Abstract
Because of the increasing emergence of cutaneous reactions from COVID-19 vaccines worldwide, we investigated the published reports of these complications. We searched the PubMed, Google Scholar, and Scopus databases and the preprint server bioRxiv for articles on cutaneous complications linked to mRNA-1273 (Moderna), BNT162b2 (Pfizer–BioNTech), and AZD1222 (AstraZeneca–Oxford University) vaccines published until 30 September 2021. Eighty studies describing a total of 1415 reactions were included. Cutaneous reactions were more prevalent in females (81.6%). Delayed large local reactions were the most common complication (40.4%), followed by local injection site reactions (16.5%), zoster (9.5%), and urticarial eruptions (9.0%). Injection site and delayed large local reactions were predominantly caused by the mRNA-1273 vaccine (79.5% and 72.0%, respectively). BNT162b2 vaccination was more closely linked to distant reactions (50.1%) than mRNA-1273 (30.0%). Zoster was the most common distant reaction. Of reactions with adequate information for both vaccine doses, 58.3% occurred after the first dose only, 26.9% after the second dose only, and 14.8% after both doses. Overall, a large spectrum of cutaneous reaction patterns occurred following the COVID-19 vaccination. Most were mild and without long-term health implications. Therefore, the occurrence of such dermatologic complications does not contraindicate subsequent vaccination.
1. Introduction
Coronavirus disease 2019 (COVID-19) vaccines are an effective tool in reducing the risk of developing COVID-19 and serious adverse outcomes. Cutaneous complications have been associated with COVID-19 vaccination [1,2]. In a US study, cutaneous adverse effects associated with the first dose of messenger RNA (mRNA) vaccines were reported by 1.9% (95% CI, 1.8–2.1%) of health care employees [3]. Of those, 83% reported no recurrent cutaneous reactions. In a prospective observational study from the UK, first-dose and second-dose skin reactions were observed in only 1.1% and 1.7% of patients, respectively, after BNT162b2 (Pfizer–BioNTech) vaccination [4]. While the cutaneous complications of these vaccines may be reported less frequently, they nonetheless impact public perception regarding vaccine safety. The objective of this systematic review was to assess the dermatologic complications of mRNA-1273 (Moderna; mRNA vaccine), BNT162b2, and AZD1222 (AstraZeneca–Oxford University; adenovirus vector vaccine) vaccination.
2. Methods
2.1. Search Strategy
A search in the PubMed, Google Scholar, and Scopus databases and the preprint server bioRxiv was conducted for articles related to cutaneous complications linked to mRNA COVID-19 vaccines. The search strategy included a combination of key terms: (‘COVID-19’ OR ‘SARS-CoV-2’) AND (‘vaccine’ OR ‘vaccination’) AND (‘skin’ OR ‘cutaneous’) AND (‘rash’ OR ‘reaction’ OR ‘eruption’ OR ‘complication’ OR ‘lesion’ OR ‘flare’ OR ‘delayed’ OR ‘urticaria’ OR ‘morbilliform’ OR ‘herpes zoster’ OR ‘chilblains’ OR ‘eczema’ OR ‘psoriasis’ OR ‘vesicular’ OR ‘bullous’). Abstracts of papers published until September 30, 2021, were reviewed. When an abstract was unavailable, we reviewed the text of the article. A manual search of references cited in the selected articles and published reviews was also used to highlight undetected studies.
2.2. Study Selection
The study selection is detailed in the Supplement (Figure S1). Eligibility assessment was performed independently by two authors (G.K and M.P.). Inclusion criteria were studies published in the English language and reporting cutaneous reactions from COVID-19 vaccines. The exclusion criteria were laboratory cell/animal studies, review/opinion articles, commentaries, consensus papers, editorials, reports missing the vaccine type, studies not focusing on cutaneous reactions, studies with incomplete clinical data (e.g., clinical features and/or course of the eruption), histopathologic studies with inadequate clinical data, and self-reported reactions. We excluded reports of reactions after booster vaccination and those associated with the CoronaVac (SinoVac) vaccine because the number was minimal (insufficient data). Any disagreements in terms of study selection were discussed among the co-authors until a consensus was reached.
2.3. Extraction of Data
We extracted the following data: study design, patient country, vaccine type(s) administered, number of persons vaccinated and the male–female ratio, type(s) of cutaneous reaction(s) observed, total reactions, reactions per vaccine type, number of reactions to first vaccine dose, number of reactions to second vaccine dose, time to onset of reaction after vaccination, time to resolution of reaction, and intervention.
2.4. Quality of Evidence Assessment
Quality rating of the studies was ranked according to the Quality Rating Scheme for Studies and Other Evidence [5] and the Oxford Centre for Evidence-based Medicine for ratings of individual studies [6]. Biases of all included studies were assessed.
3. Results
We summarize the results of 80 studies (1415 reactions; Table 1) of which 4 were registry-based, 1 was a cross-sectional national study, 1 was a retrospective study, 39 were case series, and 35 were case reports. Delayed large local reactions (DLLLs) were the most common complication (40.4%), followed by local injection site reactions (16.5%), zoster (9.5%), urticarial (9.0%), and morbilliform/diffuse erythematous eruptions (6.7%) (Table 2). In a total of 1265 patients, 618 (48.9%) were from Europe (534 from Spain; 42.2%), 592 (46.8%) from USA, 49 (3.9%) from Asia, and 6 (0.5%) from the rest of the World. There was a female predominance (81.6%). Two large studies did not report differences in types of reactions among age groups [1,2]. In these studies, the median age of participants was 44 years (interquartile range, 36–59 years) [1] and the mean age was 50.7 years [2].

Table 1.
Cutaneous reactions to mRNA and AZD1222 COVID-19 vaccines reported in the nontrial literature.

Table 2.
Cutaneous reactions by COVID-19 vaccine type.
Most reactions (55.7%) were associated with mRNA-1273 vaccination (Table 2). Injection site reactions and delayed large local reactions were predominantly caused by the mRNA-1273 vaccine (79.5% and 72.0%, respectively). BNT162b2 vaccination was more closely linked to distant reactions (334/610; 54.8%) than mRNA-1273 (191/610; 31.3%) (Table 2). Of reactions with adequate information for both vaccine doses (n = 1361), 58.3% occurred after the first dose only, 26.9% after the second dose only, and 14.8% after both doses. Potential mechanisms underlying cutaneous reactions are summarized in Table 3.

Table 3.
Suggested pathogenetic mechanisms underlying COVID-19 vaccine-related cutaneous reactions.
3.1. Quality of Evidence Assessment
The rating score of the studies included is shown in Table 1. There were only a small number of registry-based studies and cohorts [1,2,7,18,25,30], and the sample size of some outcomes (cutaneous reactions) in registries/cohorts documenting various outcomes (different types of cutaneous reactions) was small (Table 1). Reporting bias applied, as evidenced by limited data for AZD1222 and the fact that most cutaneous reactions were documented in white persons. Studies performed in health care workers confirmed reporting bias, as healthcare workers are more likely to report their reactions [1,7]. A registry-based study may have included a confirmation bias, as providers are more likely to report cases with severe or rare manifestations [1]. Biases relevant to retrospective observational studies, such as selection and information biases (e.g., short follow-up period; course of the reaction determined mainly based on the patient’s description), also applied.
3.2. Local Site Injection Reaction
Local injection site reaction can be immediate (median of 1 day after first dose) or delayed (median of 7 days after first dose) [1]. Immediate site reaction can manifest with edema/erythema and often pain. DLLL, also called the ‘COVID arm’, occurs at or near the vaccination injection site [1,2]. This reaction was more common with mRNA-1273 than BNT162b2 and AZD1222 vaccination (Table 1), and this concurs with previous studies [1,2]. In the study by Català et al., the ‘COVID arm’ was much more common in females (95.4%) and is more likely to be associated with systemic symptoms (64.6%) than other post-vaccination eruptions [2]. It can manifest as a solitary pink patch or plaque associated with erythema, induration, pruritus, and/or tenderness (Figure 1) [7,8,9,10,11,12,13,14,15,16,17]. Severe reactions with lesion sizes of >10 cm have been reported [9]. Five patients (4.9%) in a cohort presented disseminated lesions [7]. Systemic symptoms such as fever, headache, and chills can be present [1,9].
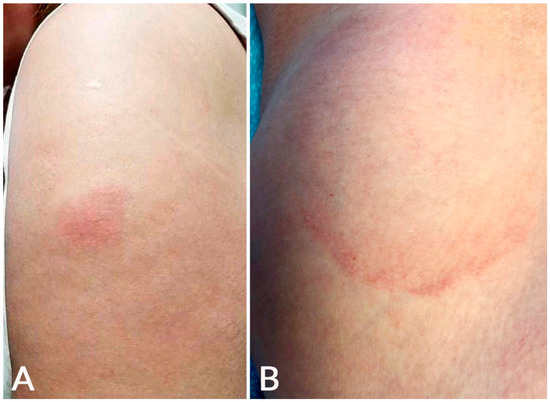
Figure 1.
Local injection site reaction: An erythematous plaque developed at the injection site on the left arm 1 day after BNT162b2 vaccination (A); delayed large, local reaction: an erythematous, annular, mildly tender plaque developed 6 days after mRNA-1273 vaccination at the injection site on the left arm (B).
Second-dose DLLLs generally occur more quickly (median of 2–3 days) [1,9,10,11]. Such reactions were fewer in the AAD/ILDS registry but not in a cohort of 103 COVID arm cases associated with BNT162b2 vaccination (54% of reactions) [1,7]. The duration of second dose DLLLs was longer than first dose examples in most studies [1,9,10]. In the American Academy of Dermatology/International League of Dermatologic Societies (AAD/ILDS) registry, the majority of patients who developed a DLLL after both doses of the mRNA-1273 or BNT162b2 vaccines showed a larger reaction after the second dose [1].
Histopathology of DLLLs showed perivascular lymphocytic infiltrates with eosinophils and scattered mast cells consistent with a delayed T-cell mediated hypersensitivity reaction [9,89]. The presence of prominently dilated vessels with edematous endothelial layers was a consistent feature [13]. ‘COVID arm’ typically resolves within one week of treatment with topical corticosteroid, oral antihistamines, and symptomatic therapy. Many cases have been treated with expectant management [2,11].
3.3. Urticaria
Urticaria can develop as an immediate hypersensitivity reaction, defined by the Centers of Disease Control and Prevention (CDC) as an onset within 4 h after injection, or can occur ≥4 h after injection. The former is a potential contraindication to the second dose. One hundred twenty-eight cases of urticarial eruption were reported in 12 studies [1,2,16,17,18,19,20,21,22,23,24,25], of which 57 (44.5%) were after BNT162b2, 47 (36.7%) after mRNA-1273, and 24 (18.8%) after AZD1222 vaccination (Figure 2). Of these cases, 11 were labeled by the CDC as part of an anaphylaxis reaction and submitted to the Vaccine Adverse Event Reporting System (VAERS) [18]. Several cases of urticaria were associated with angioedema [2]. However, in the AAD/ILDS registry, none of the 40 urticarial reactions were classified as immediate hypersensitivity reactions [1].
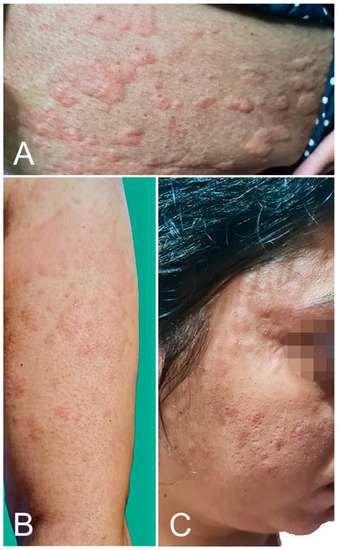
Figure 2.
Urticarial eruption: wheals over the upper limb (A), trunk (B), and face (C) developed 2 h after AZD1222 vaccination.
Anaphylaxis has developed within 150 min post-COVID-19 vaccination. It is uncommon; of 1,893,360 individuals who received the first BNT162b2 vaccine dose, the Food and Drug Administration (FDA) reported 21 patients with an anaphylactic reaction [18]. Of those, 19 were female, 2 were male, and 17 had a history of allergies or allergic reactions. The reaction occurred at a median of 13 min post-vaccination. Of 4,041,396 individuals that received the mRNA-1273 vaccine, 10 females experienced anaphylaxis after the first dose [25]. Nine of 10 patients had a history of atopic disease, and anaphylaxis occurred a median of 7.5 min post-vaccination. All patients were treated with an emergency intramuscular or subcutaneous epinephrine injection [18,25]. Mechanisms of anaphylactic reaction are shown in Table 3 [85].
3.4. Mobilliform Eruption
Twelve studies detailed 95 morbilliform/maculopapular eruptions, of which 53 (55.3%) were after BNT162b2, 31 (33.0%) after mRNA-1273, and 11 (11.7%) after AZD1222 vaccination (Table 1; Figure 3) [1,2,17,18,22,23,24,25,26,27,28,29]. In the AAD/ILDS registry, such eruptions started at a median of 3 days after the first dose and two days after the second dose [1]. In the study by Català et al., such eruptions started at a mean of 4 days after vaccination and lasted a mean of 10.3 days [2]. The authors indicated that morbilliform eruption was the earliest cutaneous reaction pattern that appeared. Half of the morbilliform eruptions were classified as grade 3 (severe) or grade 4 (very severe) in the study. In a series of five patients with morbilliform eruption, three patients had history of atopic dermatitis and one of angioedema [26]. Itching was reported in most patients [2,26].
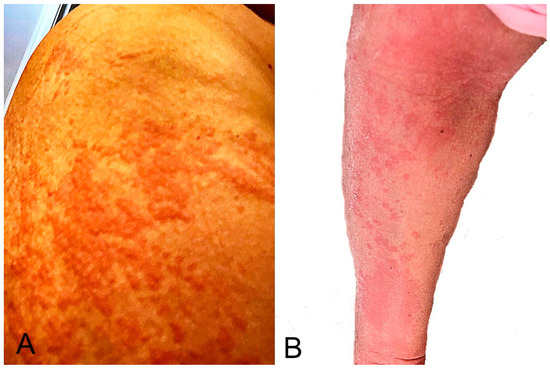
Figure 3.
Morbilliform eruption: it started around the vaccination site on the right upper arm (A) 2 days after the second mRNA-1273 dose and became generalized. Several areas showed maculopapular lesions with desquamation (B). The eruption resolved with a 5-day course of oral prednisone.
Among the cases that were not associated with anaphylaxis, most eruptions developed within 2 to 3 days post-vaccination and resolved within a week. A generalized eruption (>30% of body surface area covered) in one participant that received the BNT162b2 vaccine persisted for more than one month [28]. The patient had no significant past medical history or drug allergy. Histopathology showed lymphocytic perivascular infiltrates consistent with maculopapular eruption. A laboratory investigation showed increased liver enzymes and the second vaccine dose was not provided. Tihy et al. indicated that morbilliform eruptions shared histopathologic similarities with drug eruption [17]. Ohsawa and colleagues demonstrated similarities between the immunohistochemical features of morbilliform eruption in one case and those found in COVID-19-associated skin lesions [86]. When treatment is required, morbilliform eruptions respond to topical/systemic corticosteroids and oral antihistamines.
3.5. Varicella Zoster Virus (VZV) and Herpes Simplex Virus (HSV) Reactivation
There have been 12 reports of VZV or HSV reactivation after vaccination [1,2,30,31,32,33,34,35,36,37,38,39]. Fathy et al. published a series of 40 cases of VZV (n = 35) or HSV reactivation (n = 5) after BNT162b2 or mRNA-1273 vaccination [30]. VZV was reported at a median of 7 days and HSV reactivation at a median of 13 days following vaccination. The median onset of symptoms was 7 days post-vaccination for VZV reactivation and 13 days for HSV reactivation. The median duration of symptoms was 7 days for both groups. Two cases of zoster ophthalmicus were reported [32,33]. In several cases, healthy young individuals developed VZV after BNT162b2 or mRNA-1273 vaccination [31,37].
3.6. Pityriasis Rosea-like Eruption
Pityriasis rosea is a complication that appeared within 22 days and resolved within 12 weeks of vaccination [1,2,16,17,21,23,40,41,42,43,44,45,46,47,48,49,50]. It was associated with BNT162b2 vaccination in most patients (71.9%; 41 of 57 eruptions, Table 1). In a series of 14 patients, the median onset was 14 days after the first vaccine dose and 9 days after the second [40]. In a cross-sectional study, pityriasis rosea-like eruption was the longest-lasting cutaneous reaction pattern [2]. These authors report a similar case (Figure 4). McMahon et al. proposed that the most common histopathologic reaction pattern for pityriasis rosea and other cutaneous reactions was spongiotic dermatitis, which clinically ranged from robust papules with an overlying crust to pink papules with fine scales [89].
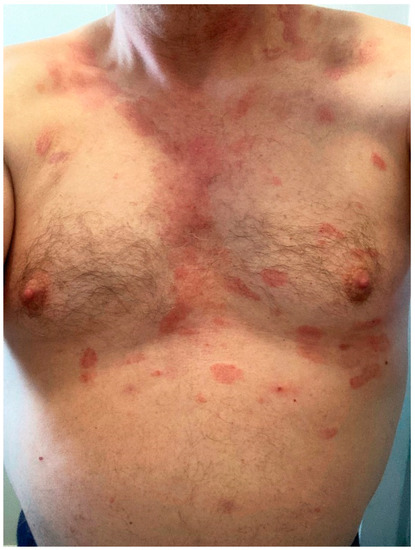
Figure 4.
Pityriasis rosea-like eruption: multiple scaly, pink, or red patches developed 20 days after mRNA-1273 vaccination. Histopathology showed features of pityriasis rosea.
3.7. Pernio, Chilblains, and Purpura
Pernio-like lesions and purpuric eruptions have been reported post-COVID-19 vaccination (Figure 5). Of the 41 cases described in 11 observational studies [1,2,21,23,24,27,51,52,53,54,55,56], 25 (61.0%) were associated with BNT162b2, 9 (22.0%) with ADZ1222, and 7 (17.1%) with mRNA-1273 vaccination (Table 1). In a series of 16 patients, purpuric manifestations appeared at a mean of 7.6 days after COVID-19 vaccination and lasted a mean of 15.7 days [2]. Pernio and purpuric manifestations were delayed-type reactions, as they typically happened days following exposure.
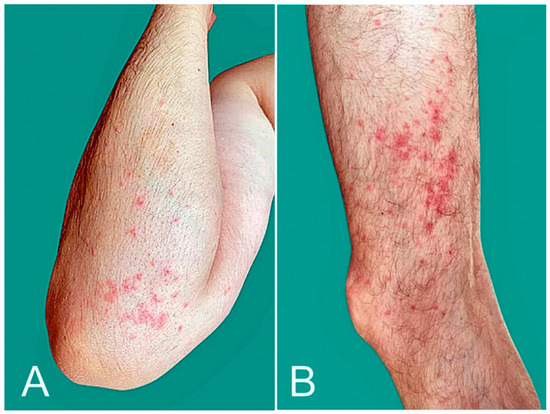
Figure 5.
Purpura: palpable purpuric papules over upper (A) and lower limbs (B) developed 10 days after AZD1222 vaccination.
3.8. Delayed Inflammatory Reaction (DIR) to Dermal Hyaluronic Acid Filler
DIR to hyaluronic acid dermal filler presents clinically as edema with inflammatory, erythematous nodules at the site of prior dermal filler injections. The AAD/ILDS registry reported one DIR after BNT162b2 and eight after mRNA-1273 vaccination [1]. Munavalli and colleagues reported three DIRs after BNT162b2 and four after mRNA-1273 vaccination [57,58]. The reactions occurred within 10 days after vaccination. Marked improvements were noted within 5 days of lisinopril 5–10 mg administration in all patients. In a patient who developed DIR after the first mRNA-1273 dose, preventive lisinopril treatment was successful before the second dose [58]. Angiotensin-converting enzyme 2 inhibitors (ACE-I), such as lisinopril, can block ACE2 receptor targeting by the SARS-CoV-2 spike protein that releases a proinflammatory cascade. This observation may explain the efficacy of lisinopril treatment in the above DIRs. A case was treated with hyaluronidase injection [59]. The American Society for Dermatologic Surgery released guidance in which it was outlined that patients with dermal fillers do not have any contraindication to receiving any COVID-19 vaccine, and that those who already received the vaccine remain candidates for the future receipt of dermal filler [90].
3.9. Unusual Reactions
3.9.1. Papulovesicular Lesions
In a series of 26 patients, the average time to onset was 6.4 days, and lesions lasted an average of 19.3 days [2].
3.9.2. Vesiculobullous Lesions
Vesiculobullous lesions have been reported [1,23]. Some cases showed features of bullous pemphigoid [50,60] or linear IgA dermatosis [60].
3.9.3. Erythromelalgia
Of 14 cases of erythromelalgia, 11 (79%) were associated with the mRNA-1273 vaccine [1].
3.9.4. Eczematous Eruption
A pruritic generalized eczematous eruption was described in three patients within 14 days post-BNT162b2 vaccination [22,61]. Two patients had a history of atopic dermatitis and another dyshidrotic eczema. Cases of localized eczematous dermatitis and hematogenous contact dermatitis have been reported [23,24].
3.9.5. Other Eruptions
Three of five erythema multiforme cases were associated with the mRNA-1273 vaccine [1,62,63]. New onset of prurigo nodularis [17], radiation recall dermatitis [64], symmetrical drug-related intertriginous and flexural exanthema (SDRIFE)-like eruption [65], Stevens-Johnson syndrome/toxic epidermal necrolysis [66,67,68], Sweet’s syndrome [69,70], vitiligo [71], vasculitis [50,72,73], livedo racemosa [27], fixed drug eruption [27], pityriasis rubra pilaris-like eruption [74], and facial pustular neutrophilic eruption [75] have been reported post-vaccination. All SJS and Sweet’s syndrome cases were managed successfully. Lastly, the inflammation of bacillus Calmette-Guérin (BCG) scars developed within 30 h of BNT162b2 or mRNA-1273 vaccination and were resolved within 4 days [76].
3.9.6. Exacerbation of Pre-Existing Skin Condition
Psoriasis vulgaris [23,77,78], generalized pustular psoriasis [79], guttate psoriasis [80], palmoplantar psoriasis [81], and cutaneous lupus [23,82] have flared or developed after vaccination. These authors report a similar case (Figure 6). Atopic dermatitis [23], Darier’s disease [83], and lichen planus [84] can also flare post-vaccination.
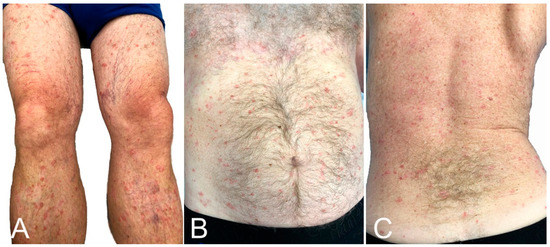
Figure 6.
Psoriasis flare: lesions were limited to legs (A), but became generalized, affecting the thighs, trunk (B,C) and upper extremities 5 days after BNT162b2 vaccination.
4. Discussion
DLLLs were the most common post-vaccination skin complication, followed by local injection site reactions, urticarial eruptions, zoster, and morbilliform eruptions. Most local reactions were associated with the mRNA-1273 vaccine and most distant reactions with BNT162b2. Zoster was the most common distant reaction. To our knowledge, this finding has not been reported. There is considerable geographic variation because most participants in the studies included were from Europe and the USA. Most patients (81.6%) that developed cutaneous reactions were female [1,2,7]. Female predominance was observed not only in US studies that included the health care workforce (consisting of 76% females [91]), which might reflect a reporting bias [1], but in European studies as well [2,7]. Some authors propose that women’s immune systems may be more reactive to coronavirus proteins, leading to a lower susceptibility to the disease and a higher reactogenicity to vaccines [2].
Most reactions were effectively managed with minimal to no long-term morbidity, and the completion of the vaccination course was recommended [1]. Anaphylactic reactions are rare with COVID-19 vaccines [18], and the incidence has been similar to what is noted with other virus-based vaccines [92]. Fatalities were not reported. CDC recommends that vaccination be contraindicated in patients who have had a severe or immediate allergic reaction to the COVID-vaccine or any of its components and that clinicians consider a referral to an allergist-immunologist in such cases [18]. As most people that experienced anaphylaxis had allergy histories [18,25,93], it is very important that clinicians screen for a history of anaphylaxis or angioedema or a proclivity to allergic reactions, e.g., a history of atopy or allergic reactions to vaccine components. Individuals with histories of allergic reactions to one or several of the COVID-19 vaccine ingredients should not receive vaccination [94]. Receiving a different COVID-19 vaccine for the second dose is appropriate for patients with a proclivity to allergy experiencing first-dose reactions. Studies have shown that heterologous prime-boost vaccines are effective and may provide higher immunogenicity than using the same vaccine for booster doses [95].
Some patients experienced reactions to mRNA vaccines, such as pernio/chilblains and erythromelalgia, which mimicked COVID-19 infection [1]. This finding suggests that the vaccine replicates the host immune response to the virus, and some components of such cutaneous reactions result from an immune response to the virus rather than direct viral effects. It is important that clinicians distinguish cutaneous reactions to vaccines from signs of COVID-19 occurring post-vaccination. However, in some cases, the development of COVID-19 after immunization cannot be excluded as a plausible cause of cutaneous reactions. Still, available data suggest that prior COVID-19 does not predetermine cutaneous reactions, or reactions of a greater severity, after vaccination [2].
This review has several limitations. The search for articles was restricted to those written in English. Many of the included studies were case reports and studies with small sample sizes that may confer publication bias. Additionally, most studies included participants from the USA and Europe, and there is a lack of data from other parts of the globe. Also, there are limited data for the AZD1222 vaccine. The above may reflect underreporting and limit the generalizability of the results. The short duration of participant selection, including the follow-up period, in large studies is an additional limitation because providers entered data at one point in time [1] and/or the study was conducted within a short period of time [2,7]. Lastly, most vaccine reactions were documented in white individuals, and this raises concerns about disparities in vaccine access, health care access after experiencing an adverse effect, the differential likelihood of reporting to registries, and/or the recognition of such reactions in patients of color [1].
Dermatologists should contribute to the improved documentation of cutaneous reactions and safety monitoring by reporting their observations to VAERS. Appropriate patient counseling regarding cutaneous reactions to COVID-19 vaccines is crucial and prevents generating concerns disproportionate to potential complications. General practitioners should be aware of such reactions and can play an important role in patient counseling. Lastly, the appropriate identification and management of vaccine reactions often requires a multispecialty approach involving dermatology, allergy, and infectious disease specialists [96].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms10030624/s1, Figure S1. Flow diagram of study selection performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines.
Author Contributions
Conceptualization: M.E.P., G.K. and E.M.; original draft preparation: G.K. and M.E.P.; review and editing: S.Y., S.B., G.K. and E.M.; supervision: E.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available on request from the authors.
Conflicts of Interest
The authors declare no conflict of interest. Eleftherios Mylonakis was involved in clinical trials on COVID-19. These trials were supported by Regeneron, NIH, and SciClone Pharmaceuticals, Inc. All funds were given to the institution, and Eleftherios Mylonakis received no direct funds.
References
- McMahon, D.E.; Amerson, E.; Rosenbach, M.; Lipoff, J.B.; Moustafa, D.; Tyagi, A.; Desai, S.R.; French, L.E.; Lim, H.W.; Thiers, B.H.; et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: A registry-based study of 414 cases. J. Am. Acad. Dermatol. 2021, 85, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Català, A.; Munoz-Santos, C.; Galvan-Casas, C.; Roncero Riesco, M.; Revilla Nebreda, D.; Solá-Truyols, A.; Giavedoni, P.; Llamas-Velasco, M.; González-Cruz, C.; Cubiró, X.; et al. Cutaneous reactions after SARS-CoV-2 vaccination: A cross-sectional Spanish nationwide study of 405 cases. Br. J. Dermatol. 2021. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.B.; Fu, X.; Hashimoto, D.; Wickner, P.; Shenoy, E.S.; Landman, A.B.; Blumenthal, K.G. Incidence of cutaneous reactions after messenger RNA COVID-19 vaccines. JAMA Dermatol. 2021, 157, 1000–1002. [Google Scholar] [CrossRef]
- Menni, C.; Klaser, K.; May, A.; Polidori, L.; Capdevila, J.; Louca, P.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Merino, J.; et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: A prospective observational study. Lancet Infect. Dis. 2021, 21, 939–949. [Google Scholar] [CrossRef]
- JAMA Network Open—Instructions for Authors: Ratings of the Quality of the Evidence. Available online: https://jamanetwork.com/journals/jamanetworkopen/pages/instructions-for-authors#SecRatingsofQuality (accessed on 17 January 2022).
- The Centre for Evidence-Based Medicine. Available online: https://www.cebm.net (accessed on 17 January 2022).
- Fernandez-Nieto, D.; Hammerle, J.; Fernandez-Escribano, M.; Moreno-Del Real, C.M.; Garcia-Abellas, P.; Carretero-Barrio, I.; Solano-Solares, E.; de la Hoz-Caballer, B.; Jimenez-Cauhe, J.; Ortega-Quijano, D.; et al. Skin manifestations of the BNT162b2 mRNA COVID-19 vaccine in healthcare workers. ‘COVID-arm’: A clinical and histological characterization. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e425–e427. [Google Scholar] [CrossRef] [PubMed]
- Juárez Guerrero, A.; Domínguez Estirado, A.; Crespo Quirós, J.; Rojas-Pérez-Ezquerra, P. Delayed cutaneous reactions after the administration of mRNA vaccines against COVID-19. J. Allergy Clin. Immunol. Pract. 2021, 9, 3811–3813. [Google Scholar] [CrossRef]
- Blumenthal, K.G.; Freeman, E.E.; Saff, R.R.; Robinson, L.B.; Wolfson, A.R.; Foreman, R.K.; Hashimoto, D.; Banerji, A.; Li, L.; Anvari, S.; et al. Delayed large local reactions to mRNA-1273 vaccine against SARS-CoV-2. N. Engl. J. Med. 2021, 384, 1273–1277. [Google Scholar] [CrossRef]
- Johnston, M.S.; Galan, A.; Watsky, K.L.; Little, A.J. Delayed localized hypersensitivity reactions to the Moderna COVID-19 vaccine: A case series. JAMA Dermatol. 2021, 157, 716–720. [Google Scholar] [CrossRef]
- Jacobson, M.A.; Zakaria, A.; Maung, Z.; Hart, C.; McCalmont, T.; Fassett, M.; Amerson, E. Incidence and characteristics of delayed injection site reaction to the mRNA-1273 SARS-CoV2 vaccine (Moderna) in a cohort of hospital employees. Clin. Infect. Dis. 2021, 74, 591–596. [Google Scholar] [CrossRef]
- Ramos, C.L.; Kelso, J.M. “COVID Arm”: Very delayed large injection site reactions to mRNA COVID-19 vaccines. J. Allergy Clin. Immunol. Pract. 2021, 9, 2480–2481. [Google Scholar] [CrossRef]
- Hoff, N.P.; Freise, N.F.; Schmidt, A.G.; Firouzi-Memarpuri, P.; Reifenberger, J.; Luedde, T.; Bölke, E.; Meller, S.; Homey, B.; Feldt, T.; et al. Delayed skin reaction after mRNA-1273 vaccine against SARS-CoV-2: A rare clinical reaction. Eur. J. Med. Res. 2021, 26, 98. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Fishman, M.; Wattenberg, D.; Gordon, M.; Lebwohl, M. “COVID arm”: A reaction to the Moderna vaccine. JAAD Case Rep. 2021, 10, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.; Bae, S.; Jung, J.; Song, W.J.; Kwon, H.S.; Kim, H.S.; Kim, S.H.; Kim, T.B.; Cho, Y.S.; Lee, J.H. Delayed local reactions after the first administration of the ChAdOx1 nCoV-19 vaccine. Allergy 2021, 76, 3520–3522. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Liew, C.F.; Oon, H.H. Cutaneous adverse effects and contraindications to COVID-19 vaccination; four cases and an illustrative review from an Asian country. Dermatol. Ther. 2021, 34, e15123. [Google Scholar] [CrossRef]
- Tihy, M.; Menzinger, S.; André, R.; Laffitte, E.; Toutous-Trellu, L.; Kaya, G. Clinicopathological features of cutaneous reactions after mRNA-based COVID-19 vaccines. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 2456–2461. [Google Scholar] [CrossRef]
- CDC COVID-19 Response Team; Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine—United States, December 14–23, 2020. Morb. Mortal. Wkly. Rep. 2021, 70, 46–51. [Google Scholar] [CrossRef]
- Sidlow, J.S.; Reichel, M.; Lowenstein, E.J. Localized and generalized urticarial allergic dermatitis secondary to SARS-CoV-2 vaccination in a series of 6 patients. JAAD Case Rep. 2021, 14, 13–16. [Google Scholar] [CrossRef]
- Kelso, J.M. Misdiagnosis of systemic allergic reactions to mRNA COVID-19 vaccines. Ann. Allergy Asthma Immunol. 2021, 127, 133–134. [Google Scholar] [CrossRef]
- Yu, J.N.; Angeles, C.B.; Lim, H.G.; Chavez, C.; Roxas-Rosete, C. Cutaneous reactions to inactivated SARS-CoV-2 vaccine and ChAdOx1-S (recombinant) vaccine against SARS-CoV-2: A case series from the Philippines. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e841–e845. [Google Scholar] [CrossRef]
- Corbeddu, M.; Diociaiuti, A.; Vinci, M.R.; Santoro, A.; Camisa, V.; Zaffina, S.; El Hachem, M. Transient cutaneous manifestations after administration of Pfizer-BioNTech COVID-19 Vaccine: An Italian single-centre case series. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e483–e485. [Google Scholar] [CrossRef]
- Niebel, D.; Wenzel, J.; Wilsmann-Theis, D.; Ziob, J.; Wilhelmi, J.; Braegelmann, C. Single-center clinico-pathological case study of 19 patients with cutaneous adverse reactions following COVID-19 vaccines. Dermatopathology 2021, 8, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Holmes, G.A.; Desai, M.; Limone, B.; Love, J.; Tawfik, M.; Wong, L.; Furukawa, B. A case series of cutaneous COVID-19 vaccine reactions at Loma Linda University Department of Dermatology. JAAD Case Rep. 2021, 16, 53–57. [Google Scholar] [CrossRef]
- CDC COVID-19 Response Team; Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Moderna COVID-19 vaccine—United States, December 21, 2020–January 10, 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Peigottu, M.F.; Ferreli, C.; Atzori, M.G.; Atzori, L. Skin adverse reactions to novel messenger RNA Coronavirus vaccination: A case series. Diseases 2021, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Annabi, E.; Dupin, N.; Sohier, P.; Garel, B.; Franck, N.; Aractingi, S.; Guégan, S.; Oulès, B. Rare cutaneous adverse effects of COVID-19 vaccines: A case series and review of the literature. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e847–e850. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, M.; Henry, D.; Finon, A.; Binois, R.; Esteve, E. Persistent maculopapular rash after the first dose of Pfizer-BioNTech COVID-19 vaccine. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e423–e425. [Google Scholar] [CrossRef] [PubMed]
- Jedlowski, P.M.; Jedlowski, M.F. Morbilliform rash after administration of Pfizer-BioNTech COVID-19 mRNA vaccine. Dermatol. Online J. 2021, 27, 20. [Google Scholar] [CrossRef]
- Fathy, R.A.; McMahon, D.E.; Lee, C.; Chamberlin, G.C.; Rosenbach, M.; Lipoff, J.B.; Tyagi, A.; Desai, S.R.; French, L.E.; Lim, H.W.; et al. Varicella zoster and herpes simplex virus reactivation post-COVID-19 vaccination: A review of 40 cases in an international dermatology registry. J. Eur. Acad. Dermatol. Venereol. 2021. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Cotter, D.; Basa, J.; Greenberg, H.L. 20 post-COVID-19 vaccine-related shingles cases seen at the Las Vegas Dermatology clinic and sent to us via social media. J. Cosmet. Dermatol. 2021, 20, 1960–1964. [Google Scholar] [CrossRef]
- Psichogiou, M.; Samarkos, M.; Mikos, N.; Hatzakis, A. Reactivation of varicella zoster virus after vaccination for SARS-CoV-2. Vaccines 2021, 9, 572. [Google Scholar] [CrossRef]
- Rodríguez-Jiménez, P.; Chicharro, P.; Cabrera, L.M.; Seguí, M.; Morales-Caballero, Á.; Llamas-Velasco, M.; Sánchez-Pérez, J. Varicella-zoster virus reactivation after SARS-CoV-2 BNT162b2 mRNA vaccination: Report of 5 cases. JAAD Case Rep. 2021, 12, 58–59. [Google Scholar] [CrossRef] [PubMed]
- Furer, V.; Zisman, D.; Kibari, A.; Rimar, D.; Paran, Y.; Elkayam, O. Herpes zoster following BNT162b2 mRNA COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: A case series. Rheumatology 2021, 60, SI90–SI95. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.H.; Wei, K.C.; Chen, A.; Wang, W.H. Herpes zoster following COVID-19 vaccine: A report of three cases. QJM Int. J. Med. 2021, 114, 531–532. [Google Scholar] [CrossRef] [PubMed]
- Alpalhão, M.; Filipe, P. Herpes zoster following SARS-CoV-2 vaccination—A series of four cases. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e750–e752. [Google Scholar] [CrossRef] [PubMed]
- van Dam, C.S.; Lede, I.; Schaar, J.; Al-Dulaimy, M.; Rösken, R.; Smits, M. Herpes zoster after COVID vaccination. Int. J. Infect. Dis. 2021, 111, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Santovito, L.S.; Pinna, G. A case of reactivation of varicella-zoster virus after BNT162b2 vaccine second dose? Inflamm. Res. 2021, 70, 935–937. [Google Scholar] [CrossRef]
- David, E.; Landriscina, A. Herpes zoster following COVID-19 vaccination. J. Drugs Dermatol. 2021, 20, 898–900. [Google Scholar] [CrossRef]
- Temiz, S.A.; Abdelmaksoud, A.; Dursun, R.; Durmaz, K.; Sadoughifar, R.; Hasan, A. Pityriasis rosea following SARS-CoV-2 vaccination: A case series. J. Cosmet. Dermatol. 2021, 20, 3080–3084. [Google Scholar] [CrossRef]
- Cyrenne, B.M.; Al-Mohammedi, F.; DeKoven, J.G.; Alhusayen, R. Pityriasis rosea-like eruptions following vaccination with BNT162b2 mRNA COVID-19 Vaccine. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e546–e548. [Google Scholar] [CrossRef] [PubMed]
- Cohen, O.G.; Clark, A.K.; Milbar, H.; Tarlow, M. Pityriasis rosea after administration of Pfizer-BioNTech COVID-19 vaccine. Hum. Vaccines Immunother. 2021, 17, 4097–4098. [Google Scholar] [CrossRef] [PubMed]
- Adya, K.A.; Inamadar, A.C.; Albadri, W. Post COVID-19 vaccination papulovesicular pityriasis rosea-like eruption in a young male. Dermatol. Ther. 2021, 34, e15040. [Google Scholar] [CrossRef]
- Dormann, H.; Grummt, S.; Karg, M. Pityriasis rosea as a possible complication of vaccination against COVID-19. Dtsch. Arztebl. Int. 2021, 118, 431. [Google Scholar] [CrossRef] [PubMed]
- Busto-Leis, J.; Servera-Negre, G.; Mayor-Ibarguren, A.; Sendagorta-Cudós, E.; Feito-Rodríguez, M.; Nuño-González, A.; Montero-Vega, M.D.; Herranz-Pinto, P. Pityriasis rosea, COVID-19 and vaccination: New keys to understand an old acquaintance. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e489–e491. [Google Scholar] [CrossRef] [PubMed]
- Carballido Vázquez, A.M.; Morgado, B. Pityriasis rosea-like eruption after Pfizer-BioNTech COVID-19 vaccination. Br. J. Dermatol. 2021, 185, e34. [Google Scholar] [CrossRef] [PubMed]
- Leerunyakul, K.; Pakornphadungsit, K.; Suchonwanit, P. Case report: Pityriasis rosea-like eruption following COVID-19 vaccination. Front. Med. 2021, 8, 752443. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, L.; Hasbani, D.; Kurban, M.; Abbas, O. Pityriasis rosea after mRNA COVID-19 vaccination. Int. J. Dermatol. 2021, 60, 1150–1151. [Google Scholar] [CrossRef]
- Bostan, E.; Jarbou, A. Atypical pityriasis rosea associated with mRNA COVID-19 vaccine. J. Med. Virol. 2021. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Larson, V.; Seidenberg, R.; Caplan, A.; Brinster, N.K.; Meehan, S.A.; Kim, R.H. Clinical and histopathological spectrum of delayed adverse cutaneous reactions following COVID-19 vaccination. J. Cutan. Pathol. 2021. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Mazzatenta, C.; Piccolo, V.; Pace, G.; Romano, I.; Argenziano, G.; Bassi, A. Purpuric lesions on the eyelids developed after BNT162b2 mRNA COVID-19 vaccine: Another piece of SARS-CoV-2 skin puzzle? J. Eur. Acad. Dermatol. Venereol. 2021, 35, e543–e545. [Google Scholar] [CrossRef] [PubMed]
- Lopez, S.; Vakharia, P.; Vandergriff, T.; Freeman, E.E.; Vasquez, R. Pernio after COVID-19 vaccination. Br. J. Dermatol. 2021, 185, 445–447. [Google Scholar] [CrossRef] [PubMed]
- Kha, C.; Itkin, A. New-onset chilblains in close temporal association to mRNA-1273 (Moderna) vaccination. JAAD Case Rep. 2021, 12, 12–14. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.W.; Dan, Y.; Wolf, M.E.; Zoccoli, C.M.; Demetriou, T.J.; Lennon, R.P. Post-vaccination COVID toes (chilblains) exacerbated by rituximab infusion suggests interferon activation as mechanism. Mil. Med. 2021. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Cazzato, G.; Romita, P.; Foti, C.; Cimmino, A.; Colagrande, A.; Arezzo, F.; Sablone, S.; Barile, A.; Lettini, T.; Resta, L.; et al. Purpuric skin rash in a patient undergoing Pfizer-BioNTech COVID-19 vaccination: Histological evaluation and perspectives. Vaccines 2021, 9, 760. [Google Scholar] [CrossRef]
- Irvine, N.J.; Wiles, B.L. Petechiae and desquamation of fingers following immunization with BTN162b2 messenger RNA (mRNA) COVID-19 vaccine. Cureus 2021, 13, e16858. [Google Scholar] [CrossRef] [PubMed]
- Munavalli, G.G.; Guthridge, R.; Knutsen-Larson, S.; Brodsky, A.; Matthew, E.; Landau, M. COVID-19/SARS-CoV-2 virus spike protein-related delayed inflammatory reaction to hyaluronic acid dermal fillers: A challenging clinical conundrum in diagnosis and treatment. Arch. Dermatol. Res. 2021, 314, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Munavalli, G.G.; Knutsen-Larson, S.; Lupo, M.P.; Geronemus, R.G. Oral angiotensin-converting enzyme inhibitors for treatment of delayed inflammatory reaction to dermal hyaluronic acid fillers following COVID-19 vaccination-a model for inhibition of angiotensin II-induced cutaneous inflammation. JAAD Case Rep. 2021, 10, 63–68. [Google Scholar] [CrossRef]
- Michon, A. Hyaluronic acid soft tissue filler delayed inflammatory reaction following COVID-19 vaccination—A case report. J. Cosmet. Dermatol. 2021, 20, 2684–2690. [Google Scholar] [CrossRef]
- Coto-Segura, P.; Fernández-Prada, M.; Mir-Bonafé, M.; García-García, B.; González-Iglesias, I.; Alonso-Penanes, P.; González-Guerrero, M.; Gutiérrez-Palacios, A.; Miranda-Martínez, E.; Martinón-Torres, F. Vesiculobullous skin reactions induced by COVID-19 mRNA vaccine: Report of four cases and review of the literature. Clin. Exp. Dermatol. 2021. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Leasure, A.C.; Cowper, S.E.; McNiff, J.; Cohen, J.M. Generalized eczematous reactions to the Pfizer-BioNTech COVID-19 vaccine. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e716–e717. [Google Scholar] [CrossRef]
- Gambichler, T.; Scholl, L.; Dickel, H.; Ocker, L.; Stranzenbach, R. Prompt onset of Rowell’s syndrome following the first BNT162b2 SARS-CoV-2 vaccination. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e415–e416. [Google Scholar] [CrossRef] [PubMed]
- Lavery, M.J.; Nawimana, S.; Parslew, R.; Stewart, L. A flare of pre-existing erythema multiforme following BNT162b2 (Pfizer-BioNTech) COVID-19 vaccine. Clin. Exp. Dermatol. 2021, 46, 1325–1327. [Google Scholar] [CrossRef]
- Soyfer, V.; Gutfeld, O.; Shamai, S.; Schlocker, A.; Merimsky, O. COVID-19 Vaccine-induced radiation recall phenomenon. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 957–961. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.N.; Wylie, G. Symmetrical drug-related intertriginous and flexural exanthema like eruption associated with COVID-19 vaccination. Clin. Exp. Dermatol. 2021. epub ahead of print. [Google Scholar] [CrossRef]
- Dash, S.; Sirka, C.S.; Mishra, S.; Viswan, P. COVID-19 vaccine induced Steven-Johnson syndrome: A case report. Clin. Exp. Dermatol. 2021. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Elboraey, M.O.; Essa, E. Stevens-Johnson syndrome post second dose of Pfizer COVID-19 vaccine: A case report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 132, e139–e142. [Google Scholar] [CrossRef]
- Bakir, M.; Almeshal, H.; Alturki, R.; Obaid, S.; Almazroo, A. Toxic epidermal necrolysis post COVID-19 vaccination—First reported case. Cureus 2021, 13, e17215. [Google Scholar] [CrossRef] [PubMed]
- Majid, I.; Mearaj, S. Sweet syndrome after Oxford-AstraZeneca COVID-19 vaccine (AZD1222) in an elderly female. Dermatol. Ther. 2021. epub ahead of print. [Google Scholar] [CrossRef]
- Torrealba-Acosta, G.; Martin, J.C.; Huttenbach, Y.; Garcia, C.R.; Sohail, M.R.; Agarwal, S.K.; Wasko, C.; Bershad, E.M.; Hirzallah, M.I. Acute encephalitis, myoclonus and Sweet syndrome after mRNA-1273 vaccine. BMJ Case Rep. 2021, 14, e243173. [Google Scholar] [CrossRef]
- Kaminetsky, J.; Rudikoff, D. New-onset vitiligo following mRNA-1273 (Moderna) COVID-19 vaccination. Clin. Case Rep. 2021, 9, e04865. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, S.; Bhatnagar, A.; Kumar, H.; Dixit, P.K.; Paliwal, G.; Suhag, D.K.; Patil, C.; Mitra, D. Leukocytoclastic vasculitis as a cutaneous manifestation of ChAdOx1 nCoV-19 corona virus vaccine (recombinant). Dermatol. Ther. 2021, 34, e15141. [Google Scholar] [CrossRef]
- Mücke, V.T.; Knop, V.; Mücke, M.M.; Ochsendorf, F.; Zeuzem, S. First description of immune complex vasculitis after COVID-19 vaccination with BNT162b2: A case report. BMC Infect. Dis. 2021, 21, 958. [Google Scholar] [CrossRef]
- Hunjan, M.K.; Roberts, C.; Karim, S.; Hague, J. Pityriasis rubra pilaris-like eruption following administration of the BNT163b2 (Pfizer-BioNTech) mRNA COVID-19 vaccine. Clin. Exp. Dermatol. 2021. epub ahead of print. [Google Scholar] [CrossRef]
- Merrill, E.D.; Kashem, S.W.; Amerson, E.H.; Pincus, L.B.; Lang, U.E.; Shinkai, K.; Chang, A.Y. Association of facial pustular neutrophilic eruption with messenger RNA-1273 SARS-CoV-2 vaccine. JAMA Dermatol. 2021, 157, 1128–1130. [Google Scholar] [CrossRef]
- Lopatynsky-Reyes, E.Z.; Acosta-Lazo, H.; Ulloa-Gutierrez, R.; Ávila-Aguero, M.L.; Chacon-Cruz, E. BCG scar local skin inflammation as a novel reaction following mRNA COVID-19 vaccines in two international healthcare workers. Cureus 2021, 13, e14453. [Google Scholar] [CrossRef]
- Bostan, E.; Elmas, L.; Yel, B.; Yalici-Armagan, B. Exacerbation of plaque psoriasis after inactivated and BNT162b2 mRNA COVID-19 vaccines: A report of two cases. Dermatol. Ther. 2021. epub ahead of print. [Google Scholar] [CrossRef]
- Krajewski, P.K.; Matusiak, L.; Szepietowski, J.C. Psoriasis flare-up associated with second dose of Pfizer-BioNTech BNT16B2b2 COVID-19 mRNA vaccine. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e632–e634. [Google Scholar] [CrossRef]
- Perna, D.; Jones, J.; Schadt, C.R. Acute generalized pustular psoriasis exacerbated by the COVID-19 vaccine. JAAD Case Rep. 2021, 17, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, M.; Schorno, P.; Hunger, R.E.; Heidemeyer, K.; Feldmeyer, L.; Yawalkar, N. New onset of mainly guttate psoriasis after COVID-19 vaccination: A case report. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e752–e755. [Google Scholar] [CrossRef] [PubMed]
- Quattrini, L.; Verardi, L.; Caldarola, G.; Peluso, G.; De Simone, C.; D’Agostino, M. New onset of remitting seronegative symmetrical synovitis with. pitting oedema and palmoplantar psoriasis flare-up after SARS-CoV-2 vaccination. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e727–e729. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.K.; Chong, B.F. Subacute cutaneous lupus erythematosus flare triggered by COVID-19 vaccine. Dermatol. Ther. 2021, 34, e15114. [Google Scholar] [CrossRef]
- Elbaek, M.V.; Vinding, G.R.; Jemec, G.B.E. Darier’s disease flare following COVID-19 vaccine. Case Rep. Dermatol. 2021, 13, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Hiltun, I.; Sarriugarte, J.; Martínez-de-Espronceda, I.; Garcés, A.; Llanos, C.; Vives, R.; Yanguas, J.I. Lichen planus arising after COVID-19 vaccination. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e414–e415. [Google Scholar] [CrossRef] [PubMed]
- Risma, K.A.; Edwards, K.M.; Hummell, D.S.; Little, F.F.; Norton, A.E.; Stallings, A.; Wood, R.A.; Milner, J.D. Potential mechanisms of anaphylaxis to COVID-19 mRNA vaccines. J. Allergy Clin. Immunol. 2021, 147, 2075–2082.e2. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, R.; Sano, H.; Ikeda, M.; Sano, S. Clinical and histopathological views of morbilliform rash after COVID-19 mRNA vaccination mimic those in SARS-CoV-2 virus infection-associated cutaneous manifestations. J. Dermatol. Sci. 2021, 103, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Afacan, E.; Ogut, B.; Ustun, P.; Şentürk, E.; Yazıcı, O.; Adışen, E. Radiation recall dermatitis triggered by inactivated COVID-19 vaccine. Clin. Exp. Dermatol. 2021. epub ahead of print. [Google Scholar] [CrossRef]
- Mungmunpuntipantip, R.; Wiwanitkit, V. COVID-19 vaccination and exanthema like eruption. Clin. Exp. Dermatol. 2021. epub ahead of print. [Google Scholar] [CrossRef]
- McMahon, D.E.; Kovarik, C.L.; Damsky, W.; Rosenbach, M.; Lipoff, J.B.; Tyagi, A.; Chamberlin, G.; Fathy, R.; Nazarian, R.M.; Desai, S.R.; et al. Clinical and pathologic correlation of cutaneous COVID-19 vaccine reactions including V-REPP: A registry-based study. J. Am. Acad. Dermatol. 2021. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- ASDS Provides Guidance Regarding SARS-CoV-2 mRNA Vaccine Side Effects. 28 December 2020. Available online: https://www.prweb.com/releases/asds_provides_guidance_regarding_sars_cov_2_mrna_vaccine_side_effects_in_dermal_filler_patients/prweb17636524.htm (accessed on 7 November 2021).
- United States Census Bureau. Your Health Care Is in Women’s Hands. Available online: https://www.census.gov/library/stories/2019/08/your-health-care-in-womens-hands.html (accessed on 15 November 2021).
- Rasmussen, T.H.; Mortz, C.G.; Georgsen, T.K.; Rasmussen, H.M.; Kjaer, H.F.; Bindslev-Jensen, C. Patients with suspected allergic reactions to COVID-19 vaccines can be safely revaccinated after diagnostic work-up. Clin. Transl. Allergy 2021, 11, e12044. [Google Scholar] [CrossRef]
- Blumenthal, K.G.; Robinson, L.B.; Camargo, C.A., Jr.; Shenoy, E.S.; Banerji, A.; Landman, A.B.; Wickner, P. Acute allergic reactions to mRNA COVID-19 vaccines. JAMA 2021, 325, 1562–1565. [Google Scholar] [CrossRef]
- Worm, M.; Bauer, A.; Wedi, B.; Treudler, R.; Pfuetzner, W.; Brockow, K.; Buhl, T.; Zuberbier, T.; Fluhr, J.; Wurpts, G.; et al. Practical recommendations for the allergological risk assessment of the COVID-19 vaccination—A harmonized statement of allergy centers in Germany. Allergol. Sel. 2021, 5, 72–76. [Google Scholar] [CrossRef]
- Liu, X.; Shaw, R.H.; Stuart, A.S.V.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): A single-blind, randomised, non-inferiority trial. Lancet 2021, 398, 856–869. [Google Scholar] [CrossRef]
- Lim, R.K.; Kalagara, S.; Chen, K.K.; Mylonakis, E.; Kroumpouzos, G. Dermatology in a multidisciplinary approach with infectious disease and obstetric medicine against COVID-19. Int. J. Womens Dermatol. 2021. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).