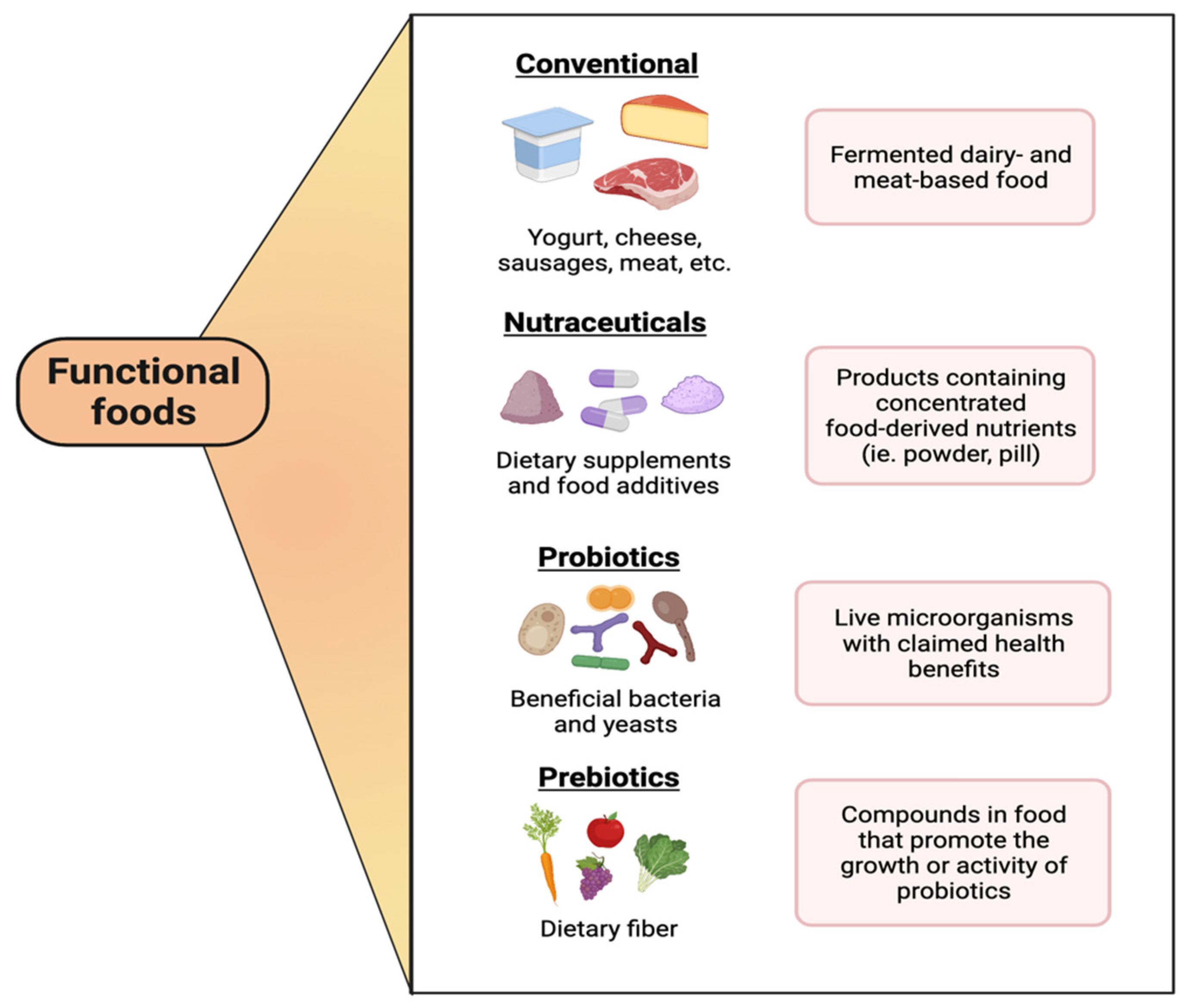
Functional Foods, Nutraceuticals and Probiotics: A Focus on Human Health
Abstract
:1. Introduction
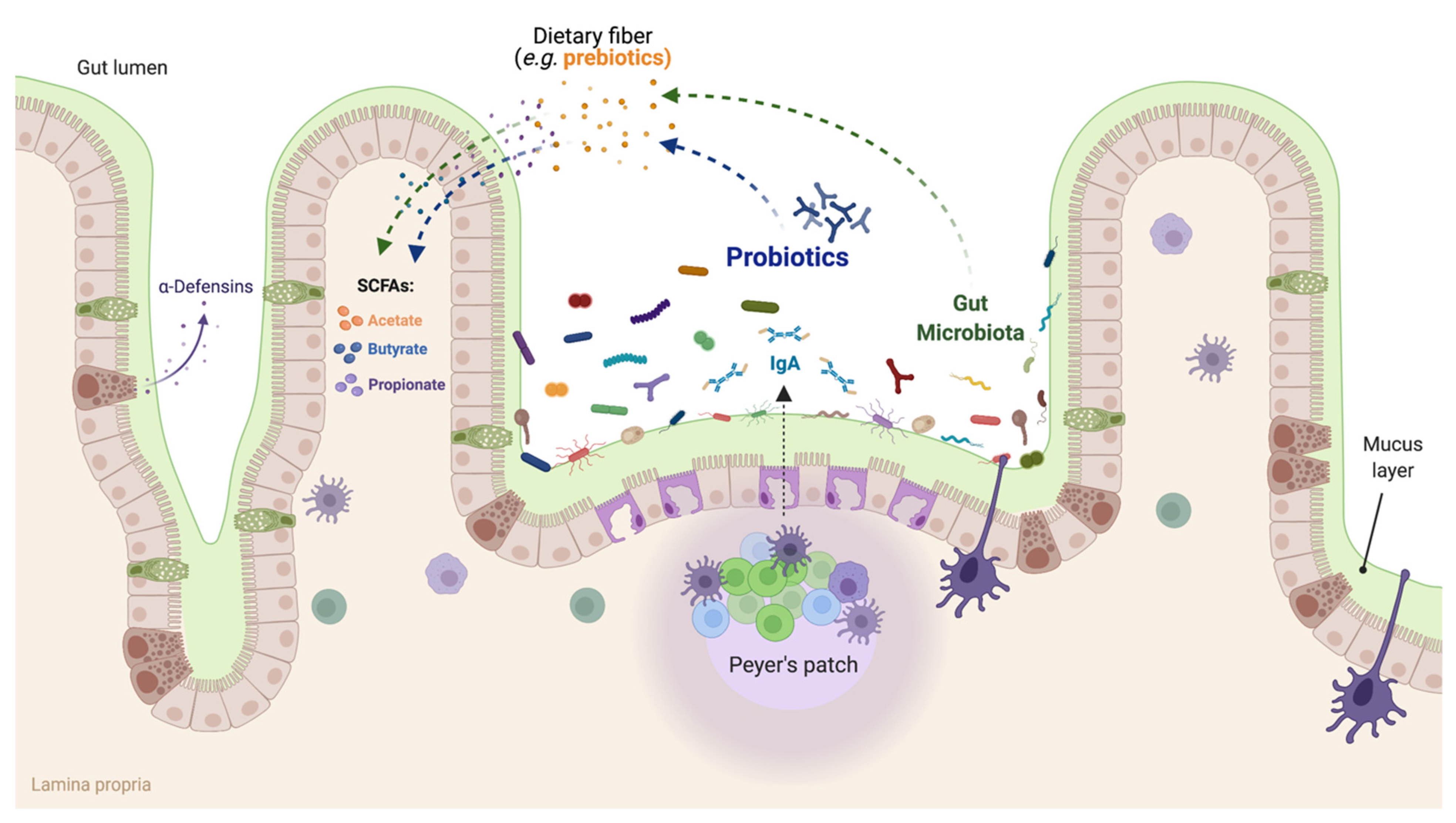
2. Proposed Mechanisms by Which Functional Foods Exert Their Beneficial Effects
3. Different Classes of Probiotic Products
3.1. Dairy-Based Functional Foods Containing Probiotics
3.1.1. Fresh and Fermented Milks
3.1.2. Yogurt
3.1.3. Cheese
3.1.4. Other Dairy Products
3.2. Plant-Based Products Containing Probiotics
3.3. Fruit-Based Products Containing Probiotics
3.4. Cereal-Based Products Containing Probiotics
3.5. Meat-Based Products Containing Probiotics
4. Fermented Foods Containing Live Probiotic Cultures
5. Microbial Biogenic Metabolites Derived from Fermentation
6. Mechanisms of Probiotic Functionality and Their Beneficial Effects
6.1. Gastrointestinal Disorders
6.1.1. Inflammatory Bowel Diseases (IBD)
6.1.2. Diarrhea
6.1.3. Food Allergy
6.1.4. Helicobacter pylori Infection
6.1.5. Lactose Intolerance
6.1.6. Cancer
7. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baker, M.T.; Lu, P.; Parrella, J.A.; Leggette, H.R. Consumer Acceptance toward Functional Foods: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 1217. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H. Regulation of “Nutraceuticals”. Science 1999, 285, 1853–1855. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granato, D.; Barba, F.J.; Kovačević, D.B.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional Foods: Product Development, Technological Trends, Efficacy Testing, and Safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Cerbo, A.; Morales-Medina, J.C.; Palmieri, B.; Pezzuto, F.; Cocco, R.; Flores, G.; Iannitti, T. Functional foods in pet nutrition: Focus on dogs and cats. Res. Veter Sci. 2017, 112, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, V.; Roy, A.; Bharadvaja, N. Current Prospects of Nutraceuticals: A Review. Curr. Pharm. Biotechnol. 2020, 21, 884–896. [Google Scholar] [CrossRef]
- Alkhatib, A. Antiviral Functional Foods and Exercise Lifestyle Prevention of Coronavirus. Nutrients 2020, 12, 2633. [Google Scholar] [CrossRef]
- Illanes, A.; Guerrero, C. Functional Foods and Feeds: Probiotics, Prebiotics, and Synbiotics. Lact. Deriv. Prebiotics A Process Perspect. 2016, 35–86. [Google Scholar] [CrossRef]
- Saulnier, D.M.; Spinler, J.; Gibson, G.R.; Versalovic, J. Mechanisms of probiosis and prebiosis: Considerations for enhanced functional foods. Curr. Opin. Biotechnol. 2009, 20, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Zommiti, M.; Feuilloley, M.G.J.; Connil, N. Update of Probiotics in Human World: A Nonstop Source of Benefactions till the End of Time. Microorganisms 2020, 8, 1907. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.C. Probiotics As Functional Foods. Nutr. Clin. Pract. 2003, 18, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peredo-Lovillo, A.; Romero-Luna, H.; Jiménez-Fernández, M. Health promoting microbial metabolites produced by gut microbiota after prebiotics metabolism. Food Res. Int. 2020, 136, 109473. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Fernández-Galilea, M.; Martínez-Fernández, L.; González-Muniesa, P.; Pérez-Chávez, A.; Martínez, J.A.; Moreno-Aliaga, M.J. Oxidative Stress and Non-Alcoholic Fatty Liver Disease: Effects of Omega-3 Fatty Acid Supplementation. Nutrients 2019, 11, 872. [Google Scholar] [CrossRef] [Green Version]
- Wieërs, G.; Belkhir, L.; Enaud, R.; Leclercq, S.; De Foy, J.-M.P.; Dequenne, I.; De Timary, P.; Cani, P.D. How Probiotics Affect the Microbiota. Front. Cell. Infect. Microbiol. 2020, 9, 454. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Xu, J.; Shuai, J.; Chen, J.; Zhang, Z.; Fang, W. The S-layer proteins of Lactobacillus crispatus strain ZJ001 is responsible for competitive exclusion against Escherichia coli O157:H7 and Salmonella typhimurium. Int. J. Food Microbiol. 2007, 115, 307–312. [Google Scholar] [CrossRef]
- Tachedjian, G.; Aldunate, M.; Bradshaw, C.S.; Cone, R.A. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res. Microbiol. 2017, 168, 782–792. [Google Scholar] [CrossRef]
- Spinler, J.K.; Taweechotipatr, M.; Rognerud, C.L.; Ou, C.N.; Tumwasorn, S.; Versalovic, J. Human-derived probiotic Lactobacillus reuteri demonstrate antimicrobial activities targeting diverse enteric bacterial pathogens. Anaerobe 2008, 14, 166–171. [Google Scholar] [CrossRef] [Green Version]
- Galdeano, C.M.; LeBlanc, A.d.M.d.; Vinderola, G.; Bonet, M.E.B.; Perdigón, G. Proposed Model: Mechanisms of Immunomodulation Induced by Probiotic Bacteria. Clin. Vaccine Immunol. 2007, 14, 485–492. [Google Scholar] [CrossRef] [Green Version]
- Vinderola, G.; Matar, C.; Perdigón, G. Milk fermentation products of L. helveticus R389 activate calcineurin as a signal to promote gut mucosal immunity. BMC Immunol. 2007, 8, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, M.; Bunt, C.R.; Mason, S.L.; Hussain, M.A. Non-dairy probiotic food products: An emerging group of functional foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 2626–2641. [Google Scholar] [CrossRef] [PubMed]
- Rastall, R.A.; Gibson, G.R. Recent developments in prebiotics to selectively impact beneficial microbes and promote intestinal health. Curr. Opin. Biotechnol. 2015, 32, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.V.; Vijayendra, S.V.N.; Reddy, O.V.S. Trends in dairy and non-dairy probiotic products—A review. J. Food Sci. Technol. 2015, 52, 6112–6124. [Google Scholar] [CrossRef] [Green Version]
- Sangwan, V.; Tomar, S.; Singh, R.; Singh, A.; Ali, B. Galactooligosaccharides: Novel Components of Designer Foods. J. Food Sci. 2011, 76, R103–R111. [Google Scholar] [CrossRef]
- Nagaoka, S. Yogurt Production. Methods Mol. Biol. 2019, 1887, 45–54. [Google Scholar] [CrossRef]
- Hurtado-Romero, A.; Del Toro-Barbosa, M.; Gradilla-Hernández, M.S.; Garcia-Amezquita, L.E.; García-Cayuela, T. Probiotic Properties, Prebiotic Fermentability, and GABA-Producing Capacity of Microorganisms Isolated from Mexican Milk Kefir Grains: A Clustering Evaluation for Functional Dairy Food Applications. Foods 2021, 10, 2275. [Google Scholar] [CrossRef]
- Zepeda-Hernández, A.; Garcia-Amezquita, L.E.; Requena, T.; García-Cayuela, T. Probiotics, prebiotics, and synbiotics added to dairy products: Uses and applications to manage type 2 diabetes. Food Res. Int. 2021, 142, 110208. [Google Scholar] [CrossRef]
- Mani-López, E.; Palou, E.; López-Malo, A. Probiotic viability and storage stability of yogurts and fermented milks prepared with several mixtures of lactic acid bacteria. J. Dairy Sci. 2014, 97, 2578–2590. [Google Scholar] [CrossRef] [Green Version]
- Falfán-Cortes, R.N.; Mora-Peñaflor, N.; Gómez-Aldapa, C.A.; Rangel-Vargas, E.; Acevedo-Sandoval, O.A.; Franco-Fernández, M.J.; Rosas, J.C. Characterization and evaluation of probiotic potential in vitro and in situ of Lacticaseibacillus paracasei isolated from tenate cheese. J. Food Prot. 2022, 85, 112–121. [Google Scholar] [CrossRef]
- Özkan, E.R.; Demirci, T.; Öztürk, H.I.; Akın, N. Screening Lactobacillus strains from artisanal Turkish goatskin casing Tulum cheeses produced by nomads via molecular and in vitro probiotic characteristics. J. Sci. Food Agric. 2021, 101, 2799–2808. [Google Scholar] [CrossRef] [PubMed]
- Klu, Y.A.K.; Chen, J. Influence of probiotics, included in peanut butter, on the fate of selected Salmonella and Listeria strains under simulated gastrointestinal conditions. J. Appl. Microbiol. 2016, 120, 1052–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aloğlu, H.; Öner, Z. Assimilation of cholesterol in broth, cream, and butter by probiotic bacteria. Eur. J. Lipid Sci. Technol. 2006, 108, 709–713. [Google Scholar] [CrossRef]
- Kim, Y.; Yoon, S.; Shin, H.; Jo, M.; Lee, S.; Kim, S.-H. Isolation of the Cholesterol-Assimilating Strain Pediococcus acidilactici LRCC5307 and Production of Low-Cholesterol Butter. Korean J. Food Sci. Anim. Resour. 2021, 41, 300–311. [Google Scholar] [CrossRef]
- Ekinci, F.Y.; Okur, O.D.; Ertekin, B.; Guzel-Seydim, Z. Effects of probiotic bacteria and oils on fatty acid profiles of cultured cream. Eur. J. Lipid Sci. Technol. 2008, 110, 216–224. [Google Scholar] [CrossRef]
- Arslan, A.A.; Gocer, E.M.C.; Demir, M.; Atamer, Z.; Hinrichs, J.; Kücükcetin, A. Viability of Lactobacillus acidophilus ATCC 4356 incorporated into ice cream using three different methods. Dairy Sci. Technol. 2016, 96, 477–487. [Google Scholar] [CrossRef] [Green Version]
- Pace, L.; Crowe, S.E. Complex Relationships Between Food, Diet, and the Microbiome. Gastroenterol. Clin. N. Am. 2016, 45, 253–265. [Google Scholar] [CrossRef] [Green Version]
- Park, K.-Y.; Jeong, J.-K.; Lee, Y.-E.; Daily, J.W. Health Benefits of Kimchi (Korean Fermented Vegetables) as a Probiotic Food. J. Med. Food 2014, 17, 6–20. [Google Scholar] [CrossRef]
- Li, X.; Liu, D. Nutritional Content Dynamics and Correlation of Bacterial Communities and Metabolites in Fermented Pickled Radishes Supplemented With Wheat Bran. Front. Nutr. 2022, 9, 840641. [Google Scholar] [CrossRef]
- Žuntar, I.; Petric, Z.; Kovačević, D.B.; Putnik, P. Safety of Probiotics: Functional Fruit Beverages and Nutraceuticals. Foods 2020, 9, 947. [Google Scholar] [CrossRef]
- Fenster, K.; Freeburg, B.; Hollard, C.; Wong, C.; Laursen, R.R.; Ouwehand, A.C. The Production and Delivery of Probiotics: A Review of a Practical Approach. Microorganisms 2019, 7, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Septembre-Malaterre, A.; Remize, F.; Poucheret, P. Fruits and vegetables, as a source of nutritional compounds and phytochemicals: Changes in bioactive compounds during lactic fermentation. Food Res. Int. 2018, 104, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T.; McDougall, G.J.; Alegria, A.; Alminger, M.; Arrigoni, E.; Aura, A.-M.; Brito, C.; Cilla, A.; El, S.N.; Karakaya, S.; et al. Mind the gap—Deficits in our knowledge of aspects impacting the bioavailability of phytochemicals and their metabolites—A position paper focusing on carotenoids and polyphenols. Mol. Nutr. Food Res. 2015, 59, 1307–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blandino, A.; Al-Aseeri, M.E.; Pandiella, S.S.; Cantero, D.; Webb, C. Cereal-based fermented foods and beverages. Food Res. Int. 2003, 36, 527–543. [Google Scholar] [CrossRef]
- Rivera-Espinoza, Y.; Navarro, Y.T.G. Non-dairy probiotic products. Food Microbiol. 2010, 27, 1–11. [Google Scholar] [CrossRef]
- Corsetti, A.; Settanni, L. Lactobacilli in sourdough fermentation. Food Res. Int. 2007, 40, 539–558. [Google Scholar] [CrossRef]
- De Vuyst, L.; Vrancken, G.; Ravyts, F.; Rimaux, T.; Weckx, S. Biodiversity, ecological determinants, and metabolic exploitation of sourdough microbiota. Food Microbiol. 2009, 26, 666–675. [Google Scholar] [CrossRef]
- De Vuyst, L.; Vancanneyt, M. Biodiversity and identification of sourdough lactic acid bacteria. Food Microbiol. 2007, 24, 120–127. [Google Scholar] [CrossRef]
- Meroth, C.B.; Walter, J.; Hertel, C.; Brandt, M.J.; Hammes, W.P. Monitoring the Bacterial Population Dynamics in Sourdough Fermentation Processes by Using PCR-Denaturing Gradient Gel Electrophoresis. Appl. Environ. Microbiol. 2003, 69, 92–96. [Google Scholar] [CrossRef] [Green Version]
- Vogel, R.F.; Böcker, G.; Stolz, P.; Ehrmann, M.; Fanta, D.; Ludwig, W.; Pot, B.; Kersters, K.; Schleifer, K.H.; Hammes, W.P. Identification of Lactobacilli from Sourdough and Description of Lactobacillus pontis sp. nov. Int. J. Syst. Bacteriol. 1994, 44, 223–229. [Google Scholar] [CrossRef] [Green Version]
- Wells, J.M. Immunomodulatory mechanisms of Lactobacilli. Microb. Cell Fact. 2011, 10 (Suppl. S1), S17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mombach, M.A.; Cabral, L.D.S.; Lima, L.R.; Ferreira, D.C.; Carneiro e Pedreira, B.; Pereira, D.H. Correction to: Association of ionophores, yeast, and bacterial probiotics alters the abundance of ruminal microbial species of pasture intensively finished beef cattle. Trop. Anim. Health Prod. 2021, 53, 401. [Google Scholar] [CrossRef] [PubMed]
- da Silva-Marques, R.P.; Zervoudakis, J.T.; Nakazato, L.; Hatamoto-Zervoudakis, L.K.; Cabral, L.D.S.; Matos, N.B.D.N.; da Silva, M.I.L.; Feliciano, A.L. Ruminal Microbial Populations and Fermentation Characteristics in Beef Cattle Grazing Tropical Forage in Dry Season and Supplemented with Different Protein Levels. Curr. Microbiol. 2019, 76, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prado, M.R.M.; Blandon, L.M.; Vandenberghe, L.; Rodrigues, C.; Castro, G.; Soccol, V.T.; Soccol, C.R. Milk kefir: Composition, microbial cultures, biological activities, and related products. Front. Microbiol. 2015, 6, 1177. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Oaxaca, D.; López-Sánchez, R.; Lozano, L.; Wacher-Rodarte, C.; Segovia, L.; Munguía, A.L. Diversity of Weissella confusa in Pozol and Its Carbohydrate Metabolism. Front. Microbiol. 2021, 12, 629449. [Google Scholar] [CrossRef]
- Pieper, R.; Boudry, C.; Bindelle, J.; Vahjen, W.; Zentek, J. Interaction between dietary protein content and the source of carbohydrates along the gastrointestinal tract of weaned piglets. Arch. Anim. Nutr. 2014, 68, 263–280. [Google Scholar] [CrossRef]
- Bikker, P.; Dirkzwager, A.; Fledderus, J.; Trevisi, P.; Le Huërou-Luron, I.; Lallès, J.P.; Awati, A. The effect of dietary protein and fermentable carbohydrates levels on growth performance and intestinal characteristics in newly weaned piglets. J. Anim. Sci. 2006, 84, 3337–3345. [Google Scholar] [CrossRef] [Green Version]
- Kiarie, E.; Nyachoti, C.M.; Slominski, B.A.; Blank, G. Growth performance, gastrointestinal microbial activity, and nutrient digestibility in early-weaned pigs fed diets containing flaxseed and carbohydrase enzyme. J. Anim. Sci. 2007, 85, 2982–2993. [Google Scholar] [CrossRef] [Green Version]
- Maldonado Galdeano, C.; Cazorla, S.I.; Lemme Dumit, J.M.; Vélez, E.; Perdigón, G. Beneficial Effects of Probiotic Consumption on the Immune System. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar] [CrossRef]
- Mu, Y.; Cong, Y. Bacillus coagulans and its applications in medicine. Benef. Microbes 2019, 10, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.-H.; Zhu, C.-X.; Quan, Y.-S.; Yang, Z.-Y.; Wu, S.; Luo, W.-W.; Tan, B.; Wang, X.-Y. Relationship between intestinal microbiota and ulcerative colitis: Mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J. Gastroenterol. 2018, 24, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Newberry, S.J.; Hempel, S.; Maher, A.R.; Wang, Z.; Miles, J.N.V.; Shanman, R.; Johnsen, B.; Shekelle, P.G. Probiotics for the Prevention and Treatment of Antibiotic-Associated Diarrhea. JAMA 2012, 307, 1959–1969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakobsson, H.E.; Abrahamsson, T.R.; Jenmalm, M.C.; Harris, K.; Quince, C.; Jernberg, C.; Björkstén, B.; Engstrand, L.; Andersson, A.F. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by Caesarean section. Gut 2013, 63, 559–566. [Google Scholar] [CrossRef] [Green Version]
- Rothenberg, D.O.; Yang, H.; Chen, M.; Zhang, W.; Zhang, L. Metabolome and Transcriptome Sequencing Analysis Reveals Anthocyanin Metabolism in Pink Flowers of Anthocyanin-Rich Tea (Camellia sinensis). Molecules 2019, 24, 1064. [Google Scholar] [CrossRef] [Green Version]
- Shu, S.-A.; Yuen, A.W.T.; Woo, E.; Chu, K.-H.; Kwan, H.-S.; Yang, G.-X.; Yang, Y.; Leung, P.S.C. Microbiota and Food Allergy. Clin. Rev. Allergy Immunol. 2019, 57, 83–97. [Google Scholar] [CrossRef]
- Lesbros-Pantoflickova, D.; Corthésy-Theulaz, I.; Blum, A.L. Helicobacter pylori and Probiotics. J. Nutr. 2007, 137, 812S–818S. [Google Scholar] [CrossRef] [Green Version]
- Goderska, K.; Pena, S.A.; Alarcon, T. Helicobacter pylori treatment: Antibiotics or probiotics. Appl. Microbiol. Biotechnol. 2018, 102, 1–7. [Google Scholar] [CrossRef]
- Leis, R.; De Castro, M.-J.; De Lamas, C.; Picáns, R.; Couce, M.L. Effects of Prebiotic and Probiotic Supplementation on Lactase Deficiency and Lactose Intolerance: A Systematic Review of Controlled Trials. Nutrients 2020, 12, 1487. [Google Scholar] [CrossRef]
- Oak, S.J.; Jha, R. The effects of probiotics in lactose intolerance: A systematic review. Crit. Rev. Food Sci. Nutr. 2018, 59, 1675–1683. [Google Scholar] [CrossRef]
- Yu, A.-Q.; Li, L. The Potential Role of Probiotics in Cancer Prevention and Treatment. Nutr. Cancer 2016, 68, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Bedada, T.L.; Feto, T.K.; Awoke, K.S.; Garedew, A.D.; Yifat, F.T.; Birri, D.J. Probiotics for cancer alternative prevention and treatment. Biomed. Pharmacother. 2020, 129, 110409. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damián, M.R.; Cortes-Perez, N.G.; Quintana, E.T.; Ortiz-Moreno, A.; Garfias Noguez, C.; Cruceño-Casarrubias, C.E.; Sánchez Pardo, M.E.; Bermúdez-Humarán, L.G. Functional Foods, Nutraceuticals and Probiotics: A Focus on Human Health. Microorganisms 2022, 10, 1065. https://doi.org/10.3390/microorganisms10051065
Damián MR, Cortes-Perez NG, Quintana ET, Ortiz-Moreno A, Garfias Noguez C, Cruceño-Casarrubias CE, Sánchez Pardo ME, Bermúdez-Humarán LG. Functional Foods, Nutraceuticals and Probiotics: A Focus on Human Health. Microorganisms. 2022; 10(5):1065. https://doi.org/10.3390/microorganisms10051065
Chicago/Turabian StyleDamián, Morayma Ramírez, Naima G. Cortes-Perez, Erika T. Quintana, Alicia Ortiz-Moreno, Cynthia Garfias Noguez, Carlos Eugenio Cruceño-Casarrubias, María Elena Sánchez Pardo, and Luis G. Bermúdez-Humarán. 2022. "Functional Foods, Nutraceuticals and Probiotics: A Focus on Human Health" Microorganisms 10, no. 5: 1065. https://doi.org/10.3390/microorganisms10051065
APA StyleDamián, M. R., Cortes-Perez, N. G., Quintana, E. T., Ortiz-Moreno, A., Garfias Noguez, C., Cruceño-Casarrubias, C. E., Sánchez Pardo, M. E., & Bermúdez-Humarán, L. G. (2022). Functional Foods, Nutraceuticals and Probiotics: A Focus on Human Health. Microorganisms, 10(5), 1065. https://doi.org/10.3390/microorganisms10051065







