Reactivity of a Recombinant Esterase from Thermus thermophilus HB27 in Aqueous and Organic Media
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cloning and Production
2.3. Analytical Methods
2.4. Effect of Several Additives on Enzyme Activity and Temperature on Enzyme Activity and Stability
2.5. Acyl Chain Length Preference
2.6. Interfacial Activation
2.7. Synthesis of (R,S) Ibuprofen Methyl- Propyl- Butyl Esters
2.8. General Procedure for the Enzymatic Hydrolysis of Esters
2.9. General Procedure for the Enzymatic Acetylation of Alcohols and Amines
2.10. HPLC-MS Analysis
3. Results and Discussion
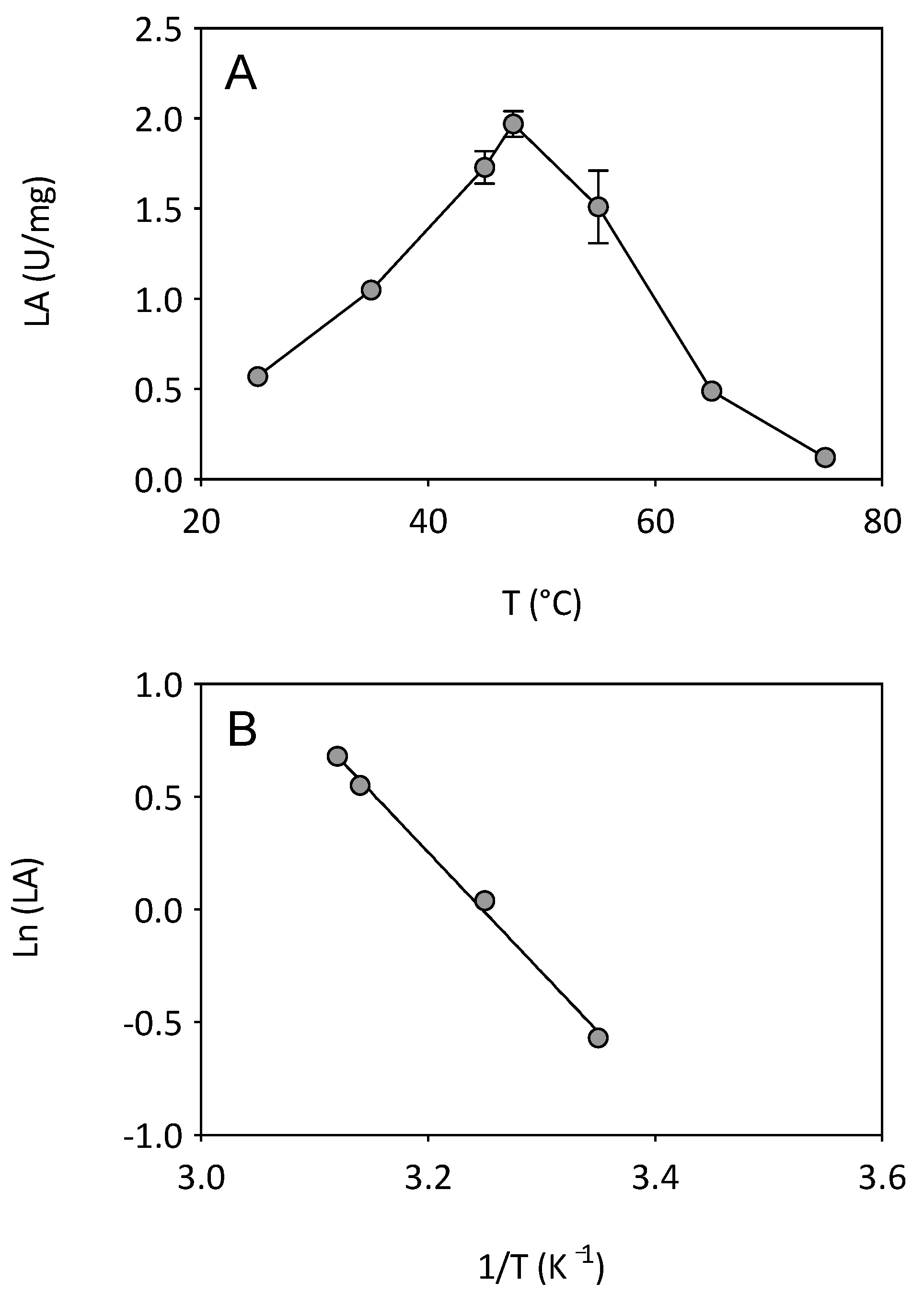
3.1. Effect of Temperature on Enzyme Activity and Stability
3.2. Effect of Detergents and Solvents
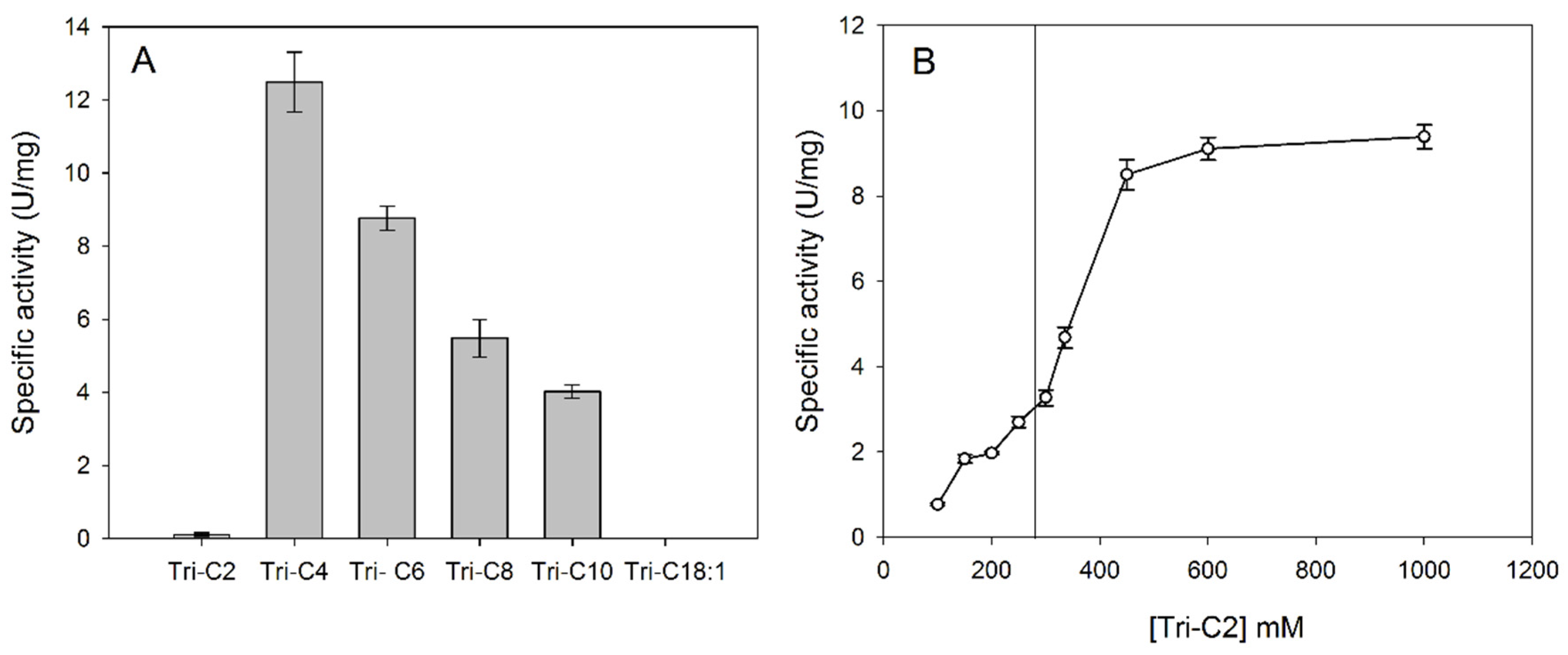
3.3. Substrate Specificity in Aqueous Solution: Reactivity on pNP Esters and Triacylglycerols
3.4. Biocatalytic Performance on Industrial/Benchmark Reactions
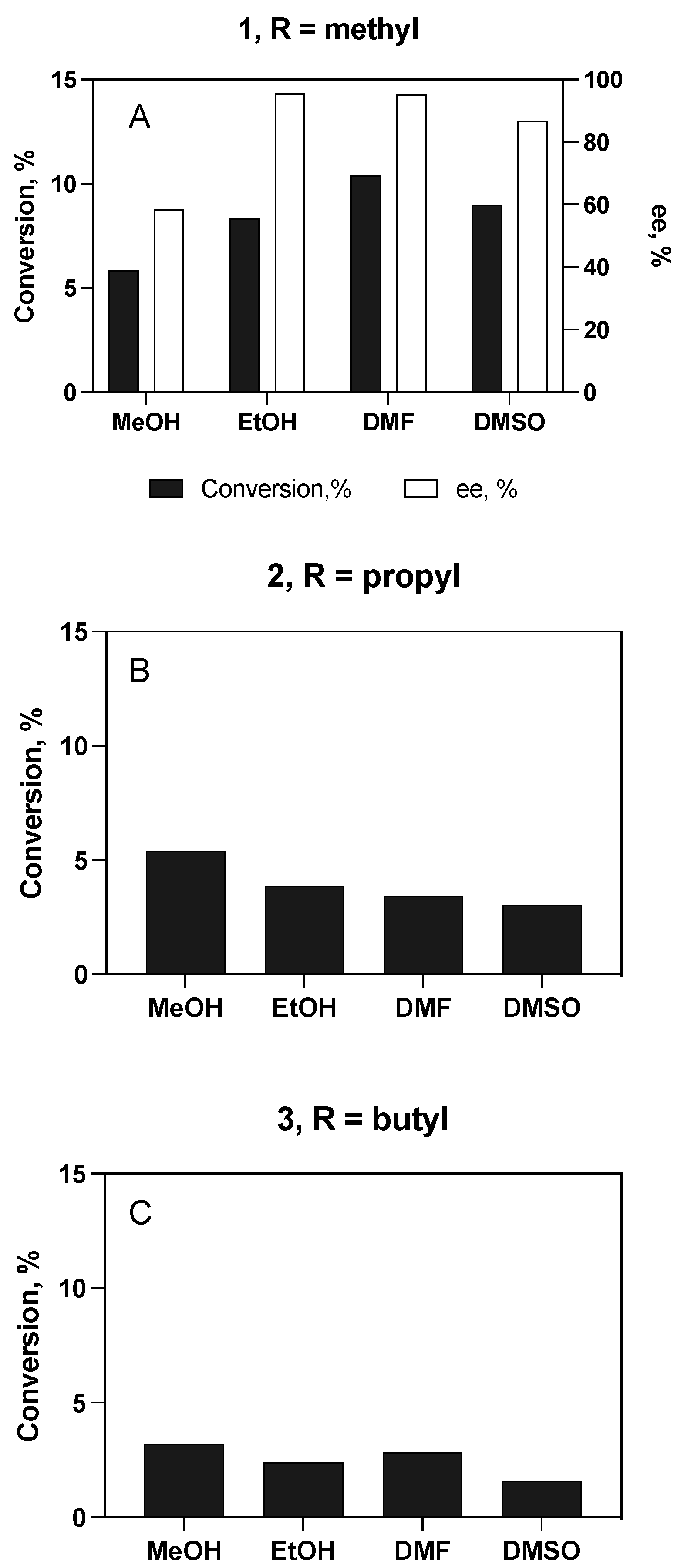
3.4.1. Enantioselective Hydrolysis of Racemic Ibuprofen Esters
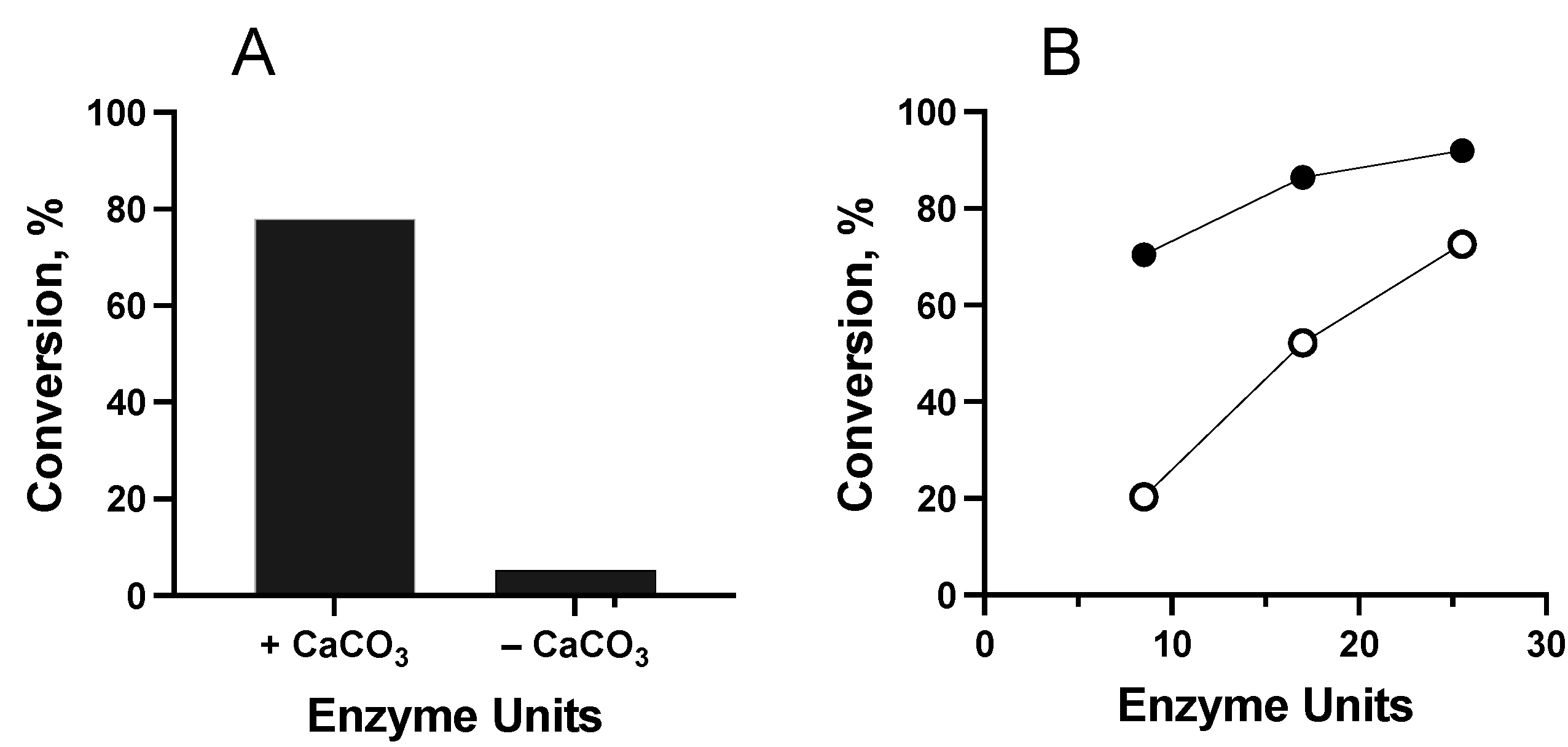
Substrate and Cosolvent Effect
Effect of Enzyme Concentration
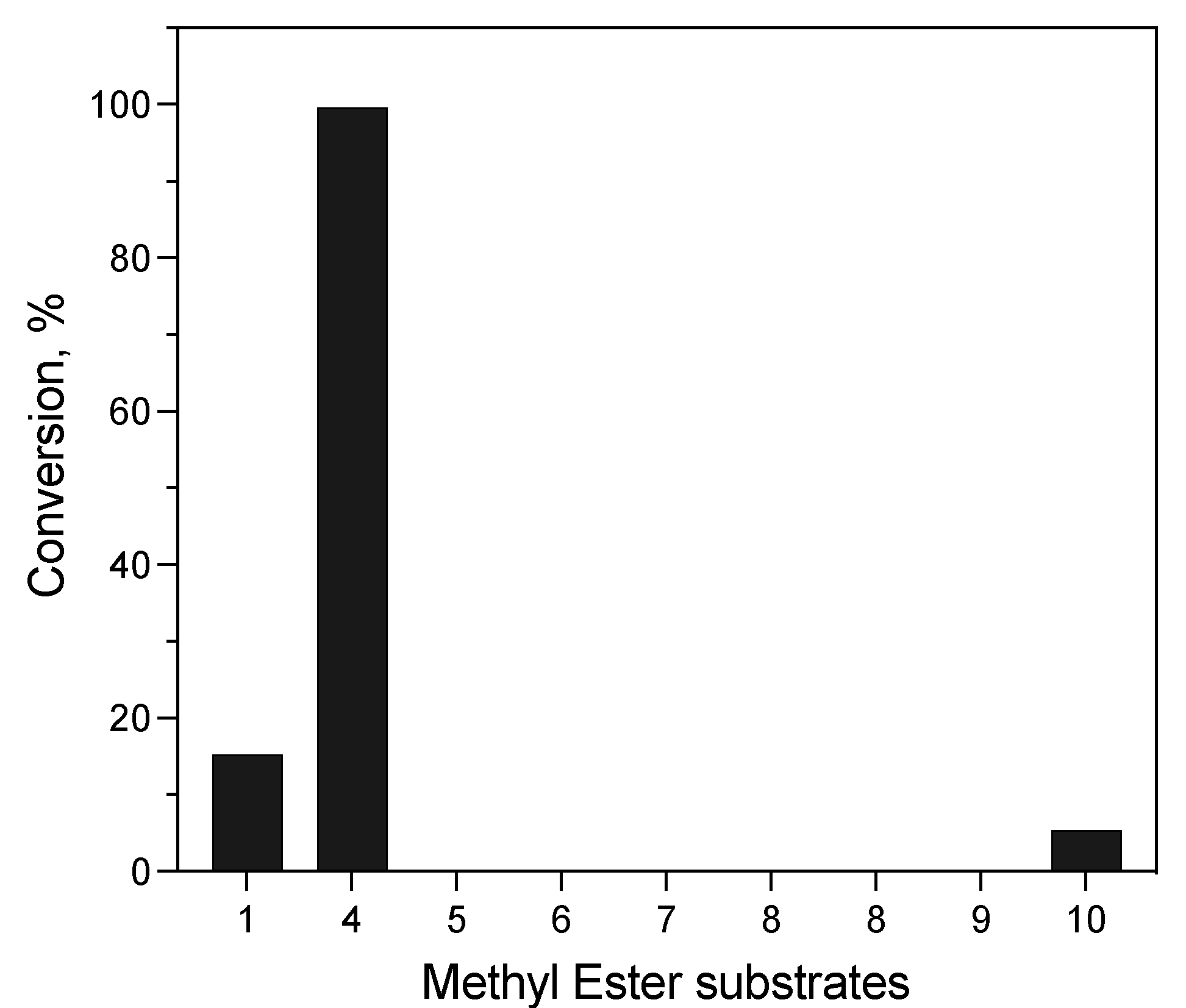
Hydrolysis of Structurally Diverse Carboxylic Esters
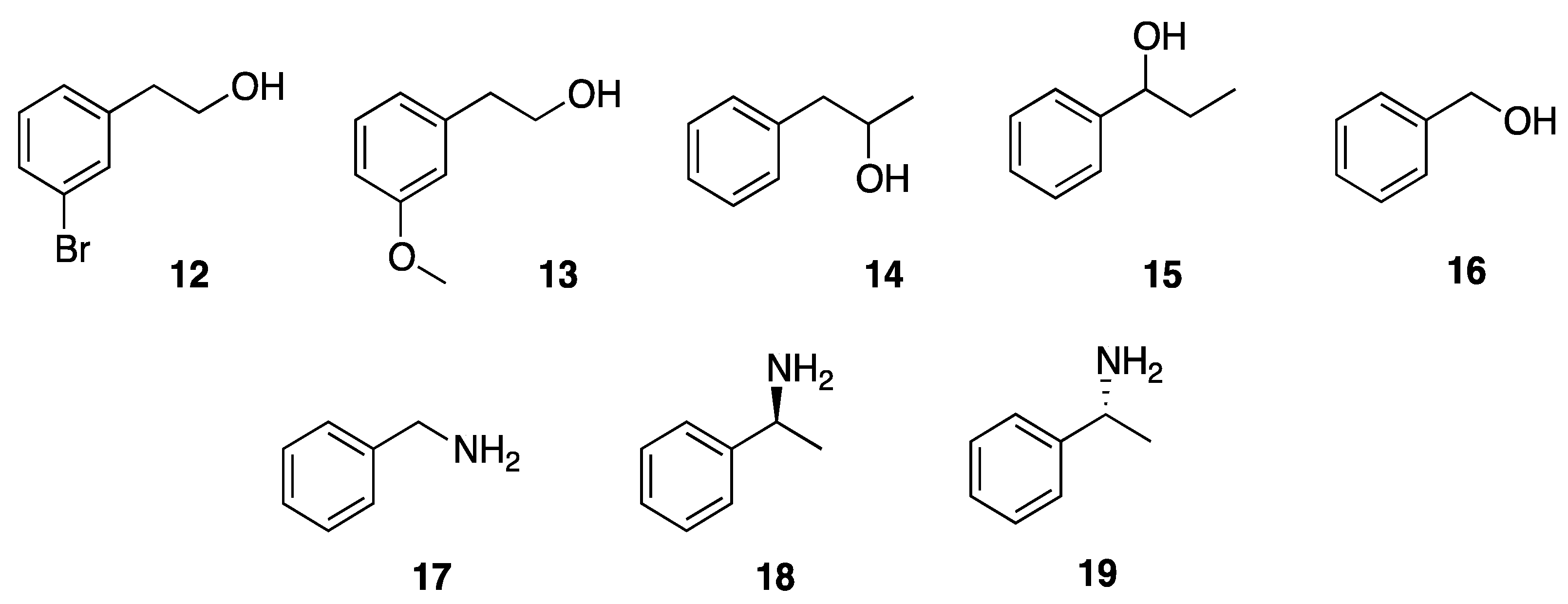
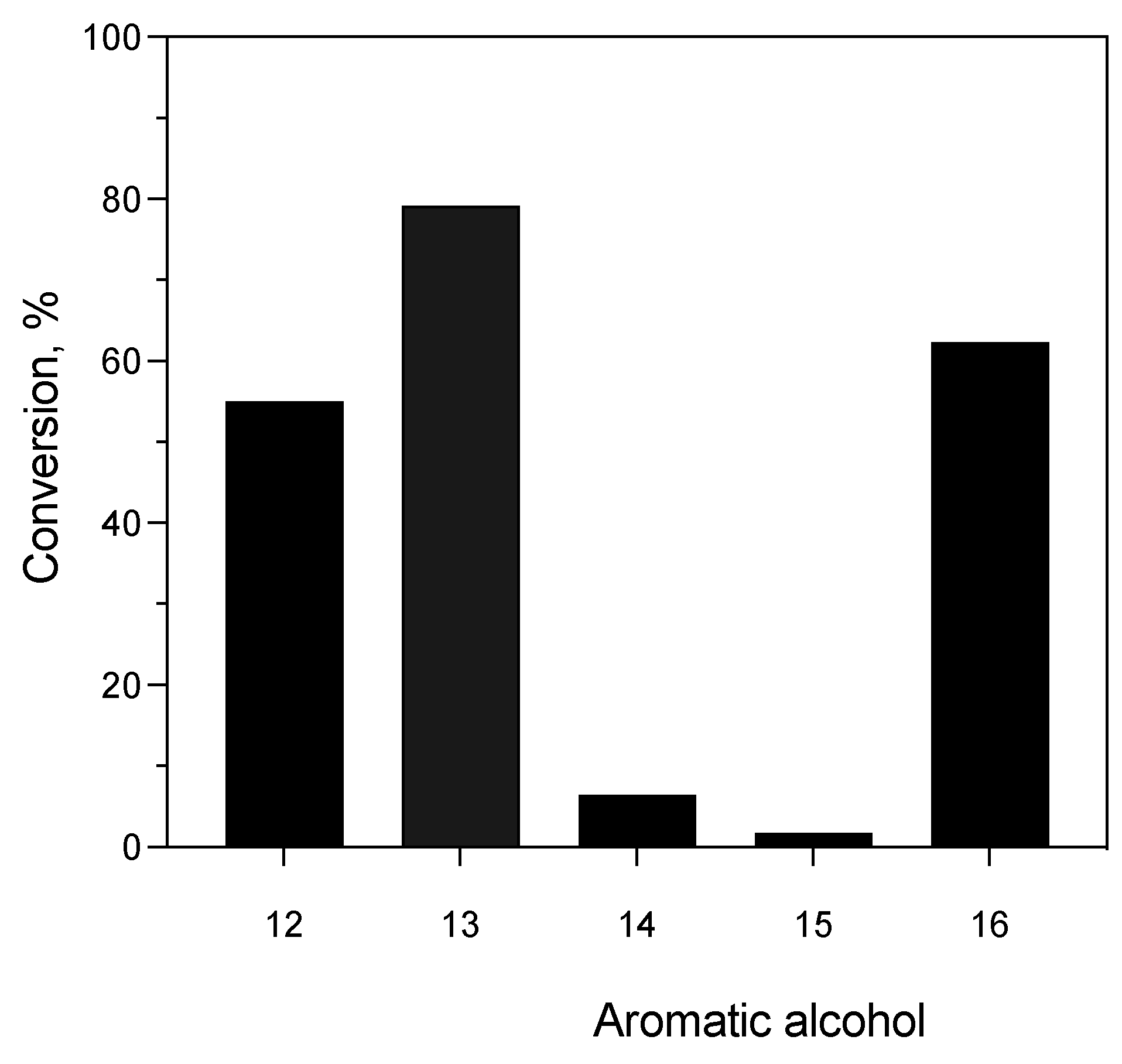
3.4.2. Enzyme-Catalyzed Acetylation Reaction
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bornscheuer, U.T. Microbial carboxyl esterases: Classification, properties and application in biocatalysis. FEMS Microbiol. Rev. 2002, 26, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Liese, A.; Seelbach, K.; Wandrey, C. Industrial Biotransformations, 2nd ed.; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Panke, S.; Held, M.; Wubbolts, M. Trends and innovations in industrial biocatalysis for the production of fine chemicals. Curr. Opin. Biotechnol. 2004, 15, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Verger, R. Interfacial activation of lipases: Facts and artifacts. Trends Biotechnol. 1997, 15, 32–38. [Google Scholar] [CrossRef]
- Panda, T.; Gowrishankar, B.S. Production and applications of esterases. Appl. Microbiol. Biotechnol. 2005, 67, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Bornscheuer, U.T.; Kazlauskas, R.J. Hydrolases in Organic Synthesis: Regio- and Stereoselective Biotransformations, 2nd ed.; Weinheim Wiley: Weinheim, Germany, 2006; ISBN 3527310290. [Google Scholar]
- Konarzycka-Bessler, M.; Jaeger, K.E. Select the best: Novel biocatalysts for industrial applications. Trends Biotechnol. 2006, 24, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Antranikian, G. Industrial relevance of thermophiles and their enzymes. In Thermophiles: Biology and Technology at High Temperatures; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- González-González, R.; Fuciños, P.; Rúa, M.L. An Overview on Extremophilic Esterases; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 9783319546841. [Google Scholar]
- Han, H.; Ling, Z.; Khan, A.; Virk, A.K.; Kulshrestha, S.; Li, X. Improvements of thermophilic enzymes: From genetic modifications to applications. Bioresour. Technol. 2019, 279, 350–361. [Google Scholar] [CrossRef]
- Kumar, S.; Dangi, A.K.; Shukla, P.; Baishya, D.; Khare, S.K. Thermozymes: Adaptive strategies and tools for their biotechnological applications. Bioresour. Technol. 2019, 278, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Singhal, P.; Singh, H.; Damle, D.; Sharma, A.K. Insight into thermophiles and their wide-spectrum applications. 3 Biotech 2016, 6, 81. [Google Scholar] [CrossRef]
- Rekadwad, B.; Gonzalez, J.M. Multidisciplinary involvement and potential of thermophiles. Folia Microbiol. 2019, 64, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Memarpoor-Yazdi, M.; Karbalaei-Heidari, H.R.; Doroodmand, M.M. Enantioselective hydrolysis of ibuprofen ethyl ester by a thermophilic immobilized lipase, ELT, from Rhodothermus marinus. Biochem. Eng. J. 2018, 130, 55–65. [Google Scholar] [CrossRef]
- Ducret, A.; Trani, M.; Lortie, R. Lipase-catalyzed enantioselective esterification of ibuprofen in organic solvents under controlled water activity. Enzym. Microb. Technol. 1998, 22, 212–216. [Google Scholar] [CrossRef]
- Chávez-Flores, D.; Salvador, J.M. Commercially viable resolution of ibuprofen. Biotechnol. J. 2009, 4, 1222–1224. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.T.; Xun, E.N.; Wang, J.X.; Wang, R.; Wei, X.F.; Wang, L.; Wang, Z. Enantioselective esterification of ibuprofen by a novel thermophilic Biocatalyst: APE1547. Biotechnol. Bioprocess Eng. 2011, 16, 638–644. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.B. Thermostable esterase from a thermoacidophilic archaeon: Purification and characterization for enzymatic resolution of a chiral compound. Biosci. Biotechnol. Biochem. 2004, 68, 2289–2298. [Google Scholar] [CrossRef] [PubMed]
- López, G.; Chow, J.; Bongen, P.; Lauinger, B.; Pietruszka, J.; Streit, W.R.; Baena, S. A novel thermoalkalostable esterase from Acidicaldus sp. strain USBA-GBX-499 with enantioselectivity isolated from an acidic hot springs of Colombian Andes. Appl. Microbiol. Biotechnol. 2014, 98, 8603–8616. [Google Scholar] [CrossRef] [PubMed]
- Fuciños, P.; Pastrana, L.; Sanromán, A.; Longo, M.A.; Hermoso, J.A.; Rúa, M.L. An esterase from Thermus thermophilus HB27 with hyper-thermoalkalophilic properties: Purification, characterisation and structural modelling. J. Mol. Catal. B Enzym. 2011, 70, 127–137. [Google Scholar] [CrossRef]
- Fuciños, P.; Domínguez, A.; Sanromán, M.A.; Longo, M.A.; Rúa, M.L.; Pastrana, L. Production of thermostable lipolytic activity by Thermus species. Biotechnol. Prog. 2005, 21, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Fuciños, P.; Atanes, E.; López-López, O.; Cerdán, M.E.; González-Siso, M.I.; Pastrana, L.; Rúa, M.L. Production and characterization of two N-terminal truncated esterases from Thermus thermophilus HB27 in a mesophilic yeast: Effect of N-terminus in thermal activity and stability. Protein Expr. Purif. 2011, 78, 120–130. [Google Scholar] [CrossRef] [PubMed]
- López-López, O.; Fuciños, P.; Pastrana, L.; Rúa, M.L.; Cerdán, M.E.; González-Siso, M.I. Heterologous expression of an esterase from Thermus thermophilus HB27 in Saccharomyces cerevisiae. J. Biotechnol. 2010, 145, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S.N.; Abrahão-Neto, J.; Cerdán, M.E.; Gombert, A.K.; González-Siso, M.I. Heterologous expression of a thermophilic esterase in Kluyveromyces yeasts. Appl. Microbiol. Biotechnol. 2011, 89, 375–385. [Google Scholar] [CrossRef]
- Fuciños, P.; Atanes, E.; López-López, O.; Solaroli, M.; Cerdán, M.E.; González-Siso, M.I.; Pastrana, L.; Rúa, M.L. Cloning, expression, purification and characterization of an oligomeric His-tagged thermophilic esterase from Thermus thermophilus HB27. Process Biochem. 2014, 49, 927–935. [Google Scholar] [CrossRef]
- López-López, O.; Cerdán, M.E.; González-Siso, M.I. Thermus thermophilus as a source of thermostable lipolytic enzymes. Microorganisms 2015, 3, 792–808. [Google Scholar] [CrossRef]
- Fuciños, P.; Abadín, C.M.; Sanromán, A.; Longo, M.A.; Pastrana, L.; Rúa, M.L. Identification of extracellular lipases/esterases produced by Thermus thermophilus HB27: Partial purification and preliminary biochemical characterisation. J. Biotechnol. 2005, 117, 233–241. [Google Scholar] [CrossRef]
- Pernas, M.A.; López, C.; Rúa, M.L.; Hermoso, J. Influence of the conformational flexibility on the kinetics and dimerisation process of two Candida rugosa lipase isoenzymes. FEBS Lett. 2001, 501, 87–91. [Google Scholar] [CrossRef]
- Kettner, C.; Obermeyer, G.; Bertl, A.; Flügge, U. Inhibition of the yeast V-type ATPase by cytosolic ADP. FEBS Lett. 2003, 535, 119–124. [Google Scholar] [CrossRef][Green Version]
- Ewis, H.; Abdelal, A.; Lu, C. Molecular cloning and characterization of two thermostable carboxyl esterases from Geobacillus stearothermophilus. Gene 2004, 329, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Xue, Y.; Zhou, C.; Tao, J.; Li, G.; Lu, J.R.; Ma, Y. A thermostable esterase from Thermoanaerobacter tengcongensis opening up a new family of bacterial lipolytic enzymes. Biochim. Biophys. Acta Proteins Proteom. 2011, 1814, 1695–1702. [Google Scholar] [CrossRef] [PubMed]
- Manco, G.; Adinolfi, E.; Pisani, F.M.; Ottolina, G.; Carrea, G.; Rossi, M. Overexpression and properties of a new thermophilic and thermostable esterase from Bacillus acidocaldarius with sequence similarity to hormone-sensitive lipase subfamily. Biochem. J. 1998, 332, 203–212. [Google Scholar] [CrossRef]
- Zieniuk, B.; Fabiszewska, A.; Białecka-Florjańczyk, E. Screening of solvents for favoring hydrolytic activity of Candida antarctica Lipase B. Bioprocess Biosyst. Eng. 2020, 43, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tan, T.; Zhang, H.; Feng, W. Analysis of the conformational stability and activity of Candida antarctica lipase B in organic solvents: Insight from molecular dynamics and quantum mechanics/simulations. J. Biol. Chem. 2010, 285, 28434–28441. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, K.E.; Dijkstra, B.W.; Reetz, M.T. Bacterial biocatalysts: Molecular biology, three-dimensional structures, and biotechnological applications of lipases. Annu. Rev. Microbiol. 1999, 53, 315–351. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.H.; Karanth, N. Lipases and lipase-catalyzed esterification reactions in nonaqueous media. Catal. Rev. Sci. Eng. 2002, 44, 499–591. [Google Scholar] [CrossRef]
- Ferrato, F.; Carriere, F.; Sarda, L.; Verger, R. A critical reevaluation of the phenomenon of interfacial activation. Methods Enzymol. 1997, 286, 327–347. [Google Scholar] [PubMed]
- Mancheño, J.M.; Pernas, M.A.; Martínez, M.J.; Ochoa, B.; Rúa, M.L.; Hermoso, J.A. Structural insights into the lipase/esterase behavior in the Candida rugosa lipases family: Crystal structure of the lipase 2 isoenzyme at 1.97 Å resolution. J. Mol. Biol. 2003, 332, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Fojan, P.; Jonson, P.H.; Petersen, M.T.; Petersen, S.B. What distinguishes an esterase from a lipase: A novel structural approach. Biochimie 2000, 82, 1033–1041. [Google Scholar] [CrossRef]
- Grochulski, P.; Li, Y.; Schrag, J.D.; Bouthillier, F.; Smith, P.; Harrison, D.; Rubin, B.; Cygler, M. Insights into interfacial activation from an open structure of Candida rugosa lipase. J. Biol. Chem. 1993, 268, 12843–12847. [Google Scholar] [CrossRef]
- Grochulski, P.; Li, Y.; Schrag, J.; Cygler, M. Two conformational states of Candida rugosa lipase. Protein Sci. 1994, 3, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Gao, B.; Zhang, L.; Lin, J.; Wang, X.; Wei, D. Template-based modeling of a psychrophilic lipase: Conformational changes, novel structural features and its application in predicting the enantioselectivity of lipase catalyzed transesterification of secondary alcohols. Biochim. Biophys. Acta Proteins Proteom. 2010, 1804, 2183–2190. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, F.; Babic, N.; Krauss, U.; Jaeger, K.-E. Classification of Lipolytic Enzymes from Bacteria. In Aerobic Utilization of Hydrocarbons, Oils, and Lipids; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Rúa, M.L.; Díaz-Mauriño, T.; Fernández, V.M.; Otero, C.; Ballesteros, A. Purification and characterization of two distinct lipases from Candida cylindracea. BBA Gen. Subj. 1993, 1156, 181–189. [Google Scholar] [CrossRef]
- Álvarez-Cao, M.E.; González, R.; Pernas, M.A.; Rúa, M.L. Contribution of the oligomeric state to the thermostability of isoenzyme 3 from Candida rugosa. Microorganisms 2018, 6, 108. [Google Scholar] [CrossRef]
- Martinelle, M.; Holmquist, M.; Hult, K. On the interfacial activation of Candida antarctica lipase A and B as compared with Humicola lanuginosa lipase. Biochim. Biophys. Acta (BBA)/Lipids Lipid Metab. 1995, 1258, 272–276. [Google Scholar] [CrossRef]
- Cajal, Y.; Svendsen, A.; Girona, V.; Patkar, S.A.; Alsina, M.A. Interfacial control of lid opening in Thermomyces lanuginosa lipase. Biochemistry 2000, 39, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Bofill, C.; Prim, N.; Mormeneo, M.; Manresa, A.; Pastor, F.I.J.; Diaz, P. Differential behaviour of Pseudomonas sp. 42A2 LipC, a lipase showing greater versatility than its counterpart LipA. Biochimie 2010, 92, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.D. Bacterial hormone-sensitive lipases (bHSLs): Emerging enzymes for biotechnological applications. J. Microbiol. Biotechnol. 2017, 27, 1907–1915. [Google Scholar] [CrossRef]
- Wang, Q.; Cen, Z.; Zhao, J. The survival mechanisms of thermophiles at high temperatures: An angle of omics. Physiology 2015, 30, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Miguel-Ruano, V.; Rivera, I.; Rajkovic, J.; Knapik, K.; Torrado, A.; Otero, J.M.; Beneventi, E.; Becerra, M.; Sánchez-Costa, M.; Hidalgo, A.; et al. Biochemical and Structural Characterization of a novel thermophilic esterase EstD11 provide catalytic insights for the HSL family. Comput. Struct. Biotechnol. J. 2021, 19, 1214–1232. [Google Scholar] [CrossRef]
- Hess, M.; Katzer, M.; Antranikian, G. Extremely thermostable esterases from the thermoacidophilic euryarchaeon Picrophilus torridus. Extremophiles 2008, 12, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Kakugawa, S.; Fushinobu, S.; Wakagi, T.; Shoun, H. Characterization of a thermostable carboxylesterase from the hyperthermophilic bacterium Thermotoga maritima. Appl. Microbiol. Biotechnol. 2007, 74, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.; Alquéres, S.; Larentis, A.; Rössle, S.; Cardoso, A.; Almeida, W.; Bisch, P.; Alves, T.; Martins, O. Cloning, expression, partial characterization and structural modeling of a novel esterase from Pyrococcus furiosus. Enzym. Microb. Technol. 2006, 39, 1128–1136. [Google Scholar] [CrossRef]
- Park, Y.J.; Choi, S.Y.; Lee, H.B. A carboxylesterase from the thermoacidophilic archaeon Sulfolobus solfataricus P1; purification, characterization, and expression. Biochim. Biophys. Acta Gen. Subj. 2006, 1760, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Feng, Y.; Ishikawa, K.; Ishida, H.; Ando, S.; Kosugi, Y.; Cao, S. Cloning, purification and properties of a hyperthermophilic esterase from archaeon Aeropyrum pernix K1. J. Mol. Catal. B Enzym. 2003, 24–25, 1–8. [Google Scholar] [CrossRef]
- Neugebauer, J.M. Detergents: An overview. Methods Enzymol. 1990, 182, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Xu, Y.; Yu, X. Purification and properties of a highly enantioselective l-menthyl acetate hydrolase from Burkholderia cepacia. J. Mol. Catal. B Enzym. 2009, 57, 27–33. [Google Scholar] [CrossRef]
- Glogauer, A.; Martini, V.P.; Faoro, H.; Couto, G.H.; Muller-Santos, M.; Monteiro, R.A.; Mitchell, D.A.; de Souza, E.M.; Pedrosa, F.O.; Krieger, N. Identification and characterization of a new true lipase isolated through metagenomic approach. Microb. Cell Factories 2011, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, K.K.; Kishen, S.; Mehta, A.; Sharma, A.; Gupta, R. Purification of high molecular weight thermotolerant esterase from Serratia sp. and its characterization. 3 Biotech 2021, 11, 308. [Google Scholar] [CrossRef] [PubMed]
 —) and 70 °C (—•—). The enzyme solution was incubated at different times between 0 and 7 h in a water bath. The pNP C12 substrate was assayed under the standard assay conditions, and residual activity was calculated; 100% KLEST-3S specific activity was 10.27 ± 0.30 U/mg.
—) and 70 °C (—•—). The enzyme solution was incubated at different times between 0 and 7 h in a water bath. The pNP C12 substrate was assayed under the standard assay conditions, and residual activity was calculated; 100% KLEST-3S specific activity was 10.27 ± 0.30 U/mg.
 —) and 70 °C (—•—). The enzyme solution was incubated at different times between 0 and 7 h in a water bath. The pNP C12 substrate was assayed under the standard assay conditions, and residual activity was calculated; 100% KLEST-3S specific activity was 10.27 ± 0.30 U/mg.
—) and 70 °C (—•—). The enzyme solution was incubated at different times between 0 and 7 h in a water bath. The pNP C12 substrate was assayed under the standard assay conditions, and residual activity was calculated; 100% KLEST-3S specific activity was 10.27 ± 0.30 U/mg.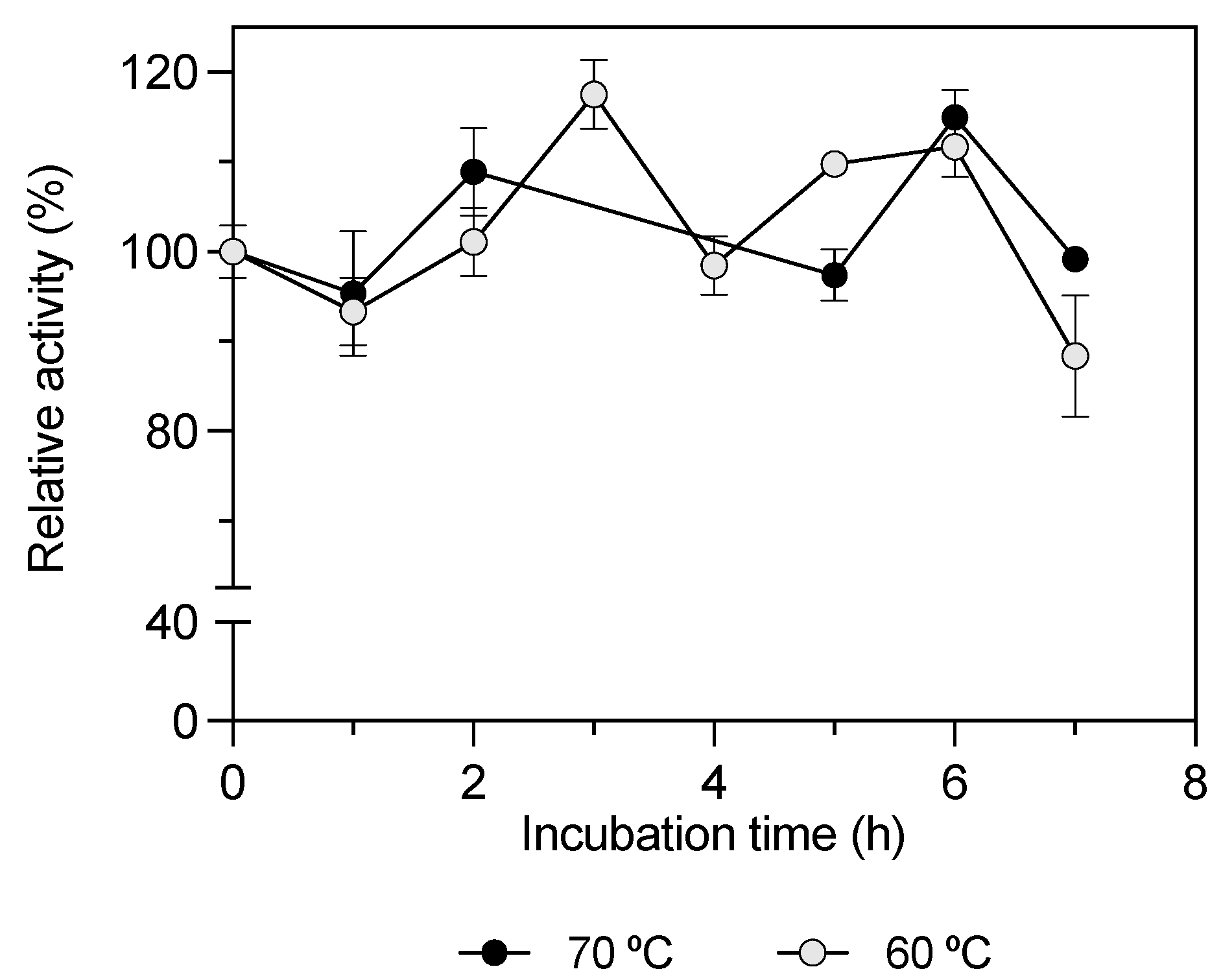




| Solvent | Residual Activity (%) |
| Control 1 (no additive) | 100.0 ± 5.8 |
| Methanol | 125.4 ± 5.4 |
| Dimethylsulfoxide (DMSO) | 104.5 ± 5.9 |
| Ethanol | 80.9 ± 4.2 |
| 2-propanol | 21.9 ± 9.3 |
| Detergent | Residual Activity (%) |
| Control 2 (no additive) | 100.0 ± 3.46 |
| Tween 20 | 55.5 ± 10.5 |
| CHAPS | 21.0 ± 2.7 |
| SDS | 38.1 ± 14.4 |
| Substrate | Vmax (U mg−1) | KM (mM) | r2 | Kcat (s−1) | kcat/KM (s−1 M−1) |
|---|---|---|---|---|---|
| pNP C8 | 3.57 ± 0.21 | 0.13 ± 0.03 | 0.9946 | 5.10 × 103 | 3.95 × 107 |
| pNP C10 | 2.85 ± 0.09 | 0.09 ± 0.01 | 0.9999 | 4.59 × 103 | 4.59 × 107 |
| pNP C12 | 2.24 ± 0.12 | 0.14 ± 0.03 | 0.9907 | 2.34 × 103 | 2.34 × 107 |
| pNP C14 | 0.21 ± 0.01 | 0.19 ± 0.04 | 0.9948 | 6.05 × 101 | 3.22 × 105 |
| Tween 20 (%, v/v) | Conversion (%) |
|---|---|
| 0 | 19.9 |
| 1 | 1.1 |
| 2.5 | 1.5 |
| Enzyme Units * | Reaction Time (h) | Conversion (%) |
| 8.5 | 6 | 2.77 |
| 34 | 6 | 11.64 |
| 51 | 24 | 32.86 |
| 51 | 40 | 29.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-González, R.; Fuciños, P.; Beneventi, E.; López-López, O.; Pampín, B.; Rodríguez, R.; González-Siso, M.I.; Cruces, J.; Rúa, M.L. Reactivity of a Recombinant Esterase from Thermus thermophilus HB27 in Aqueous and Organic Media. Microorganisms 2022, 10, 915. https://doi.org/10.3390/microorganisms10050915
González-González R, Fuciños P, Beneventi E, López-López O, Pampín B, Rodríguez R, González-Siso MI, Cruces J, Rúa ML. Reactivity of a Recombinant Esterase from Thermus thermophilus HB27 in Aqueous and Organic Media. Microorganisms. 2022; 10(5):915. https://doi.org/10.3390/microorganisms10050915
Chicago/Turabian StyleGonzález-González, Roberto, Pablo Fuciños, Elisa Beneventi, Olalla López-López, Begoña Pampín, Ramón Rodríguez, María Isabel González-Siso, Jacobo Cruces, and María Luisa Rúa. 2022. "Reactivity of a Recombinant Esterase from Thermus thermophilus HB27 in Aqueous and Organic Media" Microorganisms 10, no. 5: 915. https://doi.org/10.3390/microorganisms10050915
APA StyleGonzález-González, R., Fuciños, P., Beneventi, E., López-López, O., Pampín, B., Rodríguez, R., González-Siso, M. I., Cruces, J., & Rúa, M. L. (2022). Reactivity of a Recombinant Esterase from Thermus thermophilus HB27 in Aqueous and Organic Media. Microorganisms, 10(5), 915. https://doi.org/10.3390/microorganisms10050915








