Dinoflagellate Phosphopantetheinyl Transferase (PPTase) and Thiolation Domain Interactions Characterized Using a Modified Indigoidine Synthesizing Reporter
Abstract
1. Introduction
2. Materials and Methods
2.1. Reporter Modification
2.2. Thiolation Domain Insertion and Co-Expression
3. Results
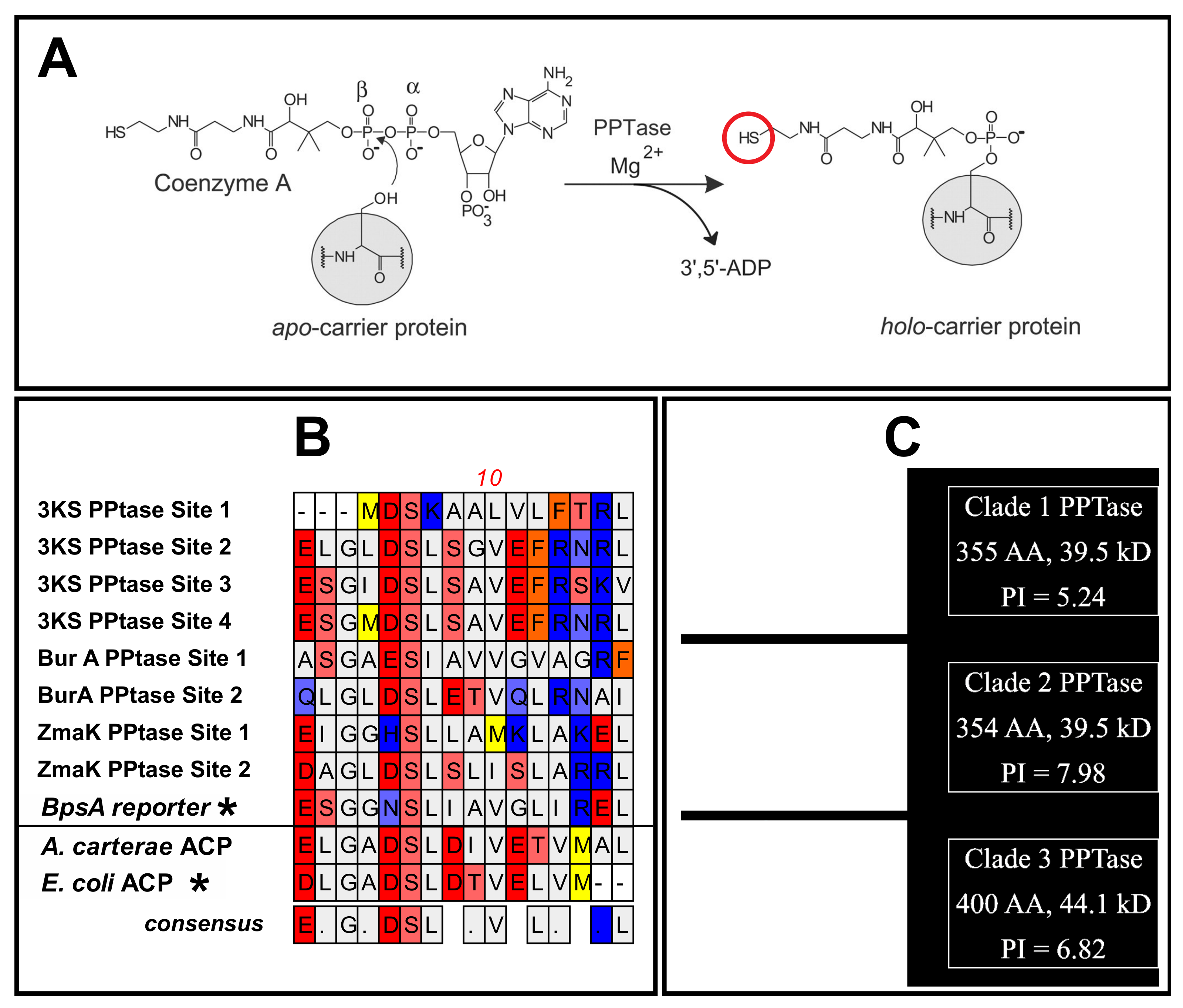
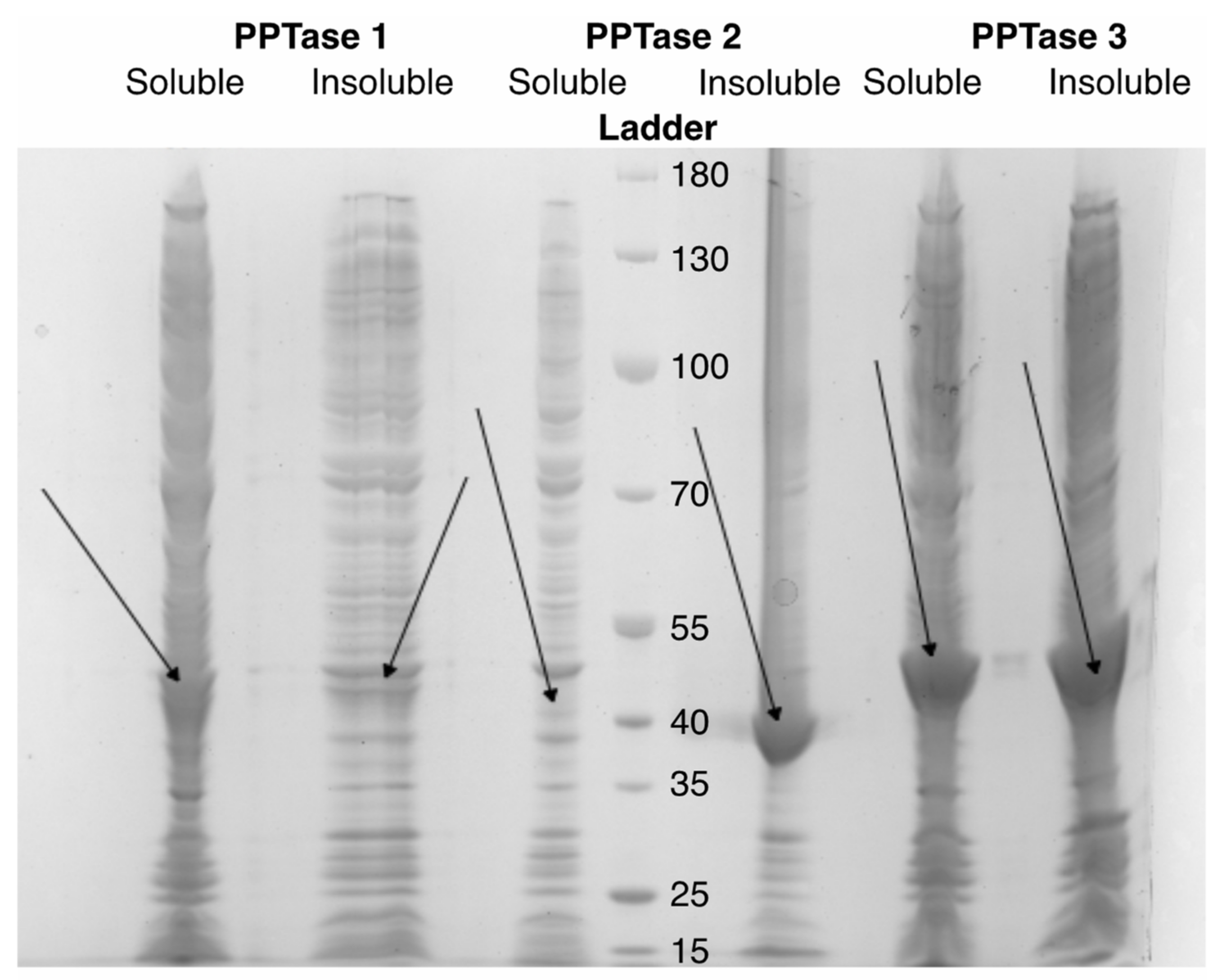
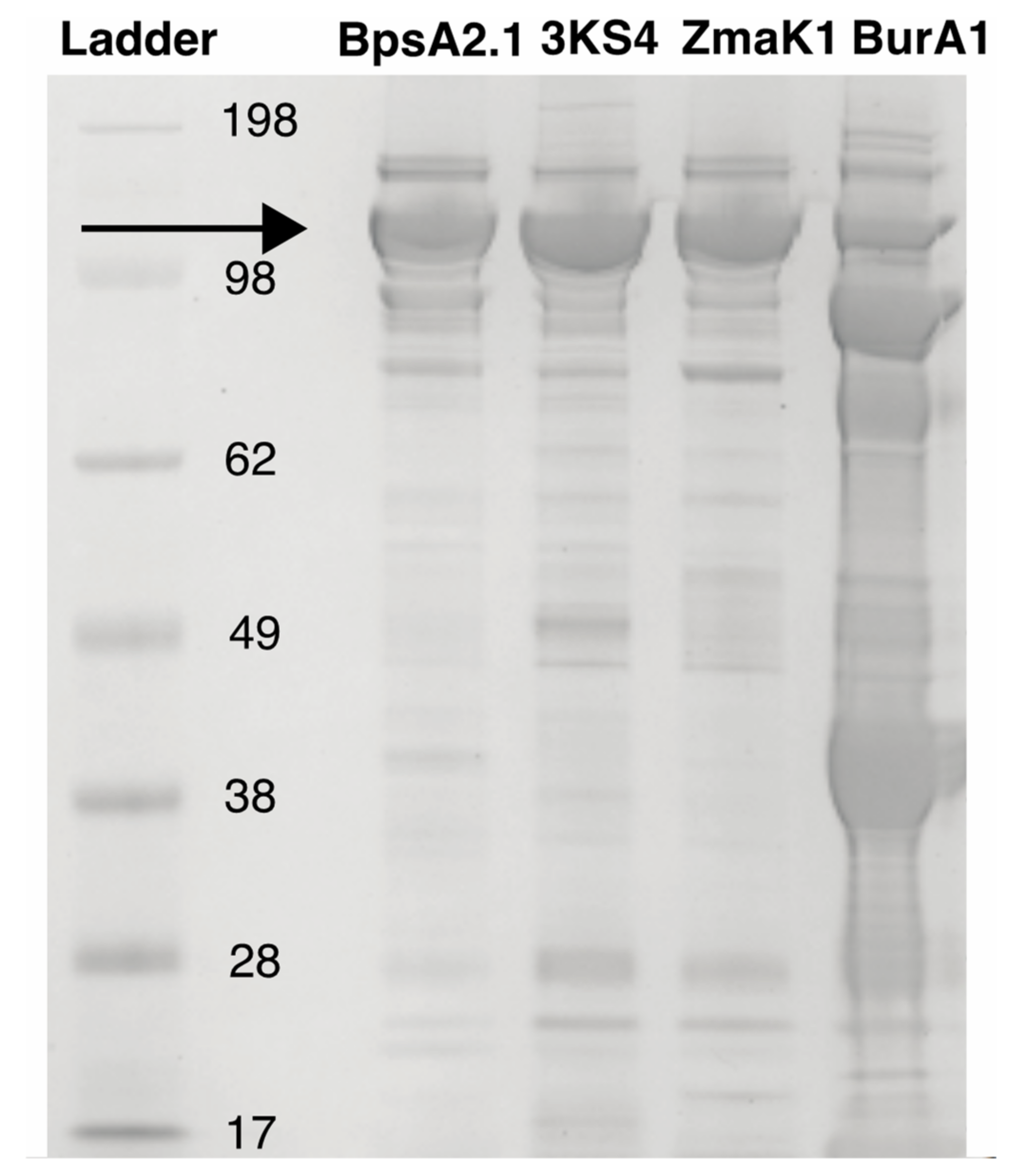
3.1. Construct Generation and Domain Insertion
3.2. Indigoidine Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deeds, J.R.; Terlizzi, D.E.; Adolf, J.E.; Stoecker, D.K.; Place, A.R. Toxic activity from cultures of Karlodinium micrum (=Gyrodinium galatheanum) (Dinophyceae)—A dinoflagellate associated with fish mortalities in an estuarine aquaculture facility. Harmful Algae 2002, 1, 169–189. [Google Scholar] [CrossRef]
- Twiner, M.J.; Flewelling, L.J.; Fire, S.E.; Bowen-Stevens, S.R.; Gaydos, J.K.; Johnson, C.K.; Landsberg, J.H.; Leighfield, T.A.; Mase-Guthrie, B.; Schwacke, L.; et al. Comparative analysis of three brevetoxin-associated bottlenose dolphin (Tursiops truncatus) mortality events in the Florida Panhandle region (USA). PLoS ONE 2012, 7, e42974. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.J.; Butawan, M.; Yordy, J.; Ball, R.; Flewelling, L.; de Wit, M.; Bonde, R.K. Sublethal red tide toxin exposure in free-ranging manatees (Trichechus manatus) affects the immune system through reduced lymphocyte proliferation responses, inflammation, and oxidative stress. Aquat. Toxicol. 2015, 161, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-Z. Neurotoxins from marine dinoflagellates: A brief review. Mar. Drugs 2008, 6, 349–371. [Google Scholar] [CrossRef] [PubMed]
- Adolf, J.E.; Krupatkina, D.; Bachvaroff, T.; Place, A.R. Karlotoxin mediates grazing by Oxyrrhis marina on strains of Karlodinium veneficum. Harmful Algae 2007, 6, 400–412. [Google Scholar] [CrossRef]
- Sheng, J.; Malkiel, E.; Katz, J.; Adolf, J.E.; Place, A.R. A dinoflagellate exploits toxins to immobilize prey prior to ingestion. Proc. Natl. Acad. Sci. USA 2010, 107, 2082–2087. [Google Scholar] [CrossRef]
- Colon, R.; Wheater, M.; Joyce, E.J.; Ste Marie, E.J.; Hondal, R.J.; Rein, K.S. The Marine Neurotoxin Brevetoxin (PbTx-2) Inhibits Karenia brevis and Mammalian Thioredoxin Reductases by Targeting Different Residues. J. Nat. Prod. 2021, 84, 2961–2970. [Google Scholar] [CrossRef]
- Chen, W.; Colon, R.; Louda, J.W.; Del Rey, F.R.; Durham, M.; Rein, K.S. Brevetoxin (PbTx-2) influences the redox status and NPQ of Karenia brevis by way of thioredoxin reductase. Harmful Algae 2018, 71, 29–39. [Google Scholar] [CrossRef]
- Beld, J.; Sonnenschein, E.C.; Vickery, C.R.; Noel, J.P.; Burkart, M.D. The phosphopantetheinyl transferases: Catalysis of a post-translational modification crucial for life. Nat. Prod. Rep. 2014, 31, 61–108. [Google Scholar] [CrossRef]
- Bentley, R.; Bennett, J.W. Constructing polyketides: From collie to combinatorial biosynthesis. Annu. Rev. Microbiol. 1999, 53, 411–446. [Google Scholar] [CrossRef]
- Khosla, C. Structures and mechanisms of polyketide synthases. J. Org. Chem. 2009, 74, 6416–6420. [Google Scholar] [CrossRef]
- Sieber, S.A.; Marahiel, M.A. Molecular mechanisms underlying nonribosomal peptide synthesis: Approaches to new antibiotics. Chem. Rev. 2005, 105, 715–738. [Google Scholar] [CrossRef]
- Khosla, C.; Kapur, S.; Cane, D.E. Revisiting the modularity of modular polyketide synthases. Curr. Opin. Chem. Biol. 2009, 13, 135–143. [Google Scholar] [CrossRef]
- Rausch, C.; Hoof, I.; Weber, T.; Wohlleben, W.; Huson, D.H. Phylogenetic analysis of condensation domains in NRPS sheds light on their functional evolution. BMC Evol. Biol. 2007, 7, 78. [Google Scholar] [CrossRef]
- Wang, H.; Fewer, D.P.; Holm, L.; Rouhiainen, L.; Sivonen, K. Atlas of nonribosomal peptide and polyketide biosynthetic pathways reveals common occurrence of nonmodular enzymes. Proc. Natl. Acad. Sci. USA 2014, 111, 9259–9264. [Google Scholar] [CrossRef]
- Franke, J.; Ishida, K.; Hertweck, C. Genomics-driven discovery of burkholderic acid, a noncanonical, cryptic polyketide from human pathogenic Burkholderia species. Angew Chem. Int. Ed. Engl. 2012, 51, 11611–11615. [Google Scholar] [CrossRef]
- Kevany, B.M.; Rasko, D.A.; Thomas, M.G. Characterization of the complete zwittermicin A biosynthesis gene cluster from Bacillus cereus. Appl. Environ. Microbiol. 2009, 75, 1144–1155. [Google Scholar] [CrossRef]
- Bachvaroff, T.R.; Place, A.R. From stop to start: Tandem gene arrangement, copy number and trans-splicing sites in the dinoflagellate Amphidinium carterae. PLoS ONE 2008, 3, e2929. [Google Scholar] [CrossRef]
- Fukatsu, T.; Onodera, K.; Ohta, Y.; Oba, Y.; Nakamura, H.; Shintani, T.; Yoshioka, Y.; Okamoto, T.; ten Lohuis, M.; Miller, D.J.; et al. Zooxanthellamide D, a polyhydroxy polyene amide from a marine dinoflagellate, and chemotaxonomic perspective of the Symbiodinium polyols. J. Nat. Prod. 2007, 70, 407–411. [Google Scholar] [CrossRef]
- Ishida, H.; Nozawa, A.; Totoribe, K.; Muramatsu, N.; Nukaya, H.; Tsuji, K.; Yamaguchi, K.; Yasumoto, T.; Kaspar, H.; Berkett, N. Brevetoxin B1, a new polyether marine toxin from the New Zealand shellfish, Austrovenus stutchburyi. Tetrahedron Lett. 1995, 36, 725–728. [Google Scholar] [CrossRef]
- Macpherson, G.R.; Burton, I.W.; LeBlanc, P.; Walter, J.A.; Wright, J.L. Studies of the biosynthesis of DTX-5a and DTX-5b by the dinoflagellate Prorocentrum maculosum: Regiospecificity of the putative Baeyer-Villigerase and insertion of a single amino acid in a polyketide chain. J. Org. Chem. 2003, 68, 1659–1664. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Van Wagoner, R.M.; Misner, I.; Tomas, C.; Wright, J.L. Structure and biosynthesis of amphidinol 17, a hemolytic compound from Amphidinium carterae. J. Nat. Prod. 2010, 73, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Place, A.R.; Yoshida, W.; Anklin, C.; Hamann, M.T. Structure and absolute configuration of karlotoxin-2, an ichthyotoxin from the marine dinoflagellate Karlodinium veneficum. J. Am. Chem. Soc. 2010, 132, 3277–3279. [Google Scholar] [CrossRef]
- Sasaki, M.; Matsumori, N.; Maruyama, T.; Nonomura, T.; Murata, M.; Tachibana, K.; Yasumoto, T. The Complete Structure of Maitotoxin, Part I: Configuration of the C1–C14 Side Chain. Angew. Chem. Int. Ed. Engl. 1996, 35, 1672–1675. [Google Scholar] [CrossRef]
- Satake, M.; Morohashi, A.; Oguri, H.; Oishi, T.; Hirama, M.; Harada, N.; Yasumoto, T. The absolute configuration of ciguatoxin. J. Am. Chem. Soc. 1997, 119, 11325–11326. [Google Scholar] [CrossRef]
- Seki, T.; Satake, M.; Mackenzie, L.; Kaspar, H.F.; Yasumoto, T. Gymnodimine, a new marine toxin of unprecedented structure isolated from New Zealand oysters and the dinoflagellate, Gymnodinium sp. Tetrahedron Lett. 1995, 36, 7093–7096. [Google Scholar] [CrossRef]
- Van Wagoner, R.M.; Deeds, J.R.; Satake, M.; Ribeiro, A.A.; Place, A.R.; Wright, J.L. Isolation and characterization of karlotoxin 1, a new amphipathic toxin from Karlodinium veneficum. Tetrahedron Lett. 2008, 49, 6457–6461. [Google Scholar] [CrossRef]
- Van Wagoner, R.M.; Deeds, J.R.; Tatters, A.O.; Place, A.R.; Tomas, C.R.; Wright, J.L. Structure and relative potency of several karlotoxins from Karlodinium veneficum. J. Nat. Prod. 2010, 73, 1360–1365. [Google Scholar] [CrossRef]
- Wright, J.L.C.; Hu, T.; McLachlan, J.L.; Needham, J.; Walter, J.A. Biosynthesis of DTX-4: Confirmation of a polyketide pathway, proof of a Baeyer—Villiger oxidation step, and evidence for an unusual carbon deletion process. J. Am. Chem. Soc. 1996, 118, 8757–8758. [Google Scholar] [CrossRef]
- Van Wagoner, R.M.; Satake, M.; Wright, J.L. Polyketide biosynthesis in dinoflagellates: What makes it different. Nat. Prod. Rep. 2014, 31, 1101–1137. [Google Scholar] [CrossRef]
- Verma, A.; Barua, A.; Ruvindy, R.; Savela, H.; Ajani, P.A.; Murray, S.A. The Genetic Basis of Toxin Biosynthesis in Dinoflagellates. Microorganisms 2019, 7, 222. [Google Scholar] [CrossRef]
- Snyder, R.V.; Gibbs, P.D.L.; Palacios, A.; Abiy, L.; Dickey, R.; Lopez, J.V.; Rein, K.S. Polyketide synthase genes from marine dinoflagellates. Mar. Biotechnol. 2003, 5, 1–12. [Google Scholar] [CrossRef]
- Beedessee, G.; Hisata, K.; Roy, M.C.; Van Dolah, F.M.; Satoh, N.; Shoguchi, E. Diversified secondary metabolite biosynthesis gene repertoire revealed in symbiotic dinoflagellates. Sci. Rep. 2019, 9, 1204. [Google Scholar] [CrossRef]
- Kohli, G.S.; John, U.; Figueroa, R.I.; Rhodes, L.L.; Harwood, D.T.; Groth, M.; Bolch, C.J.; Murray, S.A. Polyketide synthesis genes associated with toxin production in two species of Gambierdiscus (Dinophyceae). BMC Genom. 2015, 16, 410. [Google Scholar] [CrossRef]
- Kohli, G.S.; John, U.; Van Dolah, F.M.; Murray, S.A. Evolutionary distinctiveness of fatty acid and polyketide synthesis in eukaryotes. ISME J. 2016, 10, 1877–1890. [Google Scholar] [CrossRef]
- Meyer, J.M.; Rödelsperger, C.; Eichholz, K.; Tillmann, U.; Cembella, A.; McGaughran, A.; John, U. Transcriptomic characterisation and genomic glimps into the toxigenic dinoflagellate Azadinium spinosum, with emphasis on polykeitde synthase genes. BMC Genom. 2015, 16, 27. [Google Scholar] [CrossRef]
- Van Dolah, F.M.; Kohli, G.S.; Morey, J.S.; Murray, S.A. Both modular and single-domain Type I polyketide synthases are expressed in the brevetoxin-producing dinoflagellate, Karenia brevis (Dinophyceae). J. Phycol. 2017, 53, 1325–1339. [Google Scholar] [CrossRef]
- Bachvaroff, T.R.; Williams, E.P.; Jagus, R.; Place, A.R. A cryptic noncanonical multi-module PKS/NRPS found in dinoflagellates. In Proceedings of the 16th International Conference on Harmful Algae, Wellington, New Zealand, 27–31 October 2014; pp. 101–104. [Google Scholar]
- Kohli, G.S.; Campbell, K.; John, U.; Smith, K.F.; Fraga, S.; Rhodes, L.L.; Murray, S.A. Role of Modular Polyketide Synthases in the Production of Polyether Ladder Compounds in Ciguatoxin-Producing Gambierdiscus polynesiensis and G. excentricus (Dinophyceae). J. Eukaryot Microbiol. 2017, 64, 691–706. [Google Scholar] [CrossRef]
- Van Dolah, F.M.; Morey, J.S.; Milne, S.; Ung, A.; Anderson, P.E.; Chinain, M. Transcriptomic analysis of polyketide synthases in a highly ciguatoxic dinoflagellate, Gambierdiscus polynesiensis and low toxicity Gambierdiscus pacificus, from French Polynesia. PLoS ONE 2020, 15, e0231400. [Google Scholar] [CrossRef]
- Williams, E.P.; Bachvaroff, T.R.; Place, A.R. A Global Approach to Estimating the Abundance and Duplication of Polyketide Synthase Domains in Dinoflagellates. Evol. Bioinform. Online 2021, 17, 11769343211031871. [Google Scholar] [CrossRef]
- Bunkoczi, G.; Pasta, S.; Joshi, A.; Wu, X.; Kavanagh, K.L.; Smith, S.; Oppermann, U. Mechanism and substrate recognition of human holo ACP synthase. Chem. Biol. 2007, 14, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.P.; Bachvaroff, T.R.; Place, A.R. The Phosphopantetheinyl Transferases in Dinoflagellates. In Proceedings of the 18th International Conference on Harmful Algae, Harmful Algae 2018-from Ecosystems to Socioecosystems, Nantes, France, 21–26 October 2018; pp. 176–180. [Google Scholar]
- Takahashi, H.; Kumagai, T.; Kitani, K.; Mori, M.; Matoba, Y.; Sugiyama, M. Cloning and characterization of a Streptomyces single module type non-ribosomal peptide synthetase catalyzing a blue pigment synthesis. J. Biol. Chem. 2007, 282, 9073–9081. [Google Scholar] [CrossRef] [PubMed]
- Owen, J.G.; Copp, J.N.; Ackerley, D.F. Rapid and flexible biochemical assays for evaluating 4′-phosphopantetheinyl transferase activity. Biochem. J. 2011, 436, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Geerlof, A.; Lewendon, A.; Shaw, W.V. Purification and Characterization of Phosphopantetheine Adenylyltransferase from Escherichia coli. J. Biol. Chem. 1999, 274, 27105–27111. [Google Scholar] [CrossRef]
- Murugan, E.; Kong, R.; Sun, H.; Rao, F.; Liang, Z.X. Expression, purification and characterization of the acyl carrier protein phosphodiesterase from Pseudomonas Aeruginosa. Protein. Expr. Purif. 2010, 71, 132–138. [Google Scholar] [CrossRef]
- Cai, X.; Herschap, D.; Zhu, G. Functional characterization of an evolutionarily distinct phosphopantetheinyl transferase in the apicomplexan Cryptosporidium parvum. Eukaryot Cell 2005, 4, 1211–1220. [Google Scholar] [CrossRef][Green Version]
- Sonnenschein, E.C.; Pu, Y.; Beld, J.; Burkart, M.D. Phosphopantetheinylation in the green microalgae Chlamydomonas reinhardtii. J. Appl. Phycol. 2016, 28, 3259–3267. [Google Scholar] [CrossRef]
- Finking, R.; Solsbacher, J.; Konz, D.; Schobert, M.; Schafer, A.; Jahn, D.; Marahiel, M.A. Characterization of a new type of phosphopantetheinyl transferase for fatty acid and siderophore synthesis in Pseudomonas aeruginosa. J. Biol. Chem. 2002, 277, 50293–50302. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1989; p. 654. [Google Scholar]
- Studier, F.W. Protein production by auto-induction in high density shaking cultures. Protein. Expr. Purif. 2005, 41, 207–234. [Google Scholar] [CrossRef]
- Faktorová, D.; Nisbet, R.E.R.; Fernández Robledo, J.A.; Casacuberta, E.; Sudek, L.; Allen, A.E.; Ares, M.; Aresté, C.; Balestreri, C.; Barbrook, A.C.; et al. Genetic tool development in marine protists: Emerging model organisms for experimental cell biology. Nat. Methods. 2020, 17, 481–494. [Google Scholar] [CrossRef]
- Beedessee, G.; Kubota, T.; Arimoto, A.; Nishitsuji, K.; Waller, R.F.; Hisata, K.; Yamasaki, S.; Satoh, N.; Kobayashi, J.; Shoguchi, E. Integrated omics unveil the secondary metabolic landscape of a basal dinoflagellate. BMC Biol. 2020, 18, 139. [Google Scholar] [CrossRef]
- Stephens, T.G.; González-Pech, R.A.; Cheng, Y.; Mohamed, A.R.; Burt, D.W.; Bhattacharya, D.; Ragan, M.A.; Chan, C.X. Genomes of the dinoflagellate Polarella glacialis encode tandemly repeated single-exon genes with adaptive functions. BMC Biol. 2020, 18, 56. [Google Scholar] [CrossRef]
- Aranda, M.; Li, Y.; Liew, Y.J.; Baumgarten, S.; Simakov, O.; Wilson, M.C.; Piel, J.; Ashoor, H.; Bougouffa, S.; Bajic, V.B.; et al. Genomes of coral dinoflagellate symbionts highlight evolutionary adaptations conducive to a symbiotic lifestyle. Sci. Rep. 2016, 6, 39734. [Google Scholar] [CrossRef]
- Lin, S.; Cheng, S.; Song, B.; Zhong, X.; Lin, X.; Li, W.; Li, L.; Zhang, Y.; Zhang, H.; Ji, Z.; et al. The Symbiodinium kawagutii genome illuminates dinoflagellate gene expression and coral symbiosis. Science 2015, 350, 691–694. [Google Scholar] [CrossRef]
- Shoguchi, E.; Shinzato, C.; Kawashima, T.; Gyoja, F.; Mungpakdee, S.; Koyanagi, R.; Takeuchi, T.; Hisata, K.; Tanaka, M.; Fujiwara, M.; et al. Draft assembly of the Symbiodinium minutum nuclear genome reveals dinoflagellate gene structure. Curr. Biol. 2013, 23, 1399–1408. [Google Scholar] [CrossRef]
- Leblond, J.D.; Evans, T.J.; Chapman, P.J. The biochemistry of dinoflagellate lipids, with particular reference to the fatty acid and sterol composition of a Karenia brevis bloom. Phycologia 2003, 42, 324–331. [Google Scholar] [CrossRef]
- Diao, J.; Song, X.; Zhang, X.; Chen, L.; Zhang, W. Genetic Engineering of Crypthecodinium cohnii to Increase Growth and Lipid Accumulation. Front Microbiol. 2018, 9, 492. [Google Scholar] [CrossRef]
- Tuttle, R.C.; Loeblich, A.R. Genetic recombination in the dinoflagellate Crypthecodinium cohnii. Science 1974, 185, 1061–1062. [Google Scholar] [CrossRef]
- Yan, T.H.K.; Wu, Z.; Kwok, A.C.M.; Wong, J.T.Y. Knockdown of Dinoflagellate Condensin CcSMC4 Subunit Leads to S-Phase Impediment and Decompaction of Liquid Crystalline Chromosomes. Microorganisms 2020, 8, 565. [Google Scholar] [CrossRef]
- Nimmo, I.C.; Barbrook, A.C.; Lassadi, I.; Chen, J.E.; Geisler, K.; Smith, A.G.; Aranda, M.; Purton, S.; Waller, R.F.; Nisbet, R.E.R.; et al. Genetic transformation of the dinoflagellate chloroplast. eLife 2019, 8, e45292. [Google Scholar] [CrossRef]
- Lidie, K.B.; Ryan, J.C.; Barbier, M.; Van Dolah, F.M. Gene expression in Florida red tide dinoflagellate Karenia brevis: Analysis of an expressed sequence tag library and development of DNA microarray. Mar. Biotechnol. 2005, 7, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Morse, D.; Milos, P.M.; Roux, E.; Hastings, J.W. Circadian regulation of bioluminescence in Gonyaulax involves translational control. Proc. Natl. Acad. Sci. USA 1989, 86, 172–176. [Google Scholar] [CrossRef]
- Roy, S.; Jagus, R.; Morse, D. Translation and Translational Control in Dinoflagellates. Microorganisms 2018, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Shi, X.; Lin, S. Heterologous expression and cell membrane localization of dinoflagellate opsins (rhodopsin proteins) in mammalian cells. Mar. Life Sci. Technol. 2020, 2, 302–308. [Google Scholar] [CrossRef]
- Manríquez, V.; Castro Caperan, D.; Guzmán, R.; Naser, M.; Iglesia, V.; Lagos, N. First evidence of neosaxitoxin as a long-acting pain blocker in bladder pain syndrome. Int. Urogynecol. J. 2015, 26, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Mendes, A.; Reis, A.; Vasconcelos, R.; Guerra, P.; Lopes da Silva, T. Crypthecodinium cohnii with emphasis on DHA production: A review. J. Appl. Phycol. 2009, 21, 199–214. [Google Scholar] [CrossRef]
- Jiao, Y.L.; Wang, L.H.; Jiao, B.H.; Wang, S.J.; Fang, Y.W.; Liu, S. Function analysis of a new type I PKS-SAT domain by SAT-EAT domain replacement. Prikl. Biokhim. Mikrobiol. 2010, 46, 161–165. [Google Scholar]
- Wang, H.; Liang, J.; Yue, Q.; Li, L.; Shi, Y.; Chen, G.; Li, Y.Z.; Bian, X.; Zhang, Y.; Zhao, G.; et al. Engineering the acyltransferase domain of epothilone polyketide synthase to alter the substrate specificity. Microb. Cell Fact. 2021, 20, 86. [Google Scholar] [CrossRef]
- Brautaset, T.; Borgos, S.E.; Sletta, H.; Ellingsen, T.E.; Zotchev, S.B. Site-specific mutagenesis and domain substitutions in the loading module of the nystatin polyketide synthase, and their effects on nystatin biosynthesis in Streptomyces noursei. J. Biol. Chem. 2003, 278, 14913–14919. [Google Scholar] [CrossRef]
- Calcott, M.J.; Owen, J.G.; Ackerley, D.F. Efficient rational modification of non-ribosomal peptides by adenylation domain substitution. Nat. Commun. 2020, 11, 4554. [Google Scholar] [CrossRef]
- Calcott, M.J.; Ackerley, D.F. Portability of the thiolation domain in recombinant pyoverdine non-ribosomal peptide synthetases. BMC Microbiol. 2015, 15, 162. [Google Scholar] [CrossRef][Green Version]
- Butz, D.; Schmiederer, T.; Hadatsch, B.; Wohlleben, W.; Weber, T.; Süssmuth, R.D. Module extension of a non-ribosomal peptide synthetase of the glycopeptide antibiotic balhimycin produced by Amycolatopsis balhimycina. Chembiochem 2008, 9, 1195–1200. [Google Scholar] [CrossRef]
- Khosla, C.; Ebert-Khosla, S.; Hopwood, D.A. Targeted gene replacements in a Streptomyces polyketide synthase gene cluster: Role for the acyl carrier protein. Mol. Microbiol. 1992, 6, 3237–3249. [Google Scholar] [CrossRef]
- Brown, A.S.; Calcott, M.J.; Collins, V.M.; Owen, J.G.; Ackerley, D.F. The indigoidine synthetase BpsA provides a colorimetric ATP assay that can be adapted to quantify the substrate preferences of other NRPS enzymes. Biotechnol. Lett. 2020, 42, 2665–2671. [Google Scholar] [CrossRef]
- Brown, A.S.; Robins, K.J.; Ackerley, D.F. A sensitive single-enzyme assay system using the non-ribosomal peptide synthetase BpsA for measurement of L-glutamine in biological samples. Sci. Rep. 2017, 7, 41745. [Google Scholar] [CrossRef]
- Wehrs, M.; Prahl, J.P.; Moon, J.; Li, Y.; Tanjore, D.; Keasling, J.D.; Pray, T.; Mukhopadhyay, A. Production efficiency of the bacterial non-ribosomal peptide indigoidine relies on the respiratory metabolic state in S. cerevisiae. Microb. Cell Fact. 2018, 17, 193. [Google Scholar] [CrossRef]
- Owen, J.G.; Calcott, M.J.; Robins, K.J.; Ackerley, D.F. Generating Functional Recombinant NRPS Enzymes in the Laboratory Setting via Peptidyl Carrier Protein Engineering. Cell Chem. Biol. 2016, 23, 1395–1406. [Google Scholar] [CrossRef]
- Gornik, S.G.; Cassin, A.M.; MacRae, J.I.; Ramaprasad, A.; Rchiad, Z.; McConville, M.J.; Bacic, A.; McFadden, G.I.; Pain, A.; Waller, R.F. Endosymbiosis undone by stepwise elimination of the plastid in a parasitic dinoflagellate. Proc. Natl. Acad. Sci. USA 2015, 112, 5767–5772. [Google Scholar] [CrossRef]




| Primers | |||
|---|---|---|---|
| Primer Name | Sequence 5′:3′ | Length | Annealing °C |
| BpsA_outF2 | TCCAGCACCTGATGATGAAC | 20 | 58.4 |
| BpsA_outR2 | CTGGATGCCGTAGAACGAG | 19 | 59.5 |
| BpsAhindiiiR1 | GACGCCAAGCTTCGCGTTGAGCTCGCGGACGAGGCCGACGGCGATCAGCGA | 51 | 91.1 |
| BpsAhindiiiF1 | CAACGCGAAGCTTGGCGTCTCCCTGCCGCTGCAGAGCGTCCTGGAGTCC | 49 | 89.6 |
| BpsAafliiR1 | CTCGCGCTTAAGGGCCTTCTCCCAGACCGCCGCGATCTCCTTCTCCGT | 48 | 88.5 |
| BpsAafliiF1 | AGAAGGCCCTTAAGCGCGAGAACGCCTCCGTCCAGGACGACTTCTTCG | 48 | 86.4 |
| Inserts | |||
| Insert Name | Sequence 5′:3′ | Binding Site Amino Acid | |
| 3KS_1 | GAATCGGGCATGGACTCAAAAGCAGCCCTTGTTCTG | ESGMDSKAALVL | |
| 3KS_2 | GAATTGGGCTTAGATTCTTTGTCCGGCGTTGAATTT | ELGLDSLSGVEF | |
| 3KS_3 | GAAAGCGGAATTGATTCCTTGTCTGCAGTAGAGTTT | ESGIDSLSAVEF | |
| 3KS_4 | GAGAGTGGCATGGACTCATTATCTGCCGTCGAGTTT | ESGMDSLSAVEF | |
| BurA_1 | GCT TCA GGT GCA GAA TCT ATC GCT GTC GTG GGC GTG | ASGAESIAVVGV | |
| BurA_2 | CAA TTA GGA TTA GAC AGC TTG GAA ACC GTT CAA CTG | QLGLDSLETVQL | |
| ZmaK_1 | GAA ATC GGT GGG CAC TCG CTG TTA GCA ATG AAA CTT | EIGGHSLLAMKL | |
| ZmaK_2 | GAT GCC GGG TTA GAT AGC TTA TCC TTA ATT AGC TTA | DAGLDSLSLISL | |
| 5′ Linker † | AGAAGGCCCTTAAGCGCGAGAACGCCTCCGTCCAGGACGACTTCTTC | ||
| 3′ Linker † | GTCCGCGAGCTCAACGCGAAGCTTGGCGTCTCCCTGCCGCTG | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, E.; Bachvaroff, T.; Place, A. Dinoflagellate Phosphopantetheinyl Transferase (PPTase) and Thiolation Domain Interactions Characterized Using a Modified Indigoidine Synthesizing Reporter. Microorganisms 2022, 10, 687. https://doi.org/10.3390/microorganisms10040687
Williams E, Bachvaroff T, Place A. Dinoflagellate Phosphopantetheinyl Transferase (PPTase) and Thiolation Domain Interactions Characterized Using a Modified Indigoidine Synthesizing Reporter. Microorganisms. 2022; 10(4):687. https://doi.org/10.3390/microorganisms10040687
Chicago/Turabian StyleWilliams, Ernest, Tsvetan Bachvaroff, and Allen Place. 2022. "Dinoflagellate Phosphopantetheinyl Transferase (PPTase) and Thiolation Domain Interactions Characterized Using a Modified Indigoidine Synthesizing Reporter" Microorganisms 10, no. 4: 687. https://doi.org/10.3390/microorganisms10040687
APA StyleWilliams, E., Bachvaroff, T., & Place, A. (2022). Dinoflagellate Phosphopantetheinyl Transferase (PPTase) and Thiolation Domain Interactions Characterized Using a Modified Indigoidine Synthesizing Reporter. Microorganisms, 10(4), 687. https://doi.org/10.3390/microorganisms10040687







