High Genetic Diversity and Antimicrobial Resistance in Escherichia coli Highlight Arapaima gigas (Pisces: Arapaimidae) as a Reservoir of Quinolone-Resistant Strains in Brazilian Amazon Rivers
Abstract
1. Introduction
2. Materials and Methods
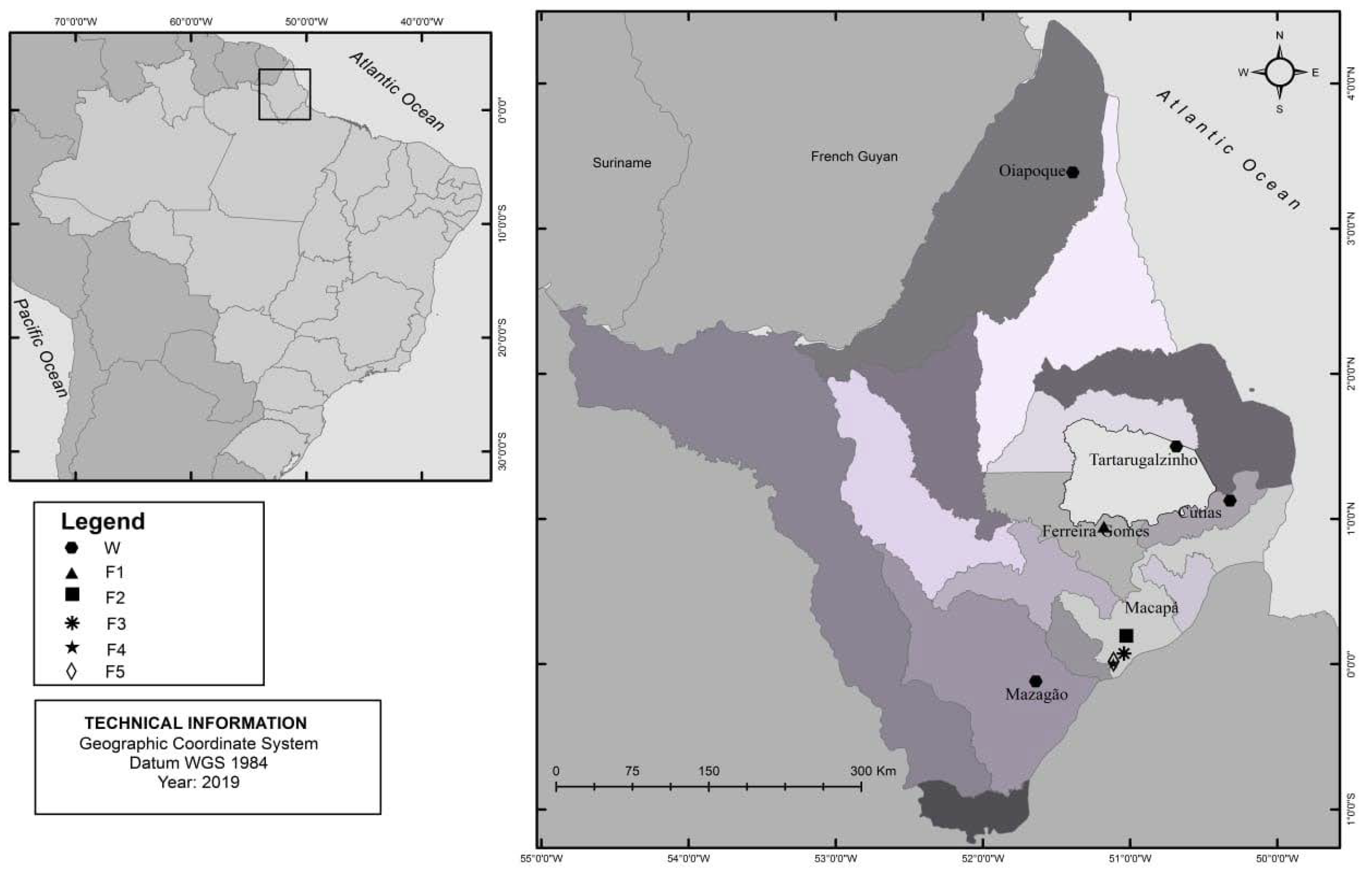
2.1. Study Setting and Sample Collection
2.2. Bacterial Isolates and Antimicrobial Susceptibility Testing
2.3. Molecular Detection of Antimicrobial Resistance and Virulence Markers
2.4. Molecular Typing by Multilocus Sequence Typing (MLST)
2.5. Molecular Typing by Pulsifield Gel Eletroforese (PFGE)
2.6. Statistical Analysis
2.7. Ethical Considerations
3. Results
3.1. Antimicrobial Resistance and Virulence Features
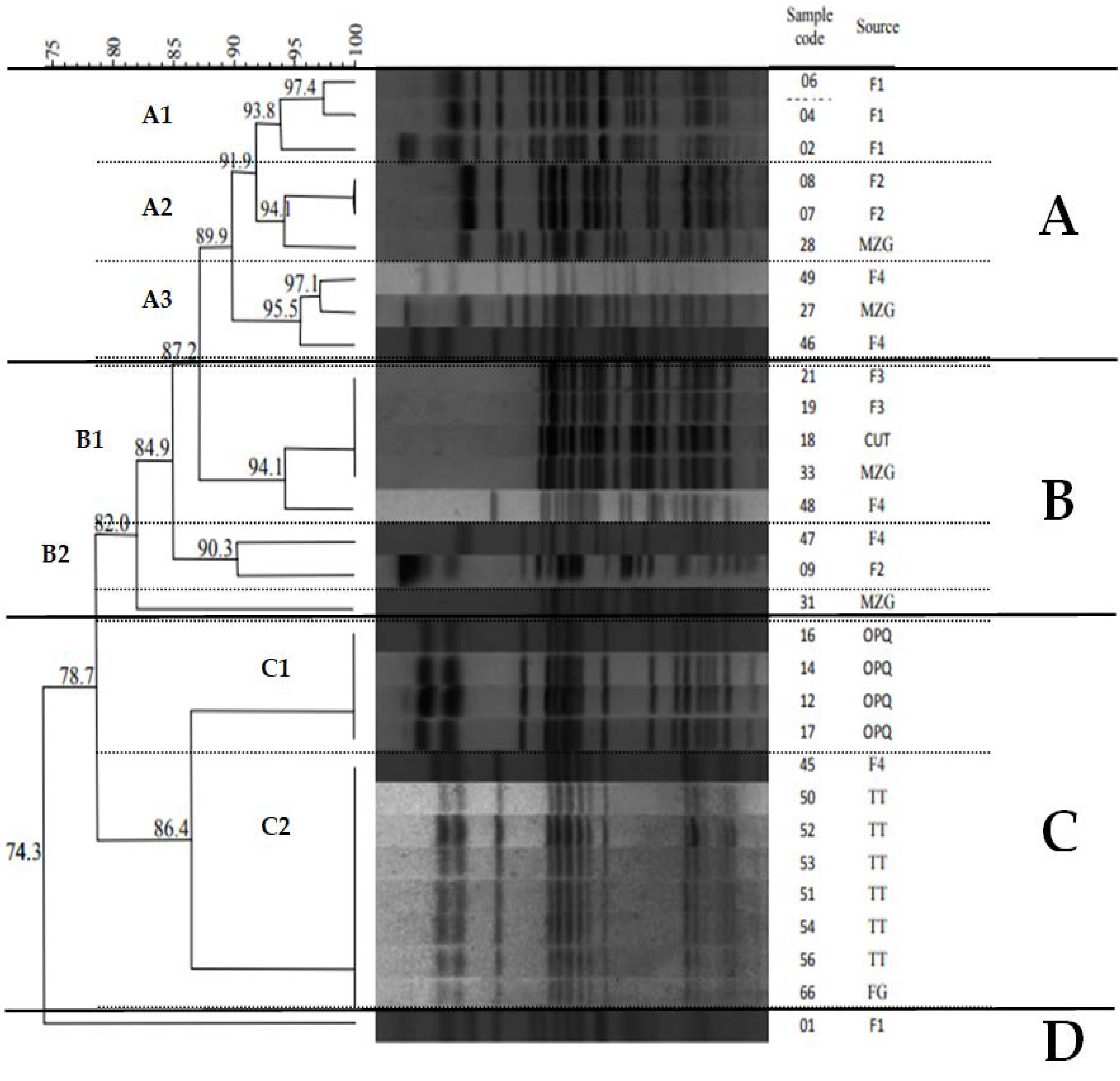
3.2. Molecular Typing by MLST and PFGE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haberecht, H.B.; Nealon, N.J.; Gilliland, J.R.; Holder, A.V.; Runyan, C.; Oppel, R.C.; Ibrahim, H.M.; Mueller, L.; Schrupp, F.; Vilchez, S.; et al. Antimicrobial-Resistant Escherichia coli from Environmental Waters in Northern Colorado. J. Environ. Public Health 2019, 2019, 3862949. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Blas, J.F.; Ovejero, C.M.; David, S.; Montero, N.; Calero-Caceres, W.; Garcillan-Barcia, M.P.; de la Cruz, F.; Muniesa, M.; Aanensen, D.M.; Gonzalez-Zorn, B. Population genomics and antimicrobial resistance dynamics of Escherichia coli in wastewater and river environments. Commun. Biol. 2021, 4, 457. [Google Scholar] [CrossRef] [PubMed]
- Berendonk, T.U.; Manaia, C.M.; Merlin, C.; Fatta-Kassinos, D.; Cytryn, E.; Walsh, F.; Bürgmann, H.; Sørum, H.; Norström, M.; Pons, M.-N.; et al. Tackling antibiotic resistance: The environmental framework. Nat. Rev. Microbiol. 2015, 13, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Xu, S.; Tang, Z.; Li, Z.; Zhang, L. Use of antimicrobials in food animals and impact of transmission of antimicrobial resistance on humans. Biosaf. Health 2021, 3, 32–38. [Google Scholar] [CrossRef]
- Proietti-Junior, A.A.; Lima, L.S.; Roges, E.M.; Rodrigues, Y.C.; Lima, K.V.B.; Rodrigues, D.P.; Tavares-Dias, M. Experimental co-infection by Aeromonas hydrophila and Aeromonas jandaei in pirarucu Arapaima gigas (Pisces: Arapaimidae). Aquac. Res. 2021, 52, 1688–1696. [Google Scholar] [CrossRef]
- Gillings, M.R.; Stokes, H.W. Are humans increasing bacterial evolvability? Trends Ecol. Evol. 2012, 27, 346–352. [Google Scholar] [CrossRef]
- Saharan, V.V.; Verma, P.; Singh, A.P. High prevalence of antimicrobial resistance in Escherichia coli, Salmonella spp. and Staphylococcus aureus isolated from fish samples in India. Aquac. Res. 2020, 51, 1200–1210. [Google Scholar] [CrossRef]
- The European Union One Health 2018 Zoonoses Report. EFSA J. 2019, 17, 5926. [CrossRef]
- Dib, A.L.; Agabou, A.; Chahed, A.; Kurekci, C.; Moreno, E.; Espigares, M.; Espigares, E. Isolation, molecular characterization and antimicrobial resistance of enterobacteriaceae isolated from fish and seafood. Food Control 2018, 88, 54–60. [Google Scholar] [CrossRef]
- Jang, J.; Hur, H.-G.; Sadowsky, M.J.; Byappanahalli, M.N.; Yan, T.; Ishii, S. Environmental Escherichia coli: Ecology and public health implications-a review. J. Appl. Microbiol. 2017, 123, 570–581. [Google Scholar] [CrossRef]
- Farfán-García, A.E.; Ariza-Rojas, S.C.; Vargas-Cárdenas, F.A.; Vargas-Remolina, L.V. Mecanismos de virulencia de Escherichia coli enteropatógena. Rev. Chil. Infectol. 2016, 33, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.-H.; Kim, D.W.; Jung, S.-M.; Cho, S.-H. Molecular Characterization of Enterotoxigenic Escherichia coli Strains Isolated from Diarrheal Patients in Korea during 2003–2011. PLoS ONE 2014, 9, e96896. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aedo, S.; Ivanova, L.; Tomova, A.; Cabello, F.C. Plasmid-Related Quinolone Resistance Determinants in Epidemic Vibrio parahaemolyticus, Uropathogenic Escherichia coli, and Marine Bacteria from an Aquaculture Area in Chile. Microb. Ecol. 2014, 68, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Tomova, A.; Ivanova, L.; Buschmann, A.H.; Godfrey, H.P.; Cabello, F.C. Plasmid-Mediated Quinolone Resistance (PMQR) Genes and Class 1 Integrons in Quinolone-Resistant Marine Bacteria and Clinical Isolates of Escherichia coli from an Aquacultural Area. Microb. Ecol. 2018, 75, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Plantamura, J.; Bousquet, A.; Védy, S.; Larréché, S.; Bigaillon, C.; Delacour, H.; Mérens, A. Molecular epidemiological of extended-spectrum β-lactamase producing Escherichia coli isolated in Djibouti. J. Infect. Dev. Ctries 2019, 13, 753–758. [Google Scholar] [CrossRef]
- Raimondi, S.; Righini, L.; Candeliere, F.; Musmeci, E.; Bonvicini, F.; Gentilomi, G.; Erjavec, M.S.; Amaretti, A.; Rossi, M. Antibiotic Resistance, Virulence Factors, Phenotyping, and Genotyping of E. coli Isolated from the Feces of Healthy Subjects. Microorganisms 2019, 7, 251. [Google Scholar] [CrossRef]
- von Wintersdorff, C.J.H.; Penders, J.; van Niekerk, J.M.; Mills, N.D.; Majumder, S.; van Alphen, L.B.; Savelkoul, P.H.M.; Wolffs, P.F.G. Dissemination of Antimicrobial Resistance in Microbial Ecosystems through Horizontal Gene Transfer. Front. Microbiol. 2016, 7, 173. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Hu, H.-W.; Chen, Q.-L.; Singh, B.K.; Yan, H.; Chen, D.; He, J.-Z. Transfer of antibiotic resistance from manure-amended soils to vegetable microbiomes. Environ. Int. 2019, 130, 104912. [Google Scholar] [CrossRef]
- Kimera, Z.I.; Mgaya, F.X.; Mshana, S.E.; Karimuribo, E.D.; Matee, M.I.N. Occurrence of Extended Spectrum Beta Lactamase (ESBL) Producers, Quinolone and Carbapenem Resistant Enterobacteriaceae Isolated from Environmental Samples along Msimbazi River Basin Ecosystem in Tanzania. Int. J. Environ. Res. Public. Health 2021, 18, 8264. [Google Scholar] [CrossRef]
- Miller, R.A.; Harbottle, H. Antimicrobial Drug Resistance in Fish Pathogens. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- Dias, M.K.R.; Sampaio, L.S.; Proietti-Junior, A.A.; Yoshioka, E.T.O.; Rodrigues, D.P.; Rodriguez, A.F.R.; Ribeiro, R.A.; Faria, F.S.E.D.V.; Ozório, R.O.A.; Tavares-Dias, M. Lethal dose and clinical signs of Aeromonas hydrophila in Arapaima gigas (Arapaimidae), the giant fish from Amazon. Vet. Microbiol. 2016, 188, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Proietti-Junior, A.A.; Lima, L.S.; Cardoso, F.M.N.; Rodrigues, D.d.P.; Dias, M.T. Bacterioses Em Alevinos de Pirarucu de Cultivo, Com Ênfase Em Edwardsielose e Aeromonose, 1st ed.; Belém, Ed.; Centro de Pesquisa Agroflorestal do Amapá—EMBRAPA: Macapá, Brazil, 2017. [Google Scholar]
- Weinstein, M.P.; Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2010; ISBN 978-1-68440-066-9. [Google Scholar]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Krumperman, P.H. Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of foods. Appl. Environ. Microbiol. 1983, 46, 165–170. [Google Scholar] [CrossRef]
- Jacoby, G.A.; Gacharna, N.; Black, T.A.; Miller, G.H.; Hooper, D.C. Temporal Appearance of Plasmid-Mediated Quinolone Resistance Genes. Antimicrob. Agents Chemother. 2009, 53, 1665–1666. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.B.; Park, C.H.; Kim, C.J.; Kim, E.-C.; Jacoby, G.A.; Hooper, D.C. Prevalence of plasmid-mediated quinolone resistance determinants over a 9-year period. Antimicrob. Agents Chemother. 2009, 53, 639–645. [Google Scholar] [CrossRef]
- Cavaco, L.M.; Hasman, H.; Xia, S.; Aarestrup, F.M. qnrD, a Novel Gene Conferring Transferable Quinolone Resistance in Salmonella enterica Serovar Kentucky and Bovismorbificans Strains of Human Origin. Antimicrob. Agents Chemother. 2009, 53, 603–608. [Google Scholar] [CrossRef]
- Park, C.H.; Robicsek, A.; Jacoby, G.A.; Sahm, D.; Hooper, D.C. Prevalence in the United States of aac(6′)-Ib-cr Encoding a Ciprofloxacin-Modifying Enzyme. Antimicrob. Agents Chemother. 2006, 50, 3953–3955. [Google Scholar] [CrossRef]
- Naas, T.; Oxacelay, C.; Nordmann, P. Identification of CTX-M-Type Extended-Spectrum-β-Lactamase Genes Using Real-Time PCR and Pyrosequencing. Antimicrob. Agents Chemother. 2007, 51, 223–230. [Google Scholar] [CrossRef]
- Eaves, D.J.; Liebana, E.; Woodward, M.J.; Piddock, L.J.V. Detection of gyrA Mutations in Quinolone-Resistant Salmonella enterica by Denaturing High-Performance Liquid Chromatography. J. Clin. Microbiol. 2002, 40, 4121–4125. [Google Scholar] [CrossRef]
- Poirel, L.; Walsh, T.R.; Cuvillier, V.; Nordmann, P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn. Microbiol. Infect. Dis. 2011, 70, 119–123. [Google Scholar] [CrossRef]
- Omar, K.B.; Barnard, T.G. Detection of diarrhoeagenic Escherichia coli in clinical and environmental water sources in South Africa using single-step 11-gene m-PCR. World J. Microbiol. Biotechnol. 2014, 30, 2663–2671. [Google Scholar] [CrossRef]
- Wirth, T.; Falush, D.; Lan, R.; Colles, F.; Mensa, P.; Wieler, L.H.; Karch, H.; Reeves, P.R.; Maiden, M.C.J.; Ochman, H.; et al. Sex and virulence in Escherichia coli: An evolutionary perspective. Mol. Microbiol. 2006, 60, 1136–1151. [Google Scholar] [CrossRef]
- Ribot, E.M.; Fair, M.A.; Gautom, R.; Cameron, D.N.; Hunter, S.B.; Swaminathan, B.; Barrett, T.J. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog. Dis. 2006, 3, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Ayres, M. BioEstat 5.0: Aplicações Estatísticas nas Áreas de Ciências Biomédicas, 4th ed.; Sociedade Civil Mamirauá; MCT-CNPq: Manaus, Brazil, 2007; ISBN 85-85924-10-1. [Google Scholar]
- Alexander, T.W.; Inglis, G.D.; Yanke, L.J.; Topp, E.; Read, R.R.; Reuter, T.; McAllister, T.A. Farm-to-fork characterization of Escherichia coli associated with feedlot cattle with a known history of antimicrobial use. Int. J. Food Microbiol. 2010, 137, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Franz, E.; Veenman, C.; van Hoek, A.H.A.M.; Husman, A.d.R.; Blaak, H. Pathogenic Escherichia coli producing Extended-Spectrum β-Lactamases isolated from surface water and wastewater. Sci. Rep. 2015, 5, 14372. [Google Scholar] [CrossRef] [PubMed]
- Onmaz, N.E.; Yildirim, Y.; Karadal, F.; Hizlisoy, H.; Al, S.; Gungor, C.; Disli, H.B.; Barel, M.; Dishan, A.; Akai Tegin, R.A.; et al. Escherichia coli O157 in fish: Prevalence, antimicrobial resistance, biofilm formation capacity, and molecular characterization. LWT 2020, 133, 109940. [Google Scholar] [CrossRef]
- de Almeida, M.V.A.; Cangussú, Í.M.; de Carvalho, A.L.S.; Brito, I.L.P.; Costa, R.A. Drug resistance, AmpC-β-lactamase and extended-spectrum β-lactamase-producing Enterobacteriaceae isolated from fish and shrimp. Rev. Inst. Med. Trop. São Paulo 2017, 59, e70. [Google Scholar] [CrossRef]
- Pavez, M.; Troncoso, C.; Osses, I.; Salazar, R.; Illesca, V.; Reydet, P.; Rodríguez, C.; Chahin, C.; Concha, C.; Barrientos, L. High prevalence of CTX-M-1 group in ESBL-producing enterobacteriaceae infection in intensive care units in southern Chile. Braz. J. Infect. Dis. 2019, 23, 102–110. [Google Scholar] [CrossRef]
- Pitout, J.D.; Laupland, K.B. Extended-spectrum β-lactamase-producing Enterobacteriaceae: An emerging public-health concern. Lancet Infect. Dis. 2008, 8, 159–166. [Google Scholar] [CrossRef]
- Rodrigues, Y.C.; Furlaneto, I.P.; Maciel, A.H.P.; Quaresma, A.J.P.G.; de Matos, E.C.O.; Conceição, M.L.; Vieira, M.C.d.S.; Brabo, G.L.d.C.; Sarges, E.d.S.N.F.; Lima, L.N.G.C.; et al. High prevalence of atypical virulotype and genetically diverse background among Pseudomonas aeruginosa isolates from a referral hospital in the Brazilian Amazon. PLoS ONE 2020, 15, e0238741. [Google Scholar] [CrossRef]
- Naeem, S.; Bilal, H.; Muhammad, H.; Khan, M.A.; Hameed, F.; Bahadur, S.; Rehman, T.U. Detection of blaNDM-1 gene in ESBL producing Escherichia coli and Klebsiella pneumoniae isolated from urine samples. J. Infect. Dev. Ctries 2021, 15, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Bilal, H.; Rehman, T.U.; Khan, M.A.; Hameed, F.; Jian, Z.G.; Han, J.; Yang, X. Molecular Epidemiology of mcr-1, blaKPC-2, and blaNDM-1 Harboring Clinically Isolated Escherichia coli from Pakistan. Infect. Drug Resist. 2021, 14, 1467–1479. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, G.A.; Strahilevitz, J.; Hooper, D.C. Plasmid-Mediated Quinolone Resistance. Microbiol. Spectr. 2014, 2, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Liu, D.; Wang, X.-H.; Wang, Y.; Zhang, B.; Wang, M.; Xu, H. Bacterial plasmid-mediated quinolone resistance genes in aquatic environments in China. Sci. Rep. 2017, 7, 40610. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.-C.; Lo, D.-Y.; Chang, S.-K.; Chou, C.-C.; Kuo, H.-C. Prevalence of plasmid-mediated quinolone resistance in Escherichia coli isolated from diseased animals in Taiwan. J. Vet. Med. Sci. 2017, 79, 730–735. [Google Scholar] [CrossRef]
- Castanheira, M.; Pereira, A.S.; Nicoletti, A.G.; Pignatari, A.C.C.; Barth, A.L.; Gales, A.C. First Report of Plasmid-Mediated qnrA1 in a Ciprofloxacin-Resistant Escherichia coli Strain in Latin America. Antimicrob. Agents Chemother. 2007, 51, 1527–1529. [Google Scholar] [CrossRef]
- Viana, A.L.M.; Cayô, R.; Avelino, C.C.; Gales, A.C.; Franco, M.C.; Minarini, L.A.R. Extended-spectrum β-lactamases in Enterobacteriaceae isolated in Brazil carry distinct types of plasmid-mediated quinolone resistance genes. J. Med. Microbiol. 2013, 62, 1326–1331. [Google Scholar] [CrossRef]
- Paiva, M.C.; Nascimento, A.M.A.; Camargo, I.L.B.C.; Lima-Bittencourt, C.I.; Nardi, R.M.D. The first report of the qnrB19, qnrS1 and aac(6´)-Ib-cr genes in urinary isolates of ciprofloxacin-resistant Escherichia coli in Brazil. Mem. Inst. Oswaldo Cruz 2012, 107, 687–689. [Google Scholar] [CrossRef][Green Version]
- Bens, C.C.P.M.; Voss, A.; Klaassen, C.H.W. Presence of a Novel DNA Methylation Enzyme in Methicillin-Resistant Staphylococcus aureus Isolates Associated with Pig Farming Leads to Uninterpretable Results in Standard Pulsed-Field Gel Electrophoresis Analysis. J. Clin. Microbiol. 2006, 44, 1875–1876. [Google Scholar] [CrossRef][Green Version]
- Riley, L.W. Pandemic lineages of extraintestinal pathogenic Escherichia coli. Clin. Microbiol. Infect. 2014, 20, 380–390. [Google Scholar] [CrossRef]
- Woodford, N.; Turton, J.F.; Livermore, D.M. Multiresistant Gram-negative bacteria: The role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol. Rev. 2011, 35, 736–755. [Google Scholar] [CrossRef] [PubMed]
- Zelendova, M.; Papagiannitsis, C.C.; Valcek, A.; Medvecky, M.; Bitar, I.; Hrabak, J.; Gelbicova, T.; Barakova, A.; Kutilova, I.; Karpiskova, R.; et al. Characterization of the Complete Nucleotide Sequences of mcr-1-Encoding Plasmids From Enterobacterales Isolates in Retailed Raw Meat Products From the Czech Republic. Front. Microbiol. 2021, 11, 604067. [Google Scholar] [CrossRef] [PubMed]
- Kubelová, M.; Koláčková, I.; Gelbíčová, T.; Florianová, M.; Kalová, A.; Karpíšková, R. Virulence Properties of mcr-1-Positive Escherichia coli Isolated from Retail Poultry Meat. Microorganisms 2021, 9, 308. [Google Scholar] [CrossRef] [PubMed]
- Wise, M.G.; Estabrook, M.A.; Sahm, D.F.; Stone, G.G.; Kazmierczak, K.M. Prevalence of mcr-type genes among colistin-resistant Enterobacteriaceae collected in 2014-2016 as part of the INFORM global surveillance program. PLoS ONE 2018, 13, e0195281. [Google Scholar] [CrossRef]
- Blaak, H.; Hamidjaja, R.A.; van Hoek, A.H.A.M.; de Heer, L.; de Roda Husman, A.M.; Schets, F.M. Detection of Extended-Spectrum Beta-Lactamase (ESBL)-Producing Escherichia coli on Flies at Poultry Farms. Appl. Environ. Microbiol. 2014, 80, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Vredenburg, J.; Varela, A.R.; Hasan, B.; Bertilsson, S.; Olsen, B.; Narciso-da-Rocha, C.; Bonnedahl, J.; Stedt, J.; Da Costa, P.M.; Manaia, C.M. Quinolone-resistant Escherichia coli isolated from birds of prey in Portugal are genetically distinct from those isolated from water environments and gulls in Portugal, Spain and Sweden: Molecular epidemiology of quinolone resistant E. coli. Environ. Microbiol. 2014, 16, 995–1004. [Google Scholar] [CrossRef]
- Reuland, E.A.; Overdevest, I.T.M.A.; al Naiemi, N.; Kalpoe, J.S.; Rijnsburger, M.C.; Raadsen, S.A.; Ligtenberg-Burgman, I.; van der Zwaluw, K.W.; Heck, M.; Savelkoul, P.H.M.; et al. High prevalence of ESBL-producing Enterobacteriaceae carriage in Dutch community patients with gastrointestinal complaints. Clin. Microbiol. Infect. 2013, 19, 542–549. [Google Scholar] [CrossRef][Green Version]
- Koga, V.L.; Maluta, R.P.; da Silveira, W.D.; Ribeiro, R.A.; Hungria, M.; Vespero, E.C.; Nakazato, G.; Kobayashi, R.K.T. Characterization of CMY-2-type beta-lactamase-producing Escherichia coli isolated from chicken carcasses and human infection in a city of South Brazil. BMC Microbiol. 2019, 19, 174. [Google Scholar] [CrossRef]
- Li, B.; Pacey, M.P.; Doi, Y. Chromosomal 16S Ribosomal RNA Methyltransferase RmtE1 in Escherichia coli Sequence Type 448. Emerg. Infect. Dis. 2000, 23, 876–878. [Google Scholar] [CrossRef]
- Aibinu, I.; Odugbemi, T.; Koenig, W.; Ghebremedhin, B. Sequence Type ST131 and ST10 Complex (ST617) predominant among CTX-M-15-producing Escherichia coli isolates from Nigeria. Clin. Microbiol. Infect. 2012, 18, E49–E51. [Google Scholar] [CrossRef][Green Version]
- Muggeo, A.; Maiga, A.; Maiga, I.; Brasme, L.; Dicko, O.A.; de Champs, C.; Guillard, T. First description of IncX3 NDM-5-producing plasmid within Escherichia coli ST448 in Mali. J. Med. Microbiol. 2020, 69, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Anyanwu, M.U.; Okpala, C.O.R.; Chah, K.F.; Shoyinka, V.S. Prevalence and Traits of Mobile Colistin Resistance Gene Harbouring Isolates from Different Ecosystems in Africa. BioMed Res. Int. 2021, 2021, 6630379. [Google Scholar] [CrossRef] [PubMed]
- Foster-Nyarko, E.; Alikhan, N.-F.; Ravi, A.; Thomson, N.M.; Jarju, S.; Kwambana-Adams, B.A.; Secka, A.; O’Grady, J.; Antonio, M.; Pallen, M.J. Genomic diversity of Escherichia coli isolates from backyard chickens and guinea fowl in the Gambia. Microb. Genomics 2021, 7, mgen000484. [Google Scholar] [CrossRef] [PubMed]
- Day, M.J.; Hopkins, K.L.; Wareham, D.W.; Toleman, M.A.; Elviss, N.; Randall, L.; Teale, C.; Cleary, P.; Wiuff, C.; Doumith, M.; et al. Extended-spectrum β-lactamase-producing Escherichia coli in human-derived and foodchain-derived samples from England, Wales, and Scotland: An epidemiological surveillance and typing study. Lancet Infect. Dis. 2019, 19, 1325–1335. [Google Scholar] [CrossRef]
- Seenama, C.; Thamlikitkul, V.; Ratthawongjirakul, P. Multilocus sequence typing and blaESBL characterization of extended-spectrum beta-lactamase-producing Escherichia coli isolated from healthy humans and swine in Northern Thailand. Infect. Drug Resist. 2019, 12, 2201–2214. [Google Scholar] [CrossRef]
- Overdevest, I. Extended-Spectrum β-Lactamase Genes of Escherichia coli in Chicken Meat and Humans, The Netherlands. Emerg. Infect. Dis. 2011, 17, 1216–1222. [Google Scholar] [CrossRef]
- Gomi, R.; Matsuda, T.; Matsumura, Y.; Yamamoto, M.; Tanaka, M.; Ichiyama, S.; Yoneda, M. Whole-Genome Analysis of Antimicrobial-Resistant and Extraintestinal Pathogenic Escherichia coli in River Water. Appl. Environ. Microbiol. 2017, 83, e02703-16. [Google Scholar] [CrossRef]
- Rayasam, S.D.G.; Ray, I.; Smith, K.R.; Riley, L.W. Extraintestinal Pathogenic Escherichia coli and Antimicrobial Drug Resistance in a Maharashtrian Drinking Water System. Am. J. Trop. Med. Hyg. 2019, 100, 1101–1104. [Google Scholar] [CrossRef]
- Salim, A.; Babu, P.; Mohan, K.; Moorthy, M.; Raj, D.; Kallampillil Thirumeni, S.; Suresh, S.; Madhavan, A.; Nair, B.G.; Chattopadhyay, S.; et al. Draft Genome Sequence of an Escherichia coli Sequence Type 155 Strain Isolated from Sewage in Kerala, India. Microbiol. Resour. Announc. 2019, 8, e01707-18. [Google Scholar] [CrossRef]
- Skurnik, D.; Clermont, O.; Guillard, T.; Launay, A.; Danilchanka, O.; Pons, S.; Diancourt, L.; Lebreton, F.; Kadlec, K.; Roux, D.; et al. Emergence of Antimicrobial-Resistant Escherichia coli of Animal Origin Spreading in Humans. Mol. Biol. Evol. 2016, 33, 898–914. [Google Scholar] [CrossRef][Green Version]
- Ramadan, H.; Soliman, A.M.; Hiott, L.M.; Elbediwi, M.; Woodley, T.A.; Chattaway, M.A.; Jenkins, C.; Frye, J.G.; Jackson, C.R. Emergence of Multidrug-Resistant Escherichia coli Producing CTX-M, MCR-1, and FosA in Retail Food from Egypt. Front. Cell. Infect. Microbiol. 2021, 11, 681588. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.-P.; Xia, J.; Yang, L.; Li, L.; Sun, J.; Liu, Y.-H.; Jiang, H.-X. Characterization of CTX-M-14-producing Escherichia coli from food-producing animals. Front. Microbiol. 2015, 6, 1136. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, W.; Wang, J.; Pan, J.; Sun, S.; Yu, Y.; Zhao, B.; Ma, Y.; Zhang, T.; Qi, J.; et al. Identification and Characterization of the First Escherichia coli Strain Carrying NDM-1 Gene in China. PLoS ONE 2013, 8, e66666. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mohsin, M.; Raza, S.; Schaufler, K.; Roschanski, N.; Sarwar, F.; Semmler, T.; Schierack, P.; Guenther, S. High Prevalence of CTX-M-15-Type ESBL-Producing E. coli from Migratory Avian Species in Pakistan. Front. Microbiol. 2017, 8, 2476. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, J.; Neuwirt, N.; Barbato, L.; Neves, P.R.; Leigue, L.; Padilha, J.; Pestana de Castro, A.F.; Gregory, L.; Lincopan, N. Identification of fluoroquinolone-resistant extended-spectrum β-lactamase (CTX-M-8)-producing Escherichia coli ST224, ST2179 and ST2308 in buffalo (Bubalus bubalis). J. Antimicrob. Chemother. 2014, 69, 2866–2869. [Google Scholar] [CrossRef] [PubMed]
- Nittayasut, N.; Yindee, J.; Boonkham, P.; Yata, T.; Suanpairintr, N.; Chanchaithong, P. Multiple and High-Risk Clones of Extended-Spectrum Cephalosporin-Resistant and blaNDM-5-Harbouring Uropathogenic Escherichia coli from Cats and Dogs in Thailand. Antibiotics 2021, 10, 1374. [Google Scholar] [CrossRef]
- Silva, K.C.; Moreno, M.; Cabrera, C.; Spira, B.; Cerdeira, L.; Lincopan, N.; Moreno, A.M. First Characterization of CTX-M-15-Producing Escherichia coli Strains Belonging to Sequence Type (ST) 410, ST224, and ST1284 from Commercial Swine in South America. Antimicrob. Agents Chemother. 2016, 60, 2505–2508. [Google Scholar] [CrossRef]
- RESET Study Group; Pietsch, M.; Irrgang, A.; Roschanski, N.; Brenner Michael, G.; Hamprecht, A.; Rieber, H.; Käsbohrer, A.; Schwarz, S.; Rösler, U.; et al. Whole genome analyses of CMY-2-producing Escherichia coli isolates from humans, animals and food in Germany. BMC Genom. 2018, 19, 601. [Google Scholar] [CrossRef]
- Ramadan, H.; Jackson, C.R.; Frye, J.G.; Hiott, L.M.; Samir, M.; Awad, A.; Woodley, T.A. Antimicrobial Resistance, Genetic Diversity and Multilocus Sequence Typing of Escherichia coli from Humans, Retail Chicken and Ground Beef in Egypt. Pathogens 2020, 9, 357. [Google Scholar] [CrossRef]
- Leão, C.; Clemente, L.; Moura, L.; Seyfarth, A.M.; Hansen, I.M.; Hendriksen, R.S.; Amaro, A. Emergence and Clonal Spread of CTX-M-65-Producing Escherichia coli From Retail Meat in Portugal. Front. Microbiol. 2021, 12, 653595. [Google Scholar] [CrossRef]
- Qiu, J.; Jiang, Z.; Ju, Z.; Zhao, X.; Yang, J.; Guo, H.; Sun, S. Molecular and Phenotypic Characteristics of Escherichia coli Isolates from Farmed Minks in Zhucheng, China. BioMed Res. Int. 2019, 2019, 3917841. [Google Scholar] [CrossRef] [PubMed]
- Kamjumphol, W.; Wongboot, W.; Suebwongsa, N.; Kluabwang, P.; Chantaroj, S.; Okada, K. Draft genome sequence of a colistin-resistant Escherichia coli ST226: A clinical strain harbouring an mcr-1 variant. J. Glob. Antimicrob. Resist. 2019, 16, 168–169. [Google Scholar] [CrossRef] [PubMed]
- Stoesser, N.; Sheppard, A.E.; Moore, C.E.; Golubchik, T.; Parry, C.M.; Nget, P.; Saroeun, M.; Day, N.P.J.; Giess, A.; Johnson, J.R.; et al. Extensive Within-Host Diversity in Fecally Carried Extended-Spectrum-Beta-Lactamase-Producing Escherichia coli Isolates: Implications for Transmission Analyses. J. Clin. Microbiol. 2015, 53, 2122–2131. [Google Scholar] [CrossRef]
- Rodrigues, C.; Machado, E.; Fernandes, S.; Peixe, L.; Novais, Â. An update on faecal carriage of ESBL-producing Enterobacteriaceae by Portuguese healthy humans: Detection of the H 30 subclone of B2-ST131 Escherichia coli producing CTX-M-27: Table 1. J. Antimicrob. Chemother. 2016, 71, 1120–1122. [Google Scholar] [CrossRef][Green Version]
- Papouskova, A.; Masarikova, M.; Valcek, A.; Senk, D.; Cejkova, D.; Jahodarova, E.; Cizek, A. Genomic analysis of Escherichia coli strains isolated from diseased chicken in the Czech Republic. BMC Vet. Res. 2020, 16, 189. [Google Scholar] [CrossRef] [PubMed]
- Dadashi, M.; Sameni, F.; Bostanshirin, N.; Yaslianifard, S.; Khosravi-Dehaghi, N.; Nasiri, M.J.; Goudarzi, M.; Hashemi, A.; Hajikhani, B. Global prevalence and molecular epidemiology of mcr-mediated colistin resistance in Escherichia coli clinical isolates: A systematic review. J. Glob. Antimicrob. Resist. 2021, S2213716521002447. [Google Scholar] [CrossRef] [PubMed]
- Martak, D.; Guther, J.; Verschuuren, T.D.; Valot, B.; Conzelmann, N.; Bunk, S.; Riccio, M.E.; Salamanca, E.; Meunier, A.; Henriot, C.P.; et al. Populations of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae are different in human-polluted environment and food items: A multicentre European study. Clin. Microbiol. Infect. 2021, S1198743X21004146. [Google Scholar] [CrossRef]
- Salgado-Caxito, M.; Benavides, J.A.; Adell, A.D.; Paes, A.C.; Moreno-Switt, A.I. Global prevalence and molecular characterization of extended-spectrum β-lactamase producing-Escherichia coli in dogs and cats—A scoping review and meta-analysis. One Health 2021, 12, 100236. [Google Scholar] [CrossRef]
- Falgenhauer, L.; zur Nieden, A.; Harpel, S.; Falgenhauer, J.; Domann, E. Clonal CTX-M-15-Producing Escherichia coli ST-949 Are Present in German Surface Water. Front. Microbiol. 2021, 12, 617349. [Google Scholar] [CrossRef]
- Fagerström, A.; Mölling, P.; Khan, F.A.; Sundqvist, M.; Jass, J.; Söderquist, B. Comparative distribution of extended-spectrum beta-lactamase–producing Escherichia coli from urine infections and environmental waters. PLoS ONE 2019, 14, e0224861. [Google Scholar] [CrossRef]
- Potel, C.; Ortega, A.; Martínez-Lamas, L.; Bautista, V.; Regueiro, B.; Oteo, J. Interspecies Transmission of the bla OXA-48 Gene from a Klebsiella pneumoniae High-Risk Clone of Sequence Type 147 to Different Escherichia coli Clones in the Gut Microbiota. Antimicrob. Agents Chemother. 2018, 62, e01699-17. [Google Scholar] [CrossRef] [PubMed]
- Tahar, S.; Nabil, M.M.; Safia, T.; Ngaiganam, E.P.; Omar, A.; Hafidha, C.; Hanane, Z.; Rolain, J.-M.; Diene, S.M. Molecular Characterization of Multidrug-Resistant Escherichia coli Isolated from Milk of Dairy Cows with Clinical Mastitis in Algeria. J. Food Prot. 2020, 83, 2173–2178. [Google Scholar] [CrossRef] [PubMed]
- Bonnedahl, J.; Stedt, J.; Waldenström, J.; Svensson, L.; Drobni, M.; Olsen, B. Comparison of Extended-Spectrum β-Lactamase (ESBL) CTX-M Genotypes in Franklin Gulls from Canada and Chile. PLoS ONE 2015, 10, e0141315. [Google Scholar] [CrossRef] [PubMed]
- Alouache, S.; Estepa, V.; Messai, Y.; Ruiz, E.; Torres, C.; Bakour, R. Characterization of ESBLs and Associated Quinolone Resistance in Escherichia coli and Klebsiella pneumoniae Isolates from an Urban Wastewater Treatment Plant in Algeria. Microb. Drug Resist. 2014, 20, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Freitas-Silva, J.; Inácio, Â.S.; Mourão, J.; Antunes, P.; Mendes, Â.; de Carvalho, A.P.; Vasconcelos, V.; Peixe, L.; da Costa, P.M. Occurrence of mcr-1 in Escherichia coli from rabbits of intensive farming. Vet. Microbiol. 2018, 227, 78–81. [Google Scholar] [CrossRef]
- Chandler, J.C.; Anders, J.E.; Blouin, N.A.; Carlson, J.C.; LeJeune, J.T.; Goodridge, L.D.; Wang, B.; Day, L.A.; Mangan, A.M.; Reid, D.A.; et al. The Role of European Starlings (Sturnus vulgaris) in the Dissemination of Multidrug-Resistant Escherichia coli among Concentrated Animal Feeding Operations. Sci. Rep. 2020, 10, 8093. [Google Scholar] [CrossRef]
- Lupo, A.; Saras, E.; Madec, J.-Y.; Haenni, M. Emergence of blaCTX-M-55 associated with fosA, rmtB and mcr gene variants in Escherichia coli from various animal species in France. J. Antimicrob. Chemother. 2018, 73, 867–872. [Google Scholar] [CrossRef]
- Belotindos, L.; Villanueva, M.; Miguel, J.; Bwalya, P.; Harada, T.; Kawahara, R.; Nakajima, C.; Mingala, C.; Suzuki, Y. Prevalence and Characterization of Quinolone-Resistance Determinants in Escherichia coli Isolated from Food-Producing Animals and Animal-Derived Food in the Philippines. Antibiotics 2021, 10, 413. [Google Scholar] [CrossRef]
- Ali, I.; Rafaque, Z.; Ahmed, I.; Tariq, F.; Graham, S.E.; Salzman, E.; Foxman, B.; Dasti, J.I. Phylogeny, sequence-typing and virulence profile of uropathogenic Escherichia coli (UPEC) strains from Pakistan. BMC Infect. Dis. 2019, 19, 620. [Google Scholar] [CrossRef]
- Miotto, M.; Ossai, S.A.; Meredith, J.E.; Barretta, C.; Kist, A.; Prudencio, E.S.; Vieira, C.R.W.; Parveen, S. Genotypic and phenotypic characterization of Escherichia coli isolated from mollusks in Brazil and the United States. Microbiol. Open 2019, 8, e00738. [Google Scholar] [CrossRef]
| Antimicrobial | Farmed (n = 16) | Wild * (n = 18) | ||
|---|---|---|---|---|
| S (%) | NS (%) | S (%) | NS * (%) | |
| AMP | 4 (25) | 12 (75.0) | 3 (16.6) | 15 (83.4) |
| SAM | 5 (31.25) | 11 (68.75) | 7 (38.9) | 11 (61.1) |
| TZP | 14 (87.25) | 2 (12.75) | 18 (100) | 0 |
| CXM | 4 (25) | 12 (75.0) | 6 (33.3) | 12 (66.7) |
| FOX | 11 (68.75) | 5 (31.25) | 7 (38.9) | 11 (61.1) |
| CAZ | 13 (81.25) | 3 (18.75) | 8 (44.4) | 10 (55.6) |
| CRO | 12 (75) | 4 (25.0) | 6 (33.3) | 12 (66.7) |
| FEP | 15 (93.75) | 1 (6.25) | 7 (38.9) | 11 (61.1) |
| CIP | 0 | 16 (100.0) | 0 | 18 (100.0) |
| Sample | Source | Resistance Profile | MDR | Molecular Profile | Virulence | ST | PFGE |
|---|---|---|---|---|---|---|---|
| 01 | F1 | AMP, CIP | − | qnrA | Commensal | 448 | D |
| 02 | F1 | AMP, SAM, CRX, CFO, CIP | + | blaCTX-M | Commensal | 448 | A1 |
| 04 | F1 | AMP, CIP | − | qnrB | Commensal | 448 | A1 |
| 06 | F1 | AMP, CIP | − | qnrB | Commensal | 448 | A1 |
| 07 | F2 | AMP, SAM, CIP | + | qnrA | Commensal | 1249 | A2 |
| 08 | F2 | AMP, SAM, CIP | + | qnrA | Commensal | 1249 | A2 |
| 09 | F2 | AMP, SAM, CIP | + | Commensal | 7973 | B2 | |
| 10 | F2 | AMP, CIP | − | Commensal | 1249 | NT | |
| 12 | OPQ | AMP, CIP | − | Commensal | 224 | C1 | |
| 14 | OPQ | AMP, CIP | − | Commensal | 224 | C1 | |
| 16 | OPQ | CIP | − | Commensal | 224 | C1 | |
| 17 | OPQ | CIP | − | Commensal | 224 | C1 | |
| 18 | CUT | CIP | − | qnrA | Commensal | 155 | B1 |
| 19 | F3 | AMP, SAM, CRX, CFO, CRO, CIP | + | blaCTX-M | ETEC | NT | B1 |
| 20 | F3 | AMP, SAM, CRX, CFO, CRO, CIP | + | blaCTX-M | ETEC | 155 | NT |
| 21 | F3 | CIP | − | qnrA | ETEC | 155 | B1 |
| 27 | MZG | AMP, SAM, CIP | + | Commensal | 4380 | A3 | |
| 28 | MZG | AMP, SAM, CIP | + | Commensal | 949 | A2 | |
| 30 | MZG | AMP, CRX, CFO, CRO, CIP | + | Commensal | NT | NT | |
| 31 | MZG | AMP, CRX, CFO, CAZ, CRO, CPM, CIP | + | blaCTX-M | Commensal | 4482 | B |
| 33 | MZG | AMP, CRX, CFO, CAZ, CRO, CPM, CIP | + | blaCTX-M | Commensal | NT | B1 |
| 45 | F4 | AMP, SAM, PPT, CRX, CFO, CAZ, CRO, CPM, CIP | + | blaCTX-M | Commensal | 226 | C2 |
| 46 | F4 | AMP, SAM, PPT, CRX, CFO, CAZ, CRO, CIP | + | blaCTX-M | Commensal | 1431 | A3 |
| 47 | F4 | CIP | − | Commensal | 224 | B2 | |
| 48 | F4 | CIP | − | Commensal | 1196 | B1 | |
| 49 | F4 | CIP | − | Commensal | 1431 | A3 | |
| 50 | TT | AMP, CRX, CFO, CAZ, CRO, CPM, CIP | + | blaCTX-M | Commensal | 7973 | C2 |
| 51 | TT | AMP, CRX, CFO, CAZ, CRO, CPM, CIP | + | blaCTX-M | Commensal | 7973 | C2 |
| 52 | TT | AMP, CRX, CFO, CAZ, CRO, CPM, CIP | + | blaCTX-M | Commensal | 7973 | C2 |
| 53 | TT | AMP, CRX, CFO, CAZ, CRO, CPM, CIP | + | blaCTX-M | Commensal | 7973 | C2 |
| 54 | TT | AMP, CRX, CFO, CAZ, CRO, CPM, CIP | + | blaCTX-M | Commensal | 7973 | C2 |
| 56 | TT | AMP, CRX, CFO, CAZ, CRO, CPM, CIP | + | blaCTX-M | Commensal | 7973 | C2 |
| 66 | FG | AMP, SAM, CRX, CFO, CAZ, CRO, CPM, CIP | + | blaCTX-M | Commensal | 7973 | C2 |
| 67 | FG | AMP, SAM, CRX, CFO, CAZ, CRO, CPM, CIP | + | blaCTX-M | Commensal | NT | NT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, L.S.; Proietti-Junior, A.A.; Rodrigues, Y.C.; da Silva Vieira, M.C.; Lima, L.N.G.C.; de Oliveira Souza, C.; Dias Gonçalves, V.; de Oliveira Lima, M.; dos Prazeres Rodrigues, D.; Lima, K.V.B. High Genetic Diversity and Antimicrobial Resistance in Escherichia coli Highlight Arapaima gigas (Pisces: Arapaimidae) as a Reservoir of Quinolone-Resistant Strains in Brazilian Amazon Rivers. Microorganisms 2022, 10, 808. https://doi.org/10.3390/microorganisms10040808
Lima LS, Proietti-Junior AA, Rodrigues YC, da Silva Vieira MC, Lima LNGC, de Oliveira Souza C, Dias Gonçalves V, de Oliveira Lima M, dos Prazeres Rodrigues D, Lima KVB. High Genetic Diversity and Antimicrobial Resistance in Escherichia coli Highlight Arapaima gigas (Pisces: Arapaimidae) as a Reservoir of Quinolone-Resistant Strains in Brazilian Amazon Rivers. Microorganisms. 2022; 10(4):808. https://doi.org/10.3390/microorganisms10040808
Chicago/Turabian StyleLima, Luciana Sampaio, Aldo Aparecido Proietti-Junior, Yan Corrêa Rodrigues, Marcelo Cleyton da Silva Vieira, Luana Nepomuceno Gondim Costa Lima, Cintya de Oliveira Souza, Verônica Dias Gonçalves, Marcelo de Oliveira Lima, Dália dos Prazeres Rodrigues, and Karla Valéria Batista Lima. 2022. "High Genetic Diversity and Antimicrobial Resistance in Escherichia coli Highlight Arapaima gigas (Pisces: Arapaimidae) as a Reservoir of Quinolone-Resistant Strains in Brazilian Amazon Rivers" Microorganisms 10, no. 4: 808. https://doi.org/10.3390/microorganisms10040808
APA StyleLima, L. S., Proietti-Junior, A. A., Rodrigues, Y. C., da Silva Vieira, M. C., Lima, L. N. G. C., de Oliveira Souza, C., Dias Gonçalves, V., de Oliveira Lima, M., dos Prazeres Rodrigues, D., & Lima, K. V. B. (2022). High Genetic Diversity and Antimicrobial Resistance in Escherichia coli Highlight Arapaima gigas (Pisces: Arapaimidae) as a Reservoir of Quinolone-Resistant Strains in Brazilian Amazon Rivers. Microorganisms, 10(4), 808. https://doi.org/10.3390/microorganisms10040808







