The Importance of Shiga Toxin-Producing Escherichia coli O145:NM[H28]/H28 Infections in Argentina, 1998–2020
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Bacterial Isolates
2.3. Phenotypic Characterization
2.4. Sequencing and Analysis
2.5. Statistical Methods
3. Results
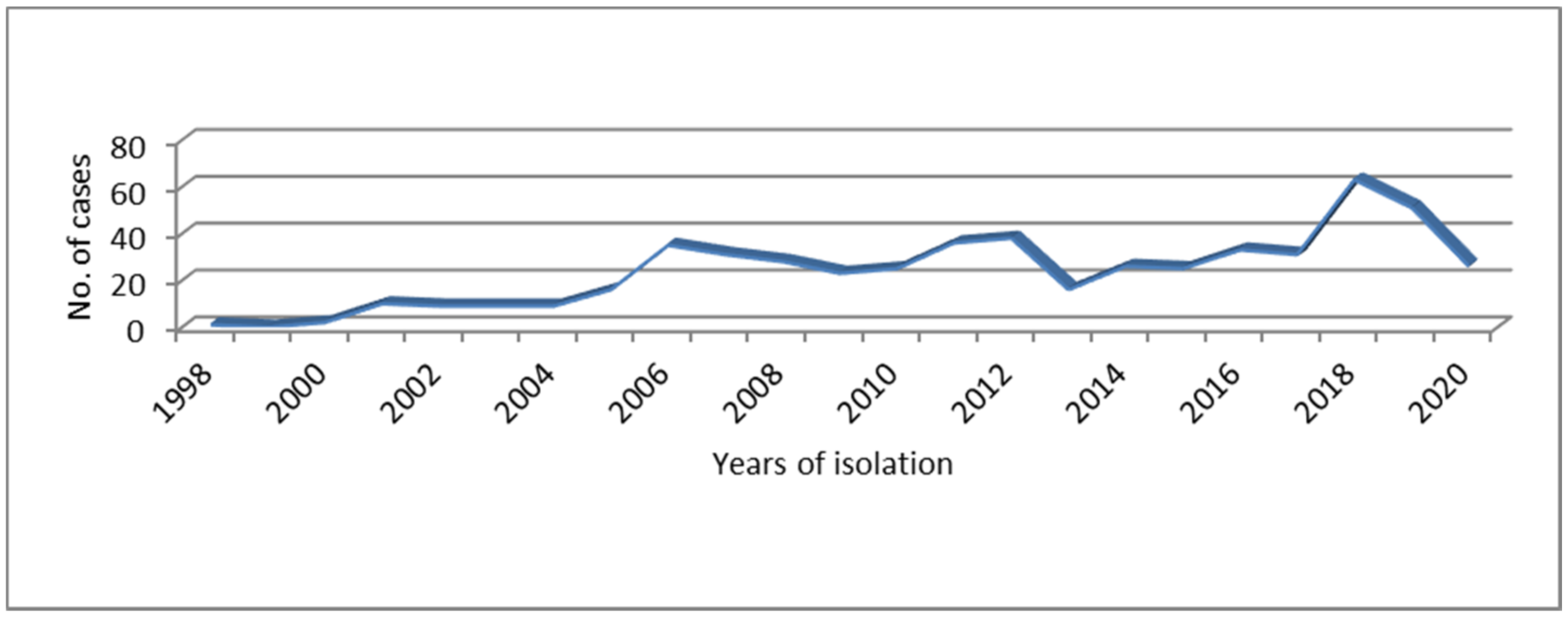
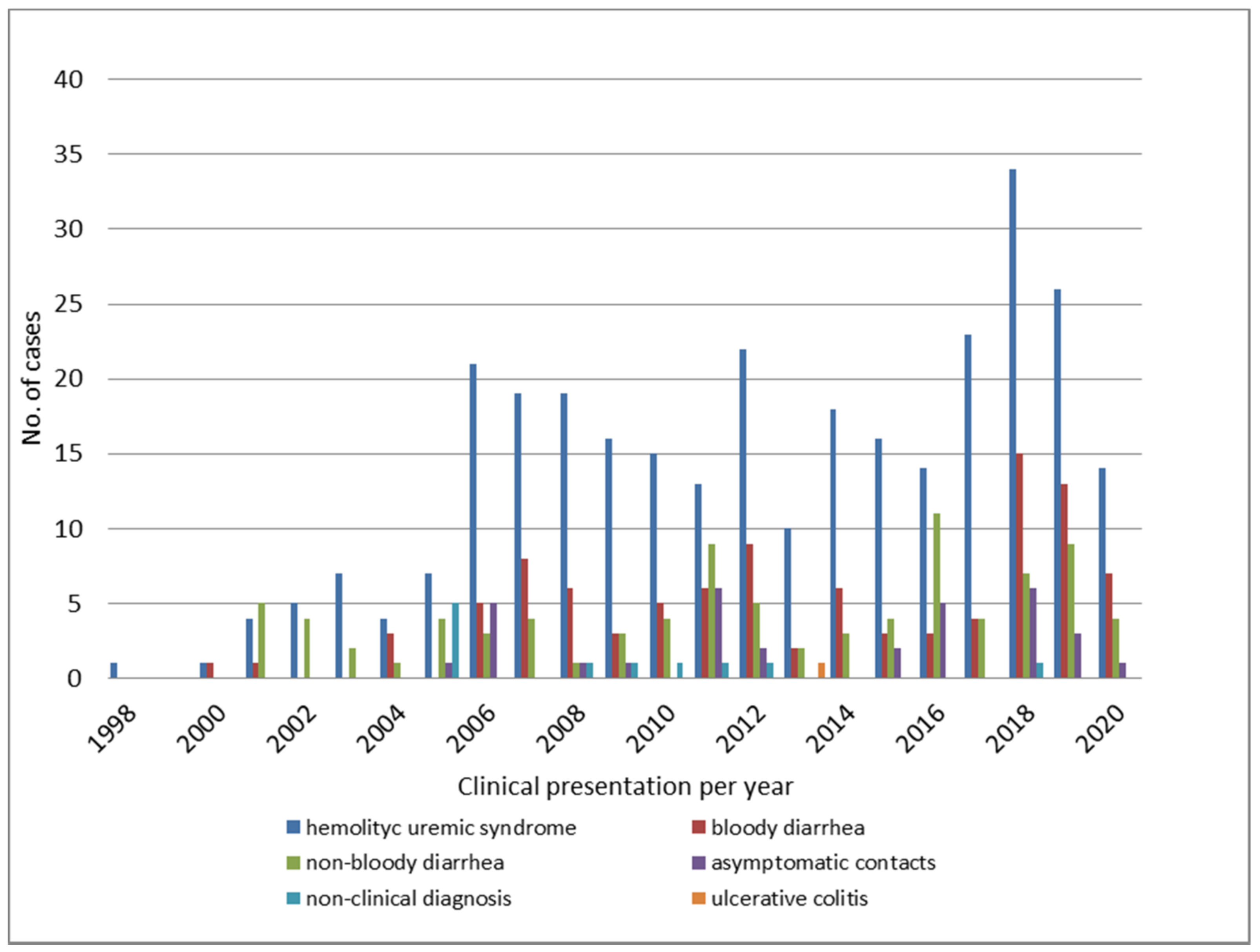
3.1. Epidemiological Features of STEC O145 Infections in Argentina
3.2. Phenotypic and Genotypic Characterization of STEC O145 in Argentina
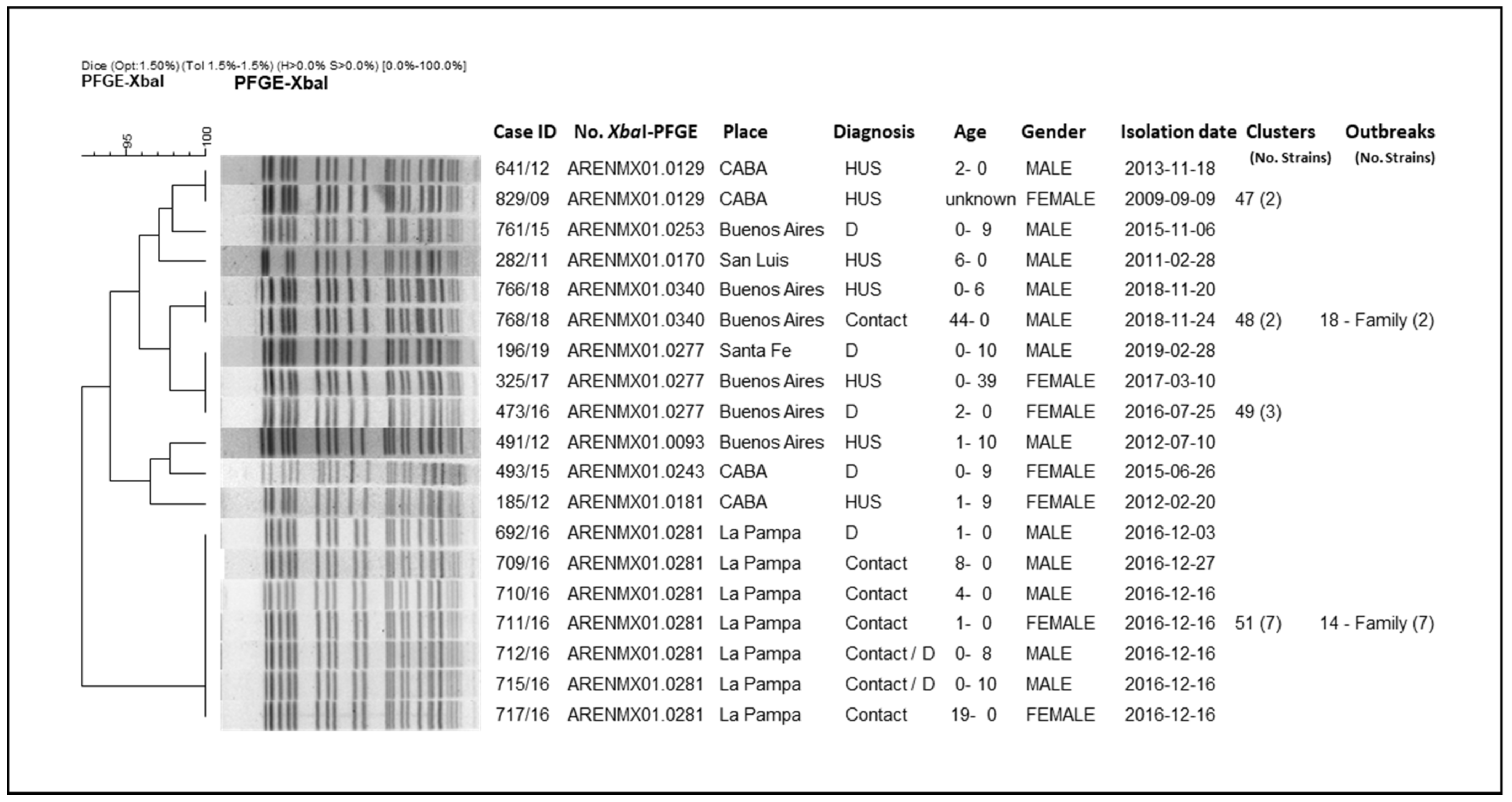
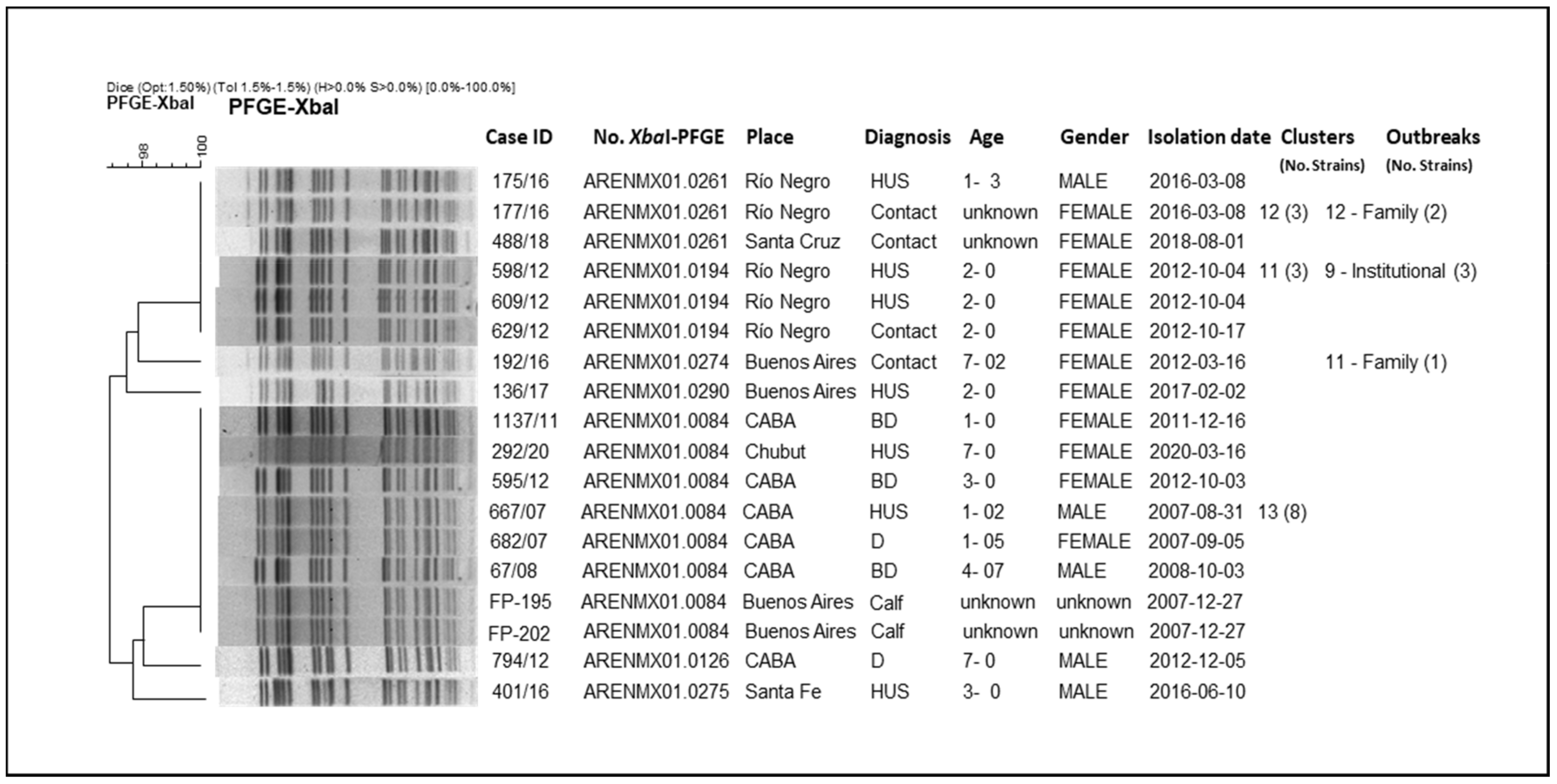
3.3. Molecular Epidemiology of Argentinian STEC O145
3.4. Sequencing and Genomic Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riley, L.W.; Remis, R.S.; Helgerson, S.D.; McGee, H.B.; Wells, J.G.; Davis, B.R.; Hebert, R.J.; Olcott, E.S.; Johnson, L.M.; Hargrett, N.T.; et al. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N. Engl. J. Med. 1983, 308, 681–685. [Google Scholar] [CrossRef]
- Tarr, P.I.; Gordon, C.A.; Chandler, W.L. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet Lond. Engl. 2005, 365, 1073–1086. [Google Scholar] [CrossRef]
- Caprioli, A.; Morabito, S.; Brugère, H.; Oswald, E. Enterohaemorrhagic Escherichia coli: Emerging issues on virulence and modes of transmission. Vet. Res. 2005, 36, 289–311. [Google Scholar] [CrossRef]
- Gyles, C.L. Shiga toxin-producing Escherichia coli: An overview. J. Anim. Sci. 2007, 85, E45–E62. [Google Scholar] [CrossRef]
- Blanco, J.E.; Blanco, M.; Alonso, M.P.; Mora, A.; Dahbi, G.; Coira, M.A.; Blanco, J. Serotypes, virulence genes, and intimin types of Shiga toxin (verotoxin)-producing Escherichia coli isolates from human patients: Prevalence in Lugo, Spain, from 1992 through 1999. J. Clin. Microbiol. 2004, 42, 311–319. [Google Scholar] [CrossRef]
- Sonntag, A.-K.; Prager, R.; Bielaszewska, M.; Zhang, W.; Fruth, A.; Tschäpe, H.; Karch, H. Phenotypic and genotypic analyses of enterohemorrhagic Escherichia coli O145 strains from patients in Germany. J. Clin. Microbiol. 2004, 42, 954–962. [Google Scholar] [CrossRef][Green Version]
- Banco de Recursos de Comunicación del Ministerio de Salud de la Nación|Boletín integrado de vigilancia N560 SE 30/2021. Available online: https://bancos.salud.gob.ar/recurso/boletin-integrado-de-vigilancia-n560-se-302021 (accessed on 2 October 2021).
- Rivas, M.; Miliwebsky, E.; Chinen, I.; Roldán, C.D.; Balbi, L.; García, B.; Fiorilli, G.; Sosa-Estani, S.; Kincaid, J.; Rangel, J.; et al. Characterization and epidemiologic subtyping of Shiga toxin-producing Escherichia coli strains isolated from hemolytic uremic syndrome and diarrhea cases in Argentina. Foodborne Pathog. Dis. 2006, 3, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Rivas, M.; Chinen, I.; Miliwebsky, E.; Masana, M. Risk Factors for Shiga Toxin-Producing Escherichia coli-Associated Human Diseases. Microbiol. Spectr. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Carlos, G. Malbrán. Manual de Procedimientos. In Diagnóstico y Caracterización de Escherichia Coli Productor de Toxina Shiga O157 y no O157 a Partir de Especímenes Clínicos; INEI-ANLIS: Buenos Aires, Argentina, 2019. [Google Scholar]
- CLSI: Clinical And Laboratory Standards Institute. Available online: https://webstore.ansi.org/sdo/clsi?gclid=CjwKCAjwhuCKBhADEiwA1HegOSWEmXu_Wr6gory5DOf1zQ8G3rvIQrycbFPF1pMQOWO7zwxCswFT7hoC5XkQAvD_BwE (accessed on 2 October 2021).
- Leotta, G.A.; Chinen, I.; Epszteyn, S.; Miliwebsky, E.; Melamed, I.C.; Motter, M.; Ferrer, M.; Marey, E.; Rivas, M. Validation of a multiplex PCR for detection of Shiga toxin-producing Escherichia coli. Rev. Argent. Microbiol. 2005, 37, 1–10. [Google Scholar]
- Fratamico, P.M.; DebRoy, C.; Miyamoto, T.; Liu, Y. PCR detection of enterohemorrhagic Escherichia coli O145 in food by targeting genes in the E. coli O145 O-antigen gene cluster and the shiga toxin 1 and shiga toxin 2 genes. Foodborne Pathog. Dis. 2009, 6, 605–611. [Google Scholar] [CrossRef]
- Bugarel, M.; Beutin, L.; Martin, A.; Gill, A.; Fach, P. Micro-array for the identification of Shiga toxin-producing Escherichia coli (STEC) seropathotypes associated with Hemorrhagic Colitis and Hemolytic Uremic Syndrome in humans. Int. J. Food Microbiol. 2010, 142, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Karch, H.; Böhm, H.; Schmidt, H.; Gunzer, F.; Aleksic, S.; Heesemann, J. Clonal structure and pathogenicity of Shiga-like toxin-producing, sorbitol-fermenting Escherichia coli O157:H-. J. Clin. Microbiol. 1993, 31, 1200–1205. [Google Scholar] [CrossRef]
- Schmidt, H.; Beutin, L.; Karch, H. Molecular analysis of the plasmid-encoded hemolysin of Escherichia coli O157:H7 strain EDL 933. Infect. Immun. 1995, 63, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Toma, C.; Martínez Espinosa, E.; Song, T.; Miliwebsky, E.; Chinen, I.; Iyoda, S.; Iwanaga, M.; Rivas, M. Distribution of putative adhesins in different seropathotypes of Shiga toxin-producing Escherichia coli. J. Clin. Microbiol. 2004, 42, 4937–4946. [Google Scholar] [CrossRef]
- Zhang, W.; Bielaszewska, M.; Kuczius, T.; Karch, H. Identification, characterization, and distribution of a Shiga toxin 1 gene variant (stx(1c)) in Escherichia coli strains isolated from humans. J. Clin. Microbiol. 2002, 40, 1441–1446. [Google Scholar] [CrossRef] [PubMed]
- Tyler, S.D.; Johnson, W.M.; Lior, H.; Wang, G.; Rozee, K.R. Identification of verotoxin type 2 variant B subunit genes in Escherichia coli by the polymerase chain reaction and restriction fragment length polymorphism analysis. J. Clin. Microbiol. 1991, 29, 1339–1343. [Google Scholar] [CrossRef]
- Scheutz, F.; Teel, L.D.; Beutin, L.; Piérard, D.; Buvens, G.; Karch, H.; Mellmann, A.; Caprioli, A.; Tozzoli, R.; Morabito, S.; et al. Multicenter evaluation of a sequence-based protocol for subtyping Shiga toxins and standardizing Stx nomenclature. J. Clin. Microbiol. 2012, 50, 2951–2963. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, V.; Brett, K.; Hornitzky, M.A.; Dowton, M.; Bettelheim, K.A.; Walker, M.J.; Djordjevic, S.P. Distribution of intimin subtypes among Escherichia coli isolates from ruminant and human sources. J. Clin. Microbiol. 2003, 41, 5022–5032. [Google Scholar] [CrossRef]
- CDC. Centers for Disease Control and Prevention. One-Day (24–48 h) Standardized Laboratory Protocol for Molecular Subtyping of Escherichia coli non-O157:H7 by Pulsed Field Gel Electrophoresis; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2010. [Google Scholar]
- Babraham Bioinformatics—FastQC A Quality Control tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 30 September 2021).
- Tritt, A.; Eisen, J.A.; Facciotti, M.T.; Darling, A.E. An integrated pipeline for de novo assembly of microbial genomes. PLoS ONE 2012, 7, e42304. [Google Scholar]
- Cooper, K.K.; Mandrell, R.E.; Louie, J.W.; Korlach, J.; Clark, T.A.; Parker, C.T.; Huynh, S.; Chain, P.S.; Ahmed, S.; Carter, M.Q. Comparative genomics of enterohemorrhagic Escherichia coli O145:H28 demonstrates a common evolutionary lineage with Escherichia coli O157:H7. BMC Genom. 2014, 15, 17. [Google Scholar] [CrossRef]
- Darling, A.C.E.; Mau, B.; Blattner, F.R.; Perna, N.T. Mauve: Multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 2004, 14, 1394–1403. [Google Scholar] [CrossRef]
- Klimke, W.; Agarwala, R.; Badretdin, A.; Chetvernin, S.; Ciufo, S.; Fedorov, B.; Kiryutin, B.; O’Neill, K.; Resch, W.; Resenchuk, S.; et al. The National Center for Biotechnology Information’s Protein Clusters Database. Nucleic Acids Res. 2009, 37, D216–D223. [Google Scholar] [CrossRef]
- Joensen, K.G.; Tetzschner, A.M.M.; Iguchi, A.; Aarestrup, F.M.; Scheutz, F. Rapid and Easy In Silico Serotyping of Escherichia coli Isolates by Use of Whole-Genome Sequencing Data. J. Clin. Microbiol. 2015, 53, 2410–2426. [Google Scholar] [CrossRef] [PubMed]
- Joensen, K.G.; Scheutz, F.; Lund, O.; Hasman, H.; Kaas, R.S.; Nielsen, E.M.; Aarestrup, F.M. Real-time whole-genome sequencing for routine typing, surveillance, and outbreak detection of verotoxigenic Escherichia coli. J. Clin. Microbiol. 2014, 52, 1501–1510. [Google Scholar] [CrossRef]
- Malberg Tetzschner, A.M.; Johnson, J.R.; Johnston, B.D.; Lund, O.; Scheutz, F. In Silico Genotyping of Escherichia coli Isolates for Extraintestinal Virulence Genes by Use of Whole-Genome Sequencing Data. J. Clin. Microbiol. 2020, 58, e01269–e20. [Google Scholar] [CrossRef]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F.; et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef] [PubMed]
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Carattoli, A.; Zankari, E.; García-Fernández, A.; Voldby Larsen, M.; Lund, O.; Villa, L.; Møller Aarestrup, F.; Hasman, H. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef]
- Larsen, M.V.; Cosentino, S.; Rasmussen, S.; Friis, C.; Hasman, H.; Marvig, R.L.; Jelsbak, L.; Sicheritz-Pontén, T.; Ussery, D.W.; Aarestrup, F.M.; et al. Multilocus sequence typing of total-genome-sequenced bacteria. J. Clin. Microbiol. 2012, 50, 1355–1361. [Google Scholar] [CrossRef]
- Arndt, D.; Grant, J.R.; Marcu, A.; Sajed, T.; Pon, A.; Liang, Y.; Wishart, D.S. PHASTER: A better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016, 44, W16–W21. [Google Scholar] [CrossRef]
- Brooks, J.T.; Sowers, E.G.; Wells, J.G.; Greene, K.D.; Griffin, P.M.; Hoekstra, R.M.; Strockbine, N.A. Non-O157 Shiga toxin-producing Escherichia coli infections in the United States, 1983–2002. J. Infect. Dis. 2005, 192, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Mathusa, E.C.; Chen, Y.; Enache, E.; Hontz, L. Non-O157 Shiga toxin-producing Escherichia coli in foods. J. Food Prot. 2010, 73, 1721–1736. [Google Scholar] [CrossRef]
- Bielaszewska, M.; Mellmann, A.; Bletz, S.; Zhang, W.; Köck, R.; Kossow, A.; Prager, R.; Fruth, A.; Orth-Höller, D.; Marejková, M.; et al. Enterohemorrhagic Escherichia coli O26:H11/H-: A new virulent clone emerges in Europe. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2013, 56, 1373–1381. [Google Scholar] [CrossRef]
- Martina Bielaszewska; Helge Karch Non-O157:H7 Shiga toxin (verocytotoxin)-producing Escherichia coli strains: Epidemiological signi®cance and microbiological diagnosis. World J. Microbiol. Biotechnol. 2000, 16, 711–718. [CrossRef]
- Banco de Recursos de Comunicación del Ministerio de Salud de la Nación|Boletín integrado de vigilancia N329 SE39-11/10/2016. Available online: https://bancos.salud.gob.ar/recurso/boletin-integrado-de-vigilancia-n329-se39-11102016 (accessed on 2 October 2021).
- Oderiz, S.; Leotta, G.A.; Galli, L. Detection and characterization of Shiga toxin-producing Escherichia coli in children treated at an inter-zonal pediatric hospital in the city of La Plata. Rev. Argent. Microbiol. 2018, 50, 341–350. [Google Scholar] [PubMed]
- Paton, J.C.; Paton, A.W. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin. Microbiol. Rev. 1998, 11, 450–479. [Google Scholar] [CrossRef]
- De Schrijver, K.; Buvens, G.; Possé, B.; Van den Branden, D.; Oosterlynck, O.; De Zutter, L.; Eilers, K.; Piérard, D.; Dierick, K.; Van Damme-Lombaerts, R.; et al. Outbreak of verocytotoxin-producing E. coli O145 and O26 infections associated with the consumption of ice cream produced at a farm, Belgium, 2007. Euro Surveill. Bull. Eur. Sur Mal. Transm. Eur. Commun. Dis. Bull. 2008, 13, 8041. [Google Scholar] [CrossRef]
- Wahl, E.; Vold, L.; Lindstedt, B.A.; Bruheim, T.; Afset, J.E. Investigation of an Escherichia coli O145 outbreak in a child day-care centre--extensive sampling and characterization of eae- and stx1-positive E. coli yields epidemiological and socioeconomic insight. BMC Infect. Dis. 2011, 11, 238. [Google Scholar] [CrossRef]
- Outbreak of Human E. coli O145 Infections Linked to Shredded Romaine Lettuce|E. coli CDC. 21 May 2010. Available online: https://www.cdc.gov/ecoli/2010/shredded-romaine-5-21-10.html (accessed on 2 October 2021).
- Multistate Outbreak of Shiga Toxin-producing Escherichia coli O145 Infections (Final Update)|Multistate Outbreak of Shiga Toxin-producing Escherichia coli O145 Infections|E. coli|CDC. Available online: https://www.cdc.gov/ecoli/2012/o145-06-12/index.html (accessed on 2 October 2021).
- Miliwebsky, E.; Deza, N.; Chinen, I.; Martinez Espinosa, E.; Gomez, D.; Pedroni, E.; Caprile, L.; Bashckier, A.; Manfredi, E.; Leotta, G.; et al. Prolonged fecal shedding of Shiga toxin-producing Escherichia coli among children attending day-care centers in Argentina. Rev. Argent. Microbiol. 2007, 39, 90–92. [Google Scholar] [PubMed]
- Karch, H.; Rüssmann, H.; Schmidt, H.; Schwarzkopf, A.; Heesemann, J. Long-term shedding and clonal turnover of enterohemorrhagic Escherichia coli O157 in diarrheal diseases. J. Clin. Microbiol. 1995, 33, 1602–1605. [Google Scholar] [CrossRef]
- Masana, M.O.; D’Astek, B.A.; Palladino, P.M.; Galli, L.; Del Castillo, L.L.; Carbonari, C.; Leotta, G.A.; Vilacoba, E.; Irino, K.; Rivas, M. Genotypic characterization of non-O157 Shiga toxin-producing Escherichia coli in beef abattoirs of Argentina. J. Food Prot. 2011, 74, 2008–2017. [Google Scholar] [CrossRef]
- Meichtri, L.; Miliwebsky, E.; Gioffré, A.; Chinen, I.; Baschkier, A.; Chillemi, G.; Guth, B.E.C.; Masana, M.O.; Cataldi, A.; Rodríguez, H.R.; et al. Shiga toxin-producing Escherichia coli in healthy young beef steers from Argentina: Prevalence and virulence properties. Int. J. Food Microbiol. 2004, 96, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Brusa, V.; Restovich, V.; Galli, L.; Teitelbaum, D.; Signorini, M.; Brasesco, H.; Londero, A.; García, D.; Padola, N.L.; Superno, V.; et al. Isolation and characterization of non-O157 Shiga toxin-producing Escherichia coli from beef carcasses, cuts and trimmings of abattoirs in Argentina. PLoS ONE 2017, 12, e0183248. [Google Scholar]
- Cap, M.; Carbonari, C.C.; D’Astek, B.A.; Zolezzi, G.; Deza, N.; Palladino, M.P.; Masana, M.; Chinen, I.; Rivas, M. Frequency, characterization and genotypic analysis of Shiga toxin-producing Escherichia coli in beef slaughterhouses of Argentina. Rev. Argent. Microbiol. 2019, 51, 32–38. [Google Scholar] [CrossRef]
- Rounds, J.M.; Rigdon, C.E.; Muhl, L.J.; Forstner, M.; Danzeisen, G.T.; Koziol, B.S.; Taylor, C.; Shaw, B.T.; Short, G.L.; Smith, K.E. Non-O157 Shiga toxin-producing Escherichia coli associated with venison. Emerg. Infect. Dis. 2012, 18, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, S.C.; Gonzalez-Escalona, N.; Kotewicz, M.L.; Fischer, M.; Kase, J.A. Genome sequencing and comparative genomics of enterohemorrhagic Escherichia coli O145:H25 and O145:H28 reveal distinct evolutionary paths and marked variations in traits associated with virulence & colonization. BMC Microbiol. 2017, 17, 183. [Google Scholar]
- Beutin, L.; Delannoy, S.; Fach, P. Sequence Variations in the Flagellar Antigen Genes fliCH25 and fliCH28 of Escherichia coli and Their Use in Identification and Characterization of Enterohemorrhagic E. coli (EHEC) O145:H25 and O145:H28. PLoS ONE 2015, 10, e0126749. [Google Scholar]
- Mellmann, A.; Bielaszewska, M.; Köck, R.; Friedrich, A.W.; Fruth, A.; Middendorf, B.; Harmsen, D.; Schmidt, M.A.; Karch, H. Analysis of collection of hemolytic uremic syndrome-associated enterohemorrhagic Escherichia coli. Emerg. Infect. Dis. 2008, 14, 1287–1290. [Google Scholar] [CrossRef]
- Gould, L.H.; Mody, R.K.; Ong, K.L.; Clogher, P.; Cronquist, A.B.; Garman, K.N.; Lathrop, S.; Medus, C.; Spina, N.L.; Webb, T.H.; et al. Increased recognition of non-O157 Shiga toxin-producing Escherichia coli infections in the United States during 2000–2010: Epidemiologic features and comparison with E. coli O157 infections. Foodborne Pathog. Dis. 2013, 10, 453–460. [Google Scholar] [CrossRef]
- Cavalcanti, A.M.F.; Hernandes, R.T.; Takagi, E.H.; Guth, B.E.C.; de Lima Ori, É.; Pinheiro, S.R.S.; de Andrade, T.S.; Oliveira, S.L.; Cergole-Novella, M.C.; Francisco, G.R.; et al. Virulence Profiling and Molecular Typing of Shiga Toxin-Producing E. coli (STEC) from Human Sources in Brazil. Microorganisms 2020, 8, 171. [Google Scholar] [CrossRef]
- Torres, A.G.; Blanco, M.; Valenzuela, P.; Slater, T.M.; Patel, S.D.; Dahbi, G.; López, C.; Barriga, X.F.; Blanco, J.E.; Gomes, T.A.T.; et al. Genes related to long polar fimbriae of pathogenic Escherichia coli strains as reliable markers to identify virulent isolates. J. Clin. Microbiol. 2009, 47, 2442–2451. [Google Scholar] [CrossRef]
- Farfan, M.J.; Torres, A.G. Molecular mechanisms that mediate colonization of Shiga toxin-producing Escherichia coli strains. Infect. Immun. 2012, 80, 903–913. [Google Scholar] [CrossRef]
- Cernicchiaro, N.; Cull, C.A.; Paddock, Z.D.; Shi, X.; Bai, J.; Nagaraja, T.G.; Renter, D.G. Prevalence of Shiga toxin-producing Escherichia coli and associated virulence genes in feces of commercial feedlot cattle. Foodborne Pathog. Dis. 2013, 10, 835–841. [Google Scholar] [CrossRef]
- Carter, M.Q.; Quinones, B.; He, X.; Zhong, W.; Louie, J.W.; Lee, B.G.; Yambao, J.C.; Mandrell, R.E.; Cooley, M.B. An Environmental Shiga Toxin-Producing Escherichia coli O145 Clonal Population Exhibits High-Level Phenotypic Variation That Includes Virulence Traits. Appl. Environ. Microbiol. 2016, 82, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Gentle, A.; Day, M.R.; Hopkins, K.L.; Godbole, G.; Jenkins, C. Antimicrobial resistance in Shiga toxin-producing Escherichia coli other than serotype O157:H7 in England, 2014-2016. J. Med. Microbiol. 2020, 69, 379–386. [Google Scholar] [CrossRef]
- Taylor, E.V.; Nguyen, T.A.; Machesky, K.D.; Koch, E.; Sotir, M.J.; Bohm, S.R.; Folster, J.P.; Bokanyi, R.; Kupper, A.; Bidol, S.A.; et al. Multistate outbreak of Escherichia coli O145 infections associated with romaine lettuce consumption, 2010. J. Food Prot. 2013, 76, 939–944. [Google Scholar] [CrossRef]
- Beier, R.C.; Franz, E.; Bono, J.L.; Mandrell, R.E.; Fratamico, P.M.; Callaway, T.R.; Andrews, K.; Poole, T.L.; Crippen, T.L.; Sheffield, C.L.; et al. Disinfectant and Antimicrobial Susceptibility Profiles of the Big Six Non-O157 Shiga Toxin-Producing Escherichia coli Strains from Food Animals and Humans. J. Food Prot. 2016, 79, 1355–1370. [Google Scholar] [CrossRef]
- Vali, L.; Hamouda, A.; Hoyle, D.V.; Pearce, M.C.; Whitaker, L.H.R.; Jenkins, C.; Knight, H.I.; Smith, A.W.; Amyes, S.G.B. Antibiotic resistance and molecular epidemiology of Escherichia coli O26, O103 and O145 shed by two cohorts of Scottish beef cattle. J. Antimicrob. Chemother. 2007, 59, 403–410. [Google Scholar] [CrossRef] [PubMed]
- WHO Integrated Global Surveillance on ESBL-Producing E. coli Using a “One Health” Approach. Available online: https://www.who.int/publications-detail-redirect/who-integrated-global-surveillance-on-esbl-producing-e.-coli-using-a-one-health-approach (accessed on 2 October 2021).
- Bai, X.; Hu, B.; Xu, Y.; Sun, H.; Zhao, A.; Ba, P.; Fu, S.; Fan, R.; Jin, Y.; Wang, H.; et al. Molecular and Phylogenetic Characterization of Non-O157 Shiga Toxin-Producing Escherichia coli Strains in China. Front. Cell. Infect. Microbiol. 2016, 6, 143. [Google Scholar] [CrossRef] [PubMed]
- D’Astek, B.A.; del Castillo, L.L.; Miliwebsky, E.; Carbonari, C.; Palladino, P.M.; Deza, N.; Chinen, I.; Manfredi, E.; Leotta, G.A.; Masana, M.O.; et al. Subtyping of Escherichia coli O157:H7 strains isolated from human infections and healthy cattle in Argentina. Foodborne Pathog. Dis. 2012, 9, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Shridhar, P.B.; Worley, J.N.; Gao, X.; Yang, X.; Noll, L.W.; Shi, X.; Bai, J.; Meng, J.; Nagaraja, T.G. Analysis of virulence potential of Escherichia coli O145 isolated from cattle feces and hide samples based on whole genome sequencing. PLoS ONE 2019, 14, e0225057. [Google Scholar] [CrossRef] [PubMed]
| # | Resistance Profile * | Origin | No Strains |
|---|---|---|---|
| 1 | AMP | 3HUS/2BD | 5 |
| 2 | AMP/CHL/S/TE | HUS | 1 |
| 3 | AMP/S | HUS | 2 |
| 4 | AMP/S/TE | HUS/unknown | 2 |
| 5 | AMP/S/TE/TMS | HUS | 5 |
| 6 | AMP/S/TMS | 3HUS/2BD | 7 |
| 7 | AMP/TE/TMS | fatal HUS | 1 |
| 8 | A/TMS | HUS/D | 2 |
| 9 | CHL/S/TE/TMS | HUS | 1 |
| 10 | CHL/TE/TMS | 2HUS/D | 3 |
| 11 | G/S/TE | HUS | 1 |
| 12 | NA/T | HUS/BD | 2 |
| 13 | S/TE | HUS/BD | 3 |
| 14 | S/TE/TMS | unknown | 1 |
| 15 | S | HUS | 1 |
| 16 | TE | 5HUS/BD/4D | 10 |
| 17 | TE/TMS | HUS/BD/D | 3 |
| Isolate No. | Origin | Place-Province/ Year of Isolation | NCBI Accession No. | No. of Scaffolds | Genome Size (bp) | N50 | G+C Content (%) |
|---|---|---|---|---|---|---|---|
| GN1228 | Homo sapiens | Santa Fe/2002 | JAJBBW000000000 | 123 | 5,239,290 | 173,877 | 50 |
| GN1229 | Homo sapiens | City of Buenos Aires/2006 | JAJBBV000000000 | 144 | 5,345,892 | 158,581 | 50 |
| GN1230 | Homo sapiens | Buenos Aires/2007 | JAJBBU000000000 | 156 | 5,251,985 | 160,369 | 50 |
| GN1231 | Homo sapiens | Neuquén/2007 | JAJBBT000000000 | 127 | 5,376,704 | 152,017 | 50 |
| GN1232 | Homo sapiens | Rio Negro/2008 | JAJBBS000000000 | 142 | 5,259,376 | 201,633 | 50 |
| Virulence Genes | Product | STEC O145 Strains 1228 1229 1230 1231 1232 | ||||
|---|---|---|---|---|---|---|
| Shiga Toxins | ||||||
| stx1a | Shiga toxin 1 subtype a | 1 | ||||
| stx2a | Shiga toxin 2 subtype a | 1 | 1 | 1 | 1 | |
| Adhesins | ||||||
| eae | Intimin | 1 | 1 | 1 | 1 | 1 |
| Iha | IrgA homologue adhesin | 1 | 1 | 1 | 1 | 1 |
| LEE encoded Type III secretory system proteins | ||||||
| Tir | Translocated intimin receptor | 1 | 1 | 1 | 1 | |
| espA | EPEC secreted protein A | 1 | 1 | 1 | 1 | 1 |
| espB | EPEC secreted protein B | 1 | 1 | 1 | 1 | 1 |
| Non-LEE encoded effector proteins | ||||||
| nleA | Non-LEE encoded effector protein A | 1 | 1 | 1 | 1 | 1 |
| nleB | Non-LEE encoded effector protein B | 1 | 1 | 1 | 1 | 1 |
| nleC | Non-LEE encoded effector protein C | 1 | 1 | 1 | 1 | |
| Phage encoded type III secretory system proteins | ||||||
| espI | E. coli-secreted protein I | 1 | 1 | 1 | 1 | 1 |
| espJ | E. coli-secreted protein J | 1 | 1 | 1 | 1 | 1 |
| Cif | Cell-cycle inhibiting factor | 1 | 1 | 1 | 1 | 1 |
| Plasmid encoded virulence factors | ||||||
| ehxA | Enterohemolysin | 1 | 1 | 1 | 1 | |
| katP | Catalase peroxidase | 1 | 1 | 1 | 1 | |
| espP | Extracellular serine protease | 1 | 1 | 1 | 1 | |
| iroN | Enterobactin siderophore receptor protein | 1 | ||||
| iucC | Aerobactin synthetase | 1 | 1 | 1 | 1 | 1 |
| iutA | Ferric aerobactin receptor | 1 | 1 | 1 | 1 | 1 |
| cma | Colicin M | 1 | ||||
| cvaC | Colicin V (Microcin) | 1 | ||||
| Antimicrobial resistance genes | ||||||
| mdfA | Multidrug transporter | 1 | 1 | 1 | 1 | 1 |
| aadA1 | Aminoglycoside resistance | 1 | ||||
| dfrA1 | Trimethoprim resistance | 1 | ||||
| pmrB | Colistin resistance | 1 | 1 | |||
| tetA | Tetracycline resistance | 1 | ||||
| sul1 | Sulphonamide resistance | 1 | ||||
| sul2 | Sulphonamide resistance | 1 | ||||
| blaTEM-1B | Beta-lactam resistance | 1 | ||||
| Others | ||||||
| Iss | Increased serum survival | 1 | 1 | 1 | 1 | 1 |
| astA | EAST-1 heat-stable toxin | 1 | 1 | 1 | 1 | 1 |
| terC | Tellurium ion resistance protein | 1 | 1 | 1 | 1 | 1 |
| toxB | Toxin B | 1 | 1 | 1 | ||
| chuA | Outer membrane hemin receptor | 1 | 1 | 1 | 1 | 1 |
| ompT | Outer membrane protease | 1 | 1 | 1 | 1 | 1 |
| traT | Outer membrane protein complement resistance | 1 | 1 | 1 | 1 | 1 |
| mchF | ABC transporter protein MchF | 1 | ||||
| neuC | Polysialic acid capsule biosynthesis protein | 1 | 1 | 1 | 1 | 1 |
| sitA | Iron transport protein | 1 | ||||
| hlyF | Hemolysin F | 1 | ||||
| Prophages | Type of Sequences | STEC O145 Strains | ||||||
|---|---|---|---|---|---|---|---|---|
| Intact | Incomplete | Questionable | 1228 | 1229 | 1230 | 1231 | 1232 | |
| Entero_YYZ_2008_NC_011356 | x x | x | x | |||||
| Stx2_c_1717_NC_011357 | x | x | ||||||
| Entero_DE3_NC_042057 | x x x x | x | x | x | x | |||
| Yersin_L_413C_NC_004745 | x x x | x | x | x | x | x | ||
| Vibrio_12B8_NC_021073 | x | x | ||||||
| Entero_phi92_NC_023693 | x x x | x | x | x | ||||
| Entero_VT2phi_272_NC_028656 | x x x | x | x | x | ||||
| Entero_mEp460_NC_019716 | x x | x | x | |||||
| Escher_P13374_NC_018846 | x | x | ||||||
| Entero_JSE_NC_012740 | x | x | ||||||
| Entero_WPhi_NC_005056 | x | x | ||||||
| Entero_P2_NC_001895 | x | x | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbonari, C.C.; Miliwebsky, E.S.; Zolezzi, G.; Deza, N.L.; Fittipaldi, N.; Manfredi, E.; Baschkier, A.; D’Astek, B.A.; Melano, R.G.; Schesi, C.; et al. The Importance of Shiga Toxin-Producing Escherichia coli O145:NM[H28]/H28 Infections in Argentina, 1998–2020. Microorganisms 2022, 10, 582. https://doi.org/10.3390/microorganisms10030582
Carbonari CC, Miliwebsky ES, Zolezzi G, Deza NL, Fittipaldi N, Manfredi E, Baschkier A, D’Astek BA, Melano RG, Schesi C, et al. The Importance of Shiga Toxin-Producing Escherichia coli O145:NM[H28]/H28 Infections in Argentina, 1998–2020. Microorganisms. 2022; 10(3):582. https://doi.org/10.3390/microorganisms10030582
Chicago/Turabian StyleCarbonari, Claudia Carolina, Elizabeth Sandra Miliwebsky, Gisela Zolezzi, Natalia Lorena Deza, Nahuel Fittipaldi, Eduardo Manfredi, Ariela Baschkier, Beatriz Alejandra D’Astek, Roberto Gustavo Melano, Carla Schesi, and et al. 2022. "The Importance of Shiga Toxin-Producing Escherichia coli O145:NM[H28]/H28 Infections in Argentina, 1998–2020" Microorganisms 10, no. 3: 582. https://doi.org/10.3390/microorganisms10030582
APA StyleCarbonari, C. C., Miliwebsky, E. S., Zolezzi, G., Deza, N. L., Fittipaldi, N., Manfredi, E., Baschkier, A., D’Astek, B. A., Melano, R. G., Schesi, C., Rivas, M., & Chinen, I. (2022). The Importance of Shiga Toxin-Producing Escherichia coli O145:NM[H28]/H28 Infections in Argentina, 1998–2020. Microorganisms, 10(3), 582. https://doi.org/10.3390/microorganisms10030582






