium solani cutinase: Stereochemical analysis. J. Mol. Biol. 1997, 268, 779–799. [Google Scholar] [CrossRef]
- Martinez, C.; de Geus, P.; Lauwereys, M.; Matthyssens, G.; Cambillau, C. Fusarium solani cutinase is a lipolytic enzyme with a catalytic serine accessible to solvent. Nature 1992, 356, 615–618. [Google Scholar] [CrossRef]
- Roussel, A.; Amara, S.; Nyyssola, A.; Mateos-Diaz, E.; Blangy, S.; Kontkanen, H.; Westerholm-Pantinen, A.; Carriere, F.; Cambillau, C. A cutinase from Trichoderma reesei with a lid-covered active site and kinetic properties of true lipases. J. Mol. Biol. 2014, 426, 3757–3772. [Google Scholar] [CrossRef]
- Lau, E.Y.; Bruice, T.C. Consequences of breaking the Asp-His hydrogen bond of the catalytic triad: Effects on the structure and dynamics of the serine esterase cutinase. Biophys. J. 1999, 77, 85–98. [Google Scholar] [CrossRef]
- Longhi, S.; Nicolas, A.; Creveld, L.; Egmond, M.; Verrips, C.T.; Vlieg, J.; Martinez, C.; Cambillau, C. Dynamics of Fusarium solani cutinase investigated through structural comparison among different crystal forms of its variant. Protein 1996, 26, 442–458. [Google Scholar] [CrossRef]
- Chen, S.; Su, L.; Chen, J.; Wu, J. Cutinase: Characteristics, preparation, and application. Biotechnol. Adv. 2013, 31, 1754–1767. [Google Scholar] [CrossRef]
- Dimarogona, M.; Nikolaivits, E.; Kanelli, M.; Christakopoulos, P.; Sandgren, M.; Topakas, E. Structural and functional studies of a Fusarium oxysporum cutinase with polyethylene terephthalate modification potential. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 2308–2317. [Google Scholar] [CrossRef]
- Silva, C.M.; Carneiro, F.; O’Neill, A.; Fonseca, L.P.; Cabral, J.S.; Guebitz, G.; Cavaco-Paulo, A. Cutinase-a new tool for biomodification of synthetic fibers. J. Polym. Sci. A Polym. Chem. 2005, 43, 2448–2450. [Google Scholar] [CrossRef]
- Singh, A.K.; Mukhopadhyay, M. Overview of fungal lipase: A review. Appl. Biochem. Biotechnol. 2012, 166, 486–520. [Google Scholar] [CrossRef]
- Ramos-Sánchez, L.B.; Cujilema-Quitio, M.C.; Julian-Ricardo, M.C.; Cordova, J.; Fickers, P. Fungal lipase production by solid-state fermentation. J. Bioprocess. Biotech. 2015, 5, 1. [Google Scholar] [CrossRef]
- Sharma, R.; Chisti, Y.; Banerjee, U.C. Production, purification, characterization, and applications of lipases. Biotechnol. Adv. 2001, 19, 627–662. [Google Scholar] [CrossRef]
- Sztajer, H.; Maliszewska, I.; Wieczorek, J. Production of exogenous lipases by bacteria, fungi, and actinomycetes. Enzym. Microb. Technol. 1988, 10, 492–497. [Google Scholar] [CrossRef]
- Ko, W.H.; Wang, I.T.; Ann, P.J. A simple method for detection of lipolytic microorganisms in soils. Soil Biol. Biochem. 2005, 37, 597–599. [Google Scholar] [CrossRef]
- Chandra, P.; Singh, R.; Arora, P.K. Microbial lipases and their industrial applications: A comprehensive review. Microb. Cell Factories 2020, 19, 169. [Google Scholar] [CrossRef] [PubMed]
- Houde, A.; Kademi, A.; Leblanc, D. Lipases and their industrial applications. Appl. Biochem. Biotechnol. 2004, 118, 155–170. [Google Scholar] [CrossRef]
- Masse, L.; Kennedy, K.J.; Chou, S.P. The effect of an enzymatic pretreatment on the hydrolysis and size reduction of fat particles in slaughterhouse wastewater. J. Chem. Technol. Biotechnol. 2001, 76, 629–635. [Google Scholar] [CrossRef]
- Takamoto, T.; Shirasaka, H.; Uyama, H.; Kobayashi, S. Lipase-catalyzed hydrolytic degradation of polyurethane in organic solvent. Chem. Lett. 2001, 30, 492–493. [Google Scholar] [CrossRef]
- Montazer, Z.; Habibi Najafi, M.B.; Levin, D.B. Challenges with verifying microbial degradation of polyethylene. Polymers 2020, 12, 123. [Google Scholar] [CrossRef]
- Mierzwa-Hersztek, M.; Gondek, K.; Kopeć, M. Degradation of polyethylene and biocomponent-derived polymer materials: An overview. J. Polym. Environ. 2019, 27, 600–611. [Google Scholar] [CrossRef]
- Gajendiran, A.; Krishnamoorthy, S.; Abraham, J. Microbial degradation of low-density polyethylene (LDPE) by Aspergillus clavatus strain JASK1 isolated from landfill soil. 3 Biotech 2016, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Bardají, D.K.R.; Moretto, J.A.S.; Furlan, J.P.R.; Stehling, E.G. A mini-review: Current advances in polyethylene biodegradation. World J. Microbiol. Biotechnol. 2020, 36, 32. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.; Manley, M. Cross-linked polyethylene. In Surgical Treatment of Hip Arthritis. Reconstruction, Replacement, and Revision; Hozack, W., Ed.; Saunders (W.B.) Co., Ltd.: Philadelphia, PA, USA, 2009; pp. 456–467. [Google Scholar]
- Ragaert, K.; Delva, L.; van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef] [PubMed]
- Harshvardhan, K.; Jha, B. Biodegradation of low-density polyethylene by marine bacteria from pelagic waters, Arabian Sea, India. Mar. Pollut. Bull. 2013, 77, 100–106. [Google Scholar] [CrossRef]
- Albertsson, A.C.; Andersson, S.O.; Karlsson, S. The mechanism of biodegradation of polyethylene. Polym. Degrad. Stab. 1987, 18, 73–87. [Google Scholar] [CrossRef]
- Santo, M.; Weitsman, R.; Sivan, A. The role of the copper-binding enzyme e laccase e in the biodegradation of polyethylene by the actinomycete Rhodococcus ruber. Int. Biodeterior. Biodegrad. 2012, 208, 1.e7. [Google Scholar] [CrossRef]
- Yoon, M.G.; Jeon, J.H.; Kim, M.N. Biodegradation of polyethylene by a soil bacterium and AlkB cloned recombinant cell. J. Bioremed. Biodegr. 2012, 3, 145. [Google Scholar] [CrossRef]
- Iiyoshi, Y.; Tsutsumi, Y.; Nishida, T. Polyethylene degradation by lignin-degrading fungi and manganese peroxidase. J. Wood Sci. 1998, 44, 222–229. [Google Scholar] [CrossRef]
- Santacruz-Juárez, E.; Buendia-Corona, R.E.; Ramírez, R.E.; Sánchez, C. Fungal enzymes for the degradation of polyethylene: Molecular docking simulation and biodegradation pathway proposal. J. Hazard. Mater. 2021, 411, 125118. [Google Scholar] [CrossRef]
- Malachová, K.; Novotný, Č.; Adamus, G.; Lotti, N.; Rybková, Z.; Soccio, M.; Šlosarčíková, P.; Verney, V.; Fava, F. Ability of Trichoderma hamatum Isolated from Plastics-Polluted Environments to Attack Petroleum-Based, Synthetic Polymer Films. Processes 2020, 8, 467. [Google Scholar] [CrossRef]
- Ojha, N.; Pradhan, N.; Singh, S.; Barla, A.; Shrivastava, A.; Khatua, P.; Bose, S. Evaluation of HDPE and LDPE degradation by fungus, implemented by statistical optimization. Sci. Rep. 2017, 7, 39515. [Google Scholar] [CrossRef]
- Sáenz, M.; Borodulina, T.; Diaz, L.; Banchon, C. Minimal Conditions to Degrade Low Density Polyethylene by Aspergillus terreus and niger. J. Ecol. Eng. 2019, 20, 44–51. [Google Scholar] [CrossRef]
- Sowmya, H.V.; Krishnappa, M.; Thippeswamy, B. Degradation of polyethylene by Trichoderma harzianum—SEM, FTIR, and NMR analyses. Environ. Monit. Assess 2014, 186, 6577–6586. [Google Scholar] [CrossRef]
- Sowmya, H.V.; Krishnappa, M.; Thippeswamy, B. Degradation of polyethylene by Penicillium simplicissimum isolated from local dumpsite of Shivamogga district. Environ. Dev. Sustain. 2015, 17, 731–745. [Google Scholar] [CrossRef]
- Shi, K.; Jing, J.; Song, L.; Su, T.; Wang, Z. Enzymatic hydrolysis of polyester: Degradation of poly (ε-caprolactone) by Candida antarctica lipase and Fusarium solani cutinase. Int. J. Biol. Macromol. 2020, 144, 183–189. [Google Scholar] [CrossRef]
- Spina, F.; Tummino, M.L.; Poli, A.; Prigione, V.; Ilieva, V.; Cocconcelli, P.; Puglisi, E.; Bracco, P.; Zanetti, M.; Varese, G.C. Low density polyethylene degradation by filamentous fungi. Environ. Pollut. 2021, 274, 116548. [Google Scholar] [CrossRef]
- Corti, A.; Sudhakar, M.; Chiellini, E. Assessment of the whole environmental degradation of oxo-biodegradable linear low density polyethylene (LLDPE) films designed for mulching applications. J. Polym. Environ. 2012, 20, 1007–1018. [Google Scholar] [CrossRef]
- Kang, B.R.; Kim, S.B.; Song, H.A.; Lee, T.K. Accelerating the biodegradation of high-density polyethylene (HDPE) using Bjerkandera adusta TBB-03 and lignocellulose substrates. Microorganisms 2019, 7, 304. [Google Scholar] [CrossRef]
- Awasthi, S.; Srivastava, N.; Singh, T.; Tiwary, D.; Mishra, P.K. Biodegradation of thermally treated low density polyethylene by fungus Rhizopus oryzae NS 5. 3 Biotech 2017, 7, 73. [Google Scholar] [CrossRef]
- Shimao, M. Biodegradation of plastics. Curr. Opin. Biotechnol. 2001, 12, 242–247. [Google Scholar] [CrossRef]
- Fujisawa, M.; Hirai, H.; Nishida, T. Degradation of polyethylene and nylon-66 by the laccase-mediator system. J. Polym. Environ. 2001, 9, 103–108. [Google Scholar] [CrossRef]
- Volkamer, A.; Griewel, A.; Grombacher, T.; Rarey, M. Analyzing the topology of active sites: On the prediction of pockets and subpockets. J. Chem. Inf. Model. 2010, 50, 2041–2052. [Google Scholar] [CrossRef]
- Volkamer, A.; Kuhn, D.; Grombacher, T.; Rippmann, F.; Rarey, M. Combining global and local measures for structure-based druggability predictions. J. Chem. Inf. Model. 2012, 52, 360–372. [Google Scholar] [CrossRef]
- Isvoran, A. Web-based computational tools used in protein surface analysis and characterization. Applications for protein–protein and protein–ligand interactions. In Exotic Properties of Carbon Nanomatter. Carbon Materials: Chemistry and Physics; Putz, M.V., Ed.; Springer: Dordrecht, The Netherlands, 2015; Volume 8, pp. 203–227. [Google Scholar]
- Bertrand, T.; Jolivalt, C.; Briozzo, P.; Caminade, E.; Joly, N.; Madzak, C.; Mougin, C. Crystal structure of a four-copper laccase complexed with an arylamine: Insights into substrate recognition and correlation with kinetics. Biochemistry 2002, 41, 7325–7333. [Google Scholar] [CrossRef] [PubMed]
- Miki, Y.; Calviño, F.R.; Pogni, R.; Giansanti, S.; Ruiz-Dueñas, F.J.; Martínez, M.J.; Basosi, R.; Romero, A.; Martínez, A.T. Crystallographic, kinetic, and spectroscopic study of the first ligninolytic peroxidase presenting a catalytic tyrosine. J. Biol. Chem. 2011, 286, 15525–15534. [Google Scholar] [CrossRef] [PubMed]
- Sang, T.; Wallis, C.J.; Hill, G.; Britovsek, G.J. Polyethylene terephthalate degradation under natural and accelerated weathering conditions. Eur. Polym. J. 2020, 136, 109873. [Google Scholar] [CrossRef]
- Raheem, A.B.; Noor, Z.Z.; Hassan, A.; Abd Hamid, M.K.; Samsudin, S.A.; Sabeen, A.H. Current developments in chemical recycling of post-consumer polyethylene terephthalate wastes for new materials production: A review. J. Clean Prod. 2019, 225, 1052–1064. [Google Scholar] [CrossRef]
- Pang, J.; Zheng, M.; Sun, R.; Wang, A.; Wang, X.; Zhang, T. Synthesis of ethylene glycol and terephthalic acid from biomass for producing PET. Green Chem. 2016, 18, 342–359. [Google Scholar] [CrossRef]
- Zimmermann, W.; Billig, S. Enzymes for the biofunctionalization of poly (ethylene terephthalate). In Biofunctionalization of Polymers and Their Applications; Nyanhongo, G.S., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 97–120. [Google Scholar]
- Fischer-Colbrie, G.; Heumann, S.; Liebminger, S.; Almansa, E.; Cavaco-Paulo, A.; Guebitz, G.M. New enzymes with potential for PET surface modification. Biocatal. Biotransform. 2004, 22, 341–346. [Google Scholar] [CrossRef]
- Malafatti-Picca, L.; de Barros Chaves, M.R.; de Castro, A.M.; Valoni, É.; de Oliveira, V.M.; Marsaioli, A.J.; de Franceschi de Angelis, D.; Attili-Angelis, D. Hydrocarbon-associated substrates reveal promising fungi for poly (ethylene terephthalate) (PET) depolymerization. Braz. J. Microbiol. 2019, 50, 633–648. [Google Scholar] [CrossRef]
- De Castro, A.M.; Carniel, A.; Nicomedes Junior, J.; da Conceição Gomes, A.; Valoni, É. Screening of commercial enzymes for poly (ethylene terephthalate) (PET) hydrolysis and synergy studies on different substrate sources. J. Ind. Microbiol. Biotechnol. 2017, 44, 835–844. [Google Scholar] [CrossRef]
- Kawai, F.; Kawabata, T.; Oda, M. Current knowledge on enzymatic PET degradation and its possible application to waste stream management and other fields. Appl. Microbiol. Biotechnol. 2019, 103, 4253–4268. [Google Scholar] [CrossRef]
- Sarkhel, R.; Sengupta, S.; Das, P.; Bhowal, A. Comparative biodegradation study of polymer from plastic bottle waste using novel isolated bacteria and fungi from marine source. J. Polym. Res. 2020, 27, 16. [Google Scholar] [CrossRef]
- Wang, X.; Lu, D.; Jönsson, L.J.; Hong, F. Preparation of a PET-hydrolyzing lipase from Aspergillus oryzae by the addition of bis(2-hydroxyethyl)terephthalate to the culture medium and enzymatic modification of PET fabrics. Eng. Life Sci. 2008, 8, 268–276. [Google Scholar] [CrossRef]
- Kanelli, M.; Vasilakos, S.; Nikolaivits, E.; Ladas, S.; Christakopoulos, P.; Topakas, E. Surface modification of poly (ethylene terephthalate) (PET) fibers by a cutinase from Fusarium oxysporum. Process Biochem. 2015, 50, 1885–1892. [Google Scholar] [CrossRef]
- Nimchua, T.; Punnapayak, H.; Zimmermann, W. Comparison of the hydrolysis of polyethylene terephthalate fibers by a hydrolase from Fusarium oxysporum LCH I and Fusarium solani f. sp. pisi. Biotechnol. J. Healthc. Nutr. Technol. 2007, 2, 361–364. [Google Scholar] [CrossRef]
- Sepperumal, U.; Markandan, M.; Palraja, I. Micromorphological and chemical changes during biodegradation of polyethylene terephthalate (PET) by Penicillium sp. J. Microbiol. Biotechnol. Res. 2013, 3, 47–53. [Google Scholar]
- Nowak, B.; Pająk, J.; Łabużek, S.; Rymarz, G.; Talik, E. Biodegradation of poly (ethylene terephthalate) modified with polyester “Bionolle®” by Penicillium funiculosum. Polimery 2011, 56, 35–44. [Google Scholar] [CrossRef]
- Taniguchi, I.; Yoshida, S.; Hiraga, K.; Miyamoto, K.; Kimura, Y.; Oda, K. Biodegradation of PET: Current status and application aspects. ACS Catal. 2019, 9, 4089–4105. [Google Scholar] [CrossRef]
- Carr, C.M.; Clarke, D.J.; Dobson, A.D.W. Microbial Polyethylene Terephthalate Hydrolases: Current and Future Perspectives. Front. Microbiol. 2020, 11, 571265. [Google Scholar] [CrossRef]
- Gao, R.; Pan, H.; Lian, J. Recent advances in the discovery, characterization, and engineering of poly (ethylene terephthalate) (PET) hydrolases. Enzym. Microb. Technol. 2021, 150, 109868. [Google Scholar] [CrossRef]
- Danso, D.; Chow, J.; Streit, W.R. Plastics: Environmental and biotechnological perspectives on microbial degradation. Appl. Environ. Microbiol. 2019, 85, e01095-19. [Google Scholar] [CrossRef]
- Ronkvist, A.M.; Xie, W.C.; Lu, W.H.; Gross, R.A. Cutinase catalyzed hydrolysis of poly(ethylene terephthalate). Macromolecules 2009, 42, 5128–5138. [Google Scholar] [CrossRef]
- Eberl, A.; Heumann, S.; Bruckner, T.; Araujo, R.; Cavaco- Paulo, A.; Kaufmann, F.; Kroutil, W.; Guebitz, G.M. Enzymatic surface hydrolysis of poly(ethylene terephthalate) and bis- (benzoyloxyethyl) terephthalate by lipase and cutinase in the presence of surface active molecules. J. Biotechnol. 2009, 143, 207–212. [Google Scholar] [CrossRef]
- Donelli, I.; Freddi, G.; Nierstrasz, V.A.; Taddei, P. Surface structure and properties of poly-(ethylene terephthalate) hydrolyzed by alkali and cutinase. Polym. Degrad. Stab. 2010, 95, 1542–1550. [Google Scholar] [CrossRef]
- Heumann, S.; Eberl, A.; Pobeheim, H.; Liebminger, S.; Fischer-Colbrie, G.; Almansa, E.; Cavaco-Paulo, A.; Gübitz, G.M. New model substrates for enzymes hydrolysing polyethyleneterephthalate and polyamide fibres. J. Biochem. Biophys. Methods 2006, 69, 89–99. [Google Scholar] [CrossRef]
- Ping, L.F.; Chen, X.Y.; Yuan, X.L.; Zhang, M.; Chai, Y.J.; Shan, S.D. Application and comparison in biosynthesis and biodegradation by Fusarium solani and Aspergillus fumigatus cutinases. Int. J. Biol. Macromol. 2017, 104, 1238–1245. [Google Scholar] [CrossRef]
- Perz, V.; Bleymaier, K.; Sinkel, C.; Kueper, U.; Bonnekessel, M.; Ribitsch, D.; Guebitz, G.M. Substrate specificities of cutinases on aliphatic–aromatic polyesters and on their model substrates. New Biotechnol. 2016, 33, 295–304. [Google Scholar] [CrossRef]
- Müller, R.J.; Schrader, H.; Profe, J.; Dresler, K.; Deckwer, W.D. Enzymatic degradation of poly(ethylene terephthalate): Rapid hydrolyse using a hydrolase from T. fusca. Macromol. Rapid Commun. 2005, 26, 1400–1405. [Google Scholar] [CrossRef]
- De Castro, A.M.; Carniel, A.; Stahelin, D.; Junior, L.S.C.; de Angeli Honorato, H.; de Menezes, S.M.C. High-fold improvement of assorted post-consumer poly (ethylene terephthalate) (PET) packages hydrolysis using Humicola insolens cutinase as a single biocatalyst. Process Biochem. 2019, 81, 85–91. [Google Scholar] [CrossRef]
- Araújo, R.; Silva, C.; O’Neill, A.; Micaelo, N.; Guebitz, G.M.; Soares, C.; Casal, M.; Cavaco-Paulo, A. Tailoring cutinase activity towards polyethylene terephthalate and polyamide 6,6 fibers. J. Biotechnol. 2007, 128, 849–857. [Google Scholar] [CrossRef]
- Griswold, K.; Mahmood, N.A.; Iverson, B.L.; Georgiou, G. Effects of codon usage versus putative 5_-mRNA structure on the expression of Fusarium solani cutinase in the Escherichia coli cytoplasm. Prot. Expr. Purif. 2003, 27, 134–142. [Google Scholar] [CrossRef]
- Carniel, A.; Valoni, É.; Junior, J.N.; da Conceição Gomes, A.; de Castro, A.M. Lipase from Candida antarctica (CALB) and cutinase from Humicola insolens act synergistically for PET hydrolysis to terephthalic acid. Process Biochem. 2017, 59, 84–90. [Google Scholar] [CrossRef]
- Gao, A.; Shen, H.; Zhang, H.; Feng, G.; Xie, K. Hydrophilic modification of polyester fabric by synergetic effect of biological enzymolysis and non-ionic surfactant, and applications in cleaner production. J. Clean Prod. 2017, 164, 277–287. [Google Scholar] [CrossRef]
- Almansa, E.; Heumann, S.; Eberl, A.; Fischer-Colbrie, G.; Martinkova, L.; Marek, J.; Cavaco-Paulo, A.; Guebitz, G.M. Enzymatic surface hydrolysis of PET enhances bonding in PVC coating. Biocatal. Biotransform. 2008, 26, 365–370. [Google Scholar] [CrossRef]
- Liebminger, S.; Eberl, A.; Sousa, F.; Heumann, S.; Fischer-Colbrie, G.; Cavaco-Paulo, A.; Guebitz, G.M. Hydrolysis of PET and bis-(benzoyloxyethyl) terephthalate with a new polyesterase from Penicillium citrinum. Biocatal. Biotransform. 2007, 25, 171–177. [Google Scholar] [CrossRef]
- Alisch-Mark, M.; Herrmann, A.; Zimmermann, W. Increase of the hydrophilicity of polyethylene terephthalate fibres by hydrolases from Thermomonospora fusca and Fusarium solani f. sp. pisi. Biotechnol. Lett. 2006, 28, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.Y.; Kellis, J.; Poulouse, A.J. Enzymatic modification of polyester. AATCC Rev. 2002, 2, 3336. [Google Scholar]
- Gouda, M.K.; Kleeberg, I.; van den Heuvel, J.; Müller, R.J.; Deckwer, W.D. Production of a polyester degrading extracellular hydrolase from Thermomonospora fusca. Biotechnol. Progr. 2002, 18, 927934. [Google Scholar] [CrossRef]
- Howard, G.T. Biodegradation of polyurethane: A review. Int. Biodeterior. Biodegrad. 2002, 49, 245–252. [Google Scholar] [CrossRef]
- Plastic Europe. Plastics—The Facts 2020: An Analysis of European Plastics Production, Demand and Waste Data. 2020. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2020/ (accessed on 19 January 2021).
- Nakajima-Kambe, T.; Shigeno-Akutsu, Y.; Nomura, N.; Onuma, F.; Nakahara, T. Microbial degradation of polyurethane, polyester polyurethanes and polyether polyurethanes. Appl. Microbiol. Biotechnol. 1999, 51, 134–140. [Google Scholar] [CrossRef]
- Gautam, R.; Bassi, A.S.; Yanful, E.K. A review of biodegradation of synthetic plastic and foams. Appl. Biochem. Biotechnol. 2007, 141, 85–108. [Google Scholar] [CrossRef]
- Mahajan, N.; Gupta, P. New insights into the microbial degradation of polyurethanes. RSC Adv. 2015, 5, 41839–41854. [Google Scholar] [CrossRef]
- Ignatyev, I.A.; Thielemans, W.; Vander Beke, B. Recycling of polymers: A review. Chem. Sus. Chem. 2014, 7, 1579–1593. [Google Scholar] [CrossRef]
- Pathirana, R.A.; Seal, K.J. Gliocladium roseum (Bainier), a potential biodeteriogen of polyester polyurethane elastomers. In Biodeterioration 5: Papers Presented at the 5th International Biodeterioration Symposium, Aberdeen, September, 1981; Oxley, T.A., Ed.; Wiley: Chichester, UK, 1983; p. c1983. [Google Scholar]
- Howard, G.T.; Blake, R.C. Growth of Pseudomonas fluorescens on a polyester–polyurethane and the purification and characterization of a polyurethanase–protease enzyme. Int. Biodeterior. Biodegrad. 1998, 42, 213–220. [Google Scholar] [CrossRef]
- Loredo-Treviño, A.; García, G.; Velasco-Téllez, A.; Rodríguez-Herrera, R.; Aguilar, C.N. Polyurethane foam as substrate for fungal strains. Adv. Biosci. Biotechnol. 2011, 2, 52. [Google Scholar] [CrossRef]
- Ru, J.; Huo, Y.; Yang, Y. Microbial degradation and valorization of plastic wastes. Front. Microbiol. 2020, 11, 442. [Google Scholar] [CrossRef]
- Matsumiya, Y.; Murata, N.; Tanabe, E.; Kubota, K.; Kubo, M. Isolation and characterization of an ether-type polyurethane-degrading micro-organism and analysis of degradation mechanism by Alternaria sp. J. Appl. Microbiol. 2010, 108, 1946–1953. [Google Scholar] [CrossRef]
- Stachelek, S.J.; Alferiev, I.; Choi, H.; Chan, C.W.; Zubiate, B.; Sacks, M.; Composto, R.; Chen, I.W.; Levy, R.J. Prevention of oxidative degradation of polyurethane by covalent attachment of di-tert-butylphenol residues. J. Biomed. Mater. Res. 2006, 78, 653–661. [Google Scholar] [CrossRef]
- Christenson, E.M.; Anderson, J.M.; Hiltner, A. Oxidative mechanisms of poly (carbonate urethane) and poly (ether urethane) biodegradation: In vivo and in vitro correlations. J. Biomed. Mater. Res. A 2004, 70, 245–255. [Google Scholar] [CrossRef]
- Kemona, A.; Piotrowska, M. Polyurethane recycling and disposal: Methods and prospects. Polymers 2020, 12, 1752. [Google Scholar] [CrossRef]
- Loredo-Treviño, A.; Gutiérrez-Sánchez, G.; Rodríguez-Herrera, R.; Aguilar, C.N. Microbial enzymes involved in polyurethane biodegradation: A review. J. Polym. Environ. 2012, 20, 258–265. [Google Scholar] [CrossRef]
- Howard, G.T. Polyurethane biodegradation. In Microbial Degradation of Xenobiotics; Singh, S.N., Ed.; Springer: Heidelberg, Germany, 2012; pp. 371–394. [Google Scholar]
- Crabbe, J.R.; Campbell, J.R.; Thompson, L.; Walz, S.L.; Schultz, W.W. Biodegradation of a colloidal ester-based polyurethane by soil fungi. Int. Biodeterior. Biodegrad. 1994, 33, 103–113. [Google Scholar] [CrossRef]
- Boubendir, A. Purification and biochemical evaluation of polyurethane degrading enzymes of fungal origin. Diss. Abstr. Int. 1993, 53, 4632. [Google Scholar]
- Saffarzadeh, N.; Moghimi, H. The study of impranil (DLN) polymer biodegradation by fungus Sarocladium kiliense. Nova Biol. Reper. 2019, 6, 20–29. [Google Scholar] [CrossRef]
- Biffinger, J.C.; Barlow, D.E.; Cockrell, A.L.; Cusick, K.D.; Hervey, W.J.; Fitzgerald, L.A.; Nadeau, L.J.; Hung, C.S.; Crookes-Goodson, W.J.; Russell, J.N., Jr. The applicability of Impranil® DLN for gauging the biodegradation of polyurethanes. Polym. Degrad. Stab. 2015, 120, 178–185. [Google Scholar] [CrossRef]
- Osman, M.; Satti, S.M.; Luqman, A.; Hasan, F.; Shah, Z.; Shah, A.A. Degradation of polyester polyurethane by Aspergillus sp. strain S45 isolated from soil. J. Polym. Environ. 2018, 26, 301–310. [Google Scholar] [CrossRef]
- Russell, J.R.; Huang, J.; Anand, P.; Kucera, K.; Sandoval, A.G.; Dantzler, K.W.; Strobel, S.A. Biodegradation of polyester polyurethane by endophytic fungi. Appl. Environ. Microbiol. 2011, 77, 6076–6084. [Google Scholar] [CrossRef]
- Gautam, R.; Bassi, A.S.; Yanful, E.K. Candida rugosa lipase-catalyzed polyurethane degradation in aqueous medium. Biotechnol. Lett. 2007, 29, 1081–1086. [Google Scholar] [CrossRef]
- Khan, S.; Nadir, S.; Shah, Z.U.; Shah, A.A.; Karunarathna, S.C.; Xu, J.; Khan, A.; Munir, S.; Hasan, F. Biodegradation of polyester polyurethane by Aspergillus tubingensis. Environ. Pollut. 2017, 225, 469–480. [Google Scholar] [CrossRef]
- Yang, S.; Xu, H.; Yan, Q.; Liu, Y.; Zhou, P.; Jiang, Z. A low molecular mass cutinase of Thielavia terrestris efficiently hydrolyzes poly (esters). J. Ind. Microbiol. Biotechnol. 2013, 40, 217–226. [Google Scholar] [CrossRef]
- Owen, S.; Otani, T.; Masaoka, S.; Ohe, T. The biodegradation of low-molecular-weight urethane compounds by a strain of Exophiala jeanselmei. Biosci. Biotechnol. Biochem. 1996, 60, 244–248. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Bakhshandeh, G.R. Recycling of PVC wastes. Polym. Degrad. Stab. 2011, 96, 404–415. [Google Scholar] [CrossRef]
- Peng, B.Y.; Chen, Z.; Chen, J.; Yu, H.; Zhou, X.; Criddle, C.S.; Zhang, Y. Biodegradation of polyvinyl chloride (PVC) in Tenebrio molitor (Coleoptera: Tenebrionidae) larvae. Environ. Int. 2020, 145, 106106. [Google Scholar] [CrossRef] [PubMed]
- Winkler, D.E. Mechanism of polyvinyl chloride degradation and stabilization. J. Polym. Sci. 1959, 35, 3–16. [Google Scholar] [CrossRef]
- Pospíšil, J.; Horák, Z.; Kruliš, Z.; Nešpůrek, S.; Kuroda, S.I. Degradation and aging of polymer blends I. Thermomechanical and thermal degradation. Polym. Degrad. Stab. 1999, 65, 405–414. [Google Scholar] [CrossRef]
- Amass, W.; Amass, A.; Tighe, B. A review of biodegradable polymers: Uses, current developments in the synthesis and characterization of biodegradable polyesters, blends of biodegradable polymers and recent advances in biodegradation studies. Polym. Int. 1998, 47, 89–144. [Google Scholar] [CrossRef]
- Roberts, W.T.; Davidson, P.M. Growth characteristics of selected fungi on polyvinyl chloride film. Appl. Environ. Microbiol. 1986, 51, 673–676. [Google Scholar] [CrossRef]
- Klrbas, Z.; Keskin, N.; Güner, A. Biodegradation of polyvinylchloride (PVC) by white rot fungi. Bull. Environ. Contam. Toxicol. 1999, 63, 335–342. [Google Scholar] [CrossRef]
- Ali, M.I.; Ahmed, S.; Robson, G.; Javed, I.; Ali, N.; Atiq, N.; Hameed, A. Isolation and molecular characterization of polyvinyl chloride (PVC) plastic degrading fungal isolates. J. Basic Microbiol. 2014, 54, 18–27. [Google Scholar] [CrossRef]
- Sakhalkar, S.; Mishra, R.L. Screening and identification of soil fungi with potential of plastic degrading ability. Indian J. Appl. Res. 2013, 3, 3. [Google Scholar] [CrossRef]
- Muthukumar, A.; Veerappapillai, S. Biodegradation of plastics: A brief review. Int. J. Pharm. Sci. Rev. Res. 2015, 31, 204–209. [Google Scholar]
- Pradeep, S.; Benjamin, S. Mycelial fungi completely remediate di (2-ethylhexyl) phthalate, the hazardous plasticizer in PVC blood storage bag. J. Hazard. Mater. 2012, 235, 69–77. [Google Scholar] [CrossRef]
- Webb, J.S.; Nixon, M.; Eastwood, I.M.; Greenhalgh, M.; Robson, G.D.; Handley, P.S. Fungal colonization and biodeterioration of plasticized polyvinyl chloride. Appl. Environ. Microbiol. 2000, 66, 3194–3200. [Google Scholar] [CrossRef]
- Gumargalieva, K.Z.; Zaikov, G.E.; Semenov, S.A.; Zhdanova, O.A. The influence of biodegradation on the loss of a plasticiser from poly (vinyl chloride). Polym. Degrad. Stab. 1999, 63, 111–112. [Google Scholar] [CrossRef]
- Mogil’nitskii, G.M.; Sagatelyan, R.T.; Kutishcheva, T.N.; Zhukova, S.V.; Kerimov, S.I.; Parfenova, T.B. Disruption of the protective properties of the polyvinyl chloride coating under the effect of microorganisms. Prot. Met. 1987, 23, 173–175. [Google Scholar]
- Sumathi, T.; Viswanath, B.; Sri Lakshmi, A.; SaiGopal, D.V.R. Production of laccase by Cochliobolus sp. isolated from plastic dumped soils and their ability to degrade low molecular weight PVC. Biochem Res Int. 2016, 2016, 9519527. [Google Scholar] [CrossRef]
- Vivi, V.K.; Martins-Franchetti, S.M.; Attili-Angelis, D. Biodegradation of PCL and PVC: Chaetomium globosum (ATCC 16021) activity. Folia Microbiol. 2019, 64, 1–7. [Google Scholar] [CrossRef]
- Kaczmarek, H.; Bajer, K. Biodegradation of plasticized poly (vinyl chloride) containing cellulose. J. Polym. Sci. B Polym. Phys. 2007, 45, 903–919. [Google Scholar] [CrossRef]
- Pardo-Rodríguez, M.L.; Zorro-Mateus, P.J.P. Biodegradation of polyvinyl chloride by Mucor sp. and Penicillium sp. isolated from soil. Rev. Investig. Desarro. Innovación 2021, 11, 387–400. [Google Scholar] [CrossRef]
- Sabev, H.A.; Handley, P.S.; Robson, G.D. Fungal colonization of soil-buried plasticized polyvinyl chloride (pPVC) and the impact of incorporated biocides. Microbiology 2006, 152, 1731–1739. [Google Scholar] [CrossRef]
- Mohanan, N.; Montazer, Z.; Sharma, P.K.; Levin, D.B. Microbial and enzymatic degradation of synthetic plastics. Front. Microbiol. 2020, 11, 2837. [Google Scholar] [CrossRef]
- Cobongela, S.Z.Z. Enzymes involved in plastic degradation. In Degradation of Plastics. Materials Research Foundations; Inamuddin, Ed.; Materials Research Forum LLC: Millersville, PA, USA, 2021; Volume 99, pp. 95–110. [Google Scholar]
- Othman, A.R.; Hasan, H.A.; Muhamad, M.H.; Ismail, N.I.; Abdullah, S.R.S. Microbial degradation of microplastics by enzymatic processes: A review. Environ. Chem. Lett. 2021, 19, 3057–3073. [Google Scholar] [CrossRef]
- Yang, S.S.; Kang, J.H.; Xie, T.R.; He, L.; Xing, D.F.; Ren, N.Q.; Ho, S.H.; Wu, W.M. Generation of high-efficient biochar for dye adsorption using frass of yellow mealworms (larvae of Tenebrio molitor Linnaeus) fed with wheat straw for insect biomass production. J. Clean Prod. 2019, 227, 33–47. [Google Scholar] [CrossRef]
- Khatoon, N.; Jamal, A.; Ali, M.I. Lignin peroxidase isoenzyme: A novel approach to biodegrade the toxic synthetic polymer waste. Environ. Technol. 2019, 40, 1366–1375. [Google Scholar] [CrossRef]
- Chaudhary, A.K.; Vijayakumar, R.P. Studies on biological degradation of polystyrene by pure fungal cultures. Environ. Dev. Sustain. 2020, 22, 4495–4508. [Google Scholar] [CrossRef]
- Pointing, S. Feasibility of bioremediation by white-rot fungi. Appl. Microbiol. Biotechnol. 2001, 57, 20–33. [Google Scholar]
- Baker, M.A.M.; Mead, J. Thermoplastics. In Handbook of Plastics, Elastomers and Composites, 4th ed.; Harper, C.A., Ed.; McGraw-Hill: New York, NY, USA, 2002; pp. 1–90. [Google Scholar]
- Karian, H. Handbook of Polypropylene and Polypropylene Composites, Revised and Expanded; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Khoironi, A.; Hadiyanto, H.; Anggoro, S.; Sudarno, S. Evaluation of polypropylene plastic degradation and microplastic identification in sediments at Tambak Lorok coastal area, Semarang. Indones. Mar. Pollut. Bull. 2020, 151, 110868. [Google Scholar] [CrossRef]
- Zheng, Y.; Yanful, E.K. A review of plastic waste degradation. Crit. Rev. Biotechnol. 2005, 25, 243–250. [Google Scholar] [CrossRef]
- Alariqi, S.A.S.; Kumar, A.P.; Rao, B.S.M.; Singh, R.P. Biodegradation of γ-sterilised biomedical polyolefins under composting and fungal culture environments. Polym. Degrad. Stab. 2006, 91, 1105–1116. [Google Scholar] [CrossRef]
- Pandey, J.K.; Singh, R.P. UV-irradiated biodegradability of ethylene-propylene copolymers, LDPE, and I-PP in composting culture environments. Biomacromolecules 2001, 2, 880–885. [Google Scholar] [CrossRef]
- Cacciari, I.; Quatrini, P.; Zirletta, G.; Mincione, E.; Vinciguerra, V.; Lupattelli, P.; Sermanni, G.G. Isotactic polypropylene biodegradation by a microbial community: Physicochemical characterization of metabolites produced. Appl. Environ. Microbiol. 1993, 59, 3695–3700. [Google Scholar] [CrossRef]
- Jeyakumar, D.; Chirsteen Doble, M. Synergistic effects of pretreatment and blending on fungi mediated biodegradation of polypropylenes. Bioresour. Technol. 2013, 148, 78–85. [Google Scholar] [CrossRef]
- Butnaru, E.; Darie-Niţă, R.N.; Zaharescu, T.; Balaeş, T.; Tănase, C.; Hitruc, G.; Doroftei, F.; Vasile, C. Gamma irradiation assisted fungal degradation of the polypropylene/biomass composites. Radiat. Phys. Chem. 2016, 125, 134–144. [Google Scholar] [CrossRef]
- Sheik, S.; Chandrashekar, K.R.; Swaroop, K.; Somashekarappa, H.M. Biodegradation of gamma irradiated low density polyethylene and polypropylene by endophytic fungi. Int. Biodeterior. Biodegrad. 2015, 105, 21–29. [Google Scholar] [CrossRef]
- Anjana, K.; Hinduja, M.; Sujitha, K.; Dharani, G. Review on plastic wastes in marine environment: Biodegradation and biotechnological solutions. Mar. Pollut. Bull. 2020, 150, 110733. [Google Scholar]
- Pires, J.P.; Miranda, G.M.; de Souza, G.L.; Fraga, F.; da Silva Ramos, A.; de Araújo, G.E.; de Lima, J.E.A. Investigation of degradation of polypropylene in soil using an enzymatic additive. Iran Polym. J. 2019, 28, 1045–1055. [Google Scholar] [CrossRef]
- Ho, B.T.; Roberts, T.K.; Lucas, S. An overview on biodegradation of polystyrene and modified polystyrene: The microbial approach. Crit. Rev. Biotechnol. 2018, 38, 308–320. [Google Scholar] [CrossRef]
- Krueger, M.C.; Seiwert, B.; Prager, A.; Zhang, S.; Abel, B.; Harms, H.; Schlosser, D. Degradation of polystyrene and selected analogues by biological Fenton chemistry approaches: Opportunities and limitations. Chemosphere 2017, 173, 520–528. [Google Scholar] [CrossRef]
- Goldman, A.S. Carbon–carbon bonds get a break. Nature 2010, 463, 435–436. [Google Scholar] [CrossRef]
- Atiq, N. Biodegradability of Synthetic Plastics Polystyrene and Styrofoam by Fungal Isolates. Ph.D. Thesis, Quaid-i-Azam University, Islamabad, Pakistan, 2011. [Google Scholar]
- Tian, L.; Kolvenbach, B.; Corvini, N.; Wang, S.; Tavanaie, N.; Wang, L.; Ma, Y.; Scheu, S.; Corvini, P.F.X.; Ji, R. Mineralisation of 14C-labelled polystyrene plastics by Penicillium variabile after ozonation pre-treatment. New Biotechnol. 2017, 38, 101–105. [Google Scholar] [CrossRef]
- Motta, O.; Proto, A.; de Carlo, F.; De Caro, F.; Santoro, E.; Brunetti, L.; Capunzo, M. Utilization of chemically oxidized polystyrene as co-substrate by filamentous fungi. Int. J. Hyg. Environ. Health 2009, 212, 61–66. [Google Scholar] [CrossRef]
- Milstein, O.; Gersonde, R.; Huttermann, A.; Chen, M.J.; Meister, J.J. Fungal biodegradation of lignopolystyrene graft copolymers. Appl. Environ. Microbiol. 1992, 58, 3225–3232. [Google Scholar] [CrossRef]
- Tahir, L.; Ali, M.I.; Zia, M.; Atiq, N.; Hasan, F.; Ahmed, S. Production and Characterization of Esterase in Lantinus tigrinus for Degradation of Polystyrene. Pol. J. Microbiol. 2013, 62, 101–108. [Google Scholar] [CrossRef]
- Datta, S.; Christena, L.R.; Rajaram, Y.R.S. Enzyme immobilization: An overview on techniques and support materials. 3 Biotech 2013, 3, 1–9. [Google Scholar] [CrossRef]
- Samak, N.A.; Jia, Y.; Sharshar, M.M.; Mu, T.; Yang, M.; Peh, S.; Xing, J. Recent advances in biocatalysts engineering for polyethylene terephthalate plastic waste green recycling. Environ. Int. 2020, 145, 106144. [Google Scholar] [CrossRef]
- Hegde, K.; Veeranki, V.D. Studies on immobilization of cutinases from Thermobifida fusca on glutaraldehyde activated chitosan beads. Biotechnol. J. Int. 2014, 4, 1049–1063. [Google Scholar] [CrossRef]
- Barth, M.; Honak, A.; Oeser, T.; Wei, R.; Belisário-Ferrari, M.R.; Then, J.; Schmidt, J.; Zimmermann, W. A dual enzyme system composed of a polyester hydrolase and a carboxylesterase enhances the biocatalytic degradation of polyethylene terephthalate films. Biotechnol. J. 2016, 11, 1082–1087. [Google Scholar] [CrossRef]
- Pellis, A.; Vastano, M.; Quartinello, F.; Herrero Acero, E.; Guebitz, G.M. His-Tag Immobilization of Cutinase 1 From Thermobifida cellulosilytica for Solvent-Free Synthesis of Polyesters. Biotechnol. J. 2017, 12, 1700322. [Google Scholar] [CrossRef]
- Ahmaditabatabaei, S.; Kyazze, G.; Iqbal, H.; Keshavarz, T. Fungal Enzymes as Catalytic Tools for Polyethylene Terephthalate (PET) Degradation. J. Fungi 2021, 7, 931. [Google Scholar] [CrossRef]
- Su, A.; Shirke, A.; Baik, J.; Zou, Y.; Gross, R. Immobilized cutinases: Preparation, solvent tolerance and thermal stability. Enzym. Microb. Technol. 2018, 116, 33–40. [Google Scholar] [CrossRef]
- Aslam, S.; Asgher, M.; Khan, N.A.; Bilal, M. Immobilization of Pleurotus nebrodensis WC 850 laccase on glutaraldehyde cross-linked chitosan beads for enhanced biocatalytic degradation of textile dyes. J. Water Process Eng. 2021, 40, 101971. [Google Scholar] [CrossRef]
- Imam, A.; Suman, S.K.; Singh, R.; Vempatapu, B.P.; Ray, A.; Kanaujia, P.K. Application of laccase immobilized rice straw biochar for anthracene degradation. Environ. Pollut. 2021, 268, 115827. [Google Scholar] [CrossRef]
- Gao, Y.; Truong, Y.B.; Cacioli, P.; Butler, P.; Kyratzis, I.L. Bioremediation of pesticide contaminated water using an organophosphate degrading enzyme immobilized on nonwoven polyester textiles. Enzym. Microb. Technol. 2014, 54, 38–44. [Google Scholar] [CrossRef]
- Jaiswal, S.; Sharma, B.; Shukla, P. Integrated approaches in microbial degradation of plastics. Environ. Technol. Innov. 2020, 17, 100567. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Vo, D.V.N.; Jeevanantham, S.; Karishma, S.; Yaashikaa, P.R. A review on catalytic-enzyme degradation of toxic environmental pollutants: Microbial enzymes. J. Hazard. Mater. 2021, 419, 126451. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, D.; Wei, N. Enzyme discovery and engineering for sustainable plastic recycling. Trends Biotechnol. 2022, 40, 22–37. [Google Scholar] [CrossRef]
- Tournier, V.; Topham, C.M.; Gilles, A.; David, B.; Folgoas, C.; Moya-Leclair, E.; Kamionka, E.; Desrousseaux, M.-L.; Texier, H.; Gavalda, S.; et al. An engineered PET depolymerase to break down and recycle plastic bottles. Nature 2020, 580, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Son, H.F.; Cho, I.J.; Joo, S.; Seo, H.; Sagong, H.Y.; Choi, S.Y.; Lee, S.Y.; Kim, K.J. Rational protein engineering of thermo-stable PETase from Ideonella sakaiensis for highly efficient PET degradation. ACS Catal. 2019, 9, 3519–3526. [Google Scholar] [CrossRef]
- Austin, H.P.; Allen, M.D.; Donohoe, B.S.; Rorrer, N.A.; Kearns, F.L.; Silveira, R.L.; Pollard, B.C.; Dominick, G.; Duman, R.; El Omari, K.; et al. Characterization and engineering of a plastic-degrading aromatic polyesterase. Proc. Natl. Acad. Sci. USA 2018, 115, E4350–E4357. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yao, M.; Li, B.; Ding, M.; He, B.; Chen, S.; Zhou, X.; Yuan, Y. Enhanced poly (ethylene terephthalate) hydrolase activity by protein engineering. Engineering 2018, 4, 888–893. [Google Scholar] [CrossRef]
- Silva, C.; Da, S.; Silva, N.; Matamá, T.; Araújo, R.; Martins, M.; Chen, S.; Chen, J.; Wu, J.; Casal, M.; et al. Engineered Thermobifida fusca cutinase with increased activity on polyester substrates. Biotechnol. J. 2011, 6, 230–1239. [Google Scholar] [CrossRef]
- Herrero Acero, E.; Ribitsch, D.; Dellacher, A.; Zitzenbacher, S.; Marold, A.; Steinkellner, G.; Gruber, K.; Schwab, H.; Guebitz, G.M. Surface engineering of a cutinase from Thermobifida cellulosilytica for improved polyester hydrolysis. Biotechnol. Bioeng. 2013, 110, 2581–2590. [Google Scholar] [CrossRef]
- Biundo, A.; Ribitsch, D.; Steinkellner, G.; Gruber, K.; Guebitz, G.M. Polyester hydrolysis is enhanced by a truncated esterase: Less is more. Biotechnol. J. 2017, 12, 1600450. [Google Scholar] [CrossRef]
- Janatunaim, R.Z.; Fibriani, A. Construction and Cloning of Plastic-degrading Recombinant Enzymes (MHETase). Recent Pat. Biotechnol. 2020, 14, 229–234. [Google Scholar] [CrossRef]
- Huy, N.D.; Le, N.T.M.; Chew, K.W.; Park, S.M.; Show, P.L. Characterization of a recombinant laccase from Fusarium oxysporum HUIB02 for biochemical application on dyes removal. Biochem. Eng. J. 2021, 168, 107958. [Google Scholar] [CrossRef]
- Zhuo, R.; He, F.; Zhang, X.; Yang, Y. Characterization of a yeast recombinant laccase rLAC-EN3-1 and its application in decolorizing synthetic dye with the coexistence of metal ions and organic solvents. Biochem. Eng. J. 2015, 93, 63–72. [Google Scholar] [CrossRef]
- Liu, S.; Xu, X.; Kang, Y.; Xiao, Y.; Liu, H. Degradation and detoxification of azo dyes with recombinant ligninolytic enzymes from Aspergillus sp. with secretory overexpression in Pichia pastoris. Royal. Soc. Open Sci. 2020, 7, 200688. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Tang, X.; Zhang, H.; Chen, W.; Chen, Y.Q. Molecular tools for gene manipulation in filamentous fungi. Appl. Microbiol. Biotechnol. 2017, 101, 8063–8075. [Google Scholar] [CrossRef]
| Enzyme | Enzyme Commission (EC) Number | Activity | Reaction Scheme |
|---|---|---|---|
| Laccases | 1.10.3.2 | Oxidoreductases | 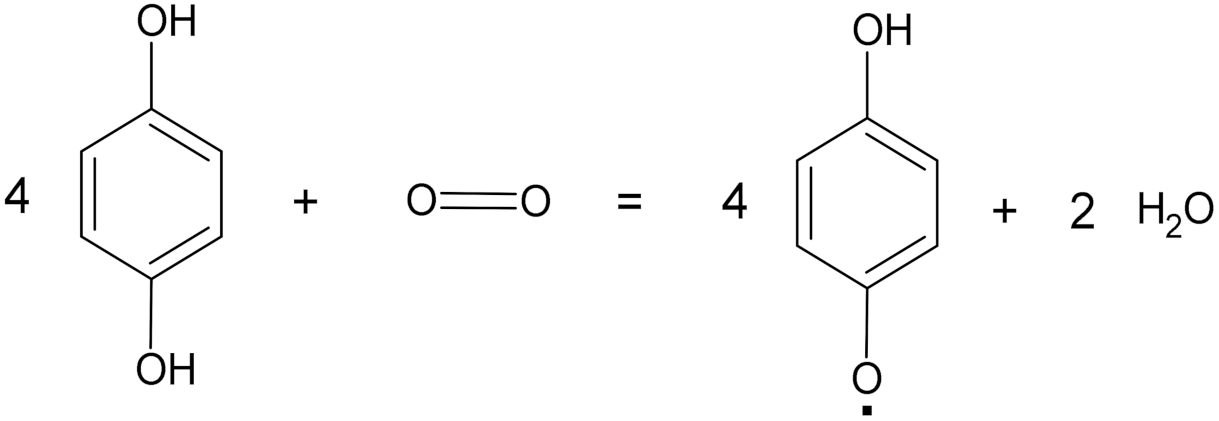 4 benzenediol + O2 = 4 benzosemiquinone + 2 water |
| Manganese peroxidases | 1.11.1.13 | Oxidoreductases |  |
| Lignin peroxidases | 1.11.1.14 | Oxidoreductases | 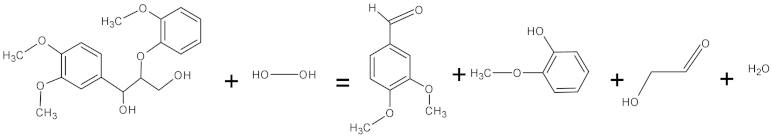 1-(3,4-dimethoxyphenyl)-2-(2-methoxyphenoxy)propane-1,3-diol + hydrogen peroxide = 3,4-dimethoxybenzaldehyde + 2-methoxyphenol + glycolaldehyde + water |
 2 (3,4-dimethoxyphenyl)methanol + hydrogen peroxide = 2 (3,4-dimethoxyphenyl)methanol radical + 2 water | |||
| Versatile peroxidase | 1.11.1.16 | Oxidoreductases |  1-(4-hydroxy-3-methoxyphenyl)-2-(2-methoxyphenoxy)propane-1,3-diol + hydrogen peroxide = 4-hydroxy-3-methoxybenzaldehyde + 2-methoxyphenol + glycolaldehyde + water |
| Versatile peroxidase | 1.11.1.16 | Oxidoreductases |  |
| Lipases | 3.1.1.3 | Hydrolases | 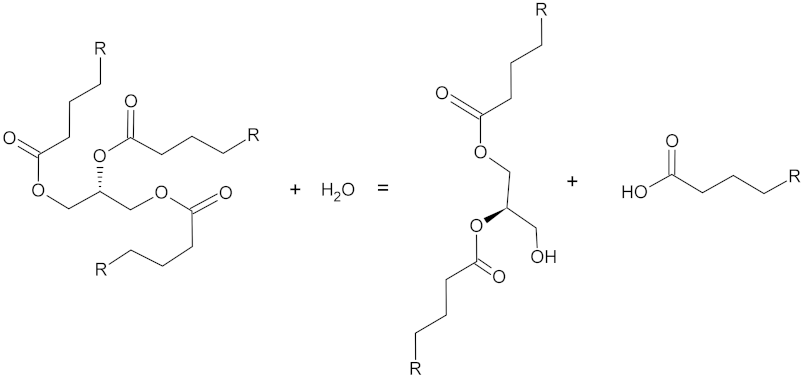 Triacylglycerol + water = diacylglycerol + a carboxylate |
| Cutinases | 3.1.1.74 | Hydrolases | 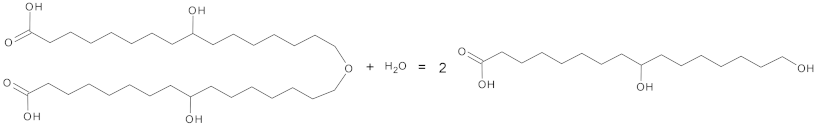 Cutin + water = 2 cutin monomers |
| Name | Plastic Demand in Europe | Molecular Weight (Daltons) | Structure |
|---|---|---|---|
| High-density polyethylene (HDPE) | 12.9% | 100,000–250,000 |  |
| Low-density polyethylene (LDPE) | 17.4% | 40,000 |  |
| Name | Plastics Demand in Europe | Molecular Weight (Daltons) | Structure |
|---|---|---|---|
| Polyethylene terephthalate (PET) | 8.4% | 20,000–50,000 |  |
| Name | Structure |
|---|---|
| Polyurethane (PU) | 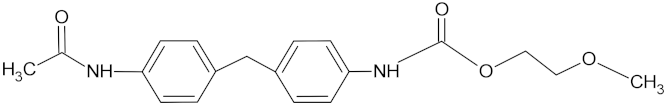 |
| Ether-PU |  |
| Ester-PU |  |
| Name | Plastics Demand in Europe | Structure |
|---|---|---|
| Polyvinylchloride (PVC) | 9.6% | 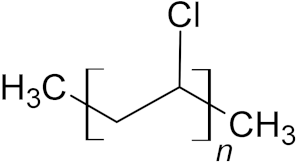 |
| Name | Plastics demand in Europe | Molecular Weight (Daltons) | Structure |
|---|---|---|---|
| Polypropylene (PP) | 19.7% | 10,000–40,000 | 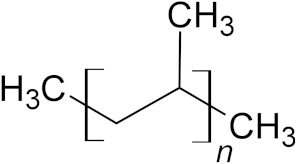 |
| Name | Plastics Demand in Europe | Molecular Weight (Daltons) | Structure |
|---|---|---|---|
| Polystyrene (PS) | 6.1% | 150,000–400,000 |  |
| Enzymes | Fungal Species | Plastic Polymers | References |
|---|---|---|---|
| Cutinase HiC | Humicola insolens | PET | [148,152,157] |
| Cutinase FoCut5a | Fusarium oxysporum | PET | [94,144] |
| Cutinase FsC | Fusarium solani pisi | PET | [43,148,151,152,153,154,155,159,160] |
| Cutinase TtcutA | Thielavia terrestris CAU709 | PUR | [193] |
| Esterases | Aspergillus fumigatus S45 | PUR | [189] |
| Aspergillus terreus | PUR | [186] | |
| Aspergillus tubingenesis | PUR | [192] | |
| Chaetomium globosum | PUR | [186] | |
| Cladosporium pseudocladosporioides T1.PL.1 | PUR | [47] | |
| Curvularia senegalensis | PUR | [185] | |
| Laccases | Aspergillus flavus PEDX3 | PE | [38] |
| Pleurotus ostreatus | PE | [48] | |
| Trichoderma harzianum | PE | [120] | |
| Laccase-like multicopper oxidases (LMCOs) | Aspergillus flavus PEDX3 | PE | [38] |
| Laccase-mediator system (LMS) | Trametes versicolor IFO 6482 | PE | [128] |
| Lipases | Aspergillus oryzae CCUG 33812 | PET | [143] |
| Aspergillus tubingenesis | PUR | [192] | |
| Candida antarctica (CALB) | PET | [140,162] | |
| Candida antarctica | PUR | [104] | |
| Candida rugosa | PUR | [191] | |
| Pichia pastoris | PET | [163] | |
| Peroxidases | Trichoderma harzianum | PE | [120] |
| Lignin peroxidases | Pleurotus ostreatus | PE | [48] |
| Manganese peroxidases | Phanerochaete chrysosporium ME-446 | PE | [115] |
| Pleurotus ostreatus | PE | [48] | |
| Polyesterases | Beauveria brongniartii | PET | [164] |
| Penicillium citrinum | PET | [165] | |
| Serine hydrolase-like enzyme | Pestalotiopsis microspora E2712A | PUR | [190] |
| Urethane hydrolases | Aspergillus terreus | PUR | [186] |
| Chaetomium globosum | PUR | [186] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Temporiti, M.E.E.; Nicola, L.; Nielsen, E.; Tosi, S. Fungal Enzymes Involved in Plastics Biodegradation. Microorganisms 2022, 10, 1180. https://doi.org/10.3390/microorganisms10061180
Temporiti MEE, Nicola L, Nielsen E, Tosi S. Fungal Enzymes Involved in Plastics Biodegradation. Microorganisms. 2022; 10(6):1180. https://doi.org/10.3390/microorganisms10061180
Chicago/Turabian StyleTemporiti, Marta Elisabetta Eleonora, Lidia Nicola, Erik Nielsen, and Solveig Tosi. 2022. "Fungal Enzymes Involved in Plastics Biodegradation" Microorganisms 10, no. 6: 1180. https://doi.org/10.3390/microorganisms10061180
APA StyleTemporiti, M. E. E., Nicola, L., Nielsen, E., & Tosi, S. (2022). Fungal Enzymes Involved in Plastics Biodegradation. Microorganisms, 10(6), 1180. https://doi.org/10.3390/microorganisms10061180







