Impact of the Introduction of a Two-Step Laboratory Diagnostic Algorithm in the Incidence and Earlier Diagnosis of Clostridioides difficile Infection
Abstract
1. Introduction
2. Methods
2.1. Study Design and Patients
2.2. Patients Design Statement
2.3. Statistical Analysis
3. Results
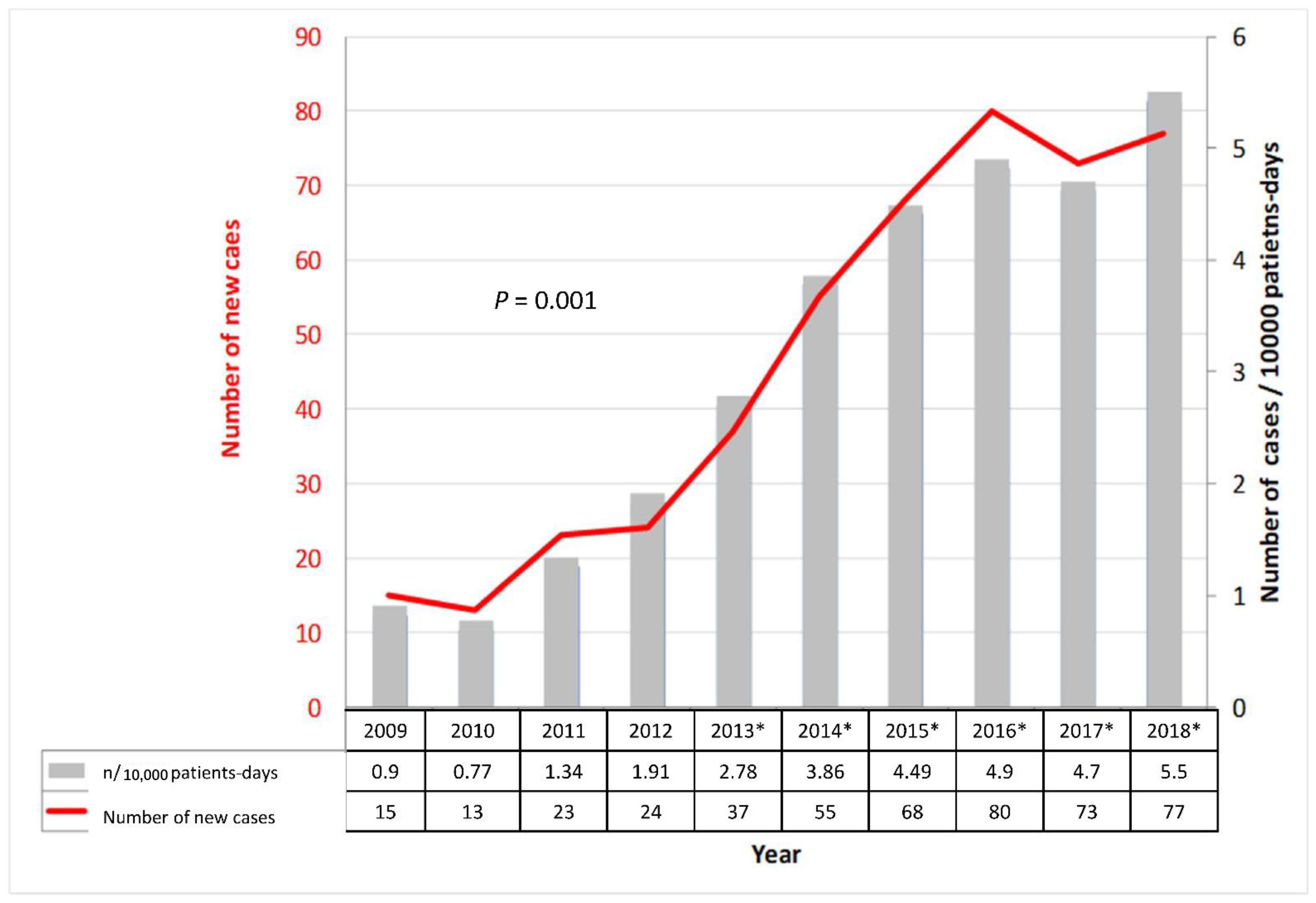
3.1. CDI Incidence along the Time Periods
3.2. Characteristics of the Patients
3.3. CDI Treatment
3.4. Clinical Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Evans, C.T.; Safdar, N. Current Trends in the Epidemiology and Outcomes of Clostridium Difficile Infection. Clin. Infect. Dis. 2015, 60, S66–S71. [Google Scholar] [CrossRef] [PubMed]
- Bogaty, C.; Lévesque, S.; Garenc, C.; Frenette, C.; Bolduc, D.; Galarneau, L.-A.; Lalancette, C.; Loo, V.; Tremblay, C.; Trudeau, M.; et al. Trends in the Use of Laboratory Tests for the Diagnosis of Clostridium Difficile Infection and Association with Incidence Rates in Quebec, Canada, 2010–2014. Am. J. Infect. Control 2017, 45, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Longtin, Y.; Trottier, S.; Brochu, G.; Paquet-Bolduc, B.; Garenc, C.; Loungnarath, V.; Beaulieu, C.; Goulet, D.; Longtin, J. Impact of the Type of Diagnostic Assay on Clostridium Difficile Infection and Complication Rates in a Mandatory Reporting Program. Clin. Infect. Dis. 2013, 56, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Guh, A.Y.; Mu, Y.; Winston, L.G.; Johnston, H.; Olson, D.; Farley, M.M.; Wilson, L.E.; Holzbauer, S.M.; Phipps, E.C.; Dumyati, G.K.; et al. Trends in U.S. Burden of Clostridioides Difficile Infection and Outcomes. N. Engl. J. Med. 2020, 382, 1320–1330. [Google Scholar] [CrossRef]
- Bauer, M.P.; Notermans, D.W.; van Benthem, B.H.B.; Brazier, J.S.; Wilcox, M.H.; Rupnik, M.; Monnet, D.L.; van Dissel, J.T.; Kuijper, E.J. Clostridium Difficile Infection in Europe: A Hospital-Based Survey. Lancet 2011, 377, 63–73. [Google Scholar] [CrossRef]
- Davies, K.A.; Longshaw, C.M.; Davis, G.L.; Bouza, E.; Barbut, F.; Barna, Z.; Delmée, M.; Fitzpatrick, F.; Ivanova, K.; Kuijper, E.; et al. Underdiagnosis of Clostridium Difficile across Europe: The European, Multicentre, Prospective, Biannual, Point-Prevalence Study of Clostridium Difficile Infection in Hospitalised Patients with Diarrhoea (EUCLID). Lancet Infect. Dis. 2014, 14, 1208–1219. [Google Scholar] [CrossRef]
- Roncarati, G.; Dallolio, L.; Leoni, E.; Panico, M.; Zanni, A.; Farruggia, P. Surveillance of Clostridium Difficile Infections: Results from a Six-Year Retrospective Study in Nine Hospitals of a North Italian Local Health Authority. Int. J. Environ. Res. Public Health 2017, 14, 61. [Google Scholar] [CrossRef]
- Rodríguez-Pardo, D.; Almirante, B.; Bartolomé, R.M.; Pomar, V.; Mirelis, B.; Navarro, F.; Soriano, A.; Sorlí, L.; Martínez-Montauti, J.; Molins, M.T.; et al. Epidemiology of Clostridium Difficile Infection and Risk Factors for Unfavorable Clinical Outcomes: Results of a Hospital-Based Study in Barcelona, Spain. J. Clin. Microbiol. 2013, 51, 1465–1473. [Google Scholar] [CrossRef]
- Crobach, M.J.T.; Planche, T.; Eckert, C.; Barbut, F.; Terveer, E.M.; Dekkers, O.M.; Wilcox, M.H.; Kuijper, E.J. European Society of Clinical Microbiology and Infectious Diseases: Update of the Diagnostic Guidance Document for Clostridium Difficile Infection. Clin. Microbiol. Infect. 2016, 22, S63–S81. [Google Scholar] [CrossRef]
- Khanafer, N.; Oltra, L.; Hulin, M.; Dauwalder, O.; Vandenesch, F.; Vanhems, P. Clostridium Difficile Infection in a French University Hospital: Eight Years of Prospective Surveillance Study. Medicine (Baltimore) 2016, 95, e3874. [Google Scholar] [CrossRef]
- Sopena, N.; Freixas, N.; Bella, F.; Pérez, J.; Hornero, A.; Limon, E.; Gudiol, F.; Pujol, M. Impact of a Training Program on the Surveillance of Clostridioides Difficile Infection. Epidemiol. Infect. 2019, 147, e231. [Google Scholar] [CrossRef]
- Thabit, A.K.; Alsolami, M.H.; Baghlaf, N.A.; Alsharekh, R.M.; Almazmumi, H.A.; Alselami, A.S.; Alsubhi, F.A. Comparison of Three Current Clostridioides Difficile Infection Guidelines: IDSA/SHEA, ESCMID, and ACG Guidelines. Infection 2019, 47, 899–909. [Google Scholar] [CrossRef]
- Fong, K.S.; Fatica, C.; Hall, G.; Procop, G.; Schindler, S.; Gordon, S.M.; Fraser, T.G. Impact of PCR Testing for Clostridium Difficile on Incident Rates and Potential on Public Reporting: Is the Playing Field Level? Infect. Control Hosp. Epidemiol. 2011, 32, 932–933. [Google Scholar] [CrossRef][Green Version]
- Guerrero, D.M.; Chou, C.; Jury, L.A.; Nerandzic, M.M.; Cadnum, J.C.; Donskey, C.J. Clinical and Infection Control Implications of Clostridium Difficile Infection with Negative Enzyme Immunoassay for Toxin. Clin. Infect. Dis. 2011, 53, 287–290. [Google Scholar] [CrossRef]
- Maharshak, N.; Barzilay, I.; Zinger, H.; Hod, K.; Dotan, I. Clostridium Difficile Infection in Hospitalized Patients with Inflammatory Bowel Disease. Medicine 2018, 97, e9772. [Google Scholar] [CrossRef]
- Revolinski, S.L.; Silvia Munoz-Price, L. Clostridium Difficile in Immunocompromised Hosts: A Review of Epidemiology, Risk Factors, Treatment, and Prevention HEALTHCARE EPIDEMIOLOGY: Robert Weinstein, Section Editor. Healthc. Epidemiol. Clin. Infect. Dis.® 2019, 2019, 2144–2153. [Google Scholar] [CrossRef]
- Zanichelli, V.; Garenc, C.; Villeneuve, J.; Moisan, D.; Frenette, C.; Loo, V.; Longtin, Y. Increased Community-Associated Clostridioides Difficile Infections in Quebec, Canada, 2008–2015. Emerg. Infect. Dis. 2020, 26, 1291–1294. [Google Scholar] [CrossRef]
- Larrainzar-Coghen, T.; Rodríguez-Pardo, D.; Fernández-Hidalgo, N.; Puig-Asensio, M.; Pigrau, C.; Ferrer, C.; Rodríguez, V.; Bartolomé, R.; Campany, D.; Almirante, B. Secular Trends in the Epidemiology of Clostridium Difficile Infection (CDI) at a Tertiary Care Hospital in Barcelona, 2006–2015: A Prospective Observational Study. Anaerobe 2018, 51, 54–60. [Google Scholar] [CrossRef]
- Beaulieu, C.; Dionne, L.L.; Julien, A.S.; Longtin, Y. Clinical Characteristics and Outcome of Patients with Clostridium Difficile Infection Diagnosed by PCR versus a Three-Step Algorithm. Clin. Microbiol. Infect. 2014, 20, 1067–1073. [Google Scholar] [CrossRef]
- Avni, T.; Babich, T.; Ben-Zvi, H.; Atamna, A.; Yahav, D.; Shepshelovich, D.; Leibovici-Weissman, Y.; Bishara, J. Molecular-Based Diagnosis of Clostridium Difficile Infection Is Associated with Reduced Mortality. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1137–1142. [Google Scholar] [CrossRef]
- Origüen, J.; Corbella, L.; Orellana, M.; Fernández-Ruiz, M.; López-Medrano, F.; San Juan, R.; Lizasoain, M.; Ruiz-Merlo, T.; Morales-Cartagena, A.; Maestro, G.; et al. Comparison of the Clinical Course of Clostridium Difficile Infection in Glutamate Dehydrogenase-Positive Toxin-Negative Patients Diagnosed by PCR to Those with a Positive Toxin Test. Clin. Microbiol. Infect. 2018, 24, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Guh, A.Y.; Hatfield, K.M.; Winston, L.G.; Martin, B.; Johnston, H.; Brousseau, G.; Farley, M.M.; Wilson, L.; Perlmutter, R.; Phipps, E.C.; et al. Toxin Enzyme Immunoassays Detect Clostridioides Difficile Infection with Greater Severity and Higher Recurrence Rates. Clin. Infect. Dis. 2019, 69, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Gentry, C.A.; Campbell, D.L.; Williams, R.J. Outcomes Associated with Recent Guideline Recommendations Removing Metronidazole for Treatment of Non-Severe Clostridioides Difficile Infection: A Retrospective, Observational, Nationwide Cohort Study. Int. J. Antimicrob. Agents 2021, 57, 106282. [Google Scholar] [CrossRef] [PubMed]
- Barbut, F.; Day, N.; Bouée, S.; Youssouf, A.; Grandvoinnet, L.; Lalande, V.; Couturier, J.; Eckert, C. Toxigenic Clostridium Difficile Carriage in General Practice: Results of a Laboratory-Based Cohort Study. Clin. Microbiol. Infect. 2019, 25, 588–594. [Google Scholar] [CrossRef]
- Reveles, K.R.; Dotson, K.M.; Gonzales-Luna, A.; Surati, D.; Endres, B.T.; Alam, M.J.; Garey, K.W. Clostridioides (Formerly Clostridium) Difficile Infection during Hospitalization Increases the Likelihood of Nonhome Patient Discharge. Clin. Infect. Dis. 2019, 68, 1887–1893. [Google Scholar] [CrossRef]
- Na’amnih, W.; Adler, A.; Miller-Roll, T.; Cohen, D.; Carmeli, Y. Risk Factors for Recurrent Clostridium Difficile Infection in a Tertiary Hospital in Israel. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1281–1288. [Google Scholar] [CrossRef]
- Appaneal, H.J.; Caffrey, A.R.; Beganovic, M.; Avramovic, S.; LaPlante, K.L. Predictors of Mortality among a National Cohort of Veterans with Recurrent Clostridium Difficile Infection. Open Forum Infect. Dis. 2018, 5, ofy175. [Google Scholar] [CrossRef]
- Mora Pinzon, M.C.; Buie, R.; Liou, J.I.; Shirley, D.K.; Evans, C.T.; Ramanathan, S.; Poggensee, L.; Safdar, N. Outcomes of Community and Healthcare-Onset Clostridium Difficile Infections. Clin. Infect. Dis. 2019, 68, 1343–1350. [Google Scholar] [CrossRef]
| Characteristic | N (%) |
|---|---|
| Age, mean (year, SD) | 68.2 ± 16.3 |
| Male gender | 241 (54.4) |
| Risk factors | |
| Chronic pulmonary disease | 95 (21.4) |
| Chronic renal disease | 119 (26.9) |
| Diabetes mellitus | 127 (28.7) |
| Heart failure | 99 (22.3) |
| Solid organ cancer | 136 (30.7) |
| Hematologic neoplasm | 47 (10.6) |
| Liver disease | 53 (12) |
| Inflammatory bowel disease | 21 (4.7) |
| HIV infection | 14 (3.2) |
| Charlson index ≥ 3 | 164 (37.1) |
| Abdominal surgery | 19 (4.3) |
| Chemotherapy | 50 (11.3) |
| Enteral nutrition | 53 (12) |
| Immunosuppressive drugs | 82 (18.5) |
| Non-CDI antibiotic use | 348 (78.6) |
| PPI use | 304 (68.6) |
| Clinical and analytical presentation | |
| Abdominal pain | 206 (46.5) |
| Fever | 108 (24.4) |
| Shock | 33 (7.4) |
| Severe case | 174 (39.7) |
| Complicated case | 66 (15) |
| Leukocytes > 15,000 cells/mm3 | 110 (25.2) |
| Albumin (g/L, SD) | 27.8 ± 6.5 |
| Creatinine (mg/dL, SD) | 1.7 ± 1.7 |
| Hemoglobin (g/dL, SD) | 10.7 ± 2.1 |
| Characteristic | Toxin + N = 297 (67%) N (%) | Toxin −/PCR + N = 146 (33%) N (%) | p Value (Unadjusted Analysis) | p Value (Adjusted Analysis) |
|---|---|---|---|---|
| Age, mean (SD) | 69.8 ± 16.1 | 64.9 ± 16.3 | 0.003 | |
| Male gender | 155 (52.2) | 86 (58.9) | 0.21 | |
| Chronic pulmonary disease | 69 (23.2) | 26 (17.8) | 0.23 | |
| Chronic renal disease | 83 (27.9) | 36 (24.7) | 0.53 | |
| Diabetes mellitus | 85 (28.6) | 42 (28.8) | 1 | |
| Heart failure | 75 (25.3) | 24 (16.4) | 0.05 | |
| Solid organ cancer | 83 (27.9) | 53 (36.3) | 0.09 | |
| Hematologic neoplasm | 27 (9.1) | 20 13.7) | 0.18 | |
| Liver disease | 28 (9.4) | 25 (17.1) | 0.03 | |
| Inflammatory bowel disease | 3 (1) | 18 (12.3) | <0.001 | 0.004 |
| HIV infection | 7 (2.4) | 7 (4.8) | 0.27 | |
| Charlson index ≥ 3 | 107 (36.1) | 57(39) | 0.21 | |
| Abdominal surgery | 11 (3.7) | 8 (5.5) | 0.53 | |
| Chemotherapy | 28 (9.4) | 22 (15.1) | 0.07 | |
| Enteral nutrition | 30 (10.1) | 23 (15.8) | 0.11 | |
| Immunosuppressive drugs | 43 (14.5) | 39 (26.7) | 0.002 | 0.004 |
| Non-CDI antibiotic use | 244 (82.2) | 104 (71.2) | 0.01 | |
| Proton pump inhibitor use | 217 (73.1) | 87 (59.6) | 0.006 | |
| Abdominal pain | 150 (50.5) | 56 (38.4) | 0.01 | |
| Fever | 73 (24.6) | 35 (24) | 0.98 | |
| Shock | 28 (9.4) | 5 (3.4) | 0.03 | |
| Leukocytes > 15,000 cells/mm3 | 91 (31.2) | 19 (13.2) | <0.001 | |
| Albumin (g/L, SD) | 27.6 ± 6.6 | 28.1 ± 6.2 | 0.48 | |
| Creatinine (mg/dL, SD) | 1.7 ± 1.7 | 1.4 ± 1.4 | 0.07 | |
| Hemoglobin (g/dL, SD) | 10.7 ± 1.9 | 10.5 ± 2.3 | 0.37 | |
| Severe presentation | 133 (45.2) | 41 (28.5) | 0.001 | 0.004 |
| Complicated case | 48 (16.3) | 18 (12.4) | 0.35 | |
| Non-CDI antibiotic suppression | 118 (40) | 36 (25.4) | 0.004 | |
| PPI suppression | 19 (6.4) | 7 (4.9) | 0.66 | |
| Recurrence | 52 (17.6) | 14 (9.6) | 0.04 | |
| Death (30d) | 42 (14.2) | 21 (14.4) | 0.95 |
| Characteristics | Deaths (30 d) N = 63 N (%) | Cured N = 380 N (%) | p Value (Unadjusted Analysis) | p Value (Adjusted Analysis) |
|---|---|---|---|---|
| Age, mean (SD) | 69.8 ± 17.7 | 67.9 ± 16.1 | 0.39 | |
| Male gender | 32 (50.8) | 209 (55) | 0.60 | |
| ICU admission | 14 (22.6) | 22(6.5) | <0.001 | <0.001 |
| Chronic pulmonary disease | 16 (25.4) | 79 (20.8) | 0.51 | |
| Chronic renal disease | 20 (31.7) | 99 (26.1) | 0.42 | |
| Diabetes mellitus | 23 (36.5) | 104 (27.4) | 0.18 | |
| Heart failure | 16 (25.4) | 83 (21.8) | 0.64 | |
| Solid organ cancer | 15 (23.8) | 121 (31.8) | 0.25 | |
| Hematologic neoplasm | 12 (19) | 35 (9.2) | 0.03 | 0.01 |
| Liver disease | 10 (15.9) | 43 (11.3) | 0.41 | |
| Inflammatory bowel disease | 3 (4.8) | 18 (4.7) | 1 | |
| HIV infection | 4 (6.3) | 10 (2.6) | 0.12 | |
| Charlson index ≥ 3 | 24 (38.1) | 140 (36.8) | 0.96 | |
| Abdominal surgery | 1 (1.6) | 18 (4.7) | 0.49 | |
| Chemotherapy | 6 (9.5) | 43 (11.3) | 1 | |
| Enteral nutrition | 9 (14.3) | 44 (11.6) | 0.68 | |
| Immunosuppressive drugs | 17 (27) | 65 (17.1) | 0.09 | |
| Non-CDI antibiotic use | 55 (87.3) | 293 (77.1) | 0.09 | |
| Proton pump inhibitor use | 39 (61.9) | 265 (69.7) | 0.27 | |
| Abdominal pain | 26 (41.3) | 180 (47.4) | 0.44 | |
| Fever | 15 (23.8) | 93 (24.5) | 1 | |
| Shock | 12 (19) | 21 (5.5) | <0.001 | |
| Ileus | 2 (3.1) | 2 (0.5) | 0.09 | |
| Toxic megacolon | 2 (3.2) | 0 | 0.02 | |
| Leukocytes > 15,000 cells/mm3 | 26 (42.6) | 64 (22.5) | 0.001 | |
| Albumin (g/L, SD) | 23.6 ± 6.5 | 28.5 ± 6.1 | <0.001 | |
| Creatinine (mg/dL, SD) | 1.7 ± 1.5 | 1.6 ± 1.6 | 0.53 | |
| Hemoglobin (g/dL, SD) | 10.2 ± 2.1 | 10.7 ± 2.1 | 0.08 | |
| Severe presentation | 33 (53.2) | 146 (38.4) | 0.05 | |
| Complicated case | 20 (32.3) | 46 (12.2) | <0.001 | 0.003 |
| CD Toxin-positive | 42 (66.7) | 255 (67.1) | 1 | |
| Vancomycin ± metronidazole | 24 (38.1) | 98 (26.4) | 0.79 | |
| Non-CDI antibiotic suppression | 15 (24.6) | 139 (37) | 0.08 | |
| PPI suppression | 2 (3.2) | 24 (6.3) | 0.55 |
| Characteristic | Recurrence N = 66 N (%) | Non-Recurrence N = 343 N (%) | p Value (Unadjusted Analysis) | p Value (Adjusted Analysis) |
|---|---|---|---|---|
| Age, mean (SD) | 71.9 ± 12.8 | 67.1 ± 16.6 | 0.05 | |
| Male gender | 36 (54.5) | 173 (55.1) | 1 | |
| UCI admission | 4 (6.7) | 18 (6.4) | 1 | |
| Chronic pulmonary disease | 17 (25.8) | 62 (19.7) | 0.27 | |
| Chronic renal disease | 23 (34.8) | 76 (24.2) | 0.07 | |
| Diabetes mellitus | 22 (33.3) | 82 (26.1) | 0.23 | |
| Heart failure | 20 (30.3) | 63 (20.1) | 0.06 | |
| Solid organ cancer | 18 (27.3) | 103 (32.8) | 0.38 | |
| Hematologic neoplasm | 6 (9.1) | 29 (9.2) | 0.97 | |
| Liver disease | 9 (13.6) | 34 (10.8) | 0.51 | |
| Inflammatory bowel disease | 2 (3) | 16 (5.1) | 0.75 | |
| VIH infection | 3 (4.5) | 7 (2.2) | 0.38 | |
| Charlson index ≥ 3 | 28 (42.4) | 112 (35.7) | 0.30 | |
| Abdominal surgery | 5 (7.6) | 13 (4.1) | 0.23 | |
| Chemotherapy | 1 (1.5) | 42 (13.1) | 0.003 | |
| Enteral nutrition | 8 (12.1) | 36 (11.5) | 0.88 | |
| Immunosuppressive drugs | 10 (15.2) | 55 (17.5) | 0.64 | |
| Non-CDI antibiotic use | 54 (81.8) | 239 (76.1) | 0.31 | |
| Proton pump inhibitor use | 56 (84.8) | 209 (66.6) | 0.003 | 0.02 |
| Abdominal pain | 36 (54.5) | 144 (45.9) | 0.19 | |
| Fever | 16 (24.2) | 77 (24.5) | 0.96 | |
| Leukocytes > 15,000 cells/mm3 | 24 (36.9) | 60 (19.4) | 0.002 | |
| Albumin (g/L, SD) | 28.1 ± 4.8 | 28.6 ± 6.4 | 0.59 | |
| Creatinine (mg/dL, SD) | 2.2 ± 2.3 | 1.4 ± 1.4 | 0.001 | |
| Hemoglobin (g/dL, SD) | 10.5 ± 1.6 | 10.7 ± 2.1 | 0.52 | |
| Severe presentation | 35 (53) | 111 (35.4) | 0.007 | 0.03 |
| Complicated case | 6 (9.1) | 40 (12.7) | 0.40 | |
| CD toxin-positive | 52 (78.8) | 203 (64.6) | 0.02 | |
| Vancomycin ± metronidazole | 19 (29.7) | 79 (25.7) | 0.51 | |
| Days of treatment (mean, SD) | 14.8 ± 7.4 | 13.7 ± 4.6 | 0.27 | |
| Non-CDI antibiotic suppression | 27 (42.2) | 112 (35.9) | 0.34 | |
| PPI suppression | 6 (9.1) | 18 (35.9) | 0.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sopena, N.; Wang-Wang, J.H.; Casas, I.; Mateu, L.; Castellà, L.; García-Quesada, M.J.; Gutierrez, S.; Llibre, J.M.; Pedro-Botet, M.L.; Fernandez-Rivas, G. Impact of the Introduction of a Two-Step Laboratory Diagnostic Algorithm in the Incidence and Earlier Diagnosis of Clostridioides difficile Infection. Microorganisms 2022, 10, 1075. https://doi.org/10.3390/microorganisms10051075
Sopena N, Wang-Wang JH, Casas I, Mateu L, Castellà L, García-Quesada MJ, Gutierrez S, Llibre JM, Pedro-Botet ML, Fernandez-Rivas G. Impact of the Introduction of a Two-Step Laboratory Diagnostic Algorithm in the Incidence and Earlier Diagnosis of Clostridioides difficile Infection. Microorganisms. 2022; 10(5):1075. https://doi.org/10.3390/microorganisms10051075
Chicago/Turabian StyleSopena, Nieves, Jun Hao Wang-Wang, Irma Casas, Lourdes Mateu, Laia Castellà, María José García-Quesada, Sara Gutierrez, Josep M. Llibre, M. Luisa Pedro-Botet, and Gema Fernandez-Rivas. 2022. "Impact of the Introduction of a Two-Step Laboratory Diagnostic Algorithm in the Incidence and Earlier Diagnosis of Clostridioides difficile Infection" Microorganisms 10, no. 5: 1075. https://doi.org/10.3390/microorganisms10051075
APA StyleSopena, N., Wang-Wang, J. H., Casas, I., Mateu, L., Castellà, L., García-Quesada, M. J., Gutierrez, S., Llibre, J. M., Pedro-Botet, M. L., & Fernandez-Rivas, G. (2022). Impact of the Introduction of a Two-Step Laboratory Diagnostic Algorithm in the Incidence and Earlier Diagnosis of Clostridioides difficile Infection. Microorganisms, 10(5), 1075. https://doi.org/10.3390/microorganisms10051075






