Whole Genome Sequence Analysis of a Novel Apilactobacillus Species from Giant Honeybee (Apis dorsata) Gut Reveals Occurrence of Genetic Elements Coding Prebiotic and Probiotic Traits
Abstract
1. Introduction
2. Materials and Methods
2.1. Culturing of Bacteria
2.2. Exopolysaccharide Production by the Isolate
2.3. Genome Sequencing
2.4. Genome Analysis
2.5. Taxonomic Evaluation
2.6. EPS Producing Genes
2.7. Submission Information
3. Results and Discussion
3.1. EPS Production
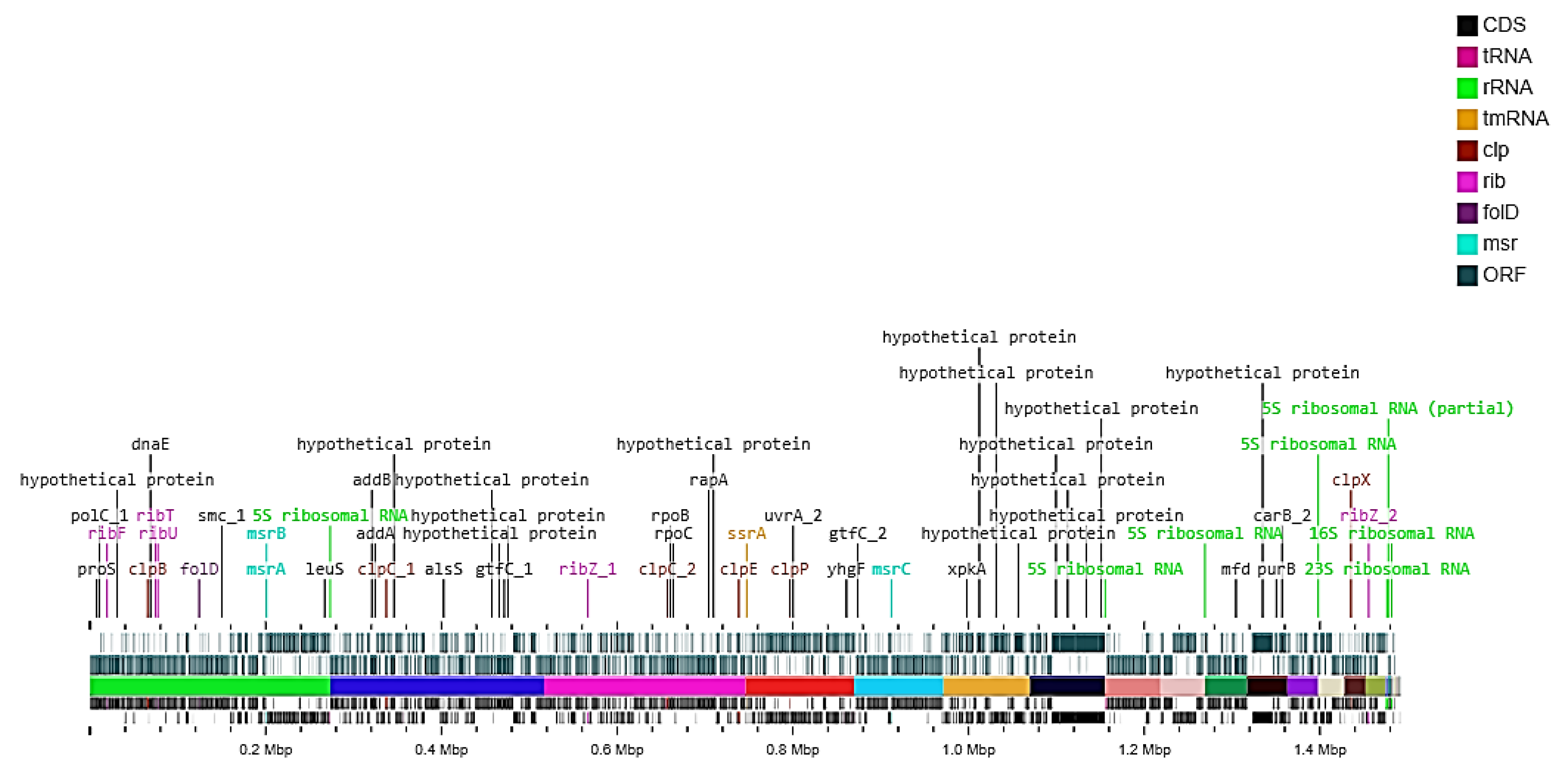
3.2. Genome Sequence and Annotation
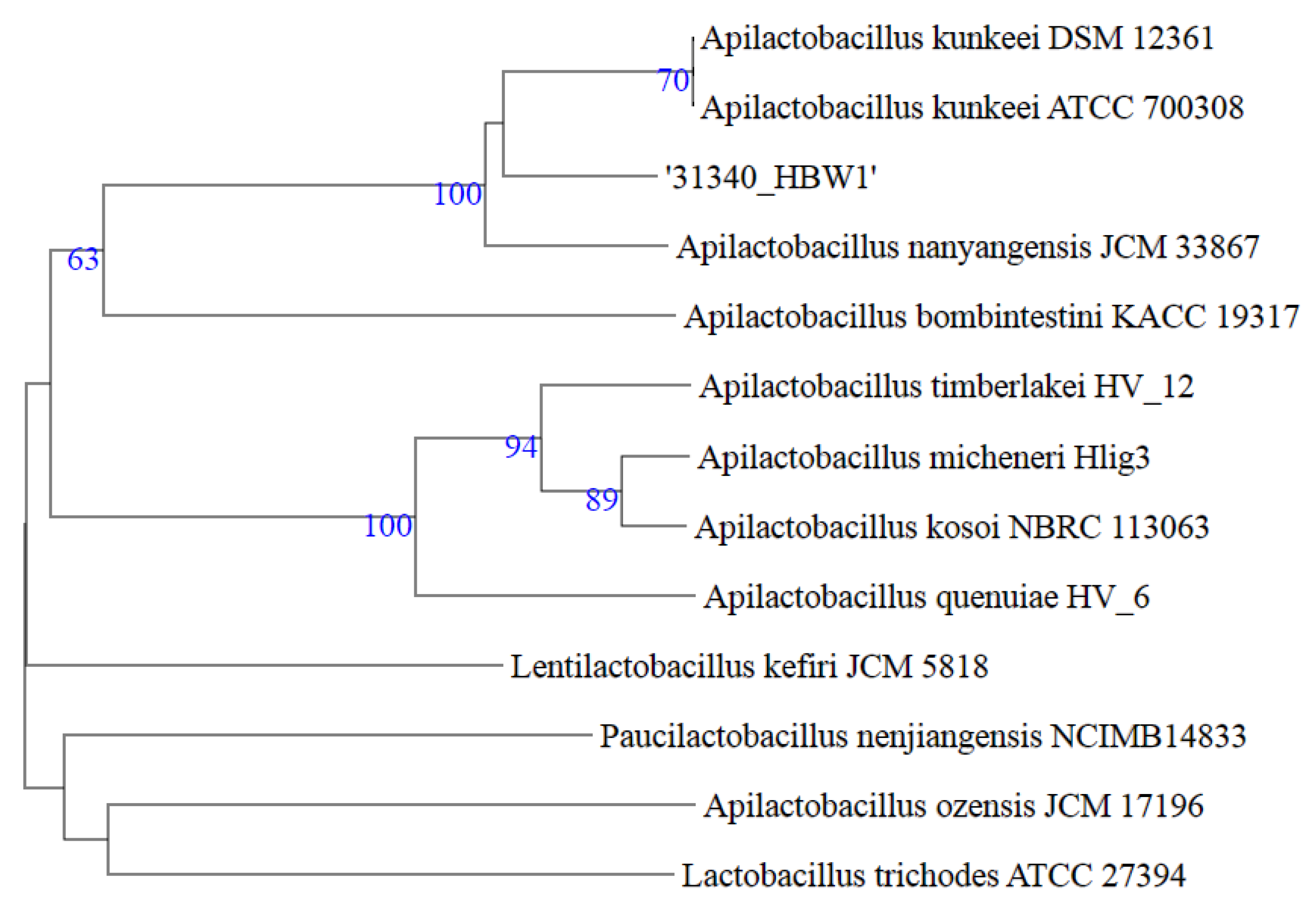
3.3. Phylogenetic and Genome Based Classification at Species Level
3.4. Genetic Traits of the Isolate HBW1 Important for Gut Function
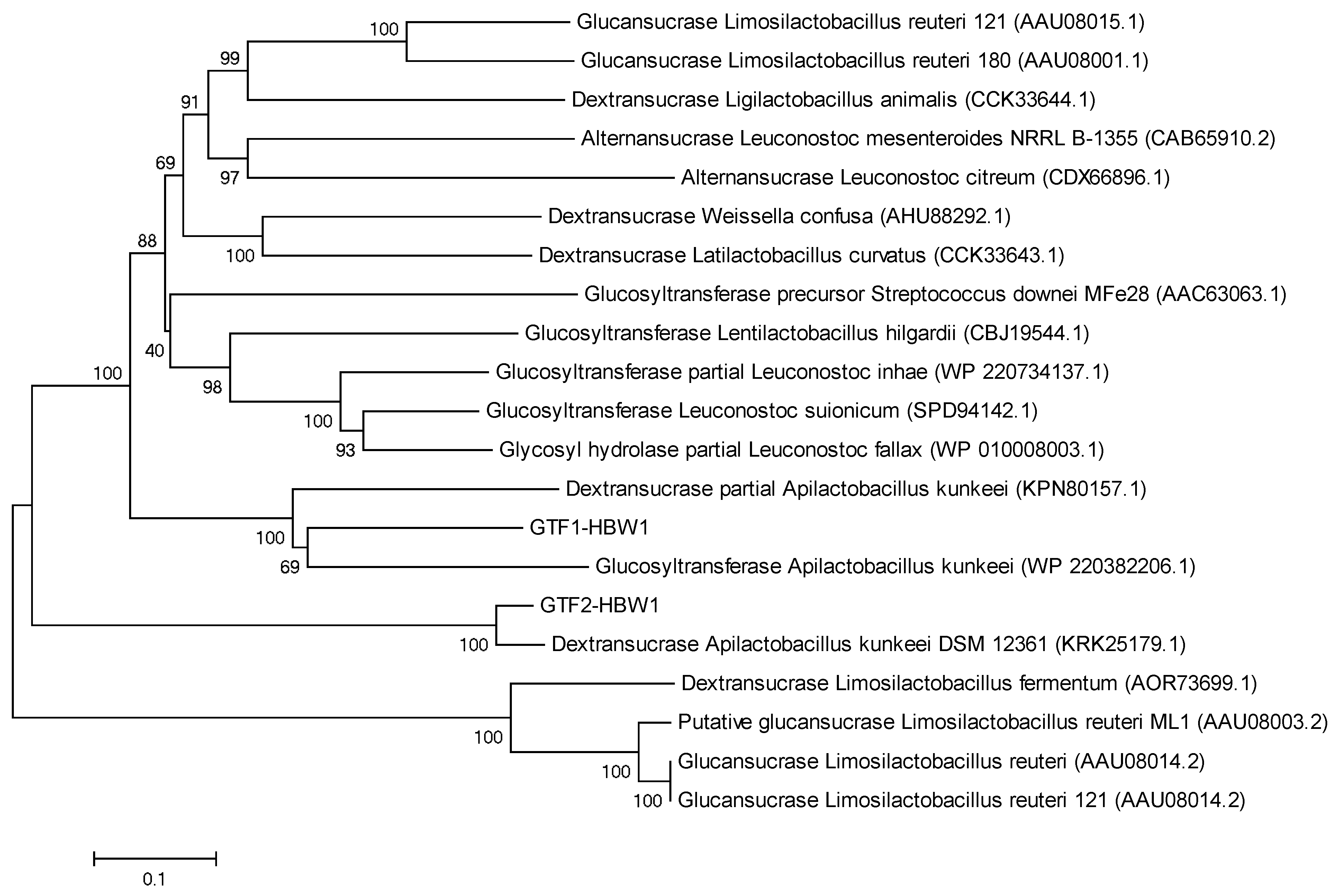
3.4.1. Putative Genes for EPS Synthesis
3.4.2. Genetic Elements Conferring Potential Probiotic Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/brain axis and the microbiota. J. Clin. Investig. 2015, 125, 926–938. [Google Scholar] [CrossRef]
- Osadchiy, V.; Martin, C.R.; Mayer, E.A. The gut–brain axis and the microbiome: Mechanisms and clinical implications. Clin. Gastroenterol. Hepatol. 2019, 17, 322–332. [Google Scholar] [CrossRef]
- Mayer, E.A.; Nance, K.; Chen, S. The Gut–Brain Axis. Annu. Rev. Med. 2022, 73, 439–453. [Google Scholar] [CrossRef]
- Berding, K.; Vlckova, K.; Marx, W.; Schellekens, H.; Stanton, C.; Clarke, G.; Jacka, F.; Dinan, T.G.; Cryan, J. Diet and the microbiota–gut–brain Axis: Sowing the seeds of good mental health. Adv. Nutr. 2021, 12, 1239–1285. [Google Scholar] [CrossRef]
- Munawar, N.; Ahsan, K.; Muhammad, K.; Ahmad, A.; Anwar, M.A.; Shah, I.; Al Ameri, A.K.; Al Mughairbi, F. Hidden role of gut microbiome dysbiosis in schizophrenia: Antipsychotics or psychobiotics as therapeutics? Int. J. Mol. Sci. 2021, 22, 7671. [Google Scholar] [CrossRef]
- Kesika, P.; Suganthy, N.; Sivamaruthi, B.S.; Chaiyasut, C. Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer’s disease. Life Sci. 2021, 264, 118627. [Google Scholar] [CrossRef]
- Munawar, N.; Ahmad, A.; Anwar, M.A.; Muhammad, K. Modulation of Gut Microbial Diversity through Non-Pharmaceutical Approaches to Treat Schizophrenia. Int. J. Mol. Sci. 2022, 23, 2625. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Roberfroid, M.B. Prebiotics and probiotics: Are they functional foods? Am. J. Clin. Nutr. 2000, 71, 1682S–1687S. [Google Scholar] [CrossRef]
- Gibson, G.; Hutkins, R.; Sanders, M.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Mohanty, D.; Misra, S.; Mohapatra, S.; Sahu, P.S. Prebiotics and synbiotics: Recent concepts in nutrition. Food Biosci. 2018, 26, 152–160. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M.M. Prebiotics and probiotics in digestive health. Clin. Gastroenterol. Hepatol. 2019, 17, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Korakli, M.; Vogel, R.F. Structure/function relationship of homopolysaccharide producing glycansucrases and therapeutic potential of their synthesised glycans. Appl. Microbiol. Biotechnol. 2006, 71, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Van Hijum, S.A.; Kralj, S.; Ozimek, L.K.; Dijkhuizen, L.; van Geel-Schutten, I.G. Structure-function relationships of glucansucrase and fructansucrase enzymes from lactic acid bacteria. Microbiol. Mol. Biol. Rev. 2006, 70, 157–176. [Google Scholar] [CrossRef]
- Drula, E.; Garron, M.-L.; Dogan, S.; Lombard, V.; Henrissat, B.; Terrapon, N. The carbohydrate-active enzyme database: Functions and literature. Nucleic Acids Res. 2022, 50, D571–D577. [Google Scholar] [CrossRef]
- Monsan, P.; Bozonnet, S.; Albenne, C.; Joucla, G.; Willemot, R.-M.; Remaud-Siméon, M. Homopolysaccharides from lactic acid bacteria. Int. Dairy J. 2001, 11, 675–685. [Google Scholar] [CrossRef]
- Monsan, P.; Remaud-Siméon, M.; André, I. Transglucosidases as efficient tools for oligosaccharide and glucoconjugate synthesis. Curr. Opin. Microbiol. 2010, 13, 293–300. [Google Scholar] [CrossRef]
- Endo, A.; Salminen, S. Honeybees and beehives are rich sources for fructophilic lactic acid bacteria. Syst. Appl. Microbiol. 2013, 36, 444–448. [Google Scholar] [CrossRef]
- Brodmann, T.; Endo, A.; Gueimonde, M.; Vinderola, G.; Kneifel, W.; de Vos, W.M.; Salminen, S.; Gómez-Gallego, C. Safety of novel microbes for human consumption: Practical examples of assessment in the European Union. Front. Microbiol. 2017, 8, 1725. [Google Scholar] [CrossRef]
- Kwong, W.K.; Moran, N.A. Gut microbial communities of social bees. Nat. Rev. Microbiol. 2016, 14, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Vásquez, A.; Forsgren, E.; Fries, I.; Paxton, R.J.; Flaberg, E.; Szekely, L.; Olofsson, T.C. Symbionts as major modulators of insect health: Lactic acid bacteria and honeybees. PLoS ONE 2012, 7, e33188. [Google Scholar]
- Filannino, P.; Di Cagno, R.; Tlais, A.Z.A.; Cantatore, V.; Gobbetti, M. Fructose-rich niches traced the evolution of lactic acid bacteria toward fructophilic species. Crit. Rev. Microbiol. 2019, 45, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.; Harris, H.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Vergalito, F.; Testa, B.; Cozzolino, A.; Letizia, F.; Succi, M.; Lombardi, S.J.; Tremonte, P.; Pannella, G.; Di Marco, R.; Sorrentino, E. Potential application of Apilactobacillus kunkeei for human use: Evaluation of probiotic and functional properties. Foods 2020, 9, 1535. [Google Scholar] [CrossRef]
- Ashfaq, I.; Amjad, H.; Ahmad, W.; Nasir, A.; Ali, A.; Ali, W.R.; Khaliq, S.; Hayat, A.; Ali, H.; Sattar, F. Growth Inhibition of Common Enteric Pathogens in the Intestine of Broilers by Microbially Produced Dextran and Levan Exopolysaccharides. Curr. Microbiol. 2020, 77, 2128–2136. [Google Scholar] [CrossRef]
- Roset, M.S.; Ciocchini, A.E.; Ugalde, R.A.; de Iannino, N.I. The Brucella abortus cyclic β-1, 2-glucan virulence factor is substituted with O-ester-linked succinyl residues. J. Bacteriol. 2006, 188, 5003–5013. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M. The RAST Server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Göker, M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef] [PubMed]
- Goris, J.; Konstantinidis, K.T.; Klappenbach, J.A.; Coenye, T.; Vandamme, P.; Tiedje, J.M. DNA–DNA hybridization values and their relationship to whole-genome sequence similarities. Int. J. Syst. Evol. Microbiol. 2007, 57, 81–91. [Google Scholar] [CrossRef]
- Yoon, S.-H.; Ha, S.-M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613. [Google Scholar] [CrossRef] [PubMed]
- Lefort, V.; Desper, R.; Gascuel, O. FastME 2.0: A comprehensive, accurate, and fast distance-based phylogeny inference program. Mol. Biol. Evol. 2015, 32, 2798–2800. [Google Scholar] [CrossRef] [PubMed]
- Farris, J.S. Estimating phylogenetic trees from distance matrices. Am. Nat. 1972, 106, 645–668. [Google Scholar] [CrossRef]
- Chun, J.; Oren, A.; Ventosa, A.; Christensen, H.; Arahal, D.R.; da Costa, M.S.; Rooney, A.P.; Yi, H.; Xu, X.-W.; De Meyer, S. Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int. J. Syst. Evol. Microbiol. 2018, 68, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Wolter, A.; Hager, A.-S.; Zannini, E.; Galle, S.; Gänzle, M.; Waters, D.M.; Arendt, E.K. Evaluation of exopolysaccharide producing Weissella cibaria MG1 strain for the production of sourdough from various flours. Food Microbiol. 2014, 37, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Saraithong, P.; Li, Y.; Saenphet, K.; Chen, Z.; Chantawannakul, P. Midgut bacterial communities in the giant Asian honeybee (Apis dorsata) across 4 developmental stages: A comparative study. Insect Sci. 2017, 24, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Gruneck, L.; Khongphinitbunjong, K.; Popluechai, S. Gut microbiota associated with two species of domesticated honey bees from Thailand. Symbiosis 2021, 83, 335–345. [Google Scholar] [CrossRef]
- Niode, N.J.; Adji, A.; Rimbing, J.; Tulung, M.; Tallei, T.E. Composition and diversity of bacteria from giant Asian honeybee Apis dorsata gut. Biodiversitas 2021, 22, 906–912. [Google Scholar] [CrossRef]
- Paris, L.; Peghaire, E.; Mone, A.; Diogon, M.; Debroas, D.; Delbac, F.; El Alaoui, H. Honeybee gut microbiota dysbiosis in pesticide/parasite co-exposures is mainly induced by Nosema ceranae. J. Invertebr. Pathol. 2020, 172, 107348. [Google Scholar] [CrossRef]
- Olofsson, T.C.; Vásquez, A. Detection and identification of a novel lactic acid bacterial flora within the honey stomach of the honey bee Apis mellifera. Curr. Micriobiol. 2008, 57, 356–363. [Google Scholar] [CrossRef]
- Nowak, A.; Szczuka, D.; Górczyńska, A.; Motyl, I.; Kręgiel, D. Characterization of Apis mellifera gastrointestinal microbiota and lactic acid bacteria for honeybee protection—A review. Cells 2021, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Gangoiti, J.; Wang, X.; Grijpstra, P.; van Leeuwen, S.S.; Pijning, T.; Dijkhuizen, L. Biochemical characterization of a GH70 protein from Lactobacillus kunkeei DSM 12361 with two catalytic domains involving branching sucrase activity. Appl. Microbiol. Biotechnol. 2018, 102, 7935–7950. [Google Scholar] [CrossRef] [PubMed]
- Bivolarski, V.; Iliev, I.; Ivanova, I.; Nikolova, M.; Salim, A.; Mihaylova, G.; Vasileva, T. Characterization of structure/prebiotic potential correlation of glucans and oligosaccharides synthetized by glucansucrases from fructophilic lactic acid bacteria from honey bee Apis mellifera. Biotechnol. Biotechnol. Equip. 2021, 35, 674–687. [Google Scholar] [CrossRef]
- Malang, S.K.; Maina, N.H.; Schwab, C.; Tenkanen, M.; Lacroix, C. Characterization of exopolysaccharide and ropy capsular polysaccharide formation by Weissella. Food Microbiol. 2015, 46, 418–427. [Google Scholar] [CrossRef]
- Molina, M.; Cioci, G.; Moulis, C.; Séverac, E.; Remaud-Siméon, M. Bacterial α-Glucan and Branching Sucrases from GH70 Family: Discovery, Structure–Function Relationship Studies and Engineering. Microorganisms 2021, 9, 1607. [Google Scholar] [CrossRef]
- Olano-Martin, E.; Mountzouris, K.C.; Gibson, G.R.; Rastall, R.A. In vitro fermentability of dextran, oligodextran and maltodextrin by human gut bacteria. Br. J. Nutr. 2000, 83, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Sarbini, S.R.; Kolida, S.; Naeye, T.; Einerhand, A.; Brison, Y.; Remaud-Simeon, M.; Monsan, P.; Gibson, G.R.; Rastall, R.A.; microbiology, E. In vitro fermentation of linear and α-1, 2-branched dextrans by the human fecal microbiota. Appl. Environ. Microbiol. 2011, 77, 5307–5315. [Google Scholar] [CrossRef]
- Goel, A.; Halami, P.M.; Tamang, J.P. Genome analysis of Lactobacillus plantarum isolated from some Indian fermented foods for bacteriocin production and probiotic marker genes. Front. Microbiol. 2020, 11, 40. [Google Scholar] [CrossRef]
- Arnoriaga-Rodríguez, M.; Mayneris-Perxachs, J.; Burokas, A.; Pérez-Brocal, V.; Moya, A.; Portero-Otin, M.; Ricart, W.; Maldonado, R.; Fernández-Real, J.M. Gut bacterial ClpB-like gene function is associated with decreased body weight and a characteristic microbiota profile. Microbiome 2020, 8, 59. [Google Scholar] [CrossRef]
- Legrand, R.; Lucas, N.; Dominique, M.; Azhar, S.; Deroissart, C.; Le Solliec, M.-A.; Rondeaux, J.; Nobis, S.; Guérin, C.; Léon, F.; et al. Commensal Hafnia alvei strain reduces food intake and fat mass in obese mice—A new potential probiotic for appetite and body weight management. Int. J. Obes. 2020, 44, 1041–1051. [Google Scholar] [CrossRef]
- Déchelotte, P.; Breton, J.; Trotin-Picolo, C.; Grube, B.; Erlenbeck, C.; Bothe, G.; Fetissov, S.O.; Lambert, G. The probiotic strain H. alvei HA4597® improves weight loss in overweight subjects under moderate hypocaloric diet: A proof-of-concept, multicenter randomized, double-blind placebo-controlled study. Nutrients 2021, 13, 1902. [Google Scholar] [CrossRef] [PubMed]
- Million, M.; Angelakis, E.; Maraninchi, M.; Henry, M.; Giorgi, R.; Valero, R.; Vialettes, B.; Raoult, D. Correlation between body mass index and gut concentrations of Lactobacillus reuteri, Bifidobacterium animalis, Methanobrevibacter smithii and Escherichia coli. Int. J. Obes. 2013, 37, 1460–1466. [Google Scholar] [CrossRef] [PubMed]
- Madeira, J.-P.; Alpha-Bazin, B.M.; Armengaud, J.; Duport, C. Methionine residues in exoproteins and their recycling by methionine sulfoxide reductase AB serve as an antioxidant strategy in Bacillus cereus. Front. Microbiol. 2017, 8, 1342. [Google Scholar] [CrossRef] [PubMed]
- De Crécy-Lagard, V.; El Yacoubi, B.; de la Garza, R.D.; Noiriel, A.; Hanson, A.D. Comparative genomics of bacterial and plant folate synthesis and salvage: Predictions and validations. BMC Genom. 2007, 8, 245. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Li, P. Biosynthesis of vitamins by probiotic bacteria. In Probiotics and Prebiotics in Human Nutrition and Health; InTech: Rijeka, Croatia, 2016; pp. 135–148. [Google Scholar]
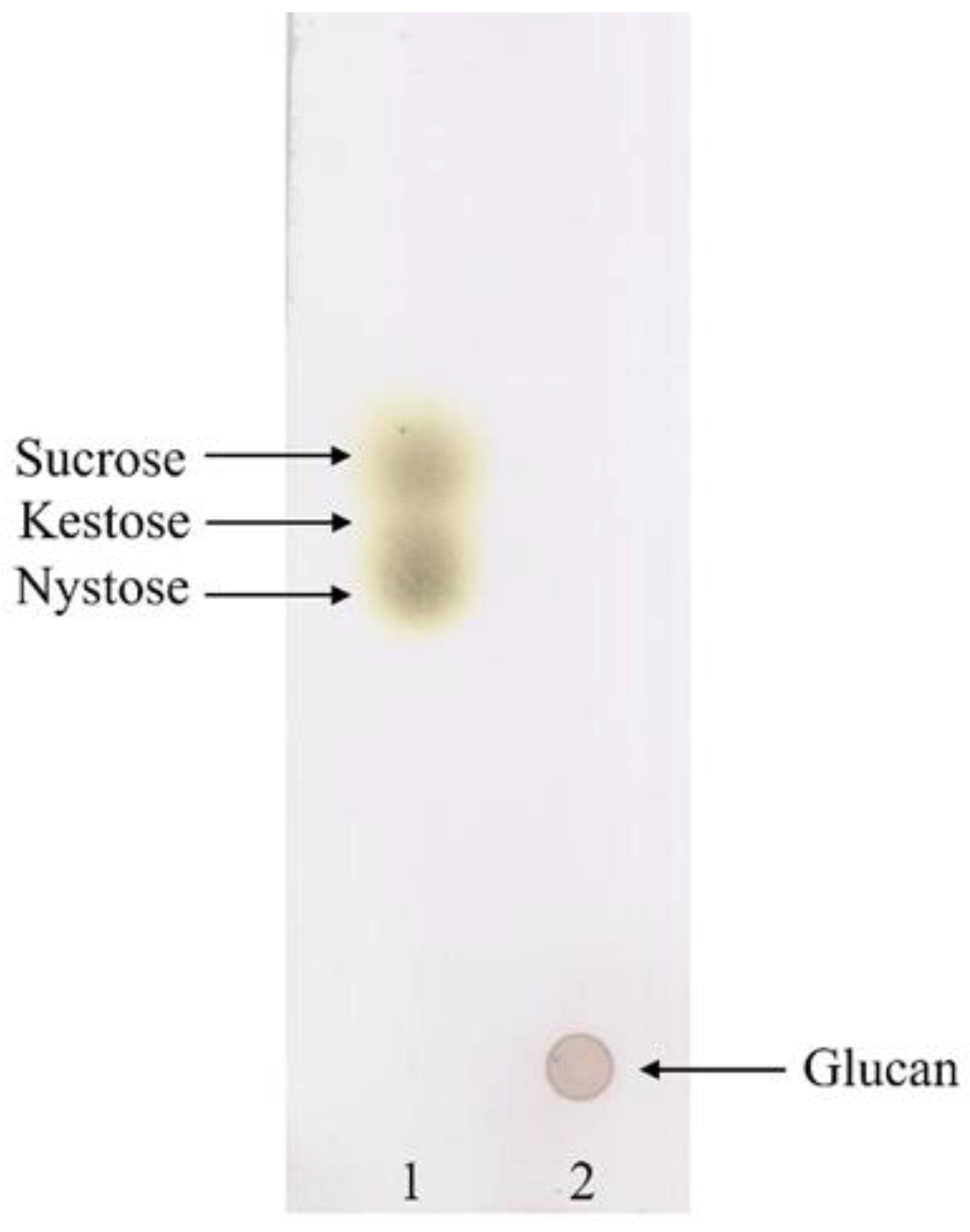

| Description | Information |
|---|---|
| Submission ID | SUB9890030 |
| Bio project ID | PRJNA740110 |
| Bio Sample | SAMN19820174 |
| Accession No | JAHQYH000000000 |
| Organism | Apilactobacillus sp. HBW1 |
| Feature | Value |
|---|---|
| Genome size | 1.49 Mb |
| Genes (total) | 1419 |
| G + C content | 37.2% |
| N50 | 229,491 |
| L50 | 3 |
| Number of contigs | 38 |
| CDSs (total) | 1344 |
| Genes (coding) | 1340 |
| CDSs (with protein) | 1340 |
| Genes (RNA) | 75 |
| rRNAs | 5, 4, 1 (5S, 16S, 23S) |
| Complete rRNAs | 4, 1, 1 (5S, 16S, 23S) |
| Partial rRNAs | 1, 3 (5S, 16S) |
| tRNAs | 62 |
| ncRNAs | 3 |
| Pseudo genes (total) | 4 |
| CDSs (without protein) | 4 |
| Pseudo genes (ambiguous residues) | 0 of 4 |
| Pseudo genes (frameshifted) | 1 of 4 |
| Pseudo genes (incomplete) | 1 of 4 |
| Pseudo genes (internal stop) | 2 of 4 |
| Subsystems | 247 |
| Carbohydrate metabolism | 70 |
| Strain Name and Accession No. | 16S% Identity | dDDH (%) | ANI (%) |
|---|---|---|---|
| Apilactobacillus kunkeei DSM 12,361 YH15T | 100.00 | 52.00 | 93.10 |
| [JXDB01000001] | |||
| Apilactobacillus apinorum Fhon13N | 98.82 | 24.50 | 82.44 |
| [KQ440395] | |||
| Apilactobacillus bombintestini BHWM-4 | 97.67 | 22.70 | 78.18 |
| [NZCP03262.1] | |||
| Apilactobacillus timberlakei HV-12 | 96.86 | 19.80 | 74.67 |
| [QUAP00000000.1] | |||
| Apilactobacillus micheneri Hlig3 | 95.45 | NA | NA |
| [KT833121] | |||
| Apilactobacillus ozensis JCM17196 | 94.38 | 22.00 | 65.38 |
| [AYYQ00000000.1] |
| Genes | Length | Strand | Putative Function | Response |
|---|---|---|---|---|
| DNA and protein protection and repair clpATPase (chaperon) | Acid and bile tolerance | |||
| clpB | 2583 bp | + | ATP-binding subunit clpB | |
| clpC | 2097 bp | + | ATP-binding subunit clpC | |
| clPE | 2172 bp | − | ATP-binding subunit clpE | |
| clpP | 594 bp | + | ATP-binding subunit clpP | |
| clpX | 1236 bp | + | ATP-binding subunit clpX | |
| Methionine sulfoxide reductase | Persistence capacity in vivo | |||
| msrA | 522 bp | − | Methionine sulfoxide reductase A | |
| msrB | 483 bp | − | Methionine sulfoxide reductase A | |
| msrC | 465 bp | − | Methionine sulfoxide reductase A | |
| Folic acid biosynthesis | Folic acid biosynthesis | |||
| folD | 858 bp | + | Methylenetetrahydrofolate dehydrogenase | |
| Riboflavin biosynthesis operon | Riboflavin biosynthesis | |||
| Ribf | 939 bp | + | Riboflavin kinase/FMN adenylyltransferase | |
| Ribu | 582 bp | + | Riboflavin transporter | |
| Ribt | 369 bp | + | Riboflavin biosynthesis RibT protein | |
| Ribz1 | 1407 bp | − | ||
| Ribz2 | 1446 bp | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, W.; Khaliq, S.; Akhtar, N.; El Arab, J.; Akhtar, K.; Prakash, S.; Anwar, M.A.; Munawar, N. Whole Genome Sequence Analysis of a Novel Apilactobacillus Species from Giant Honeybee (Apis dorsata) Gut Reveals Occurrence of Genetic Elements Coding Prebiotic and Probiotic Traits. Microorganisms 2022, 10, 904. https://doi.org/10.3390/microorganisms10050904
Ahmad W, Khaliq S, Akhtar N, El Arab J, Akhtar K, Prakash S, Anwar MA, Munawar N. Whole Genome Sequence Analysis of a Novel Apilactobacillus Species from Giant Honeybee (Apis dorsata) Gut Reveals Occurrence of Genetic Elements Coding Prebiotic and Probiotic Traits. Microorganisms. 2022; 10(5):904. https://doi.org/10.3390/microorganisms10050904
Chicago/Turabian StyleAhmad, Waqar, Shazia Khaliq, Nasrin Akhtar, Jamilah El Arab, Kalsoom Akhtar, Satya Prakash, Munir A. Anwar, and Nayla Munawar. 2022. "Whole Genome Sequence Analysis of a Novel Apilactobacillus Species from Giant Honeybee (Apis dorsata) Gut Reveals Occurrence of Genetic Elements Coding Prebiotic and Probiotic Traits" Microorganisms 10, no. 5: 904. https://doi.org/10.3390/microorganisms10050904
APA StyleAhmad, W., Khaliq, S., Akhtar, N., El Arab, J., Akhtar, K., Prakash, S., Anwar, M. A., & Munawar, N. (2022). Whole Genome Sequence Analysis of a Novel Apilactobacillus Species from Giant Honeybee (Apis dorsata) Gut Reveals Occurrence of Genetic Elements Coding Prebiotic and Probiotic Traits. Microorganisms, 10(5), 904. https://doi.org/10.3390/microorganisms10050904







