Isolation and Characterization of Commensal Bifidobacteria Strains in Gut Microbiota of Neonates Born Preterm: A Prospective Longitudinal Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Bifidobacteria Strains’ Isolation, Quantification and Identification
2.3. Strain Genotyping
2.4. Acid and Bile Tolerance Assays
2.5. Tetracycline Resistance Characterization
3. Results
3.1. Characteristics of the Infants
3.2. Bifidobacteria Colonization
3.3. Phenotypic Characterization of Bifidobacteria Strains
3.4. Genotypic Characterization and Relationships among Bifidobacteria Strains
3.5. Bifidobacteria Population Dynamics during the First Year of Life
3.6. Comparison of the Bifidobacteria Strains between Twins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mueller, S.; Saunier, K.; Hanisch, C.; Norin, E.; Alm, L.; Midtvedt, T.; Cresci, A.; Silvi, S.; Orpianesi, C.; Verdenelli, M.C.; et al. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: A cross-sectional study. Appl. Environ. Microbiol. 2006, 72, 1027–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penders, J.; Thijs, C.; Vink, C.; Stelma, F.F.; Snijders, B.; Kummeling, I.; van den Brandt, P.A.; Stobberingh, E.E. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 2006, 118, 511–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fijan, S. Microorganisms with claimed probiotic properties: An overview of recent literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767. [Google Scholar] [CrossRef]
- Papizadeh, M.; Rohani, M.; Nahrevanian, H.; Javadi, A.; Pourshafie, M.R. Probiotic characters of Bifidobacterium and Lactobacillus are a result of the ongoing gene acquisition and genome minimization evolutionary trends. Microb. Pathog. 2017, 111, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, L.; Delgado, S.; Ruas-Madiedo, P.; Sanchez, B.; Margolles, A. Bifidobacteria and Their Molecular Communication with the Immune System. Front Microbiol. 2017, 8, 2345. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.R.; Pop, M.; Deboy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic analysis of the human distal gut microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef] [Green Version]
- Manson, J.M.; Rauch, M.; Gilmore, M.S. The commensal microbiology of the gastrointestinal tract. Adv. Exp. Med. Biol. 2008, 635, 15–28. [Google Scholar]
- Shin, A.; Preidis, G.A.; Shulman, R.; Kashyap, P.C. The Gut Microbiome in Adult and Pediatric Functional Gastrointestinal Disorders. Clin. Gastroenterol. Hepatol. 2019, 17, 256–274. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [Green Version]
- Rajilic-Stojanovic, M.; Heilig, H.G.; Molenaar, D.; Kajander, K.; Surakka, A.; Smidt, H.; de Vos, W.M. Development and application of the human intestinal tract chip, a phylogenetic microarray: Analysis of universally conserved phylotypes in the abundant microbiota of young and elderly adults. Environ. Microbiol. 2009, 11, 1736–1751. [Google Scholar] [CrossRef]
- Backhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, C.; Bik, E.M.; DiGiulio, D.B.; Relman, D.A.; Brown, P.O. Development of the human infant intestinal microbiota. PLoS Biol. 2007, 5, e177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butel, M.J.; Waligora-Dupriet, A.J.; Wydau-Dematteis, S. The developing gut microbiota and its consequences for health. J. Dev. Orig. Health Dis. 2018, 9, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Palacio, S.D.; Montes, S.A.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef] [Green Version]
- Neu, J. Developmental aspects of maternal-fetal, and infant gut microbiota and implications for long-term health. Matern. Health Neonatol. Perinatol. 2015, 1, 6. [Google Scholar] [CrossRef] [Green Version]
- Turroni, F.; Milani, C.; Duranti, S.; Lugli, G.A.; Bernasconi, S.; Margolles, A.; di Pierro, F.; van Sinderen, D.; Ventura, M. The infant gut microbiome as a microbial organ influencing host well-being. Ital. J. Pediatr. 2020, 46, 16. [Google Scholar] [CrossRef]
- Dahl, C.; Stigum, H.; Valeur, J.; Iszatt, N.; Lenters, V.; Peddada, S.; Bjornholt, J.V.; Midtvedt, T.; Mandal, S.; Eggesbo, M. Preterm infants have distinct microbiomes not explained by mode of delivery, breastfeeding duration or antibiotic exposure. Int. J. Epidemiol. 2018, 47, 1658–1669. [Google Scholar] [CrossRef]
- Ho, T.T.B.; Groer, M.W.; Kane, B.; Yee, A.L.; Torres, B.A.; Gilbert, J.A.; Maheshwari, A. Dichotomous development of the gut microbiome in preterm infants. Microbiome 2018, 6, 157. [Google Scholar] [CrossRef]
- Korpela, K.; Blakstad, E.W.; Moltu, S.J.; Strommen, K.; Nakstad, B.; Ronnestad, A.E.; Braekke, K.; Iversen, P.O.; Drevon, C.A.; de Vos, W. Intestinal microbiota development and gestational age in preterm neonates. Sci. Rep. 2018, 8, 2453. [Google Scholar] [CrossRef] [Green Version]
- La Rosa, P.S.; Warner, B.B.; Zhou, Y.; Weinstock, G.M.; Sodergren, E.; Hall-Moore, C.M.; Stevens, H.J.; Bennett, W.E., Jr.; Shaikh, N.; Linneman, L.A. Patterned progression of bacterial populations in the premature infant gut. Proc. Natl. Acad. Sci. USA 2014, 111, 12522–12527. [Google Scholar] [CrossRef] [Green Version]
- Ferraris, L.; Butel, M.J.; Campeotto, F.; Vodovar, M.; Roze, J.C.; Aires, J. Clostridia in premature neonates’ gut: Incidence, antibiotic susceptibility, and perinatal determinants influencing colonization. PLoS ONE 2012, 7, e30594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roze, J.C.; Ancel, P.Y.; Marchand-Martin, L.; Rousseau, C.; Montassier, E.; Monot, C.; le Roux, K.; Butin, M.; Resche-Rigon, M.; Aires, J.; et al. Assessment of Neonatal Intensive Care Unit Practices and Preterm Newborn Gut Microbiota and 2-Year Neurodevelopmental Outcomes. JAMA Netw. Open 2020, 3, e2018119. [Google Scholar] [CrossRef] [PubMed]
- Roze, J.C.; Ancel, P.Y.; Lepage, P.; Martin-Marchand, L.; Al Nabhani, Z.; Delannoy, J.; Picaud, J.C.; Lapillonne, A.; Aires, J.; Durox, M. Nutritional strategies and gut microbiota composition as risk factors for necrotizing enterocolitis in very-preterm infants. Am. J. Clin. Nutr. 2017, 106, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Seki, D.; Mayer, M.; Hausmann, B.; Pjevac, P.; Giordano, V.; Goeral, K.; Unterasinger, L.; Klebermass-Schrehof, K.; de Paepe, K.; van de Wiele, T.; et al. Aberrant gut-microbiota-immune-brain axis development in premature neonates with brain damage. Cell Host Microbe 2021, 29, 1558–1572.e6. [Google Scholar] [CrossRef] [PubMed]
- Bommer, C.; Horn, S.; Vollmer, S. The effect of routine probiotics supplementation on preterm newborn health: A regression discontinuity analysis. Am. J. Clin. Nutr. 2020, 112, 1219–1227. [Google Scholar] [CrossRef]
- Poindexter, B.; Committee on Fetus and Newborn. Use of Probiotics in Preterm Infants. Pediatrics 2021, 147, e2021051485. [Google Scholar] [CrossRef]
- Van den Akker, C.H.P.; van Goudoever, J.B.; Shamir, R.; Domellof, M.; Embleton, N.D.; Hojsak, I.; Lapillonne, A.; Mihatsch, W.A.; Canani, R.B.; Bronsky, J.; et al. Probiotics and Preterm Infants: A Position Paper by the European Society for Paediatric Gastroenterology Hepatology and Nutrition Committee on Nutrition and the European Society for Paediatric Gastroenterology Hepatology and Nutrition Working Group for Probiotics and Prebiotics. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 664–680. [Google Scholar]
- Benno, Y.; Sawada, K.; Mitsuoka, T. The intestinal microflora of infants: Composition of fecal flora in breast-fed and bottle-fed infants. Microbiol. Immunol. 1984, 28, 975–986. [Google Scholar] [CrossRef]
- Hopkins, M.J.; Sharp, R.; Macfarlane, G.T. Variation in human intestinal microbiota with age. Dig. Liver Dis. 2002, 34 (Suppl. S2), S12–S18. [Google Scholar] [CrossRef]
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimaraes, V.; Sokol, H.; Dore, J.; Corthier, G.; Furet, J.P. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009, 9, 123. [Google Scholar] [CrossRef]
- Matsuki, T.; Watanabe, K.; Fujimoto, J.; Kado, Y.; Takada, T.; Matsumoto, K.; Tanaka, R. Quantitative PCR with 16S rRNA-gene-targeted species-specific primers for analysis of human intestinal bifidobacteria. Appl. Environ. Microbiol. 2004, 70, 167–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satokari, R.M.; Vaughan, E.E.; Akkermans, A.D.; Saarela, M.; de Vos, W.M. Polymerase chain reaction and denaturing gradient gel electrophoresis monitoring of fecal bifidobacterium populations in a prebiotic and probiotic feeding trial. Syst. Appl. Microbiol. 2001, 24, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Turroni, F.; Foroni, E.; Pizzetti, P.; Giubellini, V.; Ribbera, A.; Merusi, P.; Cagnasso, P.; Bizzarri, B.; de’Angelis, G.L.; Shanahan, F.; et al. Exploring the diversity of the bifidobacterial population in the human intestinal tract. Appl. Environ. Microbiol. 2009, 75, 1534–1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magne, F.; Hachelaf, W.; Suau, A.; Boudraa, G.; Mangin, I.; Touhami, M.; Bouziane-Nedjadi, K.; Pochart, P. A longitudinal study of infant faecal microbiota during weaning. FEMS Microbiol. Ecol. 2006, 58, 563–571. [Google Scholar] [CrossRef] [Green Version]
- Reuter, G. The Lactobacillus and Bifidobacterium microflora of the human intestine: Composition and succession. Curr. Issues Intest. Microbiol. 2001, 2, 43–53. [Google Scholar]
- Van Best, N.; Trepels-Kottek, S.; Savelkoul, P.; Orlikowsky, T.; Hornef, M.W.; Penders, J. Influence of probiotic supplementation on the developing microbiota in human preterm neonates. Gut Microbes 2020, 12, 1–16. [Google Scholar] [CrossRef]
- Westerbeek, E.A.; van den Berg, A.; Lafeber, H.N.; Knol, J.; Fetter, W.P.; van Elburg, R.M. The intestinal bacterial colonisation in preterm infants: A review of the literature. Clin. Nutr. 2006, 25, 361–368. [Google Scholar] [CrossRef]
- Yousuf, E.I.; Carvalho, M.; Dizzell, S.E.; Kim, S.; Gunn, E.; Twiss, J.; Giglia, L.; Stuart, C.; Hutton, E.K.; Morrison, K.M.; et al. Persistence of Suspected Probiotic Organisms in Preterm Infant Gut Microbiota Weeks After Probiotic Supplementation in the NICU. Front. Microbiol. 2020, 11, 574137. [Google Scholar] [CrossRef]
- Ferraris, L.; Aires, J.; Waligora-Dupriet, A.J.; Butel, M.J. New selective medium for selection of bifidobacteria from human feces. Anaerobe 2010, 16, 469–471. [Google Scholar] [CrossRef]
- Hartley, C.L.; Clements, H.M.; Linton, K.B. Escherichia coli in the faecal flora of man. J. Appl. Bacteriol. 1977, 43, 261–269. [Google Scholar] [CrossRef]
- McCartney, A.L.; Wenzhi, W.; Tannock, G.W. Molecular analysis of the composition of the bifidobacterial and lactobacillus microflora of humans. Appl. Environ. Microbiol. 1996, 62, 4608–4613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.; Qu, F.; Zhu, L.H. Isolation of genomic DNAs from plants, fungi and bacteria using benzyl chloride. Nucleic Acids Res. 1993, 21, 5279–5280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andriantsoanirina, V.; Allano, S.; Butel, M.J.; Aires, J. Tolerance of Bifidobacterium human isolates to bile, acid and oxygen. Anaerobe 2013, 21, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.S.; Yang, E.H.; Lee, S.H.; Yeon, S.W.; Kang, B.H.; Kim, T.Y. Rapid identification of potentially probiotic Bifidobacterium species by multiplex PCR using species-specific primers based on the region extending from 16S rRNA through 23S rRNA. FEMS Microbiol. Lett. 2005, 250, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Briczinski, E.P.; Roberts, R.F. Technical note: A rapid pulsed-field gel electrophoresis method for analysis of bifidobacteria. J. Dairy Sci. 2006, 89, 2424–2427. [Google Scholar] [CrossRef] [Green Version]
- Aires, J.; Thouverez, M.; Allano, S.; Butel, M.J. Longitudinal analysis and genotyping of infant dominant bifidobacterial populations. Syst. Appl. Microbiol. 2011, 34, 536–541. [Google Scholar] [CrossRef]
- Briczinski, E.P.; Loquasto, J.R.; Barrangou, R.; Dudley, E.G.; Roberts, A.M.; Roberts, R.F. Strain-specific genotyping of Bifidobacterium animalis subsp. lactis by using single-nucleotide polymorphisms, insertions, and deletions. Appl. Environ. Microbiol. 2009, 75, 7501–7508. [Google Scholar] [CrossRef] [Green Version]
- Aires, J.; Doucet-Populaire, F.; Butel, M.J. Tetracycline resistance mediated by tet(W), tet(M), and tet(O) genes of Bifidobacterium isolates from humans. Appl. Environ. Microbiol. 2007, 73, 2751–2754. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, T.M.; Scott, K.P.; Flint, H.J. Evidence for recent intergeneric transfer of a new tetracycline resistance gene, tet(W), isolated from Butyrivibrio fibrisolvens, and the occurrence of tet(O) in ruminal bacteria. Environ. Microbiol. 1999, 1, 53–64. [Google Scholar] [CrossRef]
- Arboleya, S.; Watkins, C.; Stanton, C.; Ross, R.P. Gut Bifidobacteria Populations in Human Health and Aging. Front. Microbiol. 2016, 7, 1204. [Google Scholar] [CrossRef] [Green Version]
- Turroni, F.; Duranti, S.; Milani, C.; Lugli, G.A.; van Sinderen, D.; Ventura, M. Bifidobacterium bifidum: A Key Member of the Early Human Gut Microbiota. Microorganisms 2019, 7, 544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raeisi, S.N.; Ouoba, L.I.I.; Farahmand, N.; Sutherland, J.; Ghoddus, H.B. Variation, viability and validity of bifidobacteria in fermented milk products. Food Control 2013, 34, 691–697. [Google Scholar] [CrossRef]
- Hill, C.J.; Lynch, D.B.; Murphy, K.; Ulaszewska, M.; Jeffery, I.B.; O’Shea, C.A.; Watkins, C.; Dempsey, E.; Mattivi, F.; Tuohy, K.; et al. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome 2017, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Garruti, G.; Baccetto, R.L.; Molina-Molina, E.; Bonfrate, L.; Wang, D.Q.; Portincasa, P. Bile Acid Physiology. Ann. Hepatol. 2017, 16 (Suppl. S1), S4–S14. [Google Scholar] [CrossRef] [PubMed]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.J. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef]
- Kim, M.J.; Ku, S.; Kim, S.Y.; Lee, H.H.; Jin, H.; Kang, S.; Li, R.; Johnston, T.V.; Park, M.S.; Ji, G.E. Safety Evaluations of Bifidobacterium bifidum BGN4 and Bifidobacterium longum BORI. Int. J. Mol. Sci. 2018, 19, 1422. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Hang, X.; Zhang, M.; Liu, X.; Yang, H. Analysis of newly detected tetracycline resistance genes and their flanking sequences in human intestinal bifidobacteria. Sci. Rep. 2017, 7, 6267. [Google Scholar] [CrossRef]
- Lagier, J.C.; Dubourg, G.; Million, M.; Cadoret, F.; Bilen, M.; Fenollar, F.; Levasseur, A.; Rolain, J.M.; Fournier, P.E.; Raoult, D. Culturing the human microbiota and culturomics. Nat. Rev. Microbiol. 2018, 16, 540–550. [Google Scholar] [CrossRef] [Green Version]
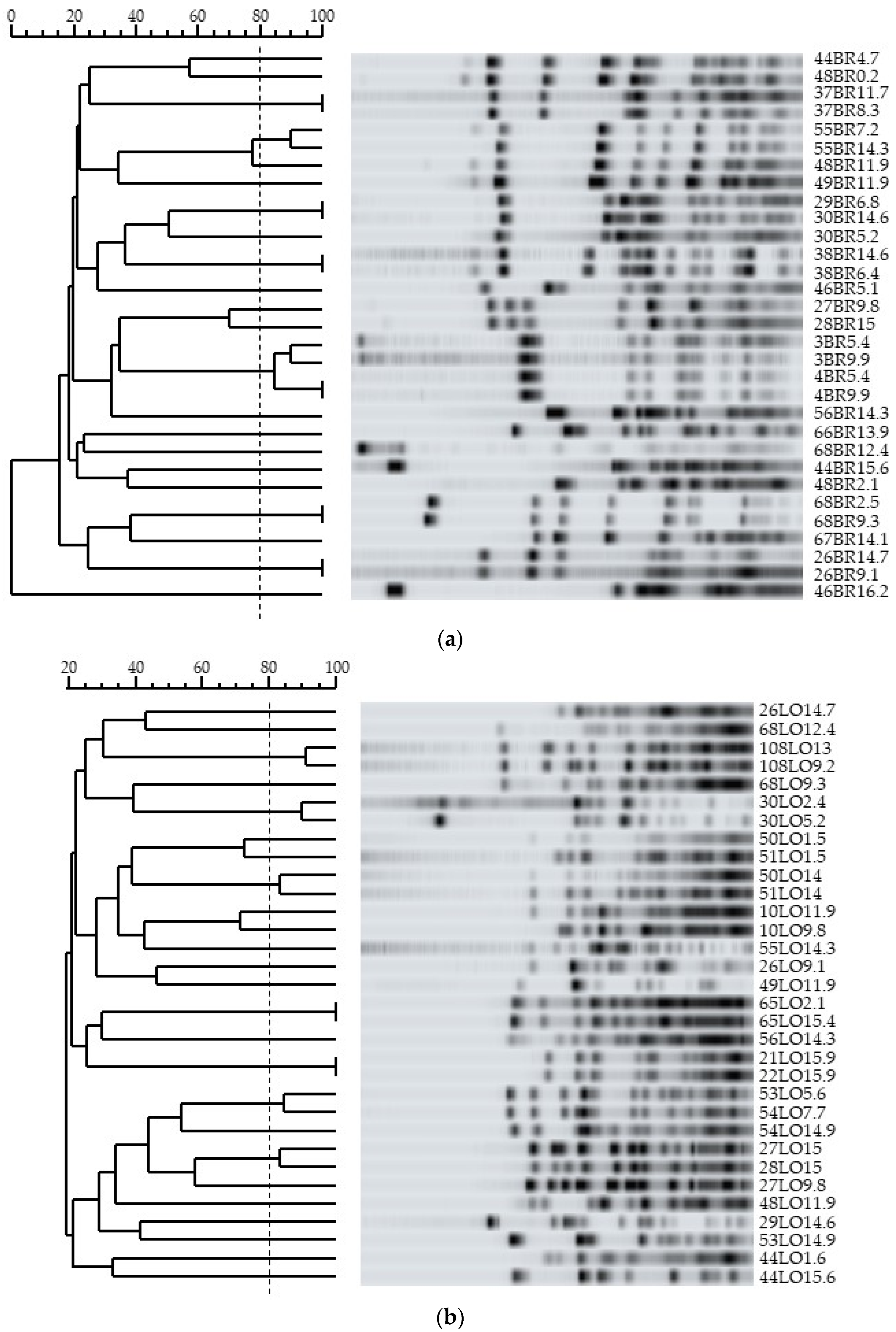
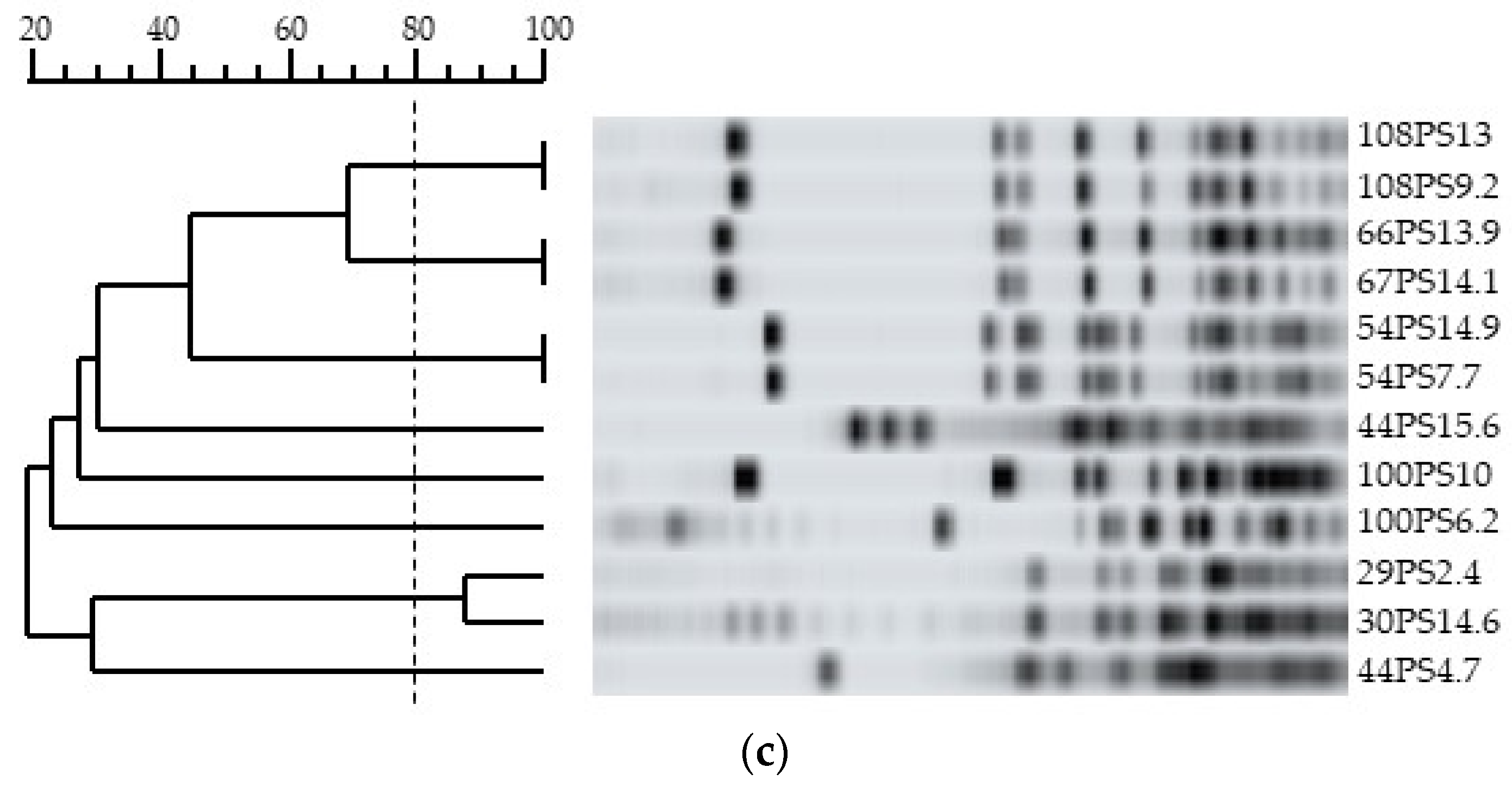

| Infants | Twins | Sampling Time (Months) | Strains | Bifidobacterium Species 1 | Level of Colonization (log10 CFU.g−1 Feces) | PFGE Pattern |
|---|---|---|---|---|---|---|
| 1 | 1; 2 | 5.4 | 3BR5.4 | breve | 9.4 | 1 |
| 9.9 | 3BR9.9 | breve | 9.3 | 1 | ||
| 2 | 1; 2 | 5.4 | 4BR5.4 | breve | 6.5 | 1 |
| 9.9 | 4BR9.9 | breve | 7.6 | 1 | ||
| 3 | 9.8 | 10LO9.8 | longum subsp. longum | 7.0 | 2 | |
| 11.9 | 10LO11.9 | longum subsp. longum | 8.5 | 3 | ||
| 4 | 4; 5 | 15.9 | 21LO15.9 | longum subsp. longum | 6.8 | 4 |
| 5 | 4; 5 | 15.9 | 22LO15.9 | longum subsp. longum | 7.3 | 4 |
| 6 | 9.1 | 26BR9.1 | breve | 9.3 | 5 | |
| 9.1 | 26LO9.1 | longum subsp. longum | 8.6 | 54 | ||
| 14.7 | 26BR14.7 | breve | 7.8 | 5 | ||
| 14.7 | 26LO14.7 | longum subsp. longum | 6.5 | 55 | ||
| 7 | 7; 8 | 9.8 | 27BR9.8 | breve | 7.9 | 6 |
| 9.8 | 27LO9.8 | longum subsp. longum | 8.3 | 7 | ||
| 15 | 27LO15 | longum subsp. longum | 8.0 | 8 | ||
| 8 | 7; 8 | 15 | 28BR15 | breve | 7.8 | 6 |
| 15 | 28LO15 | longum subsp. longum | 9.1 | 8 | ||
| 9 | 9; 10 | 2.4 | 29PS2.4 | pseudocatenulatum | 8.5 | 9 |
| 6.8 | 29BR6.8 | breve | 7.3 | 10 | ||
| 14.6 | 29LO14.6 | longum subsp. longum | 7.6 | 56 | ||
| 10 | 9; 10 | 2.4 | 30LO2.4 | longum subsp. longum | 8.2 | 57 |
| 5.2 | 30LO5.2 | longum subsp. longum | 8.5 | 57 | ||
| 5.2 | 30BR5.2 | breve | 7.9 | 11 | ||
| 14.6 | 30BR14.6 | breve | 8.8 | 10 | ||
| 14.6 | 30PS14.6 | pseudocatenulatum | 6.3 | 9 | ||
| 11 | 8.3 | 37BR8.3 | breve | 8.5 | 12 | |
| 11.1 | 37BR11.1 | breve | 9.4 | 12 | ||
| 12 | 6.4 | 38BR6.4 | breve | 5.3 | 13 | |
| 14.6 | 38BR14.6 | breve | 8.8 | 13 | ||
| 14.6 | 38LA14.6 | animalis subsp. lactis | 7.8 | ND | ||
| 13 | 1.6 | 44LO1.6 | longum subsp. longum | 8 | 14 | |
| 4.7 | 44PS4.7 | pseudocatenulatum | 8.3 | 15 | ||
| 4.7 | 44BI4.7 | bifidum | 8.5 | ND | ||
| 4.7 | 44BR4.7 | breve | 8.7 | 16 | ||
| 15.6 | 44LO15.6 | longum subsp. longum | 6.2 | 18 | ||
| 15.6 | 44PS15.6 | pseudocatenulatum | 5.8 | 19 | ||
| 15.6 | 44BR15.6 | breve | 9.0 | 17 | ||
| 15.6 | 44BI15.6 | bifidum | 7.3 | ND | ||
| 14 | 5.1 | 46BR5.1 | breve | 8.5 | 20 | |
| 16.2 | 46BR16.2 | breve | 3.9 | 21 | ||
| 15 | 15; 16 | 0.2 | 48BR0.2 | breve | 6.6 | 22 |
| 2.1 | 48BR2.1 | breve | 7.2 | 23 | ||
| 11.9 | 48BR11.9 | breve | 8.0 | 24 | ||
| 11.9 | 48LO11.9 | longum subsp. longum | 6.6 | 25 | ||
| 16 | 15; 16 | 11.9 | 49BR11.9 | breve | 8.5 | 24 |
| 11.9 | 49LO11.9 | longum subsp. longum | 8.6 | 26 | ||
| 17 | 17; 18 | 1.5 | 50LO1.5 | longum subsp. longum | 8.6 | 27 |
| 14 | 50LO14 | longum subsp. longum | 8.5 | 28 | ||
| 18 | 17; 18 | 1.5 | 51LO1.5 | longum subsp. longum | 9.0 | 29 |
| 14 | 51LO14 | longum subsp. longum | 8.8 | 28 | ||
| 14 | 51LA14 | animalis subsp. lactis | ND | ND | ||
| 19 | 19; 20 | 5.6 | 53LO5.6 | longum subsp. longum | 7.4 | 30 |
| 14.9 | 53LO14.9 | longum subsp. longum | 8.3 | 31 | ||
| 20 | 19; 20 | 7.7 | 54LO7.7 | longum subsp. longum | 7.6 | 30 |
| 7.7 | 54PS7.7 | pseudocatenulatum | 3.6 | 32 | ||
| 14.9 | 54LO14.9 | longum subsp. longum | 9.3 | 33 | ||
| 14.9 | 54PS14.9 | pseudocatenulatum | 5.6 | 32 | ||
| 21 | 21; 22 | 7.2 | 55BR7.2 | breve | 9.3 | 34 |
| 14.3 | 55BR14.3 | breve | 7.6 | 35 | ||
| 14.3 | 55LO14.3 | longum subsp. longum | 8.6 | 53 | ||
| 22 | 21; 22 | 14.3 | 56BR14.3 | breve | 7.9 | 36 |
| 14.3 | 56LO14.3 | longum subsp. longum | 8.6 | 37 | ||
| 23 | 23; 24 | 10.8 | 57BI10.8 | bifidum | 8.5 | 38 |
| 24 | 23; 24 | 15.3 | 58BI15.3 | bifidum | 8.3 | 39 |
| 25 | 2.1 | 65LO2.1 | longum subsp. longum | 9.3 | 40 | |
| 15.4 | 65LO15.4 | longum subsp. longum | 7.3 | 40 | ||
| 26 | 26; 27 | 2.4 | 66LA2.4 | animalis subsp. lactis | 5.3 | ND |
| 13.9 | 66BR13.9 | breve | 8.7 | 41 | ||
| 13.9 | 66PS13.9 | pseudocatenulatum | 9.0 | 42 | ||
| 27 | 26; 27 | 2,4 | 67LA2.4 | animalis subsp. lactis | 3.3 | ND |
| 14.1 | 67BR14.1 | breve | 8.1 | 43 | ||
| 14.1 | 67PS14.1 | pseudocatenulatum | 8.1 | 42 | ||
| 28 | 2.5 | 68BR2.5 | breve | 9.0 | 44 | |
| 9.3 | 68BR9.3 | breve | 8.8 | 44 | ||
| 9.3 | 68LO9.3 | longum subsp. longum | 7.8 | 45 | ||
| 12.4 | 68BR12.4 | breve | 8.1 | 47 | ||
| 12.4 | 68LO12.4 | longum subsp. longum | 6.5 | 46 | ||
| 29 | 6.2 | 100PS6.2 | pseudocatenulatum | 7.3 | 48 | |
| 10 | 100PS10 | pseudocatenulatum | 9.2 | 49 | ||
| 30 | 30; 31 | 2.2 | 101AD2.2 | adolescentis | 3.6 | 50 |
| 31 | 30; 31 | 2.2 | 102AD2.2 | adolescentis | 7.7 | 50 |
| 32 | 9.2 | 108LO9.2 | longum subsp. longum | 6.9 | 52 | |
| 9.2 | 108PS9.2 | pseudocatenulatum | 8.8 | 51 | ||
| 13 | 108LO13 | longum subsp. longum | 7.8 | 52 | ||
| 13 | 108PS13 | pseudocatenulatum | 9.0 | 51 |
| Intestinal Stress | Gastric Stress | ||||
|---|---|---|---|---|---|
| Infants | Strains | Species | Infants | Strains | Species |
| 2 | 4BR9.9 | breve | 13 | 44LO1.6 | longum subsp. longum |
| 7 | 27LO15 | longum subsp. longum | 13 | 44PS15.6 | pseudocatenulatum |
| 9 | 29LO14.6 | longum subsp. longum | 26 | 66BR13.9 | breve |
| 10 | 30PS14.6 | pseudocatenulatum | 28 | 68BR12.4 | breve |
| 12 | 38BR6.4 | breve | 30 | 101AD2.2 | adolescentis |
| 13 | 44BI4.7 | bifidum | |||
| 13 | 44BI15.6 | bifidum | |||
| 13 | 44BR4.7 | breve | |||
| 20 | 54LO7.7 | longum subsp. longum | |||
| 26 | 66BR13.9 | breve | |||
| 28 | 68LO12.4 | longum subsp. longum | |||
| 28 | 68BR12.4 | breve | |||
| 30 | 101AD2.2 | adolescentis | |||
| Infant | Strains | Species | tet Genes | Tetracycline MIC (mg/L) | Tetracycline Sensitivity | PFGE Pattern |
|---|---|---|---|---|---|---|
| 13 | 44LO1.6 | longum subsp. longum | - | 1 | S | 14 |
| 44PS4.7 | pseudocatenulatum | - | 1.5 | S | 15 | |
| 44BI4.7 | bifidum | - | 1.5 | S | ND | |
| 44BR4.7 | breve | - | 1 | S | 16 | |
| 44LO15.6 | longum subsp. longum | - | 1 | S | 18 | |
| 44PS15.6 | pseudocatenulatum | + | 32 | R | 19 | |
| 44BI15.6 | bifidum | - | 1.5 | S | ND | |
| 44BR15.6 | breve | - | 1.5 | S | 17 | |
| 15 | 48BR0.2 | breve | - | 0.75 | S | 22 |
| 48BR2.1 | breve | + | 16 | R | 23 | |
| 48BR11.9 | breve | - | 1.5 | S | 24 | |
| 48LO11.9 | longum subsp. longum | - | 0.5 | S | 25 | |
| 21 * | 55BR7.2 | breve | + | 16 | R | 34 |
| 55BR14.3 | breve | + | 16 | R | 35 | |
| 55LO14.3 | longum subsp. longum | - | 0.5 | S | 53 | |
| 22 * | 56BR14.3 | breve | + | 16 | R | 35 |
| 56LO14.3 | longum subsp. longum | - | 0.75 | S | 36 | |
| 23 | 57BI10.8 | bifidum | + | 1.5 | S | 37 |
| 28 | 68BR2.5 | breve | + | 24 | R | 43 |
| 68BR9.3 | breve | + | 24 | R | 44 | |
| 68LO9.3 | longum subsp. longum | + | 48 | R | 45 | |
| 68BR12.4 | breve | - | 1.5 | S | 47 | |
| 68LO12.4 | longum subsp. longum | - | 0.5 | S | 46 | |
| 29 | 100PS6.2 | pseudocatenulatum | - | 1.5 | S | 48 |
| 100PS10 | pseudocatenulatum | + | 8 | R | 49 | |
| 32 | 108LO9.2 | longum subsp. longum | + | 8 | R | 52 |
| 108PS9.2 | pseudocatenulatum | - | 1.5 | S | 51 | |
| 108LO13 | longum subsp. longum | + | 12 | R | 52 | |
| 108PS13 | pseudocatenulatum | - | 1 | S | 51 |
| Twins | Sampling Time (Months) | Species | PFGE Pattern | Tetracycline Susceptibility | Intestinal Stress | Gastric Stress |
|---|---|---|---|---|---|---|
| 1 and 2 | 5.4 | breve | 1 | S | NT | NT |
| 9.9 | breve | 1 | S | NT and T | NT | |
| 4 and 5 | 15.9 | longum subsp. longum | 4 | S | NT | NT |
| 7 and 8 | 15 | longum subsp. longum | 8 | S | T and NT | NT |
| 15 and 16 | 11.9 | breve | 24 | S | NT | NT |
| longum subsp. longum | 25 and 26 | S | NT | NT | ||
| 17 and 18 | 1.5 14 | longum subsp. longum longum subsp. longum | 27 and 29 28 | S S | NT NT | NT NT |
| 19 and 20 | 14.9 | longum subsp. longum | 31 and 33 | S | NT | NT |
| 21 and 22 | 14.3 | breve | 35 | R | NT | NT |
| longum subsp. longum | 53 and 36 | S | NT | NT | ||
| 26 and 27 | 2.4 | animalis subsp. lactis | ND | ND | ND | ND |
| 30 and 31 | 2.2 | adolescentis | 50 | S | T and NT | T and NT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wydau-Dematteis, S.; Delannoy, J.; Téolis, A.-C.; Giuseppi, A.; Campeotto, F.; Lapillonne, A.; Butel, M.-J.; Aires, J. Isolation and Characterization of Commensal Bifidobacteria Strains in Gut Microbiota of Neonates Born Preterm: A Prospective Longitudinal Study. Microorganisms 2022, 10, 654. https://doi.org/10.3390/microorganisms10030654
Wydau-Dematteis S, Delannoy J, Téolis A-C, Giuseppi A, Campeotto F, Lapillonne A, Butel M-J, Aires J. Isolation and Characterization of Commensal Bifidobacteria Strains in Gut Microbiota of Neonates Born Preterm: A Prospective Longitudinal Study. Microorganisms. 2022; 10(3):654. https://doi.org/10.3390/microorganisms10030654
Chicago/Turabian StyleWydau-Dematteis, Sandra, Johanne Delannoy, Anne-Claire Téolis, Agnès Giuseppi, Florence Campeotto, Alexandre Lapillonne, Marie-José Butel, and Julio Aires. 2022. "Isolation and Characterization of Commensal Bifidobacteria Strains in Gut Microbiota of Neonates Born Preterm: A Prospective Longitudinal Study" Microorganisms 10, no. 3: 654. https://doi.org/10.3390/microorganisms10030654
APA StyleWydau-Dematteis, S., Delannoy, J., Téolis, A.-C., Giuseppi, A., Campeotto, F., Lapillonne, A., Butel, M.-J., & Aires, J. (2022). Isolation and Characterization of Commensal Bifidobacteria Strains in Gut Microbiota of Neonates Born Preterm: A Prospective Longitudinal Study. Microorganisms, 10(3), 654. https://doi.org/10.3390/microorganisms10030654







