Molecular Characterization of Staphylococcus aureus Strains Isolated from Mobile Phones
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample and Isolated Strains
2.2. Microbiological and Biochemical Identification
2.3. Methicillin Susceptibility Testing
2.4. Detection of mecA Gene
2.5. Detection of Hospital-Acquired Methicillin Resistant Staphylococcus aureus (HA-MRSA) or Community-Acquired Methicillin-Resistant Staphylococcus aureus (CA-MRSA)
2.6. Typing with the spa Gene (spa-Typing)
2.7. Pulsed-Field Gel Electrophoresis (PFGE) Typing
2.8. Detection of Toxin and Adhesin Genes
2.9. Biofilm Analysis
2.10. Statistical Analysis
3. Results
3.1. Detection of Staphylococcus aureus Carriers
3.2. Characterization of Isolated Staphylococcus aureus Strains
3.3. Genotyping of the Strains Isolated in the MP
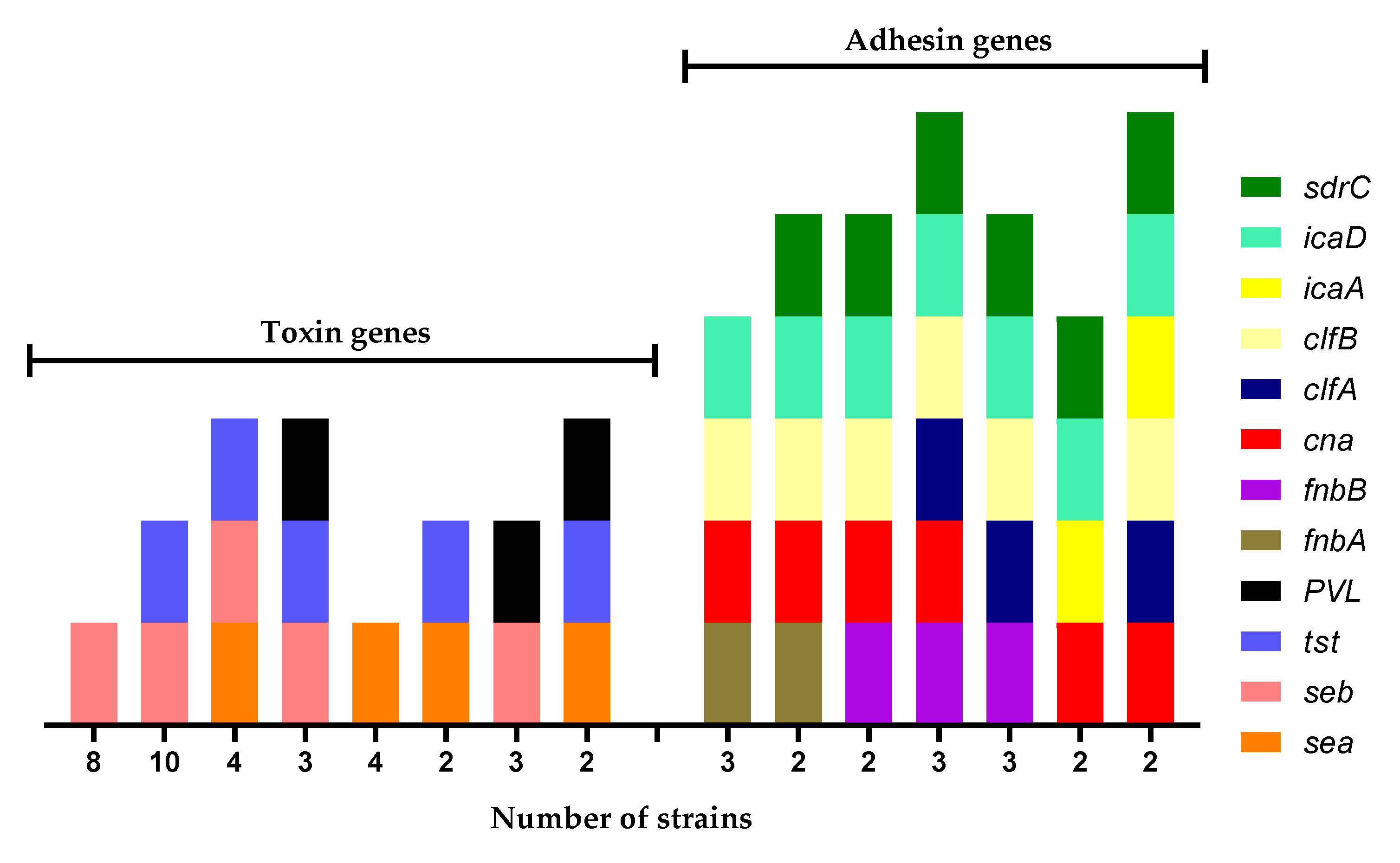
3.4. Biofilm Formation of S. aureus Strains Isolated from MP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olsen, M.; Campos, M.; Lohning, A.; Jones, P.; Legget, J.; Bannach-Brown, A.; McKirdy, S.; Alghafri, R.; Tajouri, L. Mobile phones represent a pathway for microbial transmission: A scoping review. Travel Med. Infect. Dis. 2020, 35, 101704. [Google Scholar] [CrossRef]
- Juyal, D.; Adekhandi, S.; Sharma, M.; Prakash, R.; Sharma, N.; Rana, A.; Parihar, A.; Pal, S. Mobile phones: Reservoirs for the transmission of nosocomial pathogens. Adv. Biomed. Res. 2015, 4, 144. [Google Scholar] [CrossRef] [PubMed]
- Ulger, F.; Dilek, A.; Esen, S.; Sunbul, M.; Leblebicioglu, H. Are healthcare workers’ mobile phones a potential source of nosocomial infections? Review of the literature. J. Infect. Dev. Ctries. 2015, 9, 1046–1053. [Google Scholar] [CrossRef]
- Bhat, S.S.; Hegde, S.K.; Salian, S. Potential of Mobile Phones to Serve as a Reservoir in Spread of Nosocomial Pathogens. Online J. Health Allied Sci. 2011, 10, 14. Available online: http://www.ojhas.org/issue38/2011-2-14.htm (accessed on 15 December 2021).
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed]
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef]
- Tsouklidis, N.; Kumar, R.; Heindl, S.E.; Soni, R.; Khan, S. Understanding the Fight Against Resistance: Hospital-Acquired Methicillin-Resistant Staphylococcus Aureus vs. Community-Acquired Methicillin-Resistant Staphylococcus Aureus. Cureus 2020, 12, e8867. [Google Scholar] [CrossRef]
- Missri, L.; Smiljkovski, D.; Prigent, G.; Lesenne, A.; Obadia, T.; Joumaa, M.; Chelha, R.; Chalumeau-Lemoine, L.; Obadia, E.; Galbois, A. Bacterial colonization of healthcare workers’ mobile phones in the ICU and effectiveness of sanitization. J. Occup. Environ. Hyg. 2019, 16, 97–100. [Google Scholar] [CrossRef]
- Kaiki, Y.; Kitagawa, H.; Hara, T.; Nomura, T.; Omori, K.; Shigemoto, N.; Takahashi, S.; Ohge, H. Methicillin-resistant Staphylococcus aureus contamination of hospital-use-only mobile phones and efficacy of 222-nm ultraviolet disinfection. Am. J. Infect. Control 2021, 49, 800–803. [Google Scholar] [CrossRef]
- Chang, C.-H.; Chen, S.-Y.; Lu, J.-J.; Chang, C.-J.; Chang, Y.; Hsieh, P.-H. Nasal colonization and bacterial contamination of mobile phones carried by medical staff in the operating room. PLoS ONE 2017, 12, e0175811. [Google Scholar] [CrossRef]
- Goh, Z.N.L.; Chung, P.Y. Incidence of meticillin-resistant Staphylococcus aureus contamination on mobile phones of medical students. J. Hosp. Infect. 2019, 101, 482–483. [Google Scholar] [CrossRef] [PubMed]
- Furuhata, K.; Ishizaki, N.; Sogawa, K.; Kawakami, Y.; Lee, S.-I.; Sato, M.; Fukuyama, M. Isolation, Identification and Antibacterial Susceptibility of Staphylococcus spp. Associated with the Mobile Phones of University Students. Biocontrol Sci. 2016, 21, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Modica, D.C.; Maurici, M.; D’Alò, G.L.; Mozzetti, C.; Messina, A.; Distefano, A.; Pica, F.; De Filippis, P. Taking Screenshots of the Invisible: A Study on Bacterial Contamination of Mobile Phones from University Students of Healthcare Professions in Rome, Italy. Microorganisms 2020, 8, 1075. [Google Scholar] [CrossRef] [PubMed]
- Deurenberg, R.H.; Stobberingh, E.E. The evolution of Staphylococcus aureus. Infect. Genet. Evol. 2008, 8, 747–763. [Google Scholar] [CrossRef]
- Laux, C.; Peschel, A.; Krismer, B. Staphylococcus aureus Colonization of the Human Nose and Interaction with Other Microbiome Members. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Yang, E.; Tan, J.; Eells, S.; Rieg, G.; Tagudar, G.; Miller, L. Body site colonization in patients with community-associated methicillin-resistant Staphylococcus aureus and other types of S. aureus skin infections. Clin. Microbiol. Infect. 2010, 16, 425–431. [Google Scholar] [CrossRef]
- Sollid, J.; Furberg, A.; Hanssen, A.; Johannessen, M. Staphylococcus aureus: Determinants of human carriage. Infect. Genet. Evol. 2014, 21, 531–541. [Google Scholar] [CrossRef]
- Genc, O.; Arikan, I. The relationship between hand hygiene practices and nasal Staphylococcus aureus carriage in healthcare workers. Med. Lav. 2020, 111, 54. [Google Scholar] [CrossRef]
- Hamdan-Partida, A.; Sainz-Espuñes, T.; Bustos-Martínez, J. Characterization and Persistence of Staphylococcus aureus Strains Isolated from the Anterior Nares and Throats of Healthy Carriers in a Mexican Community. J. Clin. Microbiol. 2010, 48, 1701–1705. [Google Scholar] [CrossRef]
- Hamdan-Partida, A.; González-García, S.; de la Rosa García, E.; Bustos-Martínez, J. Community-acquired methicillin-resistant Staphylococcus aureus can persist in the throat. Int. J. Med. Microbiol. 2018, 308, 469–475. [Google Scholar] [CrossRef]
- Nilsson, P.; Ripa, T. Staphylococcus aureus Throat Colonization Is More Frequent than Colonization in the Anterior Nares. J. Clin. Microbiol. 2006, 44, 3334–3339. [Google Scholar] [CrossRef]
- Foster, T.J.; Geoghegan, J.A.; Ganesh, V.K.; Höök, M. Adhesion, invasion and evasion: The many functions of the surface proteins of Staphylococcus aureus. Nat. Rev. Microbiol. 2014, 12, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Moormeier, D.E.; Bayles, K.W. Staphylococcus aureusbiofilm: A complex developmental organism. Mol. Microbiol. 2017, 104, 365–376. [Google Scholar] [CrossRef]
- Zhao, N.; Cheng, D.; Jian, Y.; Liu, Y.; Liu, J.; Huang, Q.; He, L.; Wang, H.; Miao, F.; Li, M.; et al. Molecular characteristics of Staphylococcus aureus isolates colonizing human nares and skin. Med. Microecol. 2021, 7, 100031. [Google Scholar] [CrossRef]
- Pivard, M.; Moreau, K.; Vandenesch, F. Staphylococcus aureus Arsenal to Conquer the Lower Respiratory Tract. mSphere 2021, 6, e00059-21. [Google Scholar] [CrossRef] [PubMed]
- Paharik, A.E.; Horswill, A.R. The Staphylococcal Biofilm: Adhesins, Regulation, and Host Response. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Mack, D.; Fischer, W.; Krokotsch, A.; Leopold, K.; Hartmann, R.; Egge, H.; Laufs, R. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear beta-1,6-linked glucosaminoglycan: Purification and structural analysis. J. Bacteriol. 1996, 178, 175–183. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Ravaioli, S.; Montanaro, L. Polysaccharide intercellular adhesin in biofilm: Structural and reg-ulatory aspects. Front. Cell. Infect. Microbiol. 2015, 5, 7. [Google Scholar] [CrossRef]
- Oliveira, D.; Borges, A.; Simões, M. Staphylococcus aureus Toxins and Their Molecular Activity in Infectious Diseases. Toxins 2018, 10, 252. [Google Scholar] [CrossRef]
- Zheng, Y.; Qin, C.; Zhang, X.; Zhu, Y.; Li, A.; Wang, M.; Tang, Y.; Kreiswirth, B.N.; Chen, L.; Zhang, H.; et al. The tst gene associated Staphylococcus aureus pathogenicity island facilitates its pathogenesis by promoting the secretion of inflammatory cytokines and inducing immune suppression. Microb. Pathog. 2019, 138, 103797. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standarts Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 9th ed.; Approved Standard M07-A9; CLSI: Wayne, PA, USA, 2012. [Google Scholar]
- Oliveira, D.C.; de Lencastre, H. Multiplex PCR Strategy for Rapid Identification of Structural Types and Variants of the mec Element in Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2002, 46, 2155–2161. [Google Scholar] [CrossRef] [PubMed]
- Nastaly, P.; Grinholc, M.; Bielawski, K.P. Molecular characteristics of community-associated methicillin-resistant Staphylococcus aureus strains for clinical medicine. Arch. Microbiol. 2010, 192, 603–617. [Google Scholar] [CrossRef]
- Boye, K.; Bartels, M.D.; Andersen, I.; Møller, J.; Westh, H. A new multiplex PCR for easy screening of methicillin-resistant Staphylococcus aureus SCCmec types I–V. Clin. Microbiol. Infect. 2007, 13, 725–727. [Google Scholar] [CrossRef]
- Lina, G.; Piémont, Y.; Godail-Gamot, F.; Bes, M.; Peter, M.-O.; Gauduchon, V.; Vandenesch, F.; Etienne, J. Involvement of Panton-Valentine Leukocidin-Producing Staphylococcus aureus in Primary Skin Infections and Pneumonia. Clin. Infect. Dis. 1999, 29, 1128–1132. [Google Scholar] [CrossRef] [PubMed]
- Shopsin, B.; Gomez, M.; Montgomery, S.O.; Smith, D.H.; Waddington, M.; Dodge, D.E.; Bost, D.A.; Riehman, M.; Naidich, S.; Kreiswirth, B.N. Evaluation of Protein A Gene Polymorphic Region DNA Sequencing for Typing of Staphylococcus aureus Strains. J. Clin. Microbiol. 1999, 37, 3556–3563. [Google Scholar] [CrossRef] [PubMed]
- McDougal, L.K.; Steward, C.D.; Killgore, G.E.; Chaitram, J.M.; McAllister, S.K.; Tenover, F.C. Pulsed-Field Gel Electrophoresis Typing of Oxacillin-Resistant Staphylococcus aureus Isolates from the United States: Establishing a National Database. J. Clin. Microbiol. 2003, 41, 5113–5120. [Google Scholar] [CrossRef]
- Tenover, F.C.; Arbeit, R.D.; Goering, R.V.; Mickelsen, P.A.; Murray, B.E.; Persing, D.H.; Swaminathan, B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: Criteria for bacterial strain typing. J. Clin. Microbiol. 1995, 33, 2233–2239. [Google Scholar] [CrossRef]
- Campbell, S.J.; Deshmukh, H.S.; Nelson, C.L.; Bae, I.-G.; Stryjewski, M.E.; Federspiel, J.J.; Tonthat, G.T.; Rude, T.H.; Barriere, S.L.; Corey, R.; et al. Genotypic Characteristics of Staphylococcus aureus Isolates from a Multinational Trial of Complicated Skin and Skin Structure Infections. J. Clin. Microbiol. 2008, 46, 678–684. [Google Scholar] [CrossRef]
- Jarraud, S.; Mougel, C.; Thioulouse, J.; Lina, G.; Meugnier, H.; Forey, F.; Nesme, X.; Etienne, J.; Vandenesch, F. Relationships between Staphylococcus aureus Genetic Background, Virulence Factors, agr Groups (Alleles), and Human Disease. Infect. Immun. 2002, 70, 631–641. [Google Scholar] [CrossRef]
- Peacock, S.J.; Moore, C.E.; Justice, A.; Kantzanou, M.; Story, L.; Mackie, K.; O’Neill, G.; Day, N.P.J. Virulent Combinations of Adhesin and Toxin Genes in Natural Populations of Staphylococcus aureus. Infect. Immun. 2002, 70, 4987–4996. [Google Scholar] [CrossRef] [PubMed]
- Babra, C.; Tiwari, J.; Costantino, P.; Sunagar, R.; Isloor, S.; Hegde, N.; Mukkur, T. Human methicillin-sensitive Staphylococcus aureus biofilms: Potential associations with antibiotic resistance persistence and surface polysaccharide antigens. J. Basic Microbiol. 2013, 54, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cano, H.; Santos, M.R.; Moreno, B.C. Microbiota en teléfonos móviles de médicos oftalmólogos. Arch. Soc. Esp. Oftalmol. 2018, 94, 55–59. [Google Scholar] [CrossRef]
- Simmonds, R.; Lee, D.; Hayhurst, E. Mobile phones as fomites for potential pathogens in hospitals: Microbiome analysis reveals hidden contaminants. J. Hosp. Infect. 2020, 104, 207–213. [Google Scholar] [CrossRef]
- Zakai, S.; Mashat, A.; Abumohssin, A.; Samarkandi, A.; Almaghrabi, B.; Barradah, H.; Jiman-Fatani, A. Bacterial contamination of cell phones of medical students at King Abdulaziz University, Jeddah, Saudi Arabia. J. Microsc. Ultrastruct. 2016, 4, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ruíz, F.; Carrillo-Espíndola, T.; Bustos-Martínez, J.; Hamdan-Partida, A.; Sánchez-Pérez, L.; Acosta-Gío, A. Higher prevalence of meticillin-resistant Staphylococcus aureus among dental students. J. Hosp. Infect. 2014, 86, 216–218. [Google Scholar] [CrossRef] [PubMed]
- Morubagal, R.R.; Shivappa, S.G.; Mahale, R.P.; Neelambike, S.M. Study of bacterial flora associated with mobile phones of healthcare workers and non-healthcare workers. Iran. J. Microbiol. 2017, 9, 143–151. [Google Scholar] [PubMed]
- Lin, D.; Ou, Q.; Lin, J.; Peng, Y.; Yao, Z. A meta-analysis of the rates of Staphylococcus aureus and methicillin-resistant S aureus contamination on the surfaces of environmental objects that health care workers frequently touch. Am. J. Infect. Control 2017, 45, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Kanayama, A.K.; Takahashi, H.; Yoshizawa, S.; Tateda, K.; Kaneko, A.; Kobayashi, I. Staphylococcus aureus surface contamination of mobile phones and presence of genetically identical strains on the hands of nursing personnel. Am. J. Infect. Control 2017, 45, 929–931. [Google Scholar] [CrossRef]
- Brady, R.; McDermott, C.; Fraise, A.; Verran, J.; Gibb, A. Healthcare workers’ mobile phones are rarely contaminated by MRSA in the non-clinical environment. J. Hosp. Infect. 2009, 72, 373–374. [Google Scholar] [CrossRef]
- Khivsara, A.; Sushma, T.V.; Dhanashree, B. Typing of Staphylococcus aureus from mobile phones and clinical samples. Curr. Sci. 2006, 90, 910–912. [Google Scholar]
- Pathare, N.A.; Tejani, S.; Asogan, H.; Al Mahruqi, G.; Al Fakhri, S.; Zafarulla, R.; Pathare, A.V. Comparison of Methicillin Resistant Staphylococcus aureus in Healthy Community Hospital Visitors [CA-MRSA] and Hospital Staff [HA-MRSA]. Mediterr. J. Hematol. Infect. Dis. 2015, 7, e2015053. [Google Scholar] [CrossRef] [PubMed]
- Ustun, C.; Cihangiroğlu, M. Health Care Workers’ Mobile Phones: A Potential Cause of Microbial Cross-Contamination Between Hospitals and Community. J. Occup. Environ. Hyg. 2012, 9, 538–542. [Google Scholar] [CrossRef]
- Mediavilla, J.R.; Chen, L.; Mathema, B.; Kreiswirth, B.N. Global epidemiology of community-associated methicillin resistant Staphylococcus aureus (CA-MRSA). Curr. Opin. Microbiol. 2012, 15, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Kale, P.; Dhawan, B. The changing face of community-acquired methicillin-resistant Staphylococcus aureus. Ind. J. Med. Microbiol. 2016, 34, 275–285. [Google Scholar] [CrossRef]
- Noumi, E.; Merghni, A.; Alreshidi, M.; Del Campo, R.; Adnan, M.; Haddad, O.; De Feo, V.; Snoussi, M. Phenotypic and Genotypic Characterization with MALDI-TOF-MS Based Identification of Staphylococcus spp. Isolated from Mobile Phones with their Antibiotic Susceptibility, Biofilm Formation, and Adhesion Properties. Int. J. Environ. Res. Public Health 2020, 17, 3761. [Google Scholar] [CrossRef]
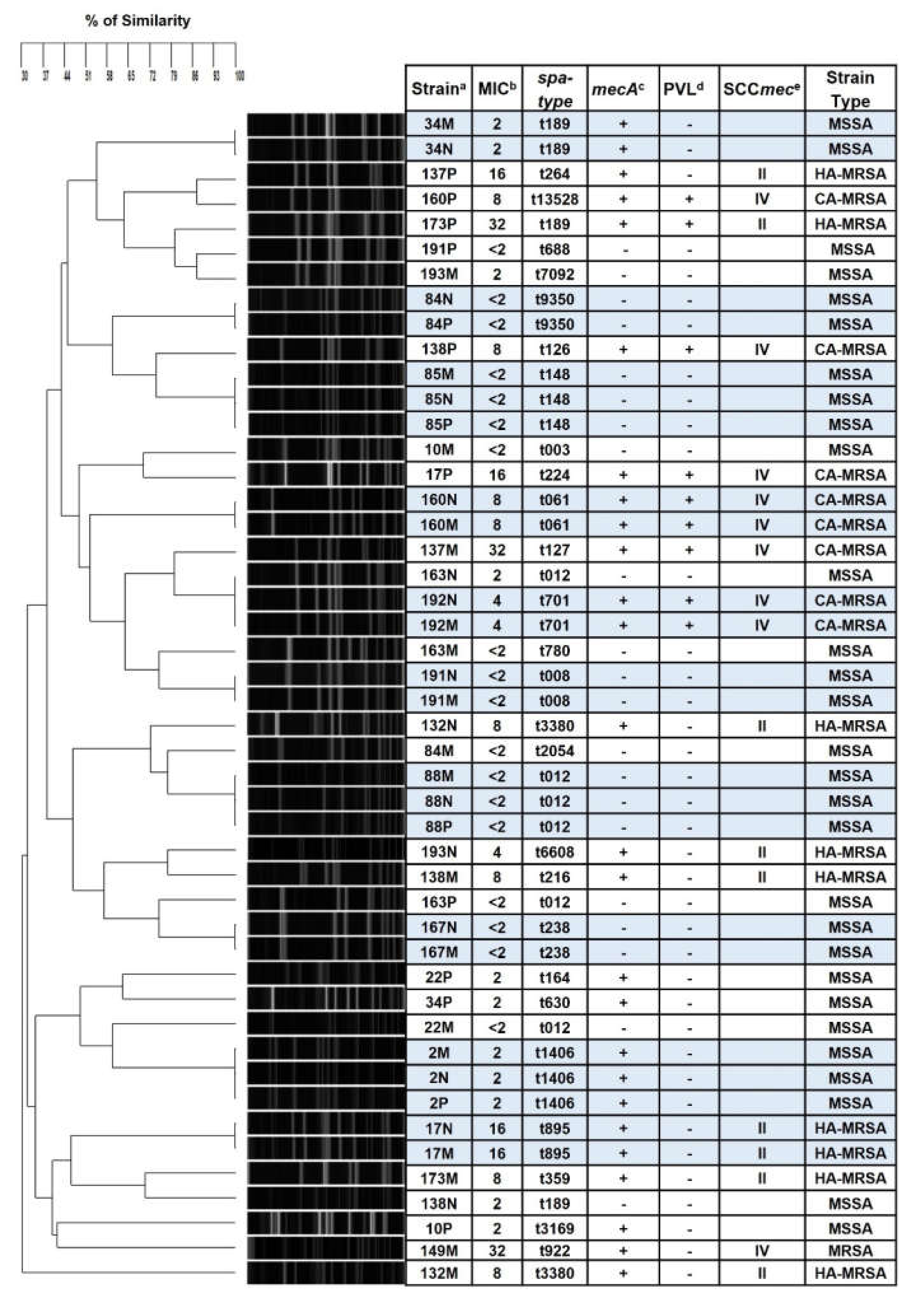
| n | Pharynx or Nose | Pharynx | Nose | Pharynx and Nose | Mobile Phone | |
|---|---|---|---|---|---|---|
| Men | 67 (33.5%) | 48 (71.6%) * | 18 (26.8%) | 16 (23.8%) | 14 (20.8%) | 7 (10.4%) |
| Women | 133 (66.5%) | 58 (43.6%) | 33 (24.8%) | 14 (10.5%) | 11 (8.2%) | 12 (9.0%) |
| Total | 200 (100%) | 106 (53.0%) | 51 (25.5%) | 30 (15.0%) | 25 (12.5%) | 19 (9.5%) |
| Toxin Genes b | Adhesin Genes b | Biofilm Foration c | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain a | sea | seb | tst | PVL | fnbA | fnbB | cna | clfA | clfB | icaA | icaD | sdrC | |
| 2N | − | + | − | − | + | − | + | − | + | − | + | − | + |
| 2P | − | − | − | − | + | − | + | − | + | − | + | − | + |
| 2M | + | − | − | − | + | − | + | − | + | − | + | − | + |
| 10P | − | + | − | − | + | + | + | − | + | − | + | − | + |
| 10M | − | + | − | − | + | − | + | + | + | − | + | − | ++ |
| 17N | − | + | − | − | + | − | + | − | + | − | + | + | + |
| 17P | − | + | − | + | + | − | + | − | + | − | + | + | + |
| 17M | − | + | + | − | − | + | − | − | + | − | + | + | + |
| 22P | − | − | − | − | + | + | − | + | + | − | + | + | +++ |
| 22M | + | − | − | − | + | + | + | + | + | − | + | + | + |
| 34N | − | − | + | − | + | + | + | − | + | − | + | + | ++ |
| 34P | − | + | + | − | + | − | + | − | − | − | + | − | +++ |
| 34M | − | − | − | − | − | + | + | − | + | − | + | + | +++ |
| 84N | − | − | − | − | − | + | + | + | + | − | + | + | +++ |
| 84P | − | + | + | − | − | + | + | + | + | − | + | + | +++ |
| 84M | − | + | + | − | − | + | + | + | + | − | + | + | ++ |
| 85N | − | + | − | − | − | + | − | + | + | − | + | + | +++ |
| 85P | − | + | − | − | − | + | − | + | + | − | + | + | +++ |
| 85M | − | + | − | − | − | + | − | − | + | − | + | + | +++ |
| 88N | − | + | + | − | − | + | + | − | + | − | + | + | ++ |
| 88P | − | + | + | − | − | − | + | − | + | − | + | + | +++ |
| 88M | − | + | + | − | − | − | + | − | + | − | + | + | ++ |
| 132N | + | − | − | − | − | − | − | − | + | + | + | + | ++ |
| 132M | + | − | − | − | − | − | − | − | + | − | + | + | + |
| 137P | + | + | − | − | − | + | − | − | + | + | + | + | + |
| 137M | + | − | + | + | − | − | − | + | + | − | + | + | + |
| 138N | + | − | + | − | − | + | − | − | + | − | + | + | +++ |
| 138P | − | + | − | + | − | + | − | + | + | − | + | + | ++ |
| 138M | − | + | + | − | − | − | − | + | + | − | + | + | +++ |
| 149M | − | + | − | − | − | − | + | + | + | − | + | + | + |
| 160N | + | − | + | + | − | − | + | − | + | + | + | + | + |
| 160P | − | + | − | + | − | + | + | − | − | + | + | + | + |
| 160M | − | − | + | + | − | − | + | − | − | + | + | + | + |
| 163N | − | + | + | − | − | − | + | − | − | + | + | + | + |
| 163P | + | − | + | − | − | − | + | + | + | + | + | + | ++ |
| 163M | − | − | + | − | − | − | + | + | − | + | + | + | + |
| 167N | + | + | + | − | − | − | + | + | − | + | + | − | + |
| 167M | + | + | + | − | − | − | + | − | + | + | + | − | + |
| 173P | − | + | + | + | − | + | + | + | − | + | + | − | ++ |
| 173M | + | + | + | − | − | − | + | + | + | + | + | + | + |
| 191N | + | − | + | − | − | + | − | + | − | + | + | + | + |
| 191P | − | + | − | − | + | − | + | + | + | + | + | + | ++ |
| 191M | − | − | + | − | − | + | − | − | + | + | + | + | + |
| 192N | − | + | + | + | − | + | + | − | + | + | + | + | ++ |
| 192M | − | + | + | + | + | + | + | + | + | + | + | + | +++ |
| 193N | − | + | + | − | − | − | + | − | + | − | + | + | +++ |
| 193M | + | + | + | − | − | − | + | − | + | + | + | + | +++ |
| Nose n = 14 | Pharynx n = 14 | Mobile Phone n = 19 | Total n = 47 | |
|---|---|---|---|---|
| Toxins | ||||
| sea | 5 (35.7%) | 2 (14.2%) | 7 (36.8%) | 14 (29.8%) |
| seb | 8 (57.0%) | 11 (78.5%) * | 11 (57.8%) | 30 (63.8%) |
| see | 0 | 0 | 0 | 0 |
| etb | 0 | 0 | 0 | 0 |
| tst | 9 (64.2%) * | 5 (35.7%) | 11 (57.8%) * | 25 (53.1%) |
| pvl | 2 (14.2%) | 3 (21.4%) | 4 (21.0%) | 9 (19.1%) |
| Adhesins | ||||
| fnbA | 3 (21.4%) | 6 (42.8%) | 4 (21.0%) | 13 (27.7%) |
| fnbB | 7 (50.0%) | 8 (57.1%) | 7 (36.8%) | 22 (46.8%) |
| cna | 10 (71.4%) | 10 (71.4%) | 13 (68.4%) | 33 (70.2%) |
| clfA | 4 (28.5%) | 7 (50.0%) * | 9 (47.3%) * | 20 (42.6%) |
| clfB | 11 (78.5%) | 11 (78.5%) | 17 (89.4%) | 39 (82.9%) |
| icaA | 6 (42.8%) | 5 (35.7%) | 7 (36.8%) | 18 (38.2%) |
| icaD | 14 (100%) | 14 (100%) | 19 (100%) | 47 (100%) |
| sdrC | 12 (85.7%) | 10 (71.4%) | 16 (84.2%) | 38 (80.8%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamdan-Partida, A.; González-García, S.; Martínez-Ruíz, F.J.; Zavala-Sánchez, M.Á.; Bustos-Hamdan, A.; Bustos-Martínez, J. Molecular Characterization of Staphylococcus aureus Strains Isolated from Mobile Phones. Microorganisms 2022, 10, 669. https://doi.org/10.3390/microorganisms10030669
Hamdan-Partida A, González-García S, Martínez-Ruíz FJ, Zavala-Sánchez MÁ, Bustos-Hamdan A, Bustos-Martínez J. Molecular Characterization of Staphylococcus aureus Strains Isolated from Mobile Phones. Microorganisms. 2022; 10(3):669. https://doi.org/10.3390/microorganisms10030669
Chicago/Turabian StyleHamdan-Partida, Aída, Samuel González-García, Francisco Javier Martínez-Ruíz, Miguel Ángel Zavala-Sánchez, Anaíd Bustos-Hamdan, and Jaime Bustos-Martínez. 2022. "Molecular Characterization of Staphylococcus aureus Strains Isolated from Mobile Phones" Microorganisms 10, no. 3: 669. https://doi.org/10.3390/microorganisms10030669
APA StyleHamdan-Partida, A., González-García, S., Martínez-Ruíz, F. J., Zavala-Sánchez, M. Á., Bustos-Hamdan, A., & Bustos-Martínez, J. (2022). Molecular Characterization of Staphylococcus aureus Strains Isolated from Mobile Phones. Microorganisms, 10(3), 669. https://doi.org/10.3390/microorganisms10030669







