Advances in the Understanding of the Lifecycle of Photosystem II
Abstract
1. Introduction: Photosystem II and Cyanobacteria
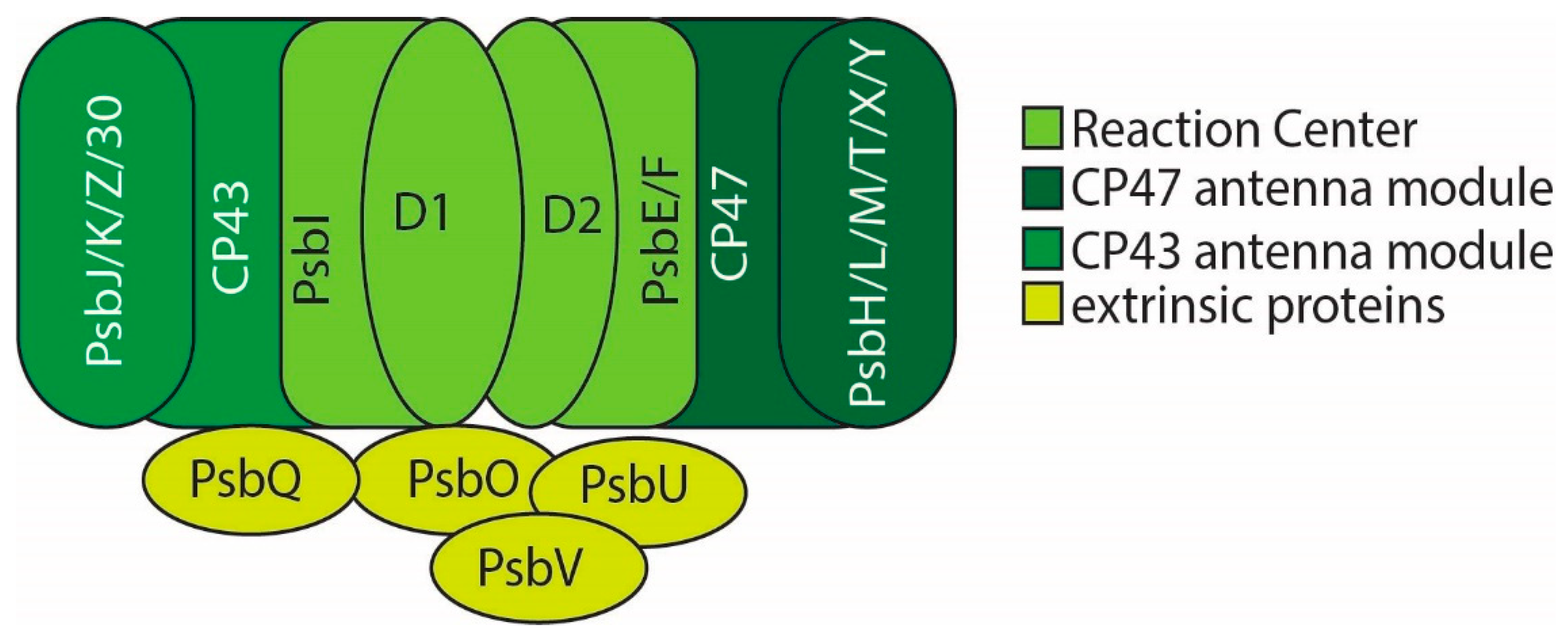
2. PSII Structure
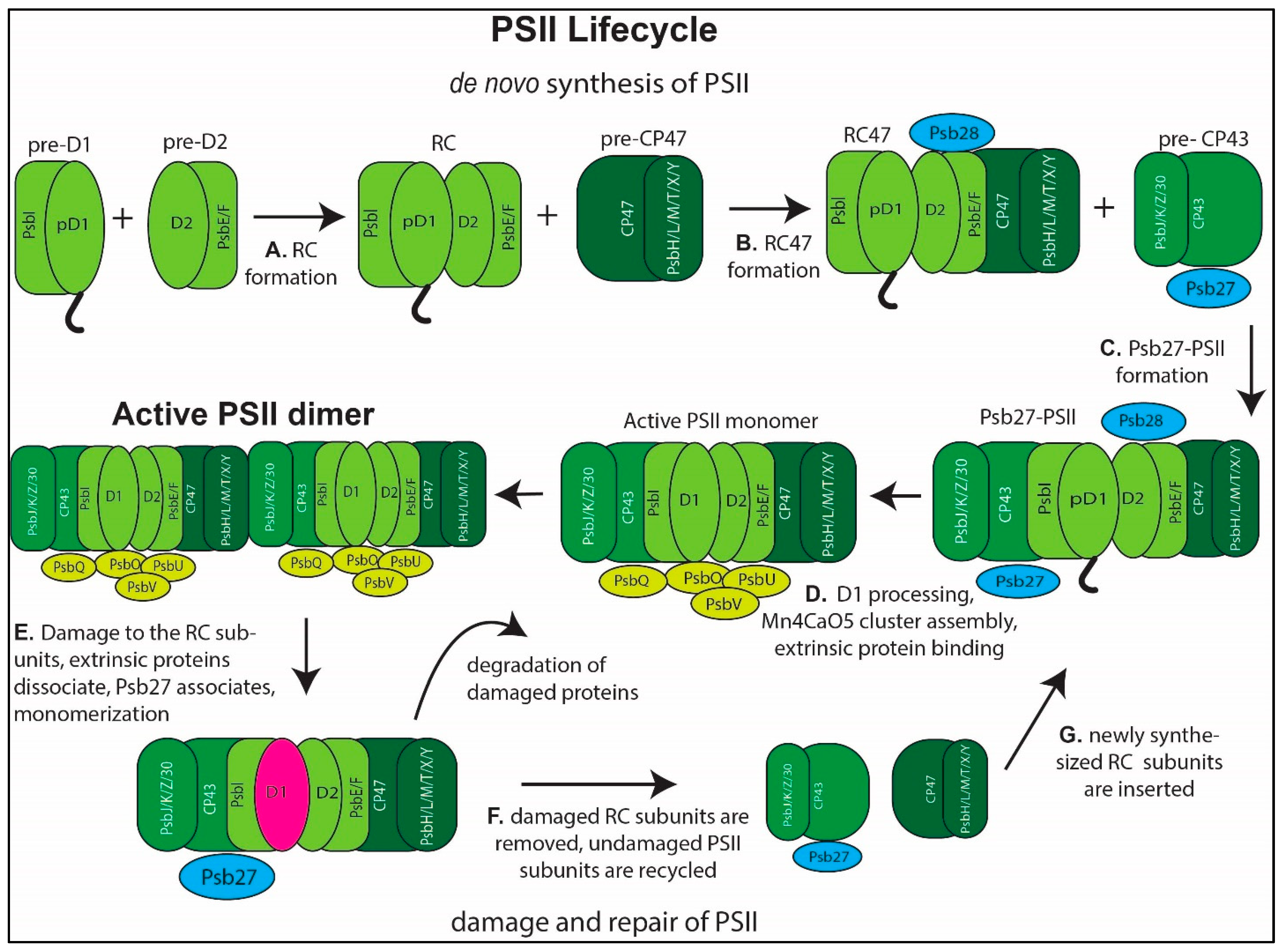
3. PSII Assembly
| Name | Function | S6803 Locus Tag | Homolog in A. thaliana or C. reinhardtii | Phenotype of Inactivation | Citations |
|---|---|---|---|---|---|
| PsbN | RC formation | smr0009 | PsbN | Not significant in cyanobacteria | [21,49,65] |
| PsbP (cyanoP) | RC formation | sll1418 | PsbP | Reduced O2 evolution, severe phenotype in low CaCl2 | [22,52,54] |
| Psb27 | Binds to CP43 during assembly | slr1645 | Psb27 | Defective photoactivation, sensitive to high light | [45,66,67,68,69,70,71,72,73,74] |
| Psb28 | Binds to CP47/cytochrome b559 in RC complex and Psb27-PSII complex. Alters electron transfer properties to increase photoprotection | sll1398 | Psb28 | Susceptible to photoinhibition in high light | [46,56,59,60,63,64,71,73] |
| Psb29 | Accumulation of FtsH2/FtsH3 | sll1414 | Psb29/Thf1 | Impaired growth in high light, lower PSII efficiency | [75,76] |
| Psb32 | ? | sll1390 | TLP18.3 | Sensitive to photoinhibition | [77,78,79,80] |
| Psb34 | Binds to RC47 and PSII-I prior to activation | ssl1498 | ? | N/A | [61,62,81] |
| Psb35 | Pre-CP47 | ssl2148 | ? | Lower CP47 accumulation, faster bleaching in dark | [57] |
| CtpA | C-terminal processing of D1 | slr0008 | CtpA | not photosynthetic, no Mn4CaO5 cluster formation | [32,66] |
| HliA | CP47 formation | ssl2542 | ? | Inhibited growth in high light | [39] |
| HliB | CP47 formation | ssr2595 | ? | Inhibited growth in high light | [39] |
| HliC/ScpB | Chlorophyll insertion | ssl1633 | CAB/HLIP/ELIP family (counterpart of OHP1/OHP2) | Inhibited growth in high light, depleted chlorophyll | [21,28,39,40,82] |
| HliD/ScpE | Chlorophyll insertion | ssr1789 | CAB/HLIP/ELIP family (counterpart to OHP1/OHP2) | Inhibited growth in high light, depleted chlorophyll | [21,28,39,40,41] |
| Pam68 | Translation of CP47 and insertion of chlorophyll | sll0933 | PAM68 | Sensitive to high light, low temperature, fluctuating light | [43,44,58] |
| PratA | Mn2+ loading to D1 and D1 processing; thylakoid and plasma membrane connection | slr2048 | LPA1 | abnormal membranes, reduced PSII accumulation | [34,35,36,37] |
| RubA | D1/D2 assembly | slr2033 | RBD1, At1g54500 | Reduced PSII level and activity | [31,83] |
| Slr0144-Slr0152 | PSII assembly associated | slr0144-slr0152 | ? | Slower growth and lower PSII activity | [47,84,85] |
| Ycf39 | Pre-D2 stabilization, chlorophyll insertion | slr0399 | HCF244 | Decrease in thermotolerance | [21,28,41,44] |
| Ycf48 | Insertion of D1, chlorophyll into D1, replacement of damaged D1, RC formation | slr2034 | HCF136 | Decrease in D1, PSII, increase in susceptibility to photoinhibition | [21,27,31,44,45,54,86] |
4. PSII Repair
5. Structural Advances in PSII Research
6. Advances in Photoactivation
7. Use of CRISPR and CRISPR Interference for Study of Photosynthetic Protein Complexes
8. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kok, B.; Forbush, B.; McGloin, M. Cooperation of charges in photosynthetic O2 evolution-I. A linear four step mechanism. Photochem. Photobiol. 1970, 11, 457–475. [Google Scholar] [CrossRef] [PubMed]
- Umena, Y.; Kawakami, K.; Shen, J.-R.; Kamiya, N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 2011, 473, 55–60. [Google Scholar] [CrossRef]
- Suga, M.; Akita, F.; Hirata, K.; Ueno, G.; Murakami, H.; Nakajima, Y.; Shimizu, T.; Yamashita, K.; Yamamoto, M.; Ago, H.; et al. Native structure of photosystem II at 1.95 A resolution viewed by femtosecond X-ray pulses. Nature 2015, 517, 99–103. [Google Scholar] [CrossRef]
- Young, I.D.; Ibrahim, M.; Chatterjee, R.; Gul, S.; Fuller, F.; Koroidov, S.; Brewster, A.S.; Tran, R.; Alonso-Mori, R.; Kroll, T.; et al. Structure of photosystem II and substrate binding at room temperature. Nature 2016, 540, 453–457. [Google Scholar] [CrossRef]
- Kern, J.; Chatterjee, R.; Young, I.D.; Fuller, F.D.; Lassalle, L.; Ibrahim, M.; Gul, S.; Fransson, T.; Brewster, A.S.; Alonso-Mori, R.; et al. Structures of the intermediates of Kok’s photosynthetic water oxidation clock. Nature 2018, 563, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Gisriel, C.J.; Wang, J.; Liu, J.; Flesher, D.A.; Reiss, K.M.; Huang, H.L.; Yang, K.R.; Armstrong, W.H.; Gunner, M.R.; Batista, V.S.; et al. High-resolution cryo-electron microscopy structure of photosystem II from the mesophilic cyanobacterium, Synechocystis sp. PCC 6803. Proc. Natl. Acad. Sci. USA 2022, 119, e2116765118. [Google Scholar] [CrossRef]
- Vass, I. Molecular mechanisms of photodamage in the Photosystem II complex. Biochim. Biophys. Acta 2012, 1817, 209–217. [Google Scholar] [CrossRef]
- Zavafer, A. A theoretical framework of the hybrid mechanism of photosystem II photodamage. Photosynth. Res. 2021, 149, 107–120. [Google Scholar] [CrossRef]
- Nixon, P.J.; Michoux, F.; Yu, J.; Boehm, M.; Komenda, J. Recent advances in understanding the assembly and repair of photosystem II. Ann. Bot. 2010, 106, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Komenda, J.; Sobotka, R.; Nixon, P.J. Assembling and maintaining the Photosystem II complex in chloroplasts and cyanobacteria. Curr. Opin. Plant. Biol. 2012, 15, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Nickelsen, J.; Rengstl, B. Photosystem II assembly: From cyanobacteria to plants. Annu. Rev. Plant Biol. 2013, 64, 609–635. [Google Scholar] [CrossRef]
- Vinyard, D.J.; Ananyev, G.M.; Dismukes, G.C. Photosystem II: The reaction center of oxygenic photosynthesis. Annu. Rev. Biochem. 2013, 82, 577–606. [Google Scholar] [CrossRef]
- Jarvi, S.; Suorsa, M.; Aro, E.M. Photosystem II repair in plant chloroplasts--Regulation, assisting proteins and shared components with photosystem II biogenesis. Biochim. Biophys. Acta 2015, 1847, 900–909. [Google Scholar] [CrossRef]
- Weisz, D.A.; Gross, M.L.; Pakrasi, H.B. The Use of Advanced Mass Spectrometry to Dissect the Life-Cycle of Photosystem II. Front. Plant Sci. 2016, 7, 617. [Google Scholar] [CrossRef] [PubMed]
- Heinz, S.; Liauw, P.; Nickelsen, J.; Nowaczyk, M. Analysis of photosystem II biogenesis in cyanobacteria. Biochim. Biophys. Acta 2016, 1857, 274–287. [Google Scholar] [CrossRef]
- Theis, J.; Schroda, M. Revisiting the photosystem II repair cycle. Plant. Signal. Behav. 2016, 11, e1218587. [Google Scholar] [CrossRef]
- Barber, J. Photosystem II: The water splitting enzyme of photosynthesis and the origin of oxygen in our atmosphere. Q. Rev. Biophys. 2016, 49, e14. [Google Scholar] [CrossRef]
- Muh, F.; Zouni, A. Structural basis of light-harvesting in the photosystem II core complex. Protein Sci. 2020, 29, 1090–1119. [Google Scholar] [CrossRef]
- Nanba, O.; Satoh, K. Isolation of a photosystem II reaction center consisting of D-1 and D-2 polypeptides and cytochrome b-559. Proc. Natl. Acad. Sci. USA 1987, 84, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, M.; Inoue, Y. A new photosystem II reaction center component (4.8 kDa protein) encoded by chloroplast genome. FEBS Lett. 1988, 241, 99–104. [Google Scholar] [CrossRef]
- Knoppova, J.; Sobotka, R.; Yu, J.; Beckova, M.; Pilny, J.; Trinugroho, J.P.; Csefalvay, L.; Bina, D.; Nixon, P.J.; Komenda, J. Assembly of D1/D2 complexes of photosystem II: Binding of pigments and a network of auxiliary proteins. Plant Physiol. 2022, kiac045. [Google Scholar] [CrossRef]
- Thornton, L.E.; Ohkawa, H.; Roose, J.L.; Kashino, Y.; Keren, N.; Pakrasi, H.B. Homologs of plant PsbP and PsbQ proteins are necessary for regulation of photosystem ii activity in the cyanobacterium Synechocystis 6803. Plant. Cell 2004, 16, 2164–2175. [Google Scholar] [CrossRef]
- Roose, J.L.; Wegener, K.M.; Pakrasi, H.B. The extrinsic proteins of Photosystem II. Photosynth. Res. 2007, 92, 369–387. [Google Scholar] [CrossRef]
- Bricker, T.M.; Roose, J.L.; Fagerlund, R.D.; Frankel, L.K.; Eaton-Rye, J.J. The extrinsic proteins of Photosystem II. Biochim. Biophys. Acta 2012, 1817, 121–142. [Google Scholar] [CrossRef]
- Gisriel, C.J.; Brudvig, G.W. Comparison of PsbQ and Psb27 in photosystem II provides insight into their roles. Photosynth. Res. 2022. [Google Scholar] [CrossRef]
- Dobakova, M.; Tichy, M.; Komenda, J. Role of the PsbI protein in photosystem II assembly and repair in the cyanobacterium Synechocystis sp. PCC 6803. Plant Physiol. 2007, 145, 1681–1691. [Google Scholar] [CrossRef]
- Komenda, J.; Nickelsen, J.; Tichy, M.; Prasil, O.; Eichacker, L.A.; Nixon, P.J. The cyanobacterial homologue of HCF136/YCF48 is a component of an early photosystem II assembly complex and is important for both the efficient assembly and repair of photosystem II in Synechocystis sp. PCC 6803. J. Biol. Chem. 2008, 283, 22390–22399. [Google Scholar] [CrossRef] [PubMed]
- Knoppova, J.; Sobotka, R.; Tichy, M.; Yu, J.; Konik, P.; Halada, P.; Nixon, P.J.; Komenda, J. Discovery of a chlorophyll binding protein complex involved in the early steps of photosystem II assembly in Synechocystis. Plant Cell 2014, 26, 1200–1212. [Google Scholar] [CrossRef]
- Muller, B.; Eichacker, L.A. Assembly of the D1 precursor in monomeric photosystem II reaction center precomplexes precedes chlorophyll a-triggered accumulation of reaction center II in barley etioplasts. Plant Cell 1999, 11, 2365–2377. [Google Scholar]
- Komenda, J.; Reisinger, V.; Muller, B.C.; Dobakova, M.; Granvogl, B.; Eichacker, L.A. Accumulation of the D2 protein is a key regulatory step for assembly of the photosystem II reaction center complex in Synechocystis PCC 6803. J. Biol. Chem. 2004, 279, 48620–48629. [Google Scholar] [CrossRef]
- Kiss, E.; Knoppova, J.; Aznar, G.P.; Pilny, J.; Yu, J.; Halada, P.; Nixon, P.J.; Sobotka, R.; Komenda, J. A Photosynthesis-Specific Rubredoxin-Like Protein Is Required for Efficient Association of the D1 and D2 Proteins during the Initial Steps of Photosystem II Assembly. Plant Cell 2019, 31, 2241–2258. [Google Scholar] [CrossRef]
- Anbudurai, P.R.; Mor, T.S.; Ohad, I.; Shestakov, S.V.; Pakrasi, H.B. The ctpA gene encodes the C-terminal processing protease for the D1 protein of the photosystem II reaction center complex. Proc. Natl. Acad. Sci. USA 1994, 91, 8082–8086. [Google Scholar] [CrossRef]
- Komenda, J.; Kuvikova, S.; Granvogl, B.; Eichacker, L.A.; Diner, B.A.; Nixon, P.J. Cleavage after residue Ala352 in the C-terminal extension is an early step in the maturation of the D1 subunit of Photosystem II in Synechocystis PCC 6803. Biochim. Biophys. Acta 2007, 1767, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Stengel, A.; Gugel, I.L.; Hilger, D.; Rengstl, B.; Jung, H.; Nickelsen, J. Initial steps of photosystem II de novo assembly and preloading with manganese take place in biogenesis centers in Synechocystis. Plant Cell 2012, 24, 660–675. [Google Scholar] [CrossRef]
- Schottkowski, M.; Gkalympoudis, S.; Tzekova, N.; Stelljes, C.; Schunemann, D.; Ankele, E.; Nickelsen, J. Interaction of the periplasmic PratA factor and the PsbA (D1) protein during biogenesis of photosystem II in Synechocystis sp. PCC 6803. J. Biol. Chem. 2009, 284, 1813–1819. [Google Scholar] [CrossRef]
- Klinkert, B.; Ossenbuhl, F.; Sikorski, M.; Berry, S.; Eichacker, L.; Nickelsen, J. PratA, a periplasmic tetratricopeptide repeat protein involved in biogenesis of photosystem II in Synechocystis sp. PCC 6803. J. Biol. Chem. 2004, 279, 44639–44644. [Google Scholar] [CrossRef]
- Rengstl, B.; Oster, U.; Stengel, A.; Nickelsen, J. An intermediate membrane subfraction in cyanobacteria is involved in an assembly network for Photosystem II biogenesis. J. Biol. Chem. 2011, 286, 21944–21951. [Google Scholar] [CrossRef]
- Garcia-Cerdan, J.G.; Furst, A.L.; McDonald, K.L.; Schunemann, D.; Francis, M.B.; Niyogi, K.K. A thylakoid membrane-bound and redox-active rubredoxin (RBD1) functions in de novo assembly and repair of photosystem II. Proc. Natl. Acad. Sci. USA 2019, 116, 16631–16640. [Google Scholar] [CrossRef]
- Komenda, J.; Sobotka, R. Cyanobacterial high-light-inducible proteins--Protectors of chlorophyll-protein synthesis and assembly. Biochim. Biophys. Acta 2016, 1857, 288–295. [Google Scholar] [CrossRef]
- Yao, D.C.; Brune, D.C.; Vavilin, D.; Vermaas, W.F. Photosystem II component lifetimes in the cyanobacterium Synechocystis sp. strain PCC 6803: Small Cab-like proteins stabilize biosynthesis intermediates and affect early steps in chlorophyll synthesis. J. Biol. Chem. 2012, 287, 682–692. [Google Scholar] [CrossRef]
- Chidgey, J.W.; Linhartova, M.; Komenda, J.; Jackson, P.J.; Dickman, M.J.; Canniffe, D.P.; Konik, P.; Pilny, J.; Hunter, C.N.; Sobotka, R. A cyanobacterial chlorophyll synthase-HliD complex associates with the Ycf39 protein and the YidC/Alb3 insertase. Plant Cell 2014, 26, 1267–1279. [Google Scholar] [CrossRef]
- Ossenbuhl, F.; Inaba-Sulpice, M.; Meurer, J.; Soll, J.; Eichacker, L.A. The Synechocystis sp. PCC 6803 oxa1 homolog is essential for membrane integration of reaction center precursor protein pD1. Plant Cell 2006, 18, 2236–2246. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, U.; Zühlke, J.; Rengstl, B.; Kreller, R.; Makarenko, E.; Rühle, T.; Schünemann, D.; Jahns, P.; Weisshaar, B.; Nickelsen, J.; et al. The Arabidopsis thylakoid protein PAM68 is required for efficient D1 biogenesis and photosystem II assembly. Plant Cell 2010, 22, 3439–3460. [Google Scholar] [CrossRef] [PubMed]
- Knoppova, J.; Komenda, J. Sequential deletions of photosystem II assembly factors Ycf48, Ycf39 and Pam68 result in progressive loss of autotrophy in the cyanobacterium Synechocystis PCC 6803. Folia Microbiol. 2019, 64, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.A.; Hervey, J.R.; Dale, A.J.; Eaton-Rye, J.J. Removal of both Ycf48 and Psb27 in Synechocystis sp. PCC 6803 disrupts Photosystem II assembly and alters Q(A)(-) oxidation in the mature complex. FEBS Lett. 2014, 588, 3751–3760. [Google Scholar] [CrossRef]
- Mabbitt, P.D.; Wilbanks, S.M.; Eaton-Rye, J.J. Structure and function of the hydrophilic Photosystem II assembly proteins: Psb27, Psb28 and Ycf48. Plant Physiol. Biochem. 2014, 81, 96–107. [Google Scholar] [CrossRef]
- Rast, A.; Rengstl, B.; Heinz, S.; Klingl, A.; Nickelsen, J. The Role of Slr0151, a Tetratricopeptide Repeat Protein from Synechocystis sp. PCC 6803, during Photosystem II Assembly and Repair. Front. Plant Sci. 2016, 7, 605. [Google Scholar] [CrossRef]
- Yu, J.; Knoppova, J.; Michoux, F.; Bialek, W.; Cota, E.; Shukla, M.K.; Straskova, A.; Pascual Aznar, G.; Sobotka, R.; Komenda, J.; et al. Ycf48 involved in the biogenesis of the oxygen-evolving photosystem II complex is a seven-bladed beta-propeller protein. Proc. Natl. Acad. Sci. USA 2018, 115, E7824–E7833. [Google Scholar] [CrossRef]
- Plochinger, M.; Schwenkert, S.; von Sydow, L.; Schroder, W.P.; Meurer, J. Functional Update of the Auxiliary Proteins PsbW, PsbY, HCF136, PsbN, TerC and ALB3 in Maintenance and Assembly of PSII. Front. Plant Sci. 2016, 7, 423. [Google Scholar] [CrossRef]
- Vermaas, W.F.; Ikeuchi, M.; Inoue, Y. Protein composition of the photosystem II core complex in genetically engineered mutants of the cyanobacterium Synechocystis sp. PCC 6803. Photosynth. Res. 1988, 17, 97–113. [Google Scholar] [CrossRef]
- van Wijk, K.J.; Roobol-Boza, M.; Kettunen, R.; Andersson, B.; Aro, E.M. Synthesis and assembly of the D1 protein into photosystem II: Processing of the C-terminus and identification of the initial assembly partners and complexes during photosystem II repair. Biochemistry 1997, 36, 6178–6186. [Google Scholar] [CrossRef]
- Knoppova, J.; Yu, J.; Konik, P.; Nixon, P.J.; Komenda, J. CyanoP is involved in the Early Steps of Photosystem II Assembly in the Cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 2016, 57, 1921–1931. [Google Scholar] [CrossRef]
- Suorsa, M.; Regel, R.E.; Paakkarinen, V.; Battchikova, N.; Herrmann, R.G.; Aro, E.M. Protein assembly of photosystem II and accumulation of subcomplexes in the absence of low molecular mass subunits PsbL and PsbJ. Eur. J. Biochem. 2004, 271, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.A.; Eaton-Rye, J.J. Characterization of a Synechocystis sp. PCC 6803 double mutant lacking the CyanoP and Ycf48 proteins of Photosystem II. Photosynth. Res. 2015, 124, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Boehm, M.; Romero, E.; Reisinger, V.; Yu, J.; Komenda, J.; Eichacker, L.A.; Dekker, J.P.; Nixon, P.J. Investigating the early stages of photosystem II assembly in Synechocystis sp. PCC 6803: Isolation of CP47 and CP43 complexes. J. Biol. Chem. 2011, 286, 14812–14819. [Google Scholar] [CrossRef]
- Boehm, M.; Yu, J.; Reisinger, V.; Beckova, M.; Eichacker, L.A.; Schlodder, E.; Komenda, J.; Nixon, P.J. Subunit composition of CP43-less photosystem II complexes of Synechocystis sp. PCC 6803: Implications for the assembly and repair of photosystem II. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 3444–3454. [Google Scholar] [CrossRef]
- Pascual-Aznar, G.; Konert, G.; Beckov, M.; Kotabov, E.; Gardian, Z.; Knoppov, J.; Bucinsk, L.; Kana, R.; Sobotka, R.; Komenda, J. Psb35 Protein Stabilizes the CP47 Assembly Module and Associated High-Light Inducible Proteins during the Biogenesis of Photosystem II in the Cyanobacterium Synechocystis sp. PCC6803. Plant. Cell Physiol. 2021, 62, 178–190. [Google Scholar] [CrossRef]
- Bucinska, L.; Kiss, E.; Konik, P.; Knoppova, J.; Komenda, J.; Sobotka, R. The Ribosome-Bound Protein Pam68 Promotes Insertion of Chlorophyll into the CP47 Subunit of Photosystem II. Plant Physiol. 2018, 176, 2931–2942. [Google Scholar] [CrossRef]
- Weisz, D.A.; Liu, H.; Zhang, H.; Thangapandian, S.; Tajkhorshid, E.; Gross, M.L.; Pakrasi, H.B. Mass spectrometry-based cross-linking study shows that the Psb28 protein binds to cytochrome b559 in Photosystem II. Proc. Natl. Acad. Sci. USA 2017, 114, 2224–2229. [Google Scholar] [CrossRef] [PubMed]
- Dobakova, M.; Sobotka, R.; Tichy, M.; Komenda, J. Psb28 protein is involved in the biogenesis of the photosystem II inner antenna CP47 (PsbB) in the cyanobacterium Synechocystis sp. PCC 6803. Plant Physiol. 2009, 149, 1076–1086. [Google Scholar] [CrossRef]
- Zabret, J.; Bohn, S.; Schuller, S.K.; Arnolds, O.; Moller, M.; Meier-Credo, J.; Liauw, P.; Chan, A.; Tajkhorshid, E.; Langer, J.D.; et al. Structural insights into photosystem II assembly. Nat. Plants 2021, 7, 524–538. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Huang, G.; You, X.; Zhu, Q.; Wang, W.; Kuang, T.; Han, G.; Sui, S.F.; Shen, J.R. Structural insights into cyanobacterial photosystem II intermediates associated with Psb28 and Tsl0063. Nat. Plants 2021, 7, 1132–1142. [Google Scholar] [CrossRef] [PubMed]
- Sakata, S.; Mizusawa, N.; Kubota-Kawai, H.; Sakurai, I.; Wada, H. Psb28 is involved in recovery of photosystem II at high temperature in Synechocystis sp. PCC 6803. Biochim. Biophys. Acta 2013, 1827, 50–59. [Google Scholar] [CrossRef]
- Beckova, M.; Gardian, Z.; Yu, J.; Konik, P.; Nixon, P.J.; Komenda, J. Association of Psb28 and Psb27 Proteins with PSII-PSI Supercomplexes upon Exposure of Synechocystis sp. PCC 6803 to High Light. Mol. Plant 2017, 10, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Kashino, Y.; Koike, H.; Yoshio, M.; Egashira, H.; Ikeuchi, M.; Pakrasi, H.B.; Satoh, K. Low-molecular-mass polypeptide components of a photosystem II preparation from the thermophilic cyanobacterium Thermosynechococcus vulcanus. Plant Cell Physiol. 2002, 43, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Roose, J.L.; Pakrasi, H.B. Evidence that D1 processing is required for manganese binding and extrinsic protein assembly into photosystem II. J. Biol. Chem. 2004, 279, 45417–45422. [Google Scholar] [CrossRef]
- Nowaczyk, M.M.; Hebeler, R.; Schlodder, E.; Meyer, H.E.; Warscheid, B.; Rogner, M. Psb27, a cyanobacterial lipoprotein, is involved in the repair cycle of photosystem II. Plant Cell 2006, 18, 3121–3131. [Google Scholar] [CrossRef]
- Roose, J.L.; Pakrasi, H.B. The Psb27 protein facilitates manganese cluster assembly in photosystem II. J. Biol. Chem. 2008, 283, 4044–4050. [Google Scholar] [CrossRef] [PubMed]
- Grasse, N.; Mamedov, F.; Becker, K.; Styring, S.; Rogner, M.; Nowaczyk, M.M. Role of novel dimeric Photosystem II (PSII)-Psb27 protein complex in PSII repair. J. Biol. Chem. 2011, 286, 29548–29555. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Huang, R.Y.; Chen, J.; Gross, M.L.; Pakrasi, H.B. Psb27, a transiently associated protein, binds to the chlorophyll binding protein CP43 in photosystem II assembly intermediates. Proc. Natl. Acad. Sci. USA 2011, 108, 18536–18541. [Google Scholar] [CrossRef]
- Liu, H.; Roose, J.L.; Cameron, J.C.; Pakrasi, H.B. A genetically tagged Psb27 protein allows purification of two consecutive photosystem II (PSII) assembly intermediates in Synechocystis 6803, a cyanobacterium. J. Biol. Chem. 2011, 286, 24865–24871. [Google Scholar] [CrossRef]
- Komenda, J.; Knoppova, J.; Kopecna, J.; Sobotka, R.; Halada, P.; Yu, J.; Nickelsen, J.; Boehm, M.; Nixon, P.J. The Psb27 assembly factor binds to the CP43 complex of photosystem II in the cyanobacterium Synechocystis sp. PCC 6803. Plant Physiol. 2012, 158, 476–486. [Google Scholar] [CrossRef]
- Nowaczyk, M.M.; Krause, K.; Mieseler, M.; Sczibilanski, A.; Ikeuchi, M.; Rogner, M. Deletion of psbJ leads to accumulation of Psb27-Psb28 photosystem II complexes in Thermosynechococcus elongatus. Biochim. Biophys. Acta 2012, 1817, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Xiao, Y.; Pi, X.; Zhao, L.; Zhu, Q.; Wang, W.; Kuang, T.; Han, G.; Sui, S.F.; Shen, J.R. Structural insights into a dimeric Psb27-photosystem II complex from a cyanobacterium Thermosynechococcus vulcanus. Proc. Natl. Acad. Sci. USA 2021, 118, e2018053118. [Google Scholar] [CrossRef]
- Keren, N.; Ohkawa, H.; Welsh, E.A.; Liberton, M.; Pakrasi, H.B. Psb29, a conserved 22-kD protein, functions in the biogenesis of Photosystem II complexes in Synechocystis and Arabidopsis. Plant Cell 2005, 17, 2768–2781. [Google Scholar] [CrossRef] [PubMed]
- Bec Kova, M.; Yu, J.; Krynicka, V.; Kozlo, A.; Shao, S.; Konik, P.; Komenda, J.; Murray, J.W.; Nixon, P.J. Structure of Psb29/Thf1 and its association with the FtsH protease complex involved in photosystem II repair in cyanobacteria. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160394. [Google Scholar] [CrossRef]
- Kashino, Y.; Lauber, W.M.; Carroll, J.A.; Wang, Q.; Whitmarsh, J.; Satoh, K.; Pakrasi, H.B. Proteomic analysis of a highly active photosystem II preparation from the cyanobacterium Synechocystis sp. PCC 6803 reveals the presence of novel polypeptides. Biochemistry 2002, 41, 8004–8012. [Google Scholar] [CrossRef]
- Wegener, K.M.; Bennewitz, S.; Oelmuller, R.; Pakrasi, H.B. The Psb32 protein aids in repairing photodamaged photosystem II in the cyanobacterium Synechocystis 6803. Mol. Plant 2011, 4, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Sirpio, S.; Allahverdiyeva, Y.; Suorsa, M.; Paakkarinen, V.; Vainonen, J.; Battchikova, N.; Aro, E.M. TLP18.3, a novel thylakoid lumen protein regulating photosystem II repair cycle. Biochem. J. 2007, 406, 415–425. [Google Scholar] [CrossRef]
- Jarvi, S.; Isojarvi, J.; Kangasjarvi, S.; Salojarvi, J.; Mamedov, F.; Suorsa, M.; Aro, E.M. Photosystem II Repair and Plant Immunity: Lessons Learned from Arabidopsis Mutant Lacking the THYLAKOID LUMEN PROTEIN 18.3. Front. Plant Sci. 2016, 7, 405. [Google Scholar] [CrossRef]
- Rahimzadeh-Karvansara, P.; Pascual-Aznar, G.; Beckova, M.; Komenda, J. Psb34 protein modulates binding of high-light-inducible proteins to CP47-containing photosystem II assembly intermediates in the cyanobacterium Synechocystis sp. PCC 6803. Photosynth. Res. 2022, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kufryk, G.; Hernandez-Prieto, M.A.; Kieselbach, T.; Miranda, H.; Vermaas, W.; Funk, C. Association of small CAB-like proteins (SCPs) of Synechocystis sp. PCC 6803 with Photosystem II. Photosynth. Res. 2008, 95, 135–145. [Google Scholar] [CrossRef]
- Calderon, R.H.; Garcia-Cerdan, J.G.; Malnoe, A.; Cook, R.; Russell, J.J.; Gaw, C.; Dent, R.M.; de Vitry, C.; Niyogi, K.K. A conserved rubredoxin is necessary for photosystem II accumulation in diverse oxygenic photoautotrophs. J. Biol. Chem. 2013, 288, 26688–26696. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liao, L.; Bo, T.; Zhao, L.; Sun, X.; Lu, X.; Norling, B.; Huang, F. Slr0151 in Synechocystis sp. PCC 6803 is required for efficient repair of photosystem II under high-light condition. J. Integr. Plant Biol. 2014, 56, 1136–1150. [Google Scholar] [CrossRef] [PubMed]
- Wegener, K.M.; Welsh, E.A.; Thornton, L.E.; Keren, N.; Jacobs, J.M.; Hixson, K.K.; Monroe, M.E.; Camp, D.G.; Smith, R.D.; Pakrasi, H.P.; et al. High sensitivity proteomics assisted discovery of a novel operon involved in the assembly of photosystem II, a membrane protein complex. J. Biol. Chem. 2008, 283, 27829–27837. [Google Scholar] [CrossRef]
- Knoppova, J.; Yu, J.; Janouškovec, J.; Halada, P.; Nixon, P.J.; Whitelegge, J.P.; Komenda, J. The Photosystem II Assembly Factor Ycf48 from the Cyanobacterium Synechocystis sp. PCC 6803 Is Lipidated Using an Atypical Lipobox Sequence. Int. J. Mol. Sci. 2021, 22, 3733. [Google Scholar] [CrossRef]
- Liu, H.; Chen, J.; Huang RY, C.; Weisz, D.; Gross, M.L.; Pakrasi, H.B. Mass spectrometry-based footprinting reveals structural dynamics of loop E of the chlorophyll-binding protein CP43 during photosystem II assembly in the cyanobacterium Synechocystis 6803. J. Biol. Chem. 2013, 288, 14212–14220. [Google Scholar] [CrossRef]
- Zhang, S.; Frankel, L.K.; Bricker, T.M. The Sll0606 protein is required for photosystem II assembly/stability in the cyanobacterium Synechocystis sp. PCC 6803. J. Biol. Chem. 2010, 285, 32047–32054. [Google Scholar] [CrossRef] [PubMed]
- Pi, X.; Zhao, S.; Wang, W.; Liu, D.; Xu, C.; Han, G.; Kuang, T.; Sui, S.-F.; Shen, J.-R. The pigment-protein network of a diatom photosystem II-light-harvesting antenna supercomplex. Science 2019, 365, eaax4406. [Google Scholar] [CrossRef]
- Bao, H.; Burnap, R.L. Photoactivation: The Light-Driven Assembly of the Water Oxidation Complex of Photosystem II. Front. Plant Sci. 2016, 7, 578. [Google Scholar] [CrossRef]
- Roose, J.L.; Kashino, Y.; Pakrasi, H.B. The PsbQ protein defines cyanobacterial Photosystem II complexes with highest activity and stability. Proc. Natl. Acad. Sci. USA 2007, 104, 2548–2553. [Google Scholar] [CrossRef]
- Shi, L.X.; Hall, M.; Funk, C.; Schroder, W.P. Photosystem II, a growing complex: Updates on newly discovered components and low molecular mass proteins. Biochim. Biophys. Acta 2012, 1817, 13–25. [Google Scholar] [CrossRef]
- Ohad, I.; Dal Bosco, C.; Herrmann, R.G.; Meurer, J. Photosystem II proteins PsbL and PsbJ regulate electron flow to the plastoquinone pool. Biochemistry 2004, 43, 2297–2308. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, H.; Niedzwiedzki, D.M.; Prado, M.; He, G.; Gross, M.L.; Blankenship, R.E. Phycobilisomes supply excitations to both photosystems in a megacomplex in cyanobacteria. Science 2013, 342, 1104–1107. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.C.; Brune, D.C.; Vermaas, W.F. Lifetimes of photosystem I and II proteins in the cyanobacterium Synechocystis sp. PCC 6803. FEBS Lett. 2012, 586, 169–173. [Google Scholar] [CrossRef]
- Vavilin, D.; Brune, D.C.; Vermaas, W. 15N-labeling to determine chlorophyll synthesis and degradation in Synechocystis sp. PCC 6803 strains lacking one or both photosystems. Biochim. Biophys. Acta 2005, 1708, 91–101. [Google Scholar] [CrossRef]
- Krynicka, V.; Shao, S.; Nixon, P.J.; Komenda, J. Accessibility controls selective degradation of photosystem II subunits by FtsH protease. Nat. Plants 2015, 1, 15168. [Google Scholar] [CrossRef] [PubMed]
- Weisz, D.A.; Johnson, V.M.; Niedzwiedzki, D.M.; Shinn, M.K.; Liu, H.; Klitzke, C.F.; Gross, M.L.; Blankenship, R.E.; Lohman, T.M.; Pakrasi, H.B. A novel chlorophyll protein complex in the repair cycle of photosystem II. Proc. Natl. Acad. Sci. USA 2019, 116, 21907–21913. [Google Scholar] [CrossRef] [PubMed]
- Beckova, M.; Sobotka, R.; Komenda, J. Photosystem II antenna modules CP43 and CP47 do not form a stable ‘no reaction centre complex’ in the cyanobacterium Synechocystis sp. PCC 6803. Photosynth. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Bentley, F.K.; Luo, H.; Dilbeck, P.; Burnap, R.L.; Eaton-Rye, J.J. Effects of inactivating psbM and psbT on photodamage and assembly of photosystem II in Synechocystis sp. PCC 6803. Biochemistry 2008, 47, 11637–11646. [Google Scholar] [CrossRef] [PubMed]
- Fagerlund, R.D.; Forsman, J.A.; Biswas, S.; Vass, I.; Davies, F.K.; Summerfield, T.C.; Eaton-Rye, J.J. Stabilization of Photosystem II by the PsbT protein impacts photodamage, repair and biogenesis. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148234. [Google Scholar] [CrossRef]
- Forsman, J.A.; Eaton-Rye, J.J. The Interaction between PsbT and the DE Loop of D1 in Photosystem II Stabilizes the Quinone-Iron Electron Acceptor Complex. Biochemistry 2021, 60, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Tokano, T.; Kato, Y.; Sugiyama, S.; Uchihashi, T.; Noguchi, T. Structural Dynamics of a Protein Domain Relevant to the Water-Oxidizing Complex in Photosystem II as Visualized by High-Speed Atomic Force Microscopy. J. Phys. Chem. B 2020, 124, 5847–5857. [Google Scholar] [CrossRef] [PubMed]
- Brinkert, K.; De Causmaecker, S.; Krieger-Liszkay, A.; Fantuzzi, A.; Rutherford, A.W. Bicarbonate-induced redox tuning in Photosystem II for regulation and protection. Proc. Natl. Acad. Sci. USA 2016, 113, 12144–12149. [Google Scholar] [CrossRef] [PubMed]
- Vass, I.; Styring, S.; Hundal, T.; Koivuniemi, A.; Aro, E.; Andersson, B. Reversible and irreversible intermediates during photoinhibition of photosystem II: Stable reduced QA species promote chlorophyll triplet formation. Proc. Natl. Acad. Sci. USA 1992, 89, 1408–1412. [Google Scholar] [CrossRef]
- Vass, I.; Styring, S. Spectroscopic characterization of triplet forming states in photosystem II. Biochemistry 1992, 31, 5957–5963. [Google Scholar] [CrossRef] [PubMed]
- Vass, I.; Styring, S. Characterization of chlorophyll triplet promoting states in photosystem II sequentially induced during photoinhibition. Biochemistry 1993, 32, 3334–3341. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.N.; Rutherford, A.W.; Krieger, A. A change in the midpoint potential of the quinone QA in Photosystem II associated with photoactivation of oxygen evolution. Biochim. Biophys. Acta BBA Bioenerg. 1995, 1229, 202–207. [Google Scholar] [CrossRef]
- Johnson, V.M.; Biswas, S.; Roose, J.L.; Pakrasi, H.B.; Liu, H. Psb27, a photosystem II assembly protein, enables quenching of excess light energy during its participation in the PSII lifecycle. Photosynth. 2022. [Google Scholar] [CrossRef]
- Lambertz, J.; Liauw, P.; Whitelegge, J.P.; Nowaczyk, M.M. Mass spectrometry analysis of the photosystem II assembly factor Psb27 revealed variations in its lipid modification. Photosynth. Res. 2021. [Google Scholar] [CrossRef]
- Dasgupta, J.; Ananyev, G.M.; Dismukes, G.C. Photoassembly of the Water-Oxidizing Complex in Photosystem II. Coord. Chem. Rev. 2008, 252, 347–360. [Google Scholar] [CrossRef]
- Hwang, H.J.; Nagarajan, A.; McLain, A.; Burnap, R.L. Assembly and disassembly of the photosystem II manganese cluster reversibly alters the coupling of the reaction center with the light-harvesting phycobilisome. Biochemistry 2008, 47, 9747–9755. [Google Scholar] [CrossRef] [PubMed]
- Radmer, R.; Cheniae, G.M. Photoactivation of the manganese catalyst of 0 2 evolution II. A two-guantum mechanism. Biochim. Biophys. Acta 1971, 253, 182–186. [Google Scholar] [CrossRef]
- Cheniae, G.M.; Martin, I.F. Photoactivation of the manganese catalyst of O 2 evolution. I. Biochemical and kinetic aspects. Biochim. Biophys. Acta 1971, 253, 167–181. [Google Scholar] [CrossRef]
- Zhang, M.; Bommer, M.; Chatterjee, R.; Hussein, R.; Yano, J.; Dau, H.; Kern, J.; Dobbek, H.; Zouni, A. Structural insights into the light-driven auto-assembly process of the water-oxidizing Mn4CaO5-cluster in photosystem II. Elife 2017, 6, e26933. [Google Scholar] [CrossRef] [PubMed]
- Gisriel, C.J.; Zhou, K.F.; Huang, H.L.; Debus, R.J.; Xiong, Y.; Brudvig, G.W. Cryo-EM Structure of Monomeric Photosystem II from Synechocystis sp. PCC 6803 Lacking the Water-Oxidation Complex. Joule 2020, 4, 2131–2148. [Google Scholar] [CrossRef]
- Narzi, D.; Guidoni, L. Structural and dynamic insights into Mn4Ca cluster-depleted Photosystem II. Phys. Chem. Chem. Phys. 2021, 23, 27428–27436. [Google Scholar] [CrossRef] [PubMed]
- Avramov, A.P.; Hwang, H.J.; Burnap, R.L. The role of Ca2+ and protein scaffolding in the formation of nature’s water oxidizing complex. Proc. Natl. Acad. Sci. USA 2020, 117, 28036–28045. [Google Scholar] [CrossRef]
- Russell, B.P.; Vinyard, D.J. Chloride facilitates Mn(III) formation during photoassembly of the Photosystem II oxygen-evolving complex. Photosynth. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Vinyard, D.J.; Badshah, S.L.; Riggio, M.R.; Kaur, D.; Fanguy, A.R.; Gunner, M.R. Photosystem II oxygen-evolving complex photoassembly displays an inverse H/D solvent isotope effect under chloride-limiting conditions. Proc. Natl. Acad. Sci. USA 2019, 116, 18917–18922. [Google Scholar] [CrossRef] [PubMed]
- Ungerer, J.; Pakrasi, H.B. Cpf1 is a versatile tool for CRISPR genome editing across diverse species of Cyanobacteria. Sci. Rep. 2016, 6, 39681. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Cengic, I.; Anfelt, J.; Hudson, E.P. Multiple Gene Repression in Cyanobacteria Using CRISPRi. ACS Synth. Biol. 2016, 5, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Behler, J.; Vijay, D.; Hess, W.R.; Akhtar, M.K. CRISPR-Based Technologies for Metabolic Engineering in Cyanobacteria. Trends Biotechnol. 2018, 36, 996–1010. [Google Scholar] [CrossRef] [PubMed]
- Santos-Merino, M.; Singh, A.K.; Ducat, D.C. New Applications of Synthetic Biology Tools for Cyanobacterial Metabolic Engineering. Front. Bioeng. Biotechnol. 2019, 7, 33. [Google Scholar] [CrossRef]
- Naduthodi, M.I.S.; Barbosa, M.J.; van der Oost, J. Progress of CRISPR-Cas Based Genome Editing in Photosynthetic Microbes. Biotechnol. J. 2018, 13, e1700591. [Google Scholar] [CrossRef]
- Yao, L.; Shabestary, K.; Bjork, S.M.; Asplund-Samuelsson, J.; Joensson, H.N.; Jahn, M.; Hudson, E.P. Pooled CRISPRi screening of the cyanobacterium Synechocystis sp. PCC 6803 for enhanced industrial phenotypes. Nat. Commun. 2020, 11, 1666. [Google Scholar] [CrossRef]
- Santos, M.; Pacheco, C.C.; Yao, L.; Hudson, E.P.; Tamagnini, P. CRISPRi as a Tool to Repress Multiple Copies of Extracellular Polymeric Substances (EPS)-Related Genes in the Cyanobacterium Synechocystis sp. PCC 6803. Life 2021, 11, 1198. [Google Scholar] [CrossRef]
- Knoot, C.J.; Biswas, S.; Pakrasi, H.B. Tunable repression of key photosynthetic processes using Cas12a CRISPR Interference in the fast-growing cyanobacterium Synechococcus sp. UTEX 2973. ACS Synth. Biol. 2020, 9, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Kirtania, P.; Hodi, B.; Mallick, I.; Vass, I.Z.; Feher, T.; Vass, I.; Kos, P.B. A single plasmid based CRISPR interference in Synechocystis 6803—A proof of concept. PLoS ONE 2019, 14, e0225375. [Google Scholar] [CrossRef]
- Liu, D.; Johnson, V.M.; Pakrasi, H.B. A Reversibly Induced CRISPRi System Targeting Photosystem II in the Cyanobacterium Synechocystis sp. PCC 6803. ACS Synth. Biol. 2020, 9, 1441–1449. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, H.; Niedzwiedzki, D.M.; Jiang, J.; Blankenship, R.E.; Gross, M.L. Fast Photochemical Oxidation of Proteins Maps the Topology of Intrinsic Membrane Proteins: Light-Harvesting Complex 2 in a Nanodisc. Anal. Chem. 2016, 88, 8827–8834. [Google Scholar] [CrossRef] [PubMed]
- Denisov, I.G.; Sligar, S.G. Nanodiscs for structural and functional studies of membrane proteins. Nat. Struct. Mol. Biol. 2016, 23, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Marty, M.T. Nanodiscs and Mass Spectrometry: Making Membranes Fly. Int. J. Mass Spectrom. 2020, 458, 116436. [Google Scholar] [CrossRef] [PubMed]
- Korotych, O.I.; Nguyen, T.T.; Reagan, B.C.; Burch-Smith, T.M.; Bruce, B.D. Poly(styrene-co-maleic acid)-mediated isolation of supramolecular membrane protein complexes from plant thylakoids. Biochim. Biophys. Acta Bioenerg. 2021, 1862, 148347. [Google Scholar] [CrossRef]
- Brady, N.G.; Workman, C.E.; Cawthon, B.; Bruce, B.D.; Long, B.K. Protein Extraction Efficiency and Selectivity of Esterified Styrene-Maleic Acid Copolymers in Thylakoid Membranes. Biomacromolecules 2021, 22, 2544–2553. [Google Scholar] [CrossRef]
- He, Q.; Vermaas, W. Chlorophyll a availability affects psbA translation and D1 precursor processing in vivo in Synechocystis sp. PCC 6803. Proc. Natl. Acad. Sci. USA 1998, 95, 5830–5835. [Google Scholar] [CrossRef]
- Chotewutmontri, P.; Williams-Carrier, R.; Barkan, A. Exploring the Link between Photosystem II Assembly and Translation of the Chloroplast psbA mRNA. Plants 2020, 9, 152. [Google Scholar] [CrossRef] [PubMed]
- Liberton, M.; Howard Berg, R.; Heuser, J.; Roth, R.; Pakrasi, H.B. Ultrastructure of the membrane systems in the unicellular cyanobacterium Synechocystis sp. strain PCC 6803. Protoplasma 2006, 227, 129–138. [Google Scholar] [CrossRef]
- van de Meene, A.M.; Hohmann-Marriott, M.F.; Vermaas, W.F.; Roberson, R.W. The three-dimensional structure of the cyanobacterium Synechocystis sp. PCC 6803. Arch. Microbiol. 2006, 184, 259–270. [Google Scholar] [CrossRef]
- Nevo, R.; Charuvi, D.; Shimoni, E.; Schwarz, R.; Kaplan, A.; Ohad, I.; Reich, Z. Thylakoid membrane perforations and connectivity enable intracellular traffic in cyanobacteria. EMBO J. 2007, 26, 1467–1473. [Google Scholar] [CrossRef]
- Huokko, T.; Ni, T.; Dykes, G.F.; Simpson, D.M.; Brownridge, P.; Conradi, F.D.; Beynon, R.J.; Nixon, P.J.; Mullineaux, C.W.; Zhang, P.; et al. Probing the biogenesis pathway and dynamics of thylakoid membranes. Nat. Commun. 2021, 12, 3475. [Google Scholar] [CrossRef]
- Rast, A.; Schaffer, M.; Albert, S.; Wan, W.; Pfeffer, S.; Beck, F.; Plitzko, J.M.; Nickelsen, J.; Engel, B.D. Biogenic regions of cyanobacterial thylakoids form contact sites with the plasma membrane. Nat. Plants 2019, 5, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Selao, T.T.; Zhang, L.; Knoppovaa, J.; Komenda, J.; Norling, B. Photosystem II Assembly Steps Take Place in the Thylakoid Membrane of the Cyanobacterium Synechocystis sp. PCC6803. Plant Cell Physiol. 2016, 57, 878. [Google Scholar] [CrossRef][Green Version]
- Zak, E.; Norling, B.; Andersson, B.; Pakrasi, H.B. Subcellular localization of the BtpA protein in the cyanobacterium Synechocystis sp. PCC 6803. Eur. J. Biochem. 1999, 261, 311–316. [Google Scholar] [CrossRef]
- Dahlgren, K.K.; Gates, C.; Lee, T.; Cameron, J.C. Proximity-based proteomics reveals the thylakoid lumen proteome in the cyanobacterium Synechococcus sp. PCC 7002. Photosynth. Res. 2021, 147, 177–195. [Google Scholar] [CrossRef]
- MacGregor-Chatwin, C.; Sener, M.; Barnett, S.F.H.; Hitchcock, A.; Barnhart-Dailey, M.C.; Maghlaoui, K.; Barber, J.; Timlin, J.A.; Schulten, K.; Hunter, C.N. Lateral Segregation of Photosystem I in Cyanobacterial Thylakoids. Plant Cell 2017, 29, 1119–1136. [Google Scholar] [CrossRef]
- Casella, S.; Huang, F.; Mason, D.; Zhao, G.Y.; Johnson, G.N.; Mullineaux, C.W.; Liu, L.N. Dissecting the Native Architecture and Dynamics of Cyanobacterial Photosynthetic Machinery. Mol. Plant 2017, 10, 1434–1448. [Google Scholar] [CrossRef]
- Mares, J.; Strunecky, O.; Bucinska, L.; Wiedermannova, J. Evolutionary Patterns of Thylakoid Architecture in Cyanobacteria. Front Microbiol. 2019, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ma, J.; Li, X.; Sui, S.F. In situ cryo-ET structure of phycobilisome-photosystem II supercomplex from red alga. Elife 2021, 10, e69635. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, V.M.; Pakrasi, H.B. Advances in the Understanding of the Lifecycle of Photosystem II. Microorganisms 2022, 10, 836. https://doi.org/10.3390/microorganisms10050836
Johnson VM, Pakrasi HB. Advances in the Understanding of the Lifecycle of Photosystem II. Microorganisms. 2022; 10(5):836. https://doi.org/10.3390/microorganisms10050836
Chicago/Turabian StyleJohnson, Virginia M., and Himadri B. Pakrasi. 2022. "Advances in the Understanding of the Lifecycle of Photosystem II" Microorganisms 10, no. 5: 836. https://doi.org/10.3390/microorganisms10050836
APA StyleJohnson, V. M., & Pakrasi, H. B. (2022). Advances in the Understanding of the Lifecycle of Photosystem II. Microorganisms, 10(5), 836. https://doi.org/10.3390/microorganisms10050836






