Essential Topics for the Regulatory Consideration of Phages as Clinically Valuable Therapeutic Agents: A Perspective from Spain
Abstract
:1. General Aspects of the Use of Phages as Antimicrobials
1.1. Advantages
- (a)
- Activity against antibiotic-resistant bacteria. Phages can infect and kill bacteria, including MDR strains [5]. This is the most obvious advantage towards recovering phage therapy to fight antimicrobial resistance today. Moreover, the composition of the phages for therapy may be designed to impose an evolutionary trade-off in which the evolution of bacterial resistance to phage results in increased susceptibility to antibiotics [6]. Under this rationale, the combination therapy of phages plus antibiotics has a remarkable potential to smartly tackle antibiotic resistance by both eliminating resistant strains and preventing the dissemination of resistance genes [7,8]. Many works have been published thus far where phage–antibiotic synergy, known as PAS effect, is reported. This suggests that combined therapy can be the safest and a more advantageous approach, as it minimizes resistance and virulence [9,10,11].
- (b)
- Specificity. The high specificity of many phages towards their host bacterial strains makes them a highly selective therapy that prevents the dysbiosis of the healthy microbiota. Contrary to antibiotics, only strains of the same genus or species—and often just one or very few strains within a species—are susceptible to infection by a given phage, protecting the normal microbiota and reducing side effects [12]. The specificity of phages lies in the bacterial receptor that the virions recognize through one or more receptor-binding proteins [13], and can be further driven by post-entry anti-phage defense mechanisms [14]. Phages can be polyvalent (i.e., with a broad host range) if they recognize a receptor present in several bacteria or, alternatively, their specificity can be very restricted if they bind receptors exclusive to a single bacterial type. Another possibility is that the phage uses a receptor that is only expressed under certain conditions which therefore restricts its infectivity (e.g., the receptor of phage lambda is the maltose receptor, which is only expressed in the presence of maltose [15]).
- (c)
- Multiplication at the site of the infection (auto-dosage). Phages can multiply at the site of the infection. Once the phages reach the targeted bacteria, they will replicate and generate progeny. Therefore, if sufficient phage particles are able to reach the infection site, phage therapy can be considered as auto-dosage treatment. Furthermore, once the infection is successfully controlled, phages would be eliminated in the absence of bacterial hosts. Thus, whenever an auto-dosed, “active” treatment is achieved, it can elicit the infection eradication by only a single administration [16].
- (d)
- Ubiquity and diversity. Phages can be found in virtually any environment [17], and they play an utmost important role in ecosystems by regulating bacterial populations [18], including the human microbiota. The main practical consequence of such ubiquity and the concomitant diversity is the ease of discovery of novel phages, which contrasts with the currently slow antibiotic discovery rate.
- (e)
- Evolvability. Phages are evolving entities and, therefore, can be optimized using directed evolution techniques. This opens up many possibilities compared to conventional treatments, which are stable chemical compounds. Phage evolvability can be exploited in many ways, such as increasing lytic capability, improving particle stability, expanding the host range, or counteracting bacterial resistance. For instance, the Appelmans’ protocol uses spontaneous mutation and recombination among phages present in a cocktail to produce phage variants capable of infecting initially non-susceptible bacterial strains [19,20].
- (f)
- Safety. Humans are carriers of many different phages forming the phageome [21,22]. Their biological functions, beyond regulating bacterial populations, are not yet entirely clear [23]. However, their widespread presence in the human body seems to be a good safety indicator. Moreover, there is evidence of phage safety from clinical trials and intake of phage-treated foods [24]. A potential concern of phage therapy could be the release of bacterial endotoxins after lysis of the targeted bacterial cells. It should be noted, however, that similar observations have been made regarding conventional therapy with certain antibiotics [16], and that the current literature does not support detrimental inflammatory reactions upon phage administration. Phages may also enter tissues that are not the specific target of the treatment, but these interactions also do not appear to produce side effects [25].
1.2. Weaknesses
- (a)
- Phage-resistance. In the same way that resistance to antibiotics emerges, bacteria can become resistant to phage infection. The most common solution to address this involves the use of cocktails of different phages, rather than a single phage, and/or the “à la carte” selection of phages for each particular infectious isolate. This makes it much less likely that the host will become resistant to all phages at the same time [26]. The so-called “step-by-step” technique is an interesting approach in which phages are isolated against phage-resistant bacterial mutants in successive screening steps to obtain other phages capable of infecting resistant variants. By this method, the natural antagonistic co-evolution that would occur upon treatment is mimicked prior to therapy, thus generating a phage cocktail able to infect both the original bacterium and the foreseeable resistant variants [27]. Moreover, the emergence of phage-resistant bacteria is not always a disadvantage, since it sometimes involves a decrease of the fitness or virulence of the bacterial host [28], or may resensitize bacteria to antibiotics [9].
- (b)
- Specificity. This feature can be a double-edged sword. Phage specificity requires careful susceptibility testing of each bacterial pathogen before treatment, which may be viewed as an issue for certain acute infections that require urgent action. In addition, this specificity may require either the development of large phage libraries and/or extensive sampling and screening efforts to provide sufficient coverage of bacterial diversity. This can be a daunting task and has posed major regulatory issues, since, according to the current framework, each individual phage should undergo review and approval. In addition, the eventual need to develop a different phage preparation for each bacterial pathogen, as a personalized medicine, reduces business profitability and can be viewed as a serious drawback by pharmaceutical companies. Again, phage cocktails targeting different receptors or different bacterial strains would be a potential solution.
- (c)
- In vivo phage activity. There is not necessarily a correlation between the in vitro and in vivo behavior of a phage, particularly regarding its propagation ability. This is due both to the complexity of body fluids and the ecological in vivo interactions [29,30]. In addition, the phage propagation is dependent on the physiological state of the bacterial host, which may not be optimal for infection in vivo (for example, depending on whether the bacterium is embedded or not within a biofilm, the expression of receptor molecules, etc.) [31]. Moreover, phages are bigger than antibiotics and, therefore, diffuse less efficiently. This limitation is aggravated in vivo, where multiple physical barriers are encountered. Therefore, the probability of infection at low phage and bacterial densities is low, and the threshold densities required to ensure phage infection may often require the administration of very high phage doses [32,33].
- (d)
- Immune response. Since phages are made of biomacromolecules, they are potentially capable of eliciting an immune reaction upon administration [34]. Generally, the immune reactions against phage components are not considered problematic for the individual under treatment, although they do have a relevant contribution to the outcome of phage therapy [35]. On one hand, the immune response potentially causes the removal of phages from the system [36], although this effect may be overcome by adjusting such parameters as dosing, administration route, etc. On the other hand, synergism with the immune antibacterial response seems relevant for therapeutic success [37], although some evidence suggests that phage therapy can also be successfully applied in immunocompromised patients [38]. To sum up, the interaction of phages with the immune system is complex and not yet well understood, although many unknown implications seem to affect the therapeutic efficacy without contradicting the presumed safety of phage therapy.
- (e)
- Gene transfer. Phages potentially have the ability to modify the genome of the host bacteria, which may increase their virulence or dissemination of antibiotic resistance genes [39]. Indeed, phages can mobilize large fragments of bacterial genomes at relatively high frequencies [40,41]. To date, it is not known whether the mobilized DNA is randomly selected or whether the transfer of some particular genes is favored, e.g., those related to virulence, survival, or fitness of the host strain. A relevant mechanism for phage-mediated transfer of particular genes is associated with lysogeny (the integration of the phage genetic material into the bacterial chromosome) [42]. Therefore, this issue could be minimized by selecting exclusively virulent phages, as well as by analyzing phage genomes in detail to ensure that they do not contain genes encoding toxins or any other undesirable genes.
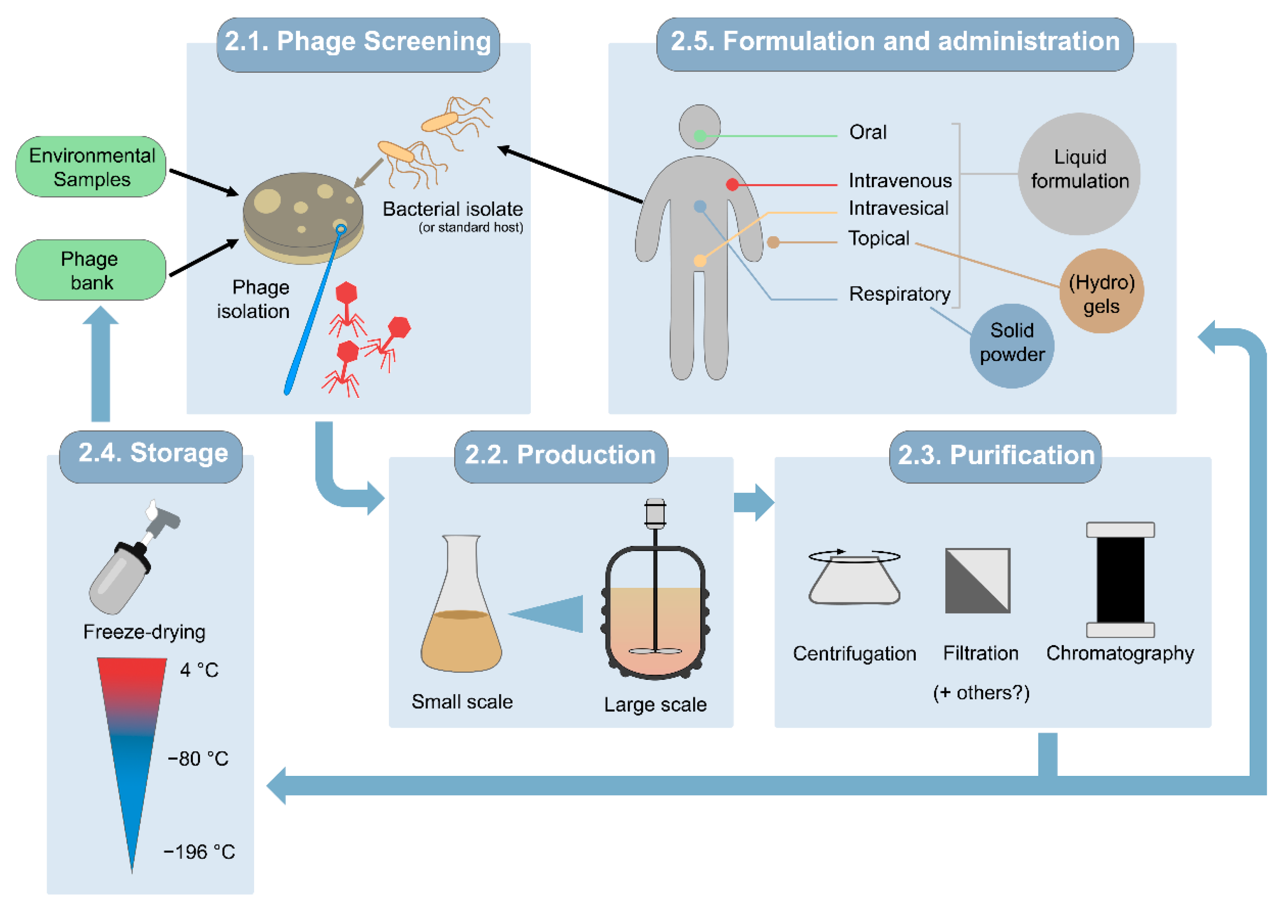
2. Obtaining Therapeutic Phage Preparations
2.1. Selection of Screening Host Strains and Phages Intended for Therapy
2.2. Small- and Large-Scale Production Processes
2.3. Purification of Phage Solutions
2.4. Storage
2.5. Formulation and Administration
- (a)
- Oral administration is appropriate for gastrointestinal infectious diseases. In some cases, oral phages given without additional protection, as water-based liquid suspensions, reportedly survived gastric passage and were recovered in the feces [60,61,62]. The formulation efforts for liquid phage suspensions are typically minimal since phages are just prepared in sterile buffers such as phosphate-buffered saline (PBS), the bacterial growth medium, standard saline, or water [61,63,64,65]. More elaborate formulations specifically intended for oral administration can improve phage survival through the extreme conditions of the gastrointestinal tract. For example, encapsulation protects phages from the highly acidic stomach environment and digestive enzymes [66]. Furthermore, their release can be triggered in a controlled manner, for example, pH-dependent release, with capsules programmed to become permeable at different pHs regarding the aimed site of action: from the stomach (pH 1–3) to the small intestine (pH 5.5–6.5) or the colon (pH 6.5–7.2) [67]. A wide range of natural and synthetic polymers are available that offer considerable plasticity for tailoring phage encapsulation and subsequent release to different biomedical applications, including polysaccharides, natural or synthetic plastic polymers, liposomes, and micelles [68,69].
- (b)
- Topical administration of phages is chosen for skin infections, wounds, burns, ulcers, and osteoarticular infections [70]. Phages have been topically administered in liquid, semi-solid, and liposome-encapsulated formulations, as well as phage-immobilized wound dressings [63]. When using liquid preparations, they may just be dripped onto the infected site or applied in a gauze soaked with the preparation. Alternatively, gel or cream formulations are suitable to overcome some of the limitations of liquid preparations, with a preference for hydrogels over organic solvent-based gels. This is especially relevant for the treatment of burn wounds, since hydrogels help keep the wound hydrated as much as they favor phage stability [63]. Commercial infection-care products can also be used as a formulation basis for topically delivering phages, but care should be taken as to whether the composition of the product reduces phage infectivity [71].
- (c)
- The local phage treatment of respiratory infections requires preparing phages either as stable liquid formulations for intranasal instillation or nebulization, or as a solid powder in an inhalable form [72]. The most popular formulations for respiratory infections are liquid suspensions, due to the relative simplicity of preparation. Nebulization of liquid phage suspensions has been tested with mixed outcomes, generally suggesting that temperature, relative humidity, the nebulization-induced mechanical stress, delivery efficiency of the system, and the nature of the phage itself greatly influence the outcome. Regarding dry powder inhalation, the methods to obtain solid phage formulations include freeze-drying or spray-drying. In general, both processes subject phages to diverse stresses that may impact their infectivity [73], but the control of key parameters and addition of suitable excipients, including polymers for encapsulation, can enhance phage preservation [74,75].
- (d)
- (e)
3. Quality Criteria for Therapeutic Phage Preparations
- (a)
- Phage identity. The identity of each phage is defined by its specific genomic sequence [79,80]. Metagenomics has already been proposed as a quality control method for some vaccines [81], and thus has also been used to assess the composition of commercial phage products [82,83]. This method allows the detection of biological contaminants while also assuring the active product identity. While random mutations during propagation are inevitable, they need to be as limited as possible by process design (e.g., minimizing subcultivation steps), and functional properties should be regularly tested with validated quality controls, as even single-nucleotide polymorphisms can lead to significant phenotypic changes. However, a highly discriminating PCR-based genotyping technique might be sufficient in some cases [79]. The maximum acceptable level of genomic divergence between the master batch and the phage population in the therapeutic product, as well as the frequency of such quality check, should be nonetheless adjusted on a case-by-case basis [79].
- (b)
- Phage Titer. The titer of each individual phage is classically assessed by the double-layer agar method. An alternative is lethality curves, in which the kinetics of phage-induced lysis are assessed by measuring the optical density of phage-infected bacterial cultures [84]. Other methods, such as qPCR and ELISA, can be used to quantify phages, but they do not necessarily quantify infectious viral particles, whereas double-layer and lethality assays do determine biological activity [79].
- (c)
- General Purity. For biopharmaceuticals, the purity and correct composition is classically assessed by high-performance liquid chromatography, combined with mass spectrometry if necessary. These methods can be used to identify phage capsid proteins, toxins, or other bacterial proteins. Because of the potential risk posed by the necessary production with pathogenic bacterial hosts, quality criteria should specify maximum levels for contaminants such as toxins or bacterial DNA, which normally must be tested with specific and appropriate molecular biology methods as specified below.
- (d)
- Toxins. Several in vitro methods have been developed for endotoxins quantification: gel-clot, turbidimetric, and chromogenic tests. Among the latter, the limulus amebocyte lysate assay is the most widely used [85]. When this assay is not applicable, e.g., due to masking effect, a reporter cell line can be used [86]. In addition, several commercial assays can be used to detect other toxic bacterial proteins, including ELISA or assays based on reporter cell lines.
- (e)
- Contaminating Nucleic Acids. Quality controls may also be required to determine the concentration of contaminating nucleic acids (i.e., non-phage nucleic acids). The presence and concentration of residual nucleic acids are typically checked by qPCR.
- (f)
- Other Quality Controls. Current regulations on sterility or general quality parameters in pharmaceutical products should also apply to phage-based pharmaceuticals [87]. Some parameters that may need to be checked are the total microbial load, pH, osmolarity, visual appearance, and/or maximum water content (in lyophilized preparations) [79].
4. Regulation for Phage Preparations
5. Clinical Trials and Prospects for Phage Therapy
6. Most Urgent Indications for the Application of Phage Therapy in Spain
- (a)
- In the case of a severe infection produced by an MDR bacterium.
- (b)
- When the infection occurs in an area reluctant to the use of antibiotics, such as in prosthetics.
- (c)
- Or, in general, whenever there is no standard of care option available, such as patients suffering from hypersensitivity to the antibiotic treatment.
6.1. Cystic Fibrosis
6.2. Osteoarticular Infections
7. General Aspects of the Use of Endolysins as Antimicrobials
8. Global Phage Therapy Market
9. Final Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; HM Government: London, UK, 2016. [Google Scholar]
- Sulakvelidze, A.; Alavidze, Z.; Morris, J.G., Jr. Bacteriophage therapy. Antimicrob. Agents Chemother. 2001, 45, 649–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCallin, S.; Sacher, J.C.; Zheng, J.; Chan, B.K. Current state of compassionate phage therapy. Viruses 2019, 11, 343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.K.; Kuo, S.C.; Chang, K.C.; Cheng, C.C.; Yu, P.Y.; Chang, C.H.; Chen, T.Y.; Tseng, C.C. Clinical Antibiotic-resistant Acinetobacter baumannii Strains with Higher Susceptibility to Environmental Phages than Antibiotic-sensitive Strains. Sci. Rep. 2017, 7, 6319. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.K.; Sistrom, M.; Wertz, J.E.; Kortright, K.E.; Narayan, D.; Turner, P.E. Phage selection restores antibiotic sensitivity in MDR Pseudomonas aeruginosa. Sci. Rep. 2016, 6, 26717. [Google Scholar] [CrossRef] [Green Version]
- North, O.I.; Brown, E.D. Phage-antibiotic combinations: A promising approach to constrain resistance evolution in bacteria. Ann. N. Y. Acad. Sci. 2021, 1496, 23–34. [Google Scholar] [CrossRef]
- Jansen, M.; Wahida, A.; Latz, S.; Kruttgen, A.; Hafner, H.; Buhl, E.M.; Ritter, K.; Horz, H.P. Enhanced antibacterial effect of the novel T4-like bacteriophage KARL-1 in combination with antibiotics against multi-drug resistant Acinetobacter baumannii. Sci. Rep. 2018, 8, 14140. [Google Scholar] [CrossRef] [Green Version]
- Gordillo Altamirano, F.; Forsyth, J.H.; Patwa, R.; Kostoulias, X.; Trim, M.; Subedi, D.; Archer, S.K.; Morris, F.C.; Oliveira, C.; Kielty, L.; et al. Bacteriophage-resistant Acinetobacter baumannii are resensitized to antimicrobials. Nat. Microbiol. 2021, 6, 157–161. [Google Scholar] [CrossRef]
- Manohar, P.; Madurantakam Royam, M.; Loh, B.; Bozdogan, B.; Nachimuthu, R.; Leptihn, S. Synergistic Effects of Phage-Antibiotic Combinations against Citrobacter amalonaticus. ACS Infect. Dis. 2022, 8, 59–65. [Google Scholar] [CrossRef]
- Blasco, L.; Ambroa, A.; Lopez, M.; Fernandez-Garcia, L.; Bleriot, I.; Trastoy, R.; Ramos-Vivas, J.; Coenye, T.; Fernandez-Cuenca, F.; Vila, J.; et al. Combined Use of the Ab105-2phiDeltaCI Lytic Mutant Phage and Different Antibiotics in Clinical Isolates of Multi-Resistant Acinetobacter baumannii. Microorganisms 2019, 7, 556. [Google Scholar] [CrossRef] [Green Version]
- Langdon, A.; Crook, N.; Dantas, G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016, 8, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boeckaerts, D.; Stock, M.; Criel, B.; Gerstmans, H.; De Baets, B.; Briers, Y. Predicting bacteriophage hosts based on sequences of annotated receptor-binding proteins. Sci. Rep. 2021, 11, 1467. [Google Scholar] [CrossRef] [PubMed]
- Hampton, H.G.; Watson, B.N.J.; Fineran, P.C. The arms race between bacteria and their phage foes. Nature 2020, 577, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Gehring, K.; Charbit, A.; Brissaud, E.; Hofnung, M. Bacteriophage lambda receptor site on the Escherichia coli K-12 LamB protein. J. Bacteriol. 1987, 169, 2103–2106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loc-Carrillo, C.; Abedon, S.T. Pros and cons of phage therapy. Bacteriophage 2011, 1, 111–114. [Google Scholar] [CrossRef] [Green Version]
- Mushegian, A.R. Are There 10(31) Virus Particles on Earth, or More, or Fewer? J. Bacteriol. 2020, 202, e00052-20. [Google Scholar] [CrossRef]
- Chibani-Chennoufi, S.; Bruttin, A.; Dillmann, M.L.; Brussow, H. Phage-host interaction: An ecological perspective. J. Bacteriol. 2004, 186, 3677–3686. [Google Scholar] [CrossRef] [Green Version]
- Burrowes, B.H.; Molineux, I.J.; Fralick, J.A. Directed in Vitro Evolution of Therapeutic Bacteriophages: The Appelmans Protocol. Viruses 2019, 11, 241. [Google Scholar] [CrossRef] [Green Version]
- Eskenazi, A.; Lood, C.; Wubbolts, J.; Hites, M.; Balarjishvili, N.; Leshkasheli, L.; Askilashvili, L.; Kvachadze, L.; van Noort, V.; Wagemans, J.; et al. Combination of pre-adapted bacteriophage therapy and antibiotics for treatment of fracture-related infection due to pandrug-resistant Klebsiella pneumoniae. Nat. Commun. 2022, 13, 302. [Google Scholar] [CrossRef]
- Navarro, F.; Muniesa, M. Phages in the Human Body. Front. Microbiol. 2017, 8, 566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manrique, P.; Bolduc, B.; Walk, S.T.; van der Oost, J.; de Vos, W.M.; Young, M.J. Healthy human gut phageome. Proc. Natl. Acad. Sci. USA 2016, 113, 10400–10405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawrence, D.; Baldridge, M.T.; Handley, S.A. Phages and Human Health: More Than Idle Hitchhikers. Viruses 2019, 11, 587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA Panel on Biological Hazards (BIOHAZ). Evaluation of the safety and efficacy of ListexTM P100 for reduction of pathogens on different ready-to-eat (RTE) food products. EFSA J. 2016, 14, 4565–4594. [Google Scholar] [CrossRef]
- Abedon, S.T.; Kuhl, S.J.; Blasdel, B.G.; Kutter, E.M. Phage treatment of human infections. Bacteriophage 2011, 1, 66–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, B.K.; Abedon, S.T.; Loc-Carrillo, C. Phage cocktails and the future of phage therapy. Future Microbiol. 2013, 8, 769–783. [Google Scholar] [CrossRef]
- Gu, J.; Liu, X.; Li, Y.; Han, W.; Lei, L.; Yang, Y.; Zhao, H.; Gao, Y.; Song, J.; Lu, R.; et al. A method for generation phage cocktail with great therapeutic potential. PLoS ONE 2012, 7, e31698. [Google Scholar] [CrossRef] [Green Version]
- Oechslin, F. Resistance Development to Bacteriophages Occurring during Bacteriophage Therapy. Viruses 2018, 10, 351. [Google Scholar] [CrossRef] [Green Version]
- Dabrowska, K. Phage therapy: What factors shape phage pharmacokinetics and bioavailability? Systematic and critical review. Med. Res. Rev. 2019, 39, 2000–2025. [Google Scholar] [CrossRef] [Green Version]
- Dabrowska, K.; Abedon, S.T. Pharmacologically Aware Phage Therapy: Pharmacodynamic and Pharmacokinetic Obstacles to Phage Antibacterial Action in Animal and Human Bodies. Microbiol. Mol. Biol. Rev. 2019, 83, e00012–e00019. [Google Scholar] [CrossRef]
- Attrill, E.L.; Claydon, R.; Lapinska, U.; Recker, M.; Meaden, S.; Brown, A.T.; Westra, E.R.; Harding, S.V.; Pagliara, S. Individual bacteria in structured environments rely on phenotypic resistance to phage. PLoS Biol. 2021, 19, e3001406. [Google Scholar] [CrossRef]
- Abedon, S.T.; Thomas-Abedon, C. Phage therapy pharmacology. Curr. Pharm. Biotechnol. 2010, 11, 28–47. [Google Scholar] [CrossRef] [PubMed]
- Jault, P.; Leclerc, T.; Jennes, S.; Pirnay, J.P.; Que, Y.A.; Resch, G.; Rousseau, A.F.; Ravat, F.; Carsin, H.; Le Floch, R.; et al. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): A randomised, controlled, double-blind phase 1/2 trial. Lancet Infect. Dis. 2019, 19, 35–45. [Google Scholar] [CrossRef]
- Perea, L.; Rodriguez-Rubio, L.; Nieto, J.C.; Zamora, C.; Canto, E.; Soriano, G.; Poca, M.; Blanco-Picazo, P.; Navarro, F.; Muniesa, M.; et al. Bacteriophages immunomodulate the response of monocytes. Exp. Biol. Med. 2021, 246, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Krut, O.; Bekeredjian-Ding, I. Contribution of the Immune Response to Phage Therapy. J. Immunol. 2018, 200, 3037–3044. [Google Scholar] [CrossRef] [PubMed]
- Hodyra-Stefaniak, K.; Miernikiewicz, P.; Drapala, J.; Drab, M.; Jonczyk-Matysiak, E.; Lecion, D.; Kazmierczak, Z.; Beta, W.; Majewska, J.; Harhala, M.; et al. Mammalian Host-Versus-Phage immune response determines phage fate in vivo. Sci. Rep. 2015, 5, 14802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roach, D.R.; Leung, C.Y.; Henry, M.; Morello, E.; Singh, D.; Di Santo, J.P.; Weitz, J.S.; Debarbieux, L. Synergy between the Host Immune System and Bacteriophage Is Essential for Successful Phage Therapy against an Acute Respiratory Pathogen. Cell Host Microbe 2017, 22, 38–47.e4. [Google Scholar] [CrossRef] [PubMed]
- Borysowski, J.; Gorski, A. Is phage therapy acceptable in the immunocompromised host. Int. J. Infect. Dis. 2008, 12, 466–471. [Google Scholar] [CrossRef] [Green Version]
- Brown-Jaque, M.; Calero-Caceres, W.; Muniesa, M. Transfer of antibiotic-resistance genes via phage-related mobile elements. Plasmid 2015, 79, 1–7. [Google Scholar] [CrossRef]
- Chen, J.; Quiles-Puchalt, N.; Chiang, Y.N.; Bacigalupe, R.; Fillol-Salom, A.; Chee, M.S.J.; Fitzgerald, J.R.; Penades, J.R. Genome hypermobility by lateral transduction. Science 2018, 362, 207–212. [Google Scholar] [CrossRef] [Green Version]
- Humphrey, S.; Fillol-Salom, A.; Quiles-Puchalt, N.; Ibarra-Chavez, R.; Haag, A.F.; Chen, J.; Penades, J.R. Bacterial chromosomal mobility via lateral transduction exceeds that of classical mobile genetic elements. Nat. Commun. 2021, 12, 6509. [Google Scholar] [CrossRef]
- Ramisetty, B.C.M.; Sudhakari, P.A. Bacterial ‘Grounded’ Prophages: Hotspots for Genetic Renovation and Innovation. Front. Genet. 2019, 10, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyman, P. Phages for Phage Therapy: Isolation, Characterization, and Host Range Breadth. Pharmaceuticals 2019, 12, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantalupo, P.G.; Calgua, B.; Zhao, G.; Hundesa, A.; Wier, A.D.; Katz, J.P.; Grabe, M.; Hendrix, R.W.; Girones, R.; Wang, D.; et al. Raw sewage harbors diverse viral populations. mBio 2011, 2, e00180-11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domingo-Calap, P.; Beamud, B.; Vienne, J.; Gonzalez-Candelas, F.; Sanjuan, R. Isolation of Four Lytic Phages Infecting Klebsiella pneumoniae K22 Clinical Isolates from Spain. Int. J. Mol. Sci. 2020, 21, 425. [Google Scholar] [CrossRef] [Green Version]
- Hatfull, G.F. The secret lives of mycobacteriophages. Adv. Virus Res. 2012, 82, 179–288. [Google Scholar] [CrossRef] [PubMed]
- Onsea, J.; Soentjens, P.; Djebara, S.; Merabishvili, M.; Depypere, M.; Spriet, I.; De Munter, P.; Debaveye, Y.; Nijs, S.; Vanderschot, P.; et al. Bacteriophage Application for Difficult-to-treat Musculoskeletal Infections: Development of a Standardized Multidisciplinary Treatment Protocol. Viruses 2019, 11, 891. [Google Scholar] [CrossRef] [Green Version]
- Lin, R.C.; Sacher, J.C.; Ceyssens, P.J.; Zheng, J.; Khalid, A.; Iredell, J.R.; Australian Phage Biobanking Network. Phage Biobank: Present Challenges and Future Perspectives. Curr. Opin. Biotechnol. 2021, 68, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Kropinski, A.M.; Mazzocco, A.; Waddell, T.E.; Lingohr, E.; Johnson, R.P. Enumeration of bacteriophages by double agar overlay plaque assay. Methods Mol. Biol. 2009, 501, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Joao, J.; Lampreia, J.; Prazeres, D.M.F.; Azevedo, A.M. Manufacturing of bacteriophages for therapeutic applications. Biotechnol. Adv. 2021, 49, 107758. [Google Scholar] [CrossRef]
- Jurac, K.; Nabergoj, D.; Podgornik, A. Bacteriophage production processes. Appl. Microbiol. Biotechnol. 2019, 103, 685–694. [Google Scholar] [CrossRef]
- Luong, T.; Salabarria, A.C.; Edwards, R.A.; Roach, D.R. Standardized bacteriophage purification for personalized phage therapy. Nat. Protoc. 2020, 15, 2867–2890. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, E.M.; Lehman, S.M.; Vandersteegen, K.; Vandenheuvel, D.; Philippe, D.L.; Cornelissen, A.; Clokie, M.R.; Garcia, A.J.; De Proft, M.; Maes, M.; et al. CIM(R) monolithic anion-exchange chromatography as a useful alternative to CsCl gradient purification of bacteriophage particles. Virology 2012, 434, 265–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortier, L.C.; Moineau, S. Phage production and maintenance of stocks, including expected stock lifetimes. Methods Mol. Biol. 2009, 501, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Zierdt, C.H. Stabilities of lyophilized Staphylococcus aureus typing bacteriophages. Appl. Environ. Microbiol. 1988, 54, 2590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourdin, G.; Schmitt, B.; Marvin Guy, L.; Germond, J.E.; Zuber, S.; Michot, L.; Reuteler, G.; Brussow, H. Amplification and purification of T4-like Escherichia coli phages for phage therapy: From laboratory to pilot scale. Appl. Environ. Microbiol. 2014, 80, 1469–1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puapermpoonsiri, U.; Ford, S.J.; van der Walle, C.F. Stabilization of bacteriophage during freeze drying. Int. J. Pharm. 2010, 389, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Tovkach, F.I.; Zhuminska, G.I.; Kushkina, A.I. Long-term preservation of unstable bacteriophages of enterobacteria. Mikrobiol. Z. 2012, 74, 60–66. [Google Scholar]
- Malik, D.J.; Sokolov, I.J.; Vinner, G.K.; Mancuso, F.; Cinquerrui, S.; Vladisavljevic, G.T.; Clokie, M.R.J.; Garton, N.J.; Stapley, A.G.F.; Kirpichnikova, A. Formulation, stabilisation and encapsulation of bacteriophage for phage therapy. Adv. Colloid Interface Sci. 2017, 249, 100–133. [Google Scholar] [CrossRef] [Green Version]
- Sarker, S.A.; Brussow, H. From bench to bed and back again: Phage therapy of childhood Escherichia coli diarrhea. Ann. N. Y. Acad. Sci. 2016, 1372, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Bruttin, A.; Brussow, H. Human volunteers receiving Escherichia coli phage T4 orally: A safety test of phage therapy. Antimicrob. Agents Chemother. 2005, 49, 2874–2878. [Google Scholar] [CrossRef] [Green Version]
- Sarker, S.A.; McCallin, S.; Barretto, C.; Berger, B.; Pittet, A.C.; Sultana, S.; Krause, L.; Huq, S.; Bibiloni, R.; Bruttin, A.; et al. Oral T4-like phage cocktail application to healthy adult volunteers from Bangladesh. Virology 2012, 434, 222–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, R.Y.K.; Morales, S.; Okamoto, Y.; Chan, H.K. Topical application of bacteriophages for treatment of wound infections. Transl. Res. 2020, 220, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Ryan, E.M.; Gorman, S.P.; Donnelly, R.F.; Gilmore, B.F. Recent advances in bacteriophage therapy: How delivery routes, formulation, concentration and timing influence the success of phage therapy. J. Pharm. Pharmacol. 2011, 63, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Eliava BioPreparations. Available online: https://phage.ge/products/?lang=en (accessed on 16 February 2022).
- Loh, B.; Gondil, V.S.; Manohar, P.; Khan, F.M.; Yang, H.; Leptihn, S. Encapsulation and Delivery of Therapeutic Phages. Appl. Environ. Microbiol. 2020, 87, e01979-20. [Google Scholar] [CrossRef] [PubMed]
- McConnell, E.L.; Fadda, H.M.; Basit, A.W. Gut instincts: Explorations in intestinal physiology and drug delivery. Int. J. Pharm. 2008, 364, 213–226. [Google Scholar] [CrossRef]
- Colom, J.; Cano-Sarabia, M.; Otero, J.; Arinez-Soriano, J.; Cortes, P.; Maspoch, D.; Llagostera, M. Microencapsulation with alginate/CaCO3: A strategy for improved phage therapy. Sci. Rep. 2017, 7, 41441. [Google Scholar] [CrossRef]
- Puapermpoonsiri, U.; Spencer, J.; van der Walle, C.F. A freeze-dried formulation of bacteriophage encapsulated in biodegradable microspheres. Eur. J. Pharm. Biopharm. 2009, 72, 26–33. [Google Scholar] [CrossRef]
- Duplessis, C.A.; Biswas, B. A Review of Topical Phage Therapy for Chronically Infected Wounds and Preparations for a Randomized Adaptive Clinical Trial Evaluating Topical Phage Therapy in Chronically Infected Diabetic Foot Ulcers. Antibiotics 2020, 9, 377. [Google Scholar] [CrossRef]
- Merabishvili, M.; Monserez, R.; van Belleghem, J.; Rose, T.; Jennes, S.; De Vos, D.; Verbeken, G.; Vaneechoutte, M.; Pirnay, J.P. Stability of bacteriophages in burn wound care products. PLoS ONE 2017, 12, e0182121. [Google Scholar] [CrossRef] [Green Version]
- Chang, R.Y.K.; Wallin, M.; Lin, Y.; Leung, S.S.Y.; Wang, H.; Morales, S.; Chan, H.K. Phage therapy for respiratory infections. Adv. Drug Deliv. Rev. 2018, 133, 76–86. [Google Scholar] [CrossRef]
- Bodier-Montagutelli, E.; Morello, E.; L’Hostis, G.; Guillon, A.; Dalloneau, E.; Respaud, R.; Pallaoro, N.; Blois, H.; Vecellio, L.; Gabard, J.; et al. Inhaled phage therapy: A promising and challenging approach to treat bacterial respiratory infections. Expert Opin. Drug Deliv. 2017, 14, 959–972. [Google Scholar] [CrossRef] [PubMed]
- Malik, D.J. Bacteriophage Encapsulation Using Spray Drying for Phage Therapy. Curr. Issues Mol. Biol. 2021, 40, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Golshahi, L.; Lynch, K.H.; Dennis, J.J.; Finlay, W.H. In vitro lung delivery of bacteriophages KS4-M and PhiKZ using dry powder inhalers for treatment of Burkholderia cepacia complex and Pseudomonas aeruginosa infections in cystic fibrosis. J. Appl. Microbiol. 2011, 110, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Speck, P.; Smithyman, A. Safety and efficacy of phage therapy via the intravenous route. FEMS Microbiol. Lett. 2016, 363, fnv242. [Google Scholar] [CrossRef] [PubMed]
- Ujmajuridze, A.; Chanishvili, N.; Goderdzishvili, M.; Leitner, L.; Mehnert, U.; Chkhotua, A.; Kessler, T.M.; Sybesma, W. Adapted Bacteriophages for Treating Urinary Tract Infections. Front. Microbiol. 2018, 9, 1832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Letkiewicz, S.; Lusiak-Szelachowska, M.; Miedzybrodzki, R.; Zaczek, M.; Weber-Dabrowska, B.; Gorski, A. Low Immunogenicity of Intravesical Phage Therapy for Urogenitary Tract Infections. Antibiotics 2021, 10, 627. [Google Scholar] [CrossRef]
- Pirnay, J.P.; Blasdel, B.G.; Bretaudeau, L.; Buckling, A.; Chanishvili, N.; Clark, J.R.; Corte-Real, S.; Debarbieux, L.; Dublanchet, A.; De Vos, D.; et al. Quality and safety requirements for sustainable phage therapy products. Pharm. Res. 2015, 32, 2173–2179. [Google Scholar] [CrossRef] [Green Version]
- Philipson, C.W.; Voegtly, L.J.; Lueder, M.R.; Long, K.A.; Rice, G.K.; Frey, K.G.; Biswas, B.; Cer, R.Z.; Hamilton, T.; Bishop-Lilly, K.A. Characterizing Phage Genomes for Therapeutic Applications. Viruses 2018, 10, 188. [Google Scholar] [CrossRef] [Green Version]
- Hoper, D.; Freuling, C.M.; Muller, T.; Hanke, D.; von Messling, V.; Duchow, K.; Beer, M.; Mettenleiter, T.C. High definition viral vaccine strain identity and stability testing using full-genome population data--The next generation of vaccine quality control. Vaccine 2015, 33, 5829–5837. [Google Scholar] [CrossRef]
- McCallin, S.; Sarker, S.A.; Sultana, S.; Oechslin, F.; Brussow, H. Metagenome analysis of Russian and Georgian Pyophage cocktails and a placebo-controlled safety trial of single phage versus phage cocktail in healthy Staphylococcus aureus carriers. Environ. Microbiol. 2018, 20, 3278–3293. [Google Scholar] [CrossRef]
- Villarroel, J.; Larsen, M.V.; Kilstrup, M.; Nielsen, M. Metagenomic Analysis of Therapeutic PYO Phage Cocktails from 1997 to 2014. Viruses 2017, 9, 328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajnovic, D.; Munoz-Berbel, X.; Mas, J. Fast phage detection and quantification: An optical density-based approach. PLoS ONE 2019, 14, e0216292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abate, W.; Sattar, A.A.; Liu, J.; Conway, M.E.; Jackson, S.K. Evaluation of recombinant factor C assay for the detection of divergent lipopolysaccharide structural species and comparison with Limulus amebocyte lysate-based assays and a human monocyte activity assay. J. Med. Microbiol. 2017, 66, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, H.; Gornicec, J.; Neuper, T.; Parigiani, M.A.; Wallner, M.; Duschl, A.; Horejs-Hoeck, J. Biological Activity of Masked Endotoxin. Sci. Rep. 2017, 7, 44750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shintani, H. Validation Study of Rapid Assays of Bioburden, Endotoxins and Other Contamination. Biocontrol Sci. 2016, 21, 63–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patey, O.; McCallin, S.; Mazure, H.; Liddle, M.; Smithyman, A.; Dublanchet, A. Clinical Indications and Compassionate Use of Phage Therapy: Personal Experience and Literature Review with a Focus on Osteoarticular Infections. Viruses 2018, 11, 18. [Google Scholar] [CrossRef] [Green Version]
- Brussow, H. Hurdles for Phage Therapy to Become a Reality-An Editorial Comment. Viruses 2019, 11, 557. [Google Scholar] [CrossRef] [Green Version]
- Sarker, S.A.; Sultana, S.; Reuteler, G.; Moine, D.; Descombes, P.; Charton, F.; Bourdin, G.; McCallin, S.; Ngom-Bru, C.; Neville, T.; et al. Oral Phage Therapy of Acute Bacterial Diarrhea with Two Coliphage Preparations: A Randomized Trial in Children from Bangladesh. EBioMedicine 2016, 4, 124–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leitner, L.; Ujmajuridze, A.; Chanishvili, N.; Goderdzishvili, M.; Chkonia, I.; Rigvava, S.; Chkhotua, A.; Changashvili, G.; McCallin, S.; Schneider, M.P.; et al. Intravesical bacteriophages for treating urinary tract infections in patients undergoing transurethral resection of the prostate: A randomised, placebo-controlled, double-blind clinical trial. Lancet Infect. Dis. 2021, 21, 427–436. [Google Scholar] [CrossRef]
- Pirnay, J.P.; Kutter, E. Bacteriophages: It’s a medicine, Jim, but not as we know it. Lancet Infect. Dis. 2021, 21, 309–311. [Google Scholar] [CrossRef]
- Pirnay, J.P.; Merabishvili, M.; Van Raemdonck, H.; De Vos, D.; Verbeken, G. Bacteriophage Production in Compliance with Regulatory Requirements. Methods Mol. Biol. 2018, 1693, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Pirnay, J.P.; Verbeken, G.; Ceyssens, P.J.; Huys, I.; De Vos, D.; Ameloot, C.; Fauconnier, A. The Magistral Phage. Viruses 2018, 10, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fauconnier, A. Regulating phage therapy: The biological master file concept could help to overcome regulatory challenge of personalized medicines. EMBO Rep. 2017, 18, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Verbeken, G.; Pirnay, J.P.; Lavigne, R.; Jennes, S.; De Vos, D.; Casteels, M.; Huys, I. Call for a dedicated European legal framework for bacteriophage therapy. Arch. Immunol. Ther. Exp. 2014, 62, 117–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelfrene, E.; Sebris, Z.; Cavaleri, M. Comment on Fauconnier, A. Phage Therapy Regulation: From Night to Dawn. Viruses 2019, 11, 771. [Google Scholar] [CrossRef] [Green Version]
- Harper, D.R. Criteria for Selecting Suitable Infectious Diseases for Phage Therapy. Viruses 2018, 10, 177. [Google Scholar] [CrossRef] [Green Version]
- Wright, A.; Hawkins, C.H.; Anggard, E.E.; Harper, D.R. A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy. Clin. Otolaryngol. 2009, 34, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Djebara, S.; Maussen, C.; De Vos, D.; Merabishvili, M.; Damanet, B.; Pang, K.W.; De Leenheer, P.; Strachinaru, I.; Soentjens, P.; Pirnay, J.P. Processing Phage Therapy Requests in a Brussels Military Hospital: Lessons Identified. Viruses 2019, 11, 265. [Google Scholar] [CrossRef] [Green Version]
- Lood, C.; Boeckaerts, D.; Stock, M.; De Baets, B.; Lavigne, R.; van Noort, V.; Briers, Y. Digital phagograms: Predicting phage infectivity through a multilayer machine learning approach. Curr. Opin. Virol. 2021, 52, 174–181. [Google Scholar] [CrossRef]
- Farrell, P.M. The prevalence of cystic fibrosis in the European Union. J. Cyst. Fibros. 2008, 7, 450–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Causape, C.; de Dios-Caballero, J.; Cobo, M.; Escribano, A.; Asensio, O.; Oliver, A.; Del Campo, R.; Canton, R.; Sole, A.; Cortell, I.; et al. Antibiotic resistance and population structure of cystic fibrosis Pseudomonas aeruginosa isolates from a Spanish multi-centre study. Int. J. Antimicrob. Agents 2017, 50, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, B.S.; Flume, P.A. Nontuberculous Mycobacteria in Cystic Fibrosis. Semin. Respir. Crit. Care Med. 2018, 39, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Osmani, M.; Sotello, D.; Alvarez, S.; Odell, J.A.; Thomas, M. Mycobacterium abscessus infections in lung transplant recipients: 15-year experience from a single institution. Transpl. Infect. Dis. 2018, 20, e12835. [Google Scholar] [CrossRef]
- Beam, E.; Osmon, D. Prosthetic Joint Infection Update. Infect. Dis. Clin. N. Am. 2018, 32, 843–859. [Google Scholar] [CrossRef]
- Hoyle, N.; Zhvaniya, P.; Balarjishvili, N.; Bolkvadze, D.; Nadareishvili, L.; Nizharadze, D.; Wittmann, J.; Rohde, C.; Kutateladze, M. Phage therapy against Achromobacter xylosoxidans lung infection in a patient with cystic fibrosis: A case report. Res. Microbiol. 2018, 169, 540–542. [Google Scholar] [CrossRef] [PubMed]
- Dedrick, R.M.; Guerrero-Bustamante, C.A.; Garlena, R.A.; Russell, D.A.; Ford, K.; Harris, K.; Gilmour, K.C.; Soothill, J.; Jacobs-Sera, D.; Schooley, R.T.; et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat. Med. 2019, 25, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Law, N.; Logan, C.; Yung, G.; Furr, C.L.; Lehman, S.M.; Morales, S.; Rosas, F.; Gaidamaka, A.; Bilinsky, I.; Grint, P.; et al. Successful adjunctive use of bacteriophage therapy for treatment of multidrug-resistant Pseudomonas aeruginosa infection in a cystic fibrosis patient. Infection 2019, 47, 665–668. [Google Scholar] [CrossRef]
- Lebeaux, D.; Merabishvili, M.; Caudron, E.; Lannoy, D.; Van Simaey, L.; Duyvejonck, H.; Guillemain, R.; Thumerelle, C.; Podglajen, I.; Compain, F.; et al. A Case of Phage Therapy against Pandrug-Resistant Achromobacter xylosoxidans in a 12-Year-Old Lung-Transplanted Cystic Fibrosis Patient. Viruses 2021, 13, 60. [Google Scholar] [CrossRef]
- Aslam, S.; Courtwright, A.M.; Koval, C.; Lehman, S.M.; Morales, S.; Furr, C.L.; Rosas, F.; Brownstein, M.J.; Fackler, J.R.; Sisson, B.M.; et al. Early clinical experience of bacteriophage therapy in 3 lung transplant recipients. Am. J. Transpl. 2019, 19, 2631–2639. [Google Scholar] [CrossRef]
- Tan, X.; Chen, H.; Zhang, M.; Zhao, Y.; Jiang, Y.; Liu, X.; Huang, W.; Ma, Y. Clinical Experience of Personalized Phage Therapy Against Carbapenem-Resistant Acinetobacter baumannii Lung Infection in a Patient with Chronic Obstructive Pulmonary Disease. Front. Cell. Infect. Microbiol 2021, 11, 631585. [Google Scholar] [CrossRef] [PubMed]
- Ferry, T.; Boucher, F.; Fevre, C.; Perpoint, T.; Chateau, J.; Petitjean, C.; Josse, J.; Chidiac, C.; L’Hostis, G.; Leboucher, G.; et al. Innovations for the treatment of a complex bone and joint infection due to XDR Pseudomonas aeruginosa including local application of a selected cocktail of bacteriophages. J. Antimicrob. Chemother. 2018, 73, 2901–2903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferry, T.L.G.; Fevre, C.; Herry, Y.; Conrad, A.; Josse, J.; Batailler, C.; Chidiac, C.; Medina, M.; Lustig, S.; Laurent, F.; et al. Salvage Debridement, Antibiotics and Implant Retention (“DAIR”) with Local Injection of a Selected Cocktail of Bacteriophages: Is It an Option for an Elderly Patient with Relapsing Staphylococcus aureus Prosthetic-Joint Infection. Open Forum Infect. Dis. 2018, 5, ofy269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tkhilaishvili, T.; Winkler, T.; Muller, M.; Perka, C.; Trampuz, A. Bacteriophages as Adjuvant to Antibiotics for the Treatment of Periprosthetic Joint Infection Caused by Multidrug-Resistant Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2019, 64, e00924-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fish, R.; Kutter, E.; Bryan, D.; Wheat, G.; Kuhl, S. Resolving Digital Staphylococcal Osteomyelitis Using Bacteriophage-A Case Report. Antibiotics 2018, 7, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vázquez, R.; García, E.; García, P. Phage lysins for fighting bacterial respiratory infections: A new generation of antimicrobials. Front. Immunol. 2018, 9, 2252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutierrez, D.; Briers, Y. Lysins breaking down the walls of Gram-negative bacteria, no longer a no-go. Curr. Opin. Biotechnol. 2021, 68, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Pastagia, M.; Schuch, R.; Fischetti, V.A.; Huang, D.B. Lysins: The arrival of pathogen-directed anti-infectives. J. Med. Microbiol. 2013, 62, 1506–1516. [Google Scholar] [CrossRef] [PubMed]
- Dams, D.; Briers, Y. Enzybiotics: Enzyme-based antibacterials as therapeutics. Adv. Exp. Med. Biol. 2019, 1148, 233–253. [Google Scholar] [CrossRef]
- Schuch, R.; Lee, H.M.; Schneider, B.C.; Sauve, K.L.; Law, C.; Khan, B.K.; Rotolo, J.A.; Horiuchi, Y.; Couto, D.E.; Raz, A.; et al. Combination therapy with lysin CF-301 and antibiotic is superior to antibiotic alone for treating methicillin-resistant Staphylococcus aureus-induced murine bacteremia. J. Infect. Dis. 2014, 209, 1469–1478. [Google Scholar] [CrossRef]
- Loeffler, J.M.; Djurkovic, S.; Fischetti, V.A. Phage lytic enzyme Cpl-1 as a novel antimicrobial for pneumococcal bacteremia. Infect. Immun. 2003, 71, 6199–6204. [Google Scholar] [CrossRef] [Green Version]
- Rashel, M.; Uchiyama, J.; Ujihara, T.; Uehara, Y.; Kuramoto, S.; Sugihara, S.; Yagyu, K.; Muraoka, A.; Sugai, M.; Hiramatsu, K.; et al. Efficient elimination of multidrug-resistant Staphylococcus aureus by cloned lysin derived from bacteriophage phi MR11. J. Infect. Dis. 2007, 196, 1237–1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischetti, V.A. Bacteriophage endolysins: A novel anti-infective to control Gram-positive pathogens. Int. J. Med. Microbiol. 2010, 300, 357–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasina, D.V.; Antonova, N.P.; Grigoriev, I.V.; Yakimakha, V.S.; Lendel, A.M.; Nikiforova, M.A.; Pochtovyi, A.A.; Remizov, T.A.; Usachev, E.V.; Shevlyagina, N.V.; et al. Discovering the Potentials of Four Phage Endolysins to Combat Gram-Negative Infections. Front. Microbiol. 2021, 12, 748718. [Google Scholar] [CrossRef] [PubMed]
- Lysando. Available online: https://www.lysando.com/systemic-applications.html (accessed on 16 February 2022).
- Ghose, C.; Euler, C.W. Gram-Negative Bacterial Lysins. Antibiotics 2020, 9, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerstmans, H.; Grimon, D.; Gutierrez, D.; Lood, C.; Rodriguez, A.; van Noort, V.; Lammertyn, J.; Lavigne, R.; Briers, Y. A VersaTile-driven platform for rapid hit-to-lead development of engineered lysins. Sci. Adv. 2020, 6, eaaz1136. [Google Scholar] [CrossRef] [PubMed]
- ContraFect. Contrafect Announces First Gram-Negative Product Candidate CF_370, a Direct Lytic Agent Targeting Pseudomonas aeruginosa. Globe Newswire, 18 December 2019. [Google Scholar]
- Jun, S.Y.; Jang, I.J.; Yoon, S.; Jang, K.; Yu, K.S.; Cho, J.Y.; Seong, M.W.; Jung, G.M.; Yoon, S.J.; Kang, S.H. Pharmacokinetics and tolerance of the phage endolysin-based candidate drug SAL200 after a single intravenous administration among healthy volunteers. Antimicrob. Agents Chemother. 2017, 61, e02629-02616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fowler, V.G., Jr.; Das, A.F.; Lipka-Diamond, J.; Schuch, R.; Pomerantz, R.; Jauregui-Peredo, L.; Bressler, A.; Evans, D.; Moran, G.J.; Rupp, M.E.; et al. Exebacase for patients with Staphylococcus aureus bloodstream infection and endocarditis. J. Clin. Investig. 2020, 130, 3750–3760. [Google Scholar] [CrossRef] [Green Version]
- Abdelkader, K.; Gerstmans, H.; Saafan, A.; Dishisha, T.; Briers, Y. The preclinical and clinical progress of bacteriophages and their lytic enzymes: The parts are easier than the whole. Viruses 2019, 11, 96. [Google Scholar] [CrossRef] [Green Version]
- ContraFect. Available online: https://www.contrafect.com/pipeline/exebacase (accessed on 21 February 2022).
- Intron Biotechnology. Phase IIa Clinical Study of N-Rephasin® SAL200. Available online: https://clinicaltrials.gov/ct2/show/NCT03089697 (accessed on 21 February 2022).
- ContraFect. Direct Lysis of Staph Aureus Resistant Pathogen Trial of Exebacase (DISRUPT). Available online: https://www.clinicaltrials.gov/ct2/show/NCT04160468?term=CF-301&draw=1 (accessed on 21 February 2022).
- Palaniappan, R.; Dayanithi, G. Therapeutic Efficacy of Bacteriophages. In Bacteriophages in Therapeutics; IntechOpen: London, UK, 2021. [Google Scholar]
- Keswani, C. Agri-Based Bioeconomy: Reintegrating Trans-Disciplinary Research and Sustainable Development Goals, 1st ed.; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Bacteriophage Market by Product Type (dsDNA Bacteriophage, ssDNA Bacteriophage, ssRNA Bacteriophage), by Application (Clinical Application, Food & Beverages, Phage Display, Phage Therapy, Environmental Application, Veterinary)-Growth, Future Prospects & Competitive Analysis, 2018–2026. ID: 16969; 2018. Available online: https://www.credenceresearch.com/report/bacteriophage-market (accessed on 21 February 2022).
- Global Antimicrobial Resistance Market Size, Trends & Growth Opportunity, by Disease, by Pathogen, by Drug Class, by Region and Forecast till 2027. ID: 5398519; August 2021. Available online: https://www.researchandmarkets.com/reports/5398519/global-antimicrobial-resistance-market-size (accessed on 21 February 2022).
- Fauconnier, A. Phage Therapy Regulation: From Night to Dawn. Viruses 2019, 11, 352. [Google Scholar] [CrossRef] [Green Version]
- Telum Therapeutics. Available online: https://telumtherapeutics.com/our-science/pipeline/ (accessed on 20 January 2022).
- EIT Health. Available online: https://eithealth.eu/news-article/phagomed-acquired-by-biontech/ (accessed on 21 February 2022).
- PR Newswire. Available online: https://www.prnewswire.com/news-releases/micreos-secures-32-million-for-its-endolysin-based-platform-as-sustainable-alternative-to-antibiotics-301388573.html (accessed on 21 February 2022).
| Disease | Pathogen(s) | Treatment | Status | References |
|---|---|---|---|---|
| Diabetic foot ulcers | Staphylococcus aureus | Topical phage cocktail | Not yet recruiting (expected start date: June 2022) | NCT02664740 |
| Invasive infection in patients with inactive Crohn’s disease | E. coli | Oral phage cocktail | Recruiting (estimated completion: June 2023) | NCT03808103 |
| Chronic airway infection in cystic fibrosis patients | P. aeruginosa | Nebulized phage therapy | Recruiting (estimated completion: December 2022) | NCT04684641 |
| Diabetic foot ulcers | P. aeruginosa, S. aureus and/or Acinetobacter baumannii | Topical phage cocktail | Recruiting (estimated completion: December 2021 | NCT04803708 |
| Prosthetic joint infections | Several pathogens | Combined antibiotic/personalized phage therapy | Not yet recruiting (estimated start date: October 2022) | NCT04787250 |
| Chronic airway infection in cystic fibrosis patients | P. aeruginosa | Nebulized phage cocktail | Not yet recruiting | NCT05010577 |
| Wound infections in burned patients | S. aureus, P. aeruginosa or Klebsiella pneumoniae | Topical phage cocktail | Not yet recruiting (estimated start date: January 2022) | NCT04323475 |
| Pressure injury infections | S. aureus, P. aeruginosa, K. pneumoniae | Topical phage cocktail in combination with antibiotics | Not yet recruiting (estimated start date: January 2022) | NCT04815798 |
| Urinary tract infections | E. coli or K. pneumoniae | Personalized phage therapy administered through intravenous or intravesical route | Recruiting (estimated completion: September 2023) | NCT04287478 |
| Tonsillitis | Several pathogens | Nebulized phage cocktail | Phase 3. Active, not recruiting (estimated completion: December 2024) | NCT04682964 |
| Chronic airway infection in cystic fibrosis patients | P. aeruginosa | Inhaled phage cocktail | Recruiting (estimated completion: March 2022) | NCT04596319 |
| Disease | Pathogen(s) | Treatment | Outcome | References |
|---|---|---|---|---|
| CF with chronic MDR lung infection | Achromobacter xylosoxidans | Inhalation, orally | Dyspnea resolved and cough reduced. Lung function improved | [107] |
| CF with disseminated infection, lung transplantation | M. abscessus | Intravenous | Sternal wound closure, improved liver function, substantial resolution of infected skin nodules | [108] |
| CF with MDR pneumonia, persistent respiratory failure, and colistin-induced renal failure | P. aeruginosa | Intravenous | Pneumonia clinically resolved, no sputum production, return to baseline renal function, white blood cell count normalized | [109] |
| CF with persistent lung infection, lung transplantation | A. xylosoxidans | Inhalation | Respiratory condition improved; sputum cultures positive but with low bacteria concentration | [110] |
| Lung transplant recipient patients with MRD resistant infections | P. aeruginosa and Burkholderia dolosa | Intravenous, inhalation | Two patients were discharged from the hospital off ventilator support. A third patient infection relapsed and died | [111] |
| COPD with drug-resistant pneumonia | A. baumannii | Inhalation | Sputum/ blood and bronchoalveolar lavage fluid negative, restoration sinus rhythm, lung function improved | [112] |
| Prosthesis infection | S. aureus | Local | Bacteria removed, rapid healing | [113] |
| Osteomyelitis | P. aeruginosa | Local | No clinical signs of persistent infection | [114] |
| Infection of the right knee and chronic osteomyelitis of the femur after injury | P. aeruginosa | Local | No pain, soft tissue at the surgical site unremarkable, mobility satisfactory | [115] |
| Osteomyelitis of the distal phalanx | S. aureus | Local | The ulcer healed, re-ossification of the distal phalanx, erythema and edema decreased | [116] |
| Fracture-related infection | K. pneumoniae | Local | Skin graft vascularized and viable, the sinus tract closed and dry, pus no longer discharged from the pin sites of the external fixator, restored muscle function | [20] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez, R.; Díez-Martínez, R.; Domingo-Calap, P.; García, P.; Gutiérrez, D.; Muniesa, M.; Ruiz-Ruigómez, M.; Sanjuán, R.; Tomás, M.; Tormo-Mas, M.Á.; et al. Essential Topics for the Regulatory Consideration of Phages as Clinically Valuable Therapeutic Agents: A Perspective from Spain. Microorganisms 2022, 10, 717. https://doi.org/10.3390/microorganisms10040717
Vázquez R, Díez-Martínez R, Domingo-Calap P, García P, Gutiérrez D, Muniesa M, Ruiz-Ruigómez M, Sanjuán R, Tomás M, Tormo-Mas MÁ, et al. Essential Topics for the Regulatory Consideration of Phages as Clinically Valuable Therapeutic Agents: A Perspective from Spain. Microorganisms. 2022; 10(4):717. https://doi.org/10.3390/microorganisms10040717
Chicago/Turabian StyleVázquez, Roberto, Roberto Díez-Martínez, Pilar Domingo-Calap, Pedro García, Diana Gutiérrez, Maite Muniesa, María Ruiz-Ruigómez, Rafael Sanjuán, María Tomás, María Ángeles Tormo-Mas, and et al. 2022. "Essential Topics for the Regulatory Consideration of Phages as Clinically Valuable Therapeutic Agents: A Perspective from Spain" Microorganisms 10, no. 4: 717. https://doi.org/10.3390/microorganisms10040717
APA StyleVázquez, R., Díez-Martínez, R., Domingo-Calap, P., García, P., Gutiérrez, D., Muniesa, M., Ruiz-Ruigómez, M., Sanjuán, R., Tomás, M., Tormo-Mas, M. Á., & García, P. (2022). Essential Topics for the Regulatory Consideration of Phages as Clinically Valuable Therapeutic Agents: A Perspective from Spain. Microorganisms, 10(4), 717. https://doi.org/10.3390/microorganisms10040717










