Abstract
To feed a world population, which will reach 9.7 billion in 2050, agricultural production will have to increase by 35–56%. Therefore, more food is urgently needed. Yield improvements for any given crop would require adequate fertilizer, water, and plant protection from pests and disease, but their further abuse will be economically disadvantageous and will have a negative impact on the environment. Using even more agricultural inputs is simply not possible, and the availability of arable land will be increasingly reduced due to climate changes. To improve agricultural production without further consumption of natural resources, farmers have a powerful ally: the beneficial microorganisms inhabiting the rhizosphere. However, to fully exploit the benefits of these microorganisms and therefore to widely market microbial-based products, there are still gaps that need to be filled, and here we will describe some critical issues that should be better addressed.
1. Introduction
The beneficial microorganisms interacting with plants support their growth through various mechanisms: (i) improvement of nutrient availability; (ii) enhancement of root development; (iii) reduction of toxic compounds levels in the soil; (iv) improvement of resistance to both biotic and abiotic stresses [1]. Plants can grow around the Yellowstone’s hot springs, in deserts, under flooding, and in salty and contaminated soils thanks to their genetic plasticity but also to the contribution of microbial communities helping them overcome these challenges [2,3,4]. In addition, certain plant diseases seemed to be rarer in some plants, leading to hypothesize that the disease suppression could also be due to living microorganisms [5,6]. Studies carried out to date suggest that plants completely free of microorganisms represent an exception. Furthermore, in recent years, new beneficial microorganisms potentially applicable for agricultural production have been identified [7]. The best known and most ancient beneficial microorganisms are the Rhizobia. These microorganisms, described for the first time by Martin Beijerinck in 1888 [8], inhabit the roots of leguminous plants and provide them with fixed nitrogen. In the last few years, the knowledge of microorganisms’ diversity and molecular mechanisms that regulate plant–microorganism interactions has improved considerably [9]. The number of recognized microbes interacting with plants, including the beneficial ones, is large and continuously growing, and these microbes can be classified in different ways [10]. Beneficial microorganisms can act as free-living bacteria, symbiotic (that establish specific symbiotic relationships with plants, i.e., Rhizobia and Frankia), and endophytes (that colonize the interior plant tissues). Within them, a group of soil bacteria called plant growth-promoting bacteria (PGPB) stimulates the growth and health of the plant through different mechanisms [1]. These mechanisms include: (i) production of plant hormones; (ii) nitrogen fixation; (iii) solubilization of inorganic phosphate and mineralization of organic phosphate; (iv) antagonism against pathogens by production of antibiotics, enzymes, and fungicidal compounds; and (v) competition with detrimental microorganisms causing disease to crops. The effects of human-caused climate changes are becoming more and more evident as we see more record-breaking heat waves, intense droughts, shifts in rainfall patterns and an increase in average temperatures. These environmental changes touch every step of crop production [11,12]. Studies concerning the effects of climate changes on the living organisms inhabiting our planet have highlighted that in the future the strong acceleration of these changes will impact more and more on the growth and productivity of plants [13,14]. Furthermore, to cope with the increasing world population, an improved food production is required. However, this objective cannot be achieved by intensifying the exploitation of natural resources, which will be increasingly limited [15]. The application of efficient and low-cost PGPB, which need minimum external energy and chemicals, could be a valid strategy to improve the response of plants to stress and to make the best use of available natural resources [16,17]. Although considerable efforts have been made in recent years to clarify the mechanisms of action of these microorganisms, several aspects still remain to be clarified. Several factors can limit the success of PGPB and some of them are the following: (i) less than 2% of microbes interacting with plants are cultivable in the laboratory; (ii) PGPB products are often crop-specific; (iii) the effectiveness of PGPB is generally assessed using single microorganisms instead of an appropriate consortium and without clarifying the roles and relationships between the various microorganisms; (iv) PGPB performances are not evaluated under different climatic and agronomic conditions; (v) the stability and persistence of PGPB in the host plants are often time-dependent. The latter has many similarities with human gut microbial communities, which are closely interconnected with human health. Indeed, several strategic therapies to restore and/or maintain the eubiotic state of the microbial intestinal ecosystem are under investigation. In this perspective paper, we discuss and highlight some of the gaps preventing full exploitation of PGPB abilities for sustainable crop production.
2. Linking Plant Diversity with Microbial Diversity
The microbial community structure is modulated by various factors, whose influence varies according to the ecosystem in which the host plant grows [18]. The main drivers of microbial community diversity in agricultural ecosystems are: farming practices, environmental conditions, soil type, and plant species; whereas in natural ecosystems, these communities are mainly modulated by biotic interactions, plant species, and plant diversity [19]. Microbial communities and their host plants have closely linked identities: plant’s genotypic differences strongly influence the structure of plant microbial communities. To better explain this relationship and to analyse the effect of reduced plant genetic diversity on soil microbial abundance, the identification of plant candidate genes regulating the interaction with beneficial microbial communities could be an efficient strategy [20]. In species-rich plant communities, the aboveground litterfall and the belowground fine root mortality lead to the availability of greater amounts of carbon and nutrient resources for soil microorganisms. Microbial diversity and biomass are also stimulated by diverse root exudates, whose production is influenced by plant genotypes and development stages (seedling, vegetative, bolting, and flowering) [21]. The exudates released by roots might have a double effect: they stimulate the interaction with some particular microorganisms (i.e., beneficial microorganisms) and act as antimicrobial agents for other ones (i.e., pathogenic microorganisms). Therefore, the diversity and quantity of exudates released can influence the type of interaction that plants establish with the microorganisms with which they come into contact. On the other hand, interaction with PGPB can induce changes in the type of exudates, which in turn modulates the response of the host plant to various external stimuli [22]. In addition, different plant tissues can host distinct microbial communities [23]. For instance, since shoot tissues are exposed to challenging environmental factors as compared to roots, a high degree of adaptation is required for microbial colonization, and only a few microbial groups adapted to environmental variations will be successful. Moreover, it was observed that microorganisms, such as PGPB, containing key genes responsible for the health status of the host plant, are common in different plant species [24]. However, there is a knowledge gap concerning the taxonomy of PGPB, and this is probably due to the following reasons: (i) these microorganisms belongs to many different phyla, and most of their phylogenetic analyses have been carried out within their specific genus and not within a group; (ii) the microbial classification is initially carried out only evaluating the morphological, biochemical, and functional characteristics through culture-based methods, without considering the genetic features, thus leading to incorrect classifications of many taxa [24]. Therefore, a careful classification of microbial community associated with a given plant genotype is essential to recognize the efficiency of PGPB as bio-inoculants under various environmental conditions.
3. Competence and Persistence of Microorganisms in the Field: Effect of Inoculant Strains on the Resident Microflora
Various critical factors can influence the efficacy of PGPB-based bioinoculants, including soil health, colonization efficiency, and persistence in the soil [25]. One of the main factors influencing soil health is the carbon transformation [26]. The decomposition of plant residues and other organic matter, the cultivation intensity, and tillage lead to carbon transformation, which in turn directly affects the composition of microbial communities [27]. The efficiency of PGPB strongly depends on their ability to compete with autochthonous microorganisms present in the soil, and to adhere and colonize the external and/or internal part of plant tissues [28]. In these processes, the formation of biofilms by PGPB can play an important role. To assess the ability of PGPB to colonize roots and persist in the soils, several techniques can be used, including microbial enumerations by culture-based methods, DNA-based methods, and microscopy-based techniques. To ensure good results in microbiological analyses and to obtain representative samples for each treatment to be analysed, correct soil sampling in the laboratory, greenhouse trials, and field experiments is a critical step [22]. Temporal and spatial aspects could also be considered during sampling. To reduce the environmental impact, the cultivation of a specific crop over a consistent number of years and in the same soils is the most prominent factor [29]. The simplest approach used to overcome spatial variables is to collect the soil samples at random, ensuring that each sample has the same opportunity to be selected. For rhizosphere studies, soil attached to the roots should be carefully removed and collected. To evaluate external and internal root colonization, plant roots should be washed in sterile water or phosphate buffered saline and then homogenized in the same buffer. To study microbial endophytes, sterilization of the roots’ surface is necessary before carrying out the homogenization procedure. The procedure widely used to evaluate the persistence of inoculated microorganisms in soil and/or rhizosphere is the culture-dependent method [22]. However, with this method, it is not possible to evaluate the totality of microorganisms present in the soil and/or rhizosphere, because only 0.1 to 1.0% of soil microorganisms are culturable [30]. Moreover, this method is useful if the experiments are carried out in sterile conditions, without the interference of soil autochthonous microbial populations. Culture-based methods may be complemented with culture-independent approaches, such as PCR-based methods and next-generation sequencing, to examine the variations in the microbial community after inoculation treatment [22,31]. Therefore, a combination of culture-dependent methods and molecular approaches could be used to track inoculated strains or microbial consortia in natural habitats. The limiting step in both direct and indirect methods is the DNA extraction: due to the adhesion of DNA to soil particles, the extracted DNA will mostly represent the dominant species. It has been observed that to fully exploit the abilities of PGPB, it is very important to reach and maintain a high-density population on the roots of the host plants [32].
Nevertheless, it is also possible that microorganisms never colonize the plant despite their high density. Furthermore, there are also cases in which microorganisms induce the plant to synthesize molecules necessary for their nutritional needs. A particular example concerns the really useful pathogen Agrobacterium. Through a rare interkingdom DNA transfer, this bacterium moves some of its genes into its host’s genome, thereby inducing the host cells to proliferate. The result is uncontrolled cell growth leading to a tumour or excessive production of roots. The proliferating plant tissues produce opines, which are compounds that Agrobacterium and a few other organisms can use as a source of nutrients [33,34]. The most commonly used method to detect bacteria inside plant tissues involves the labelling of the cells with fluorescent systems, such as GFP, and their detection by fluorescence microscopy [22]. However, the use of GFP-tagged microbial strains is not applicable in field trials since the tagged microbial cells could be released into the environment. In addition, the autofluorescence of the plant cell walls makes the visualization of labelled microorganisms difficult in situ. Therefore, efforts should be made to find a suitable system to track PGPB interacting with plants under field conditions.
4. Plant Phenotyping to Analyse the Effects of Beneficial Microbes
PGPB efficiency is also greatly affected by plant age and developmental stage [33].
These parameters have greater effects on microorganisms present in the plant compartments than in the soil [35]. However, the mechanisms that regulate the distribution and differentiation of microbial communities during the various phases of plant development in the field are not yet well known. The microbial differentiation may be influenced by plant factors (such as root growth, physiology, architecture, morphology, and exudates), environmental factors (such as air, dust, rainfall, and temperature), edaphic factors, and fertilization regimes [36]. Plant phenotyping technologies based on non-destructive image analyses are useful instruments for addressing and understanding the complex plant-environment dynamics represented. The information resulting from the application of these technologies can be exploited for the genetic improvement of crops or for the analyses of the beneficial effects of microbial communities’ presence [37,38,39,40,41,42,43]. The development of new innovative approaches (visible imaging, fluorescence imaging, thermal infrared imaging, imaging spectroscopy, and other techniques) allowed to reach accuracy and precision in phenotyping analyses [35,37,39,43]. In particular, visible imaging provides information regarding plant growth morphology and allows the analysis of changes in phenotypic characteristics and the plant’s biomass. Another technology commonly used for plant phenotyping is spectroscopy imaging [35,38,43]. One of the parameters measured with this technology is the vegetation indices, the most popular of which is the normalized difference vegetation index, which is used to assess the general health status of crops. Early stress symptoms of plant diseases or the plant water status can be monitored through the use of thermal imaging, which is the most commonly used system for the analysis of stomatal activity, as the stomatal conductance increases with rising temperature [38,43]. Fluorescence imaging, consisting of the imaging of fluorescence signals obtained by illuminating samples with visible or UV (ultraviolet) light, is primarily used to study the effect of environmental conditions on photosynthesis and its associated metabolism [43]. Finally, modern optical 3D structural tomography and functional imaging techniques, such as Nuclear Magnetic Resonance Imaging and Positron Emission Tomography, have greatly improved living plant visualization in a non-destructive manner. Phenotyping can take place under laboratory, greenhouse or field conditions. In laboratory experiments, environmental factors can be controlled and varied during the experiments, but only a limited number of environmental factors can be investigated [42]. Anyway, laboratory experiments contribute to understanding specific plant dynamics in detail. This information should be then related to field conditions, which are more complex, highly variable and fluctuating in time and space, and therefore could better support scientists and breeders in the analyses of crop features [42,44,45,46].
5. Model Plants to Identify Plant and Microbial Candidate Genes Governing Plant-Microbe Interaction
The genetic features needed for an efficient association between plants and microbial communities are complex and still poorly understood. Even though it was observed that genotypic differences of the host plants significantly affect root associated microbial communities, plant breeding programs so far did not pay attention to the analysis of the microbial communities associated with them. In particular, no genetic loci affecting the establishment of association with the microbial communities have been identified. Basic studies have detected few plant molecular pathways so far, only those associated with shaping the plant-microbe interaction, and only for a few model cultivars [47]. It was shown that the plant-associated microorganisms are able to affect different plant traits, such as nutrient uptake, flowering time, and stress resistance [48]. Indeed, recent studies with microorganisms isolated from soil suggest that the capacity of plants to mobilize phosphorus (P) improves when they are associated with specific microbial communities [49]. Moreover, it was discovered that rhizosphere microbial communities influenced the flowering time of Arabidopsis thaliana, suggesting that microorganisms play a key role in plant functioning [50]. In addition, several studies concerning the beneficial effects of microorganisms on plant response to stresses, and in particular to the abiotic ones have also been reported [51,52,53]. Therefore, to modify specific plant traits, a combined approach of breeding plants and engineering the microbial community associated with them could be an effective approach [54,55]. However, due to the lack of integration between physiological data and those concerning the interaction with microbial communities, the targeted microbiome modification remains an arduous process. To design synthetic microbial communities for higher crop productivity, it is important to understand how plants and microbes communicate with each other, and which plant genes allow crops to shape the rhizosphere microbial community [56,57]. Modern technologies such as next-generation sequencing (NGS), omics approaches (metagenomics, transcriptomics, proteomics, metabolomics), and computational tools enable the understanding of molecular aspects of the plant-microbes interactions governing the plant traits. Recently, several reports investigated the influence of host genotype on different facets of the microbial communities. Genetic information about these interactions is becoming available for several crops and associated microbes [58,59]. In this regard, the CRISPR (clustered regularly interspaced short palindromic repeats)-based genome editing, is an ideal technology to obtain mutant plants or microbes differing from the parental ones only for a point mutation, without introducing exogenous DNA sequences [60,61,62]. The use of complementary sequencing and transcriptomics techniques currently available can lead to the production of a lot of sequence data, several mutants, even for the main crops, and to identification of specific genetic loci involved in plant-microbe interactions under field conditions. The CRISPR technology could exploit this information to introduce targeted genetic modifications in both plants and microbes, thus leading to the development of improved plants/microbes usable for sustainable agricultural practices [63,64,65]. However, a major obstacle to the genetic improvement of plants is represented by the lack of adequate legislation.
6. Application of Stress Conditions to Analyse Microbial Traits and Plant Phenotype under Conditions as Close as Possible to Those Found in the Open Field
The ultimate action of PGPB on plant growth and health depends on several factors, including the ability to survive, colonize, and establish interactions with the host plants.
The evaluation of these parameters is especially important for PGPB application in field conditions, where several variables hindering their success come into play.
The most commonly used approach for the selection of PGPB is to isolate the different strains and assess their growth promoting traits under aseptic conditions. However, this approach is limited since it does not take into account that in real conditions the PGPB efficiency depends not only on the individual traits analysed but also on their interaction with other factors, which can be associated with both plants and the environment. Therefore, to identify efficient PGPB, it is essential to evaluate as many variables as possible in greenhouse experiments, in order to simulate the real field conditions [66,67,68,69]. Moreover, considering that the environmental conditions have strong influence on the microbial communities associated with plants, having information about the place of origin of the tested strains can be helpful for field application [67,68]. The use of throughput sequencing of nucleic acids for molecular characterization of PGPB under real field conditions might be an effective approach to select PGPB candidates [69]. Plants living in extreme environments, such as areas characterized by high temperatures, high salinity, and low level of nutrients, have adapted to those conditions. This adaptation is the result of their genetic plasticity but is also due to the action of the microorganisms associated with them. Since these microorganisms have experienced the same adaptation as their host plants, they could represent an optimal source for the selection of PGPB usable to improve plant growth under real field conditions where they are frequently subjected to different abiotic stress conditions [67,70].
7. Effects of Climatic Conditions on Microbial Inoculants
It is now well recognized that climate changes negatively affect most of the living organisms of our planet, including plants and microorganisms [71]. The ever more sudden alterations in environmental conditions can induce changes in the physiology of plants, leading to a different distribution of fundamental elements, such as C and N, in the different compartments of the plants [71]. A different distribution of C and N within plants can cause alterations in the exudates released by the roots into the environment. The ability of beneficial bacteria, such as PGPB, to colonize the roots of the host plants is strongly influenced by root exudates, which have a complex composition and include molecules acting as chemoattractants. The recognition of root exudates represents the first step of the recruitment and colonization processes that allow PGPB to colonize the host plants. Therefore, climate changes negatively impact the composition of microbial communities in the soil and the activities of microbes interacting with the plants [72,73,74,75]. Moreover, climate changes can directly influence the microbial composition of soils. In particular, the scarcity of rain can lead to a reduction in the biomass of microorganisms present in the rhizosphere. Among them, there are PGPB, which help the host plants to counteract the negative effects caused by stress conditions (abiotic and biotic). As plant-associated bacteria depend on root exudates or plant metabolites and are substantially influenced by environmental parameters, it is plausible that these microbial communities will be increasingly affected by the extreme conditions associated with climate changes [76,77]. Therefore, understanding how climate influences, either directly by altering environmental conditions or indirectly by changing plant physiology, microbial community composition is a major challenge in the future, when the use of sustainable agriculture will be imperative.
8. Find the Best Practice for Application of PGPB in the Field
An important parameter that must be evaluated before using bioinoculants in agricultural practices is its vitality during the storage and application steps.
To ensure the activity of bio-inoculants, the use of microbial consortia, instead of single strains, to inoculate the host plants can be useful. Applying consortia, there will be a good probability that at least some of the microorganisms contained in the bioinoculants will survive [78]. However, even in the case of microbial consortia, it is important to evaluate how the environmental stresses can affect the vitality of bioinoculants. To this aim, various parameters, including bioinoculants formulation and delivery, should be monitored [79,80,81]. The identification of the right procedure for inoculant formulation is crucial to provide stabilization and protection of consortia during transport, storage, and application. This procedure should ensure the survival of a sufficient number of microbial cells able to exert their positive effect in the host plants. Bioinoculants industry has developed several systems that involve the use of different carriers (solid, slurry and liquid) and additives (i.e., adhesives and surfactants) [82]. Another important factor to consider is the release procedure, which can consist of the release of microbial suspensions (made in water, oils, or emulsions) directly into soils or applied on the seeds. Although the direct application in the soils allows to obtain a higher concentration of PGPB compared to the treatment on seeds [83], it is associated with a higher risk of contamination and to the loss of metabolic activities of PGPB contained therein [69]. Microbial encapsulation with polymeric hydrogels can be used to overcome these limitations. An alternative system is represented by solid formulations, in which solid carriers, such as peat, charcoal, vermiculite, cellulose and polymers, are used.
The use of alginate microbeads to encapsulate PGPB has yielded very promising results [84,85], as it is a reproducible method. However, even if this method is effective in laboratory experiments, it is economically disadvantageous when used on a large scale, such as open field applications. An alternative system, to increase inoculant survival rate and reduce contamination, could be the reduction of the moisture content of PGPB formulation by using a fluidized bed dryer (FBD) equipment. This system has a high drying rate, and the material is dried in a very short time and remains free-flowing and uniform [86,87]. One of the main disadvantages of this process is that it operates at about 37 °C to 40 °C, and therefore is more suitable for mesophilic organisms. To ensure a fair cell density during storage and to increase formulation efficiency, the alginate or FBD methods could be promising systems. To date, one of the main obstacles to the use of microbial-based bioinoculants is the lack of data regarding their efficacy across a range of agricultural field settings. Moreover, systems to evaluate the effective colonization of the host plants and the persistence of PGPB in soils over time have not yet been developed.
9. Functional Collaboration Combining Expertise in Basic Science, Development, Testing, and Marketing
To fully exploit the potentiality of PGPB as bio-inoculants for sustainable agriculture productions, the integration of biology, chemistry, physics, engineering, microbiology, biotechnology, genomics, computer science, and many other disciplines is a promising strategy. Using this approach, the selection of most suitable and effective strains can be performed for the production of high-quality bio-inoculants having higher performance and wide applicability. For example, in an area where phosphate utilization is the major concern, it is relevant to select the inoculant having the property of phosphate-solubilizing activity [88]. Moreover, the solid substrate-based bio-inoculants usually have a short shelf life (about 6 months) and are highly sensitive to direct sunlight, thus requiring specific storage conditions [10]. Therefore, the selection of bio-inoculants that maintain their potential efficacy beyond the period of 6 months and do not require temperature-controlled storage is desirable. Furthermore, since the lack of awareness regarding the use and role of bio-inoculants has hindered their mass spreading among the different stakeholders, the use of appropriate market information could be a successful strategy to overcome this gap [89]. To date, the demand for bio-inoculants is still not very high, and this is probably due to the implementation of policies that are either too restrictive or improper, especially in the countries of the European Community [90,91]. In the USA, APHIS regulates living organisms that could impact animal and plant health and that can be biocontrol organisms or have biopesticidal properties (https://www.aphis.usda.gov/aphis/home (accessed on 14 April 2022)). In Latin America, public policies are encouraging the use of alternatives to agrochemicals (pesticides and fertilisers) through the development of regulatory frameworks for product evaluation and approval. Colombia is the only country with specific legislation on biological inputs, while Argentina and Brazil have both recently developed a national programme to promote bio-inputs [92]. Therefore, the implementation of proper and uniform policies could favour the production of bio-inoculants and help to overcome the difficulties in registering new products.
10. Conclusions
To enhance plant protection and agriculture production in a sustainable way, there is a need for new tools to increase our knowledge on the mechanisms that regulate plant-microbiome interactions. In this context, an in-depth analysis, also based on model plants and microorganisms, will allow to better understand how microbes can contribute to the well-being of plants and how these beneficial effects can be harnessed for agricultural application. Furthermore, the development of specific and easy methodologies for the evaluation of PGP activities that inoculated strains have on the soil could help to clarify the processes that take place during plant-soil-microbe interactions. The potential application of beneficial microbes in agriculture seems unlimited, but more attention to the transfer of discoveries from lab to field, as well as technical, regulatory, and marketing issues, is mandatory. An integrated approach to address the multi-faceted nature of these challenges is most likely to succeed (Figure 1).
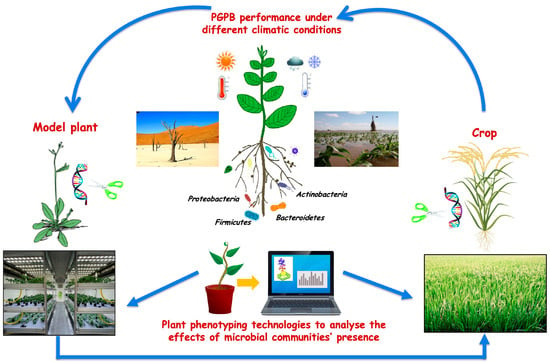
Figure 1.
Plant Growth Promoting Bacteria (PGPB) for sustainable agriculture: link between laboratory and field experiments.
Author Contributions
Conceptualization, F.M. and C.B.; writing—original draft preparation, F.M. and C.B.; writing—review and editing, R.D. and C.B.; funding acquisition, R.D. and C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the grant “MICRO4Legumes” (Il microbioma vegetale simbionte come strumento per il miglioramento delle leguminose foraggere), D.M.n.89267 (MIUR, Italian Ministry of Agriculture). This work was also partially supported by the grants “E-Crops” (Tecnologie per l’Agricoltura Digitale Sostenibile), ARS01_01136 (MIUR, Italian Ministry of Agriculture), and “Bio-Memory” (La rete delle bio-banche del CNR per il bio-monitoraggio, la conservazione della biodiversità, la sostenibilità agro-alimentare e ambientale, e il benessere), SAC.AD002.173 (CNR, National Research Council).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Marco Petruzziello for technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Glick, B.R.; Gamalero, E. Recent developments in the study of plant microbiomes. Microorganisms 2021, 9, 1533. [Google Scholar] [CrossRef]
- Xu, S.; Wang, J.; Guo, Z.; He, Z.; Shi, S. Genomic convergence in the adaptation to extreme environments. Plant Comm. 2020, 1, 100117. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Verma, J.P. Does plant—Microbe interaction confer stress tolerance in plants: A review? Microbiol. Res. 2018, 207, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Chevin, L.-M.; Hoffmann, A.A. Evolution of phenotypic plasticity in extreme environments. Phil. Trans. R. Soc. B 2017, 372, 20160138. [Google Scholar] [CrossRef] [PubMed]
- Lutz, S.; Thuerig, B.; Oberhaensli, T.; Mayerhofer, J.; Fuchs, J.G.; Widmer, F.; Freimoser, F.M.; Ahrens, C.H. Harnessing the microbiomes of suppressive composts for plant protection: From metagenomes to beneficial microorganisms and reliable diagnostics. Front. Microbiol. 2020, 11, 1810. [Google Scholar] [CrossRef] [PubMed]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of action of microbial biological control agents against plant diseases: Relevance beyond efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef] [Green Version]
- Chandran, H.; Meena, M.; Swapnil, P. Plant growth-promoting rhizobacteria as a green alternative for sustainable agriculture. Sustainability 2021, 13, 10986. [Google Scholar] [CrossRef]
- Beijerinck, M.W. Die Bakterien der Papilionaceenknollchen. Bot. Ztg. 1888, 46, 725–804. [Google Scholar]
- Berg, G.; Kusstatscher, P.; Abdelfattah, A.; Cernava, T.; Smalla, K. Microbiome modulation-toward a better understanding of plant microbiome response to microbial inoculants. Front. Microbiol. 2021, 12, 650610. [Google Scholar] [CrossRef]
- Maitra, S.; Brestic, M.; Bhadra, P.; Shankar, T.; Praharaj, S.; Palai, J.B.; Shah, M.M.R.; Barek, V.; Ondrisik, P.; Skalický, M.; et al. Bioinoculants-Natural biological resources for sustainable plant production. Microorganisms 2022, 10, 51. [Google Scholar] [CrossRef]
- Di Benedetto, N.A.; Corbo, M.R.; Campaniello, D.; Cataldi, M.P.; Bevilacqua, A.; Sinigaglia, M.; Flagella, Z. The role of plant growth promoting bacteria in improving nitrogen use efficiency for sustainable crop production: A focus on wheat. AIMS Microbiology 2017, 3, 413–434. [Google Scholar] [CrossRef]
- Jia, J.; Dai, Z.; Li, F.; Liu, Y. How will global environmental changes affect the growth of alien plants? Front. Plant Sci. 2016, 7, 1623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pareek, A.; Dhankher, O.P.; Foyer, C.H. Mitigating the impact of climate change on plant productivity and ecosystem sustainability. J. Exp. Bot. 2020, 71, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Lemanceau, P.; Blouin, M.; Muller, D.; Moënne-Loccoz, Y. Let the core microbiota be functional. Trends Plant Sci. 2017, 22, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Dulanto, P.N.; Thiry, A.A.A.; Glorio-Paulet, P.; Vögler, O.; Carvalho, F.P. Increasing the impact of science and technology to provide more people with healthier and safer food. Food Energy Secur. 2020, 10, e259. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.d.C.; Flores, A.; Rojas-Sánchez, B.; Urtis-Flores, C.A.; Morales-Cedeño, L.R.; Valencia-Marin, M.F.; Chávez-Avila, S.; Rojas-Solis, D.; Santoyo, G. Plant growth-promoting bacteria as bioinoculants: Attributes and challenges for sustainable crop improvement. Agronomy 2021, 11, 1167. [Google Scholar] [CrossRef]
- de Souza, R.; Ambrosini, A.; Passaglia, L.M.P. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef]
- Dastogeer, K.M.G.; Tumpa, F.H.; Sultana, A.; Akter, M.A.; Chakraborty, A. Plant microbiome-an account of the factors that shape community composition and diversity. Curr. Plant Biol. 2020, 23, 100161. [Google Scholar] [CrossRef]
- Santoyo, G.; Hernández-Pacheco, C.; Hernández-Salmerón, J.; Hernández-León, R. The role of abiotic factors modulating the plant-microbe-soil interactions: Toward sustainable agriculture. A review. Span. J. Agric. Res. 2017, 15, e03R01. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Chen, H.Y.H.; Chen, X.; Huang, Z. Meta-analysis shows positive effects of plant diversity on microbial biomass and respiration. Nat. Commun. 2019, 10, 1332. [Google Scholar] [CrossRef] [Green Version]
- Santoyo, G. How plants recruit their microbiome? New insights into beneficial interactions. J. Adv. Res. 2021; in press. [Google Scholar] [CrossRef]
- Romano, I.; Ventorino, V.; Pepe, O. Effectiveness of plant beneficial microbes: Overview of the methodological approaches for the assessment of root colonization and persistence. Front. Plant Sci. 2020, 11, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, J.; Johnson, C.; Santos-Medellín, C.; Lurie, E.; Podishetty, N.K.; Bhatnagar, S.; Eisen, J.A.; Sundaresan, V. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc. Natl. Acad. Sci. USA 2015, 112, E911–E920. [Google Scholar] [CrossRef] [Green Version]
- Carro, L.; Nouioui, I. Taxonomy and systematics of plant probiotic bacteria in the genomic era. AIMS Microbiol. 2017, 3, 383–412. [Google Scholar] [CrossRef] [PubMed]
- Bertola, M.; Ferrarini, A.; Visioli, G. Improvement of soil microbial diversity through sustainable agricultural practices and its evaluation by -omics approaches: A perspective for the environment, food quality and human safety. Microorganisms 2021, 9, 1400. [Google Scholar] [CrossRef] [PubMed]
- Lombard, N.; Prestat, E.; van Elsas, J.D.; Simonet, P. Soil-specific limitations for access and analysis of soil microbial communities by metagenomics. FEMS Microbiol. Ecol. 2011, 78, 31–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, R.; Li, W.; Dong, W.; Tian, Y.; Hu, C.; Liu, B. Tillage changes vertical distribution of soil bacterial and fungal communities. Front. Microbiol. 2018, 9, 699. [Google Scholar] [CrossRef]
- Grover, M.; Bodhankar, S.; Sharma, A.; Sharma, P.; Singh, J.; Nain, L. PGPR mediated alterations in root traits: Way toward sustainable crop production. Front. Sustain. Food Syst. 2021, 4, 618230. [Google Scholar] [CrossRef]
- Barros, E.; Lezar, S.; Anttonen, M.J.; van Dijk, J.P.; Röhlig, R.M.; Kok, E.J.; Engel, K.H. Comparison of two GM maize varieties with a near-isogenic non-GM variety using transcriptomics, proteomics and metabolomics. Plant Biotechnol. J. 2010, 8, 436–451. [Google Scholar] [CrossRef]
- Bodor, A.; Bounedjoum, N.; Vincze, G.E.; Erdeiné Kis, Á.; Laczi, K.; Bende, G.; Szilágyi, Á.; Kovács, T.; Perei, K.; Rákhely, G. Challenges of unculturable bacteria: Environmental perspectives. Rev. Environ. Sci. Biotechnol. 2020, 19, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Lahlali, R.; Ibrahim, D.S.S.; Belabess, Z.; Kadir Roni, M.Z.; Radouane, N.; Vicente, C.S.L.; Menéndez, E.; Mokrini, F.; Barka, E.A.; Galvão de Melo E Mota, M.; et al. High-throughput molecular technologies for unraveling the mystery of soil microbial community: Challenges and future prospects. Heliyon 2021, 7, e08142. [Google Scholar] [CrossRef]
- Hassan, M.K.; McInroy, J.A.; Kloepper, J.W. The interactions of rhizodeposits with plant growth-promoting rhizobacteria in the rhizosphere: A review. Agriculture 2019, 9, 142. [Google Scholar] [CrossRef] [Green Version]
- Subramoni, S.; Nathoo, N.; Klimov, E.; Yuan, Z. Agrobacterium tumefaciens responses to plant-derived signaling molecules. Front. Plant Sci. 2014, 5, 322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Z.; Williams, M. A really useful pathogen, Agrobacterium tumefaciens. Plant Cell 2012, 24, tpc-112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, C.; Singh, B.K.; He, J.; Han, Y.; Li, P.; Wan, L.; Meng, G.; Liu, S.; Wang, J.; Wu, C.; et al. Plant developmental stage drives the differentiation in ecological role of the maize microbiome. Microbiome 2021, 9, 171. [Google Scholar] [CrossRef]
- Wagner, M.R.; Lundberg, D.S.; del Rio, T.G.; Tringe, S.G.; Dangl, J.L.; Mitchell-Olds, T. Host genotype and age shape the leaf and root microbiomes of a wild perennial plant. Nat. Commun. 2016, 7, 12151. [Google Scholar] [CrossRef] [Green Version]
- Watt, M.; Fiorani, F.; Usadel, B.; Rascher, U.; Muller, O.; Schurr, U. Phenotyping: New windows into the plant for breeders. Ann. Rev. Plant Biol. 2020, 71, 689–712. [Google Scholar] [CrossRef]
- Walter, A.; Liebisch, F.; Hund, A. Plant phenotyping: From bean weighing to image analysis. Plant Methods 2015, 11, 14. [Google Scholar] [CrossRef] [Green Version]
- Pieruschka, R.; Schurr, U. Plant phenotyping: Past, present, and future. Plant Phenomics 2019, 2019, 7507131. [Google Scholar] [CrossRef]
- Busby, P.E.; Soman, C.; Wagner, M.R.; Friesen, M.L.; Kremer, J.; Bennett, A.; Morsy, M.; Eisen, J.A.; Leach, J.E.; Dangl, J.L. Research priorities for harnessing plant microbiomes in sustainable agriculture. PLoS Biol. 2017, 15, e2001793. [Google Scholar] [CrossRef]
- Rahaman, M.; Chen, D.; Gillani, Z.; Klukas, C.; Chen, M. Advanced phenotyping and phenotype data analysis for the study of plant growth and development. Front. Plant Sci. 2015, 6, 619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rascher, U.; Blossfeld, S.; Fiorani, F.; Jahnke, S.; Jansen, M.; Kuhn, A.J.; Matsubara, S.; Märtin, L.L.A.; Merchant, A.; Metzner, R.; et al. Non-invasive approaches for phenotyping of enhanced performance traits in bean. Funct. Plant Biol. 2011, 38, 968–983. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Zhang, Q.; Huang, D. A review of imaging techniques for plant phenotyping. Sensors 2014, 14, 20078–20111. [Google Scholar] [CrossRef]
- Rascher, U.; Nedbal, L. Dynamics of photosynthesis in fluctuating light-commentary. Curr. Opin. Plant Biol. 2006, 9, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Schurr, U.; Walter, A.; Rascher, U. Functional dynamics of plant growth and photosynthesis—From steady-state to dynamics—From homogeneity to heterogeneity. Plant Cell Environ. 2006, 29, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Blumwald, E. Genetic engineering for modern agriculture: Challenges and perspectives. Ann. Rev. Plant Biol. 2010, 61, 443–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razzaq, A.; Kaur, P.; Akhter, N.; Wani, S.H.; Saleem, F. Next-Generation breeding strategies for climate-ready crops. Front. Plant Sci. 2021, 12, 620420. [Google Scholar] [CrossRef]
- Wei, Z.; Jousset, A. Plant breeding goes microbial. Trends Plant Sci. 2017, 22, 555–558. [Google Scholar] [CrossRef]
- Richardson, A.E.; Simpson, R.J. Soil microorganisms mediating phosphorus availability update on microbial phosphorus. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef] [Green Version]
- Lu, T.; Ke, M.; Lavoie, M.; Jin, Y.; Fan, X.; Zhang, Z.; Fu, Z.; Sun, L.; Gillings, M.; Peñuelas, J.; et al. Rhizosphere microorganisms can influence the timing of plant flowering. Microbiome 2018, 6, 231. [Google Scholar] [CrossRef]
- Sandrini, M.; Nerva, L.; Sillo, F.; Balestrini, R.; Chitarra, W.; Zampieri, E. Abiotic stress and belowground microbiome: The potential of omics approaches. Int. J. Mol. Sci. 2022, 23, 1091. [Google Scholar] [CrossRef] [PubMed]
- Bianco, C.; Defez, R. Medicago truncatula improves salt tolerance when nodulated by an indole-3-acetic acid-overproducing Sinorhizobium meliloti strain. J. Exp. Bot. 2009, 60, 3097–3107. [Google Scholar] [CrossRef] [PubMed]
- Bianco, C.; Defez, R. Improvement of phosphate solubilization and Medicago plant yield by an indole-3-acetic acid-overproducing strain of Sinorhizobium meliloti. Appl. Environ. Microbiol. 2010, 76, 4626–4632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Hidalgo, P.; Maymon, M.; Pule-Meulenberg, F.; Hirsch, A.M. Engineering root microbiomes for healthier crops and soils using beneficial, environmentally safe bacteria. Can. J. Microbiol. 2019, 65, 91–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orozco-Mosqueda, M.d.C.; Rocha-Granados, M.d.C.; Glick, B.R.; Santoyo, G. Microbiome engineering to improve biocontrol and plant growth-promoting mechanisms. Microbiol. Res. 2018, 208, 25–31. [Google Scholar] [CrossRef]
- Levy, A.; Conway, J.M.; Dangl, J.L.; Woyke, T. Elucidating bacterial gene functions in the plant microbiome. Cell Host Microbe 2018, 24, 475–485. [Google Scholar] [CrossRef] [Green Version]
- Ravanbakhsh, M.; Kowalchuk, G.A.; Jousset, A. Targeted plant hologenome editing for plant trait enhancement. New Phytol. 2021, 229, 1067–1077. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Garrido-Oter, R.; Münch, P.C.; Weiman, A.; Dröge, J.; Pan, Y.; McHardy, A.C.; Schulze-Lefert, P. Structure and function of the bacterial root microbiota in wild and domesticated barley. Cell Host Microbe 2015, 17, 392–403. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Jaramillo, J.E.; Carrión, V.J.; de Hollander, M.; Raaijmakers, J.M. The wild side of plant microbiomes. Microbiome 2018, 6, 143. [Google Scholar] [CrossRef] [Green Version]
- Knott, G.J.; Doudna, J.A. CRISPR-Cas guides the future of genetic engineering. Science 2018, 361, 866–869. [Google Scholar] [CrossRef] [Green Version]
- Zaidi, S.S.; Mukhtar, M.S.; Mansoor, S. Genome editing: Targeting susceptibility genes for plant disease resistance. Trends Biotechnol. 2018, 36, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.S.; Mahas, A.; Vanderschuren, H.; Mahfouz, M.M. Engineering crops of the future: CRISPR approaches to develop climate-resilient and disease-resistant plants. Genome Biol. 2020, 21, 289. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Borrill, P. Blurring the boundaries between cereal crops and model plants. New Phytol. 2020, 228, 1721–1727. [Google Scholar] [CrossRef]
- Shelake, R.M.; Pramanik, D.; Kim, J. Exploration of plant-microbe interactions for sustainable agriculture in CRISPR Era. Microorganisms 2019, 7, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherif-Silini, H.; Silini, A.; Chenari Bouket, A.; Alenezi, F.N.; Luptakova, L.; Bouremani, N.; Nowakowska, J.A.; Oszako, T.; Belbahri, L. Tailoring next generation plant growth promoting microorganisms as versatile tools beyond soil desalinization: A road map towards field application. Sustainability 2021, 13, 4422. [Google Scholar] [CrossRef]
- Bonatelli, M.L.; Vieira Lacerda-Junior, G.; Bueno dos Reis Junior, F.; Fernandes-Junior, P.I.; Melo, I.S.; Quecine, M.C. Beneficial plant-associated microorganisms from semiarid regions and seasonally dry environments: A review. Front. Microbiol. 2021, 11, 553223. [Google Scholar] [CrossRef] [PubMed]
- Mokrani, S.; Nabti, E.; Cruz, C. Current advances in plant growth promoting bacteria alleviating salt stress for sustainable agriculture. Appl. Sci. 2020, 10, 7025. [Google Scholar] [CrossRef]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef]
- Goswami, M.; Deka, S. Plant growth-promoting rhizobacteria-alleviators of abiotic stresses in soil: A review. Pedosphere 2020, 30, 40–61. [Google Scholar] [CrossRef]
- Cavicchioli, R.; Ripple, W.J.; Timmis, K.N.; Azam, F.; Bakken, L.R.; Baylis, M.; Behrenfeld, M.J.; Boetius, A.; Boyd, P.W.; Classen, A.T.; et al. Scientists’ warning to humanity: Microorganisms and climate change. Nat. Rev. Microbiol. 2019, 17, 569–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Compant, S.; van der Heijden, M.G.A.; Sessitsch, A. Climate change effects on beneficial plant-microorganism interactions. FEMS Microbiol. Ecol. 2010, 73, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Bano, A.; Khan, N. Climate change and salinity effects on crops and chemical communication between plants and plant growth-promoting microorganisms under stress. Front. Sustain. Food Syst. 2021, 5, 618092. [Google Scholar] [CrossRef]
- Fiodor, A.; Singh, S.; Pranaw, K. The contrivance of plant growth promoting microbes to mitigate climate change impact in agriculture. Microorganisms 2021, 9, 1841. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Nazari, M.; Antar, M.; Msimbira, L.A.; Naamala, J.; Lyu, D.; Rabileh, M.; Zajonc, J.; Smith, D.L. PGPR in agriculture: A sustainable approach to increasing climate change resilience. Front. Sustain. Food Syst. 2021, 5, 667546. [Google Scholar] [CrossRef]
- Abdelaal, K.; AlKahtani, M.; Attia, K.; Hafez, Y.; Király, L.; Künstler, A. The role of plant growth-promoting bacteria in alleviating the adverse effects of drought on plants. Biology 2021, 10, 520. [Google Scholar] [CrossRef]
- Trivedi, P.; Batista, B.D.; Bazany, K.E.; Singh, B.K. Plant–microbiome interactions under achanging world: Responses, consequences and perspectives. New Phytol. 2022. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Kumar, N.; Shandilya, C.; Mohapatra, S.; Bhayana, S.; Varma, A. Revisiting plant–microbe interactions and microbial consortia application for enhancing sustainable agriculture: A review. Front. Microbiol. 2020, 11, 560406. [Google Scholar] [CrossRef]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R.; Hernandez, J. Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Lur, H.; Liu, C. From lab to farm: Elucidating the beneficial roles of photosynthetic bacteria in sustainable agriculture. Microorganisms 2021, 9, 2453. [Google Scholar] [CrossRef]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant growth promoting rhizobacteria (PGPR) as green bioinoculants: Recent developments, constraints, and prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Mitter, E.K.; Tosi, M.; Obregón, D.; Dunfield, K.E.; Germida, J.J. Rethinking crop nutrition in times of modern microbiology: Innovative biofertilizer technologies. Front. Sustain. Food Syst. 2021, 5, 606815. [Google Scholar] [CrossRef]
- Bamdad, H.; Papari, S.; Lazarovits, G.; Berruti, F. Soil amendments for sustainable agriculture: Microbial organic fertilizers. Soil Use Manag. 2021, 38, 94–120. [Google Scholar] [CrossRef]
- Germanà, M.A.; Micheli, M.; Chiancone, B.; Bianco, C.; Casales, F.G.; Defez, R. Biotization of encapsulated in vitro derived propagules of Carrizo citrange. Acta Hortic. 2015, 1065, 663–669. [Google Scholar] [CrossRef]
- Saberi Riseh, R.; Ebrahimi-Zarandi, M.; Gholizadeh Vazvani, M.; Skorik, Y.A. Reducing drought stress in plants by encapsulating plant growth-promoting bacteria with polysaccharides. Int. J. Mol. Sci. 2021, 22, 12979. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.K.; Gupta, A.; Singh, M.; Mehrotra, P.; Brahmaprakash, G.P. Bioformulation and fluid bed drying: A new approach towards an improved biofertilizer formulation. In Eco-Friendly Agro-Biological Techniques for Enhancing Crop Productivity; Segar, R., Singh, A., Eds.; Springer: Singapore, 2018; pp. 47–62. [Google Scholar] [CrossRef]
- Berninger, T.; López, O.G.; Bejarano, A.; Preininger, C.; Sessitsch, A. Maintenance and assessment of cell viability in formulation of non-sporulating bacterial inoculants. Microb. Biotechnol. 2018, 11, 277–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalayu, G. Phosphate solubilizing microorganisms: Promising approach as biofertilizers. Int. J. Agron. 2019, 2019, 4917256. [Google Scholar] [CrossRef]
- Sanches Santos, M.; Nogueira, M.A.; Hungria, M. Microbial inoculants: Reviewing the past, discussing the present and previewing an outstanding future for the use of beneficial bacteria in agriculture. AMB Express 2019, 9, 205. [Google Scholar] [CrossRef]
- Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; de Lourdes Bastos, M.; Bories, G.; Chesson, A.; Cocconcelli, P.S.; Flachowsky, G.; Gropp, J.; et al. Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA J. 2018, 16, 5206. [Google Scholar] [CrossRef]
- Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying Down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003 (Text with EEA Relevance). Available online: http://data.europa.eu/eli/reg/2019/1009/oj (accessed on 14 April 2022).
- Goulet, F. Biological inputs and agricultural policies in South America: Between disruptive innovation and continuity. Perspective 2021, 55, 1–4. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).