An Evaluation of Aluminum Tolerant Pseudomonas aeruginosa A7 for In Vivo Suppression of Fusarium Wilt of Chickpea Caused by Fusarium oxysporum f. sp. ciceris and Growth Promotion of Chickpea
Abstract
1. Introduction
2. Materials and Methods
2.1. The Isolation, Morphological and Biochemical Characterization of Aluminum-Tolerant Bacteria from the Rhizoids of Polytrichum sp.
2.2. Molecular Identification of the Strain
2.3. In Vitro Antagonistic Activity of the Strain A7
2.4. Plant Growth-Promoting (PGP) Activities of the Strain A7
2.5. Growth Profile of Strain A7 on Different Concentrations of Aluminum, Iron, and Other Stressed Conditions
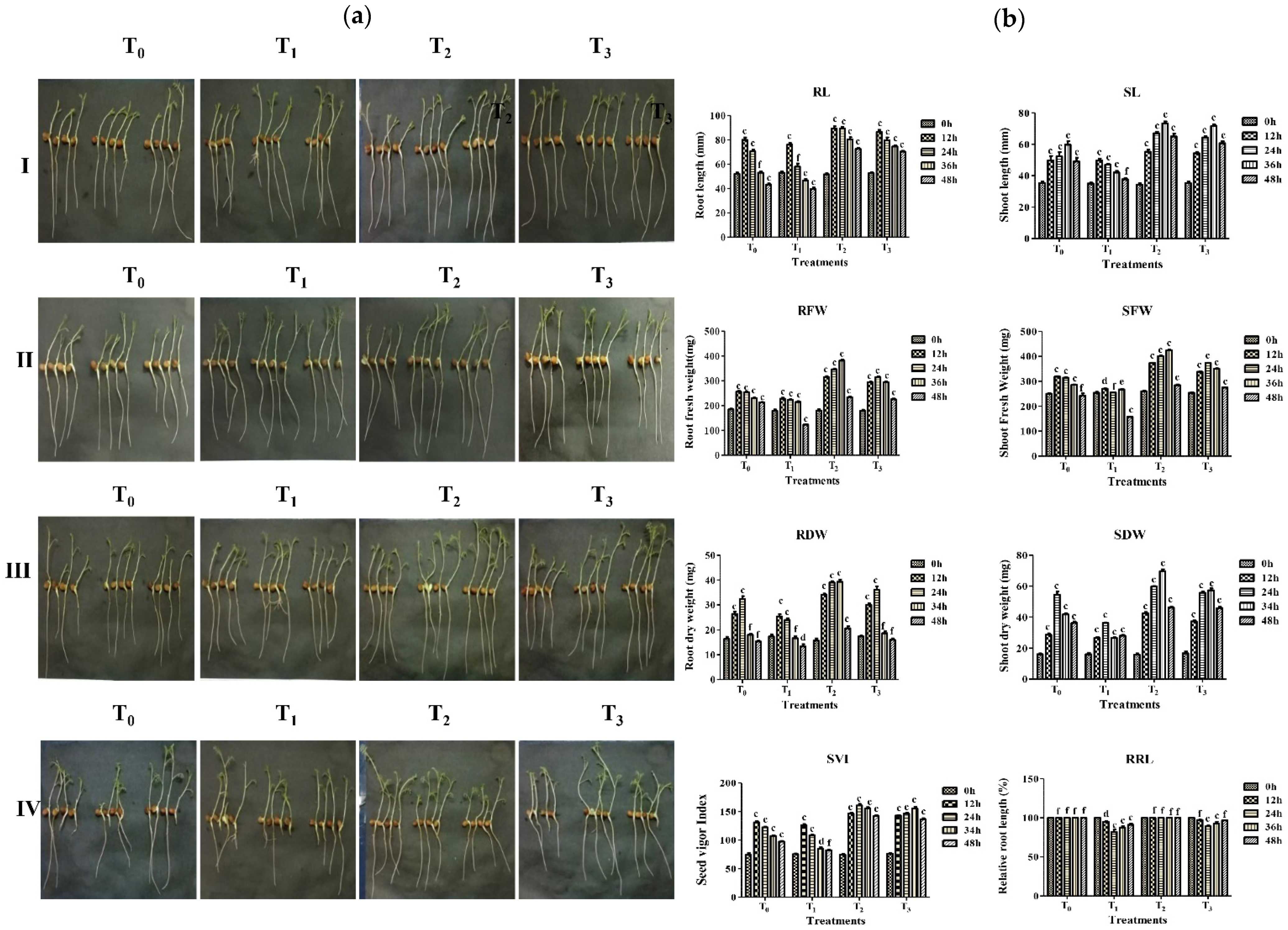
2.6. In Vitro Protective Effect of Strain A7 on Aluminum Toxicity in Chickpea Growth
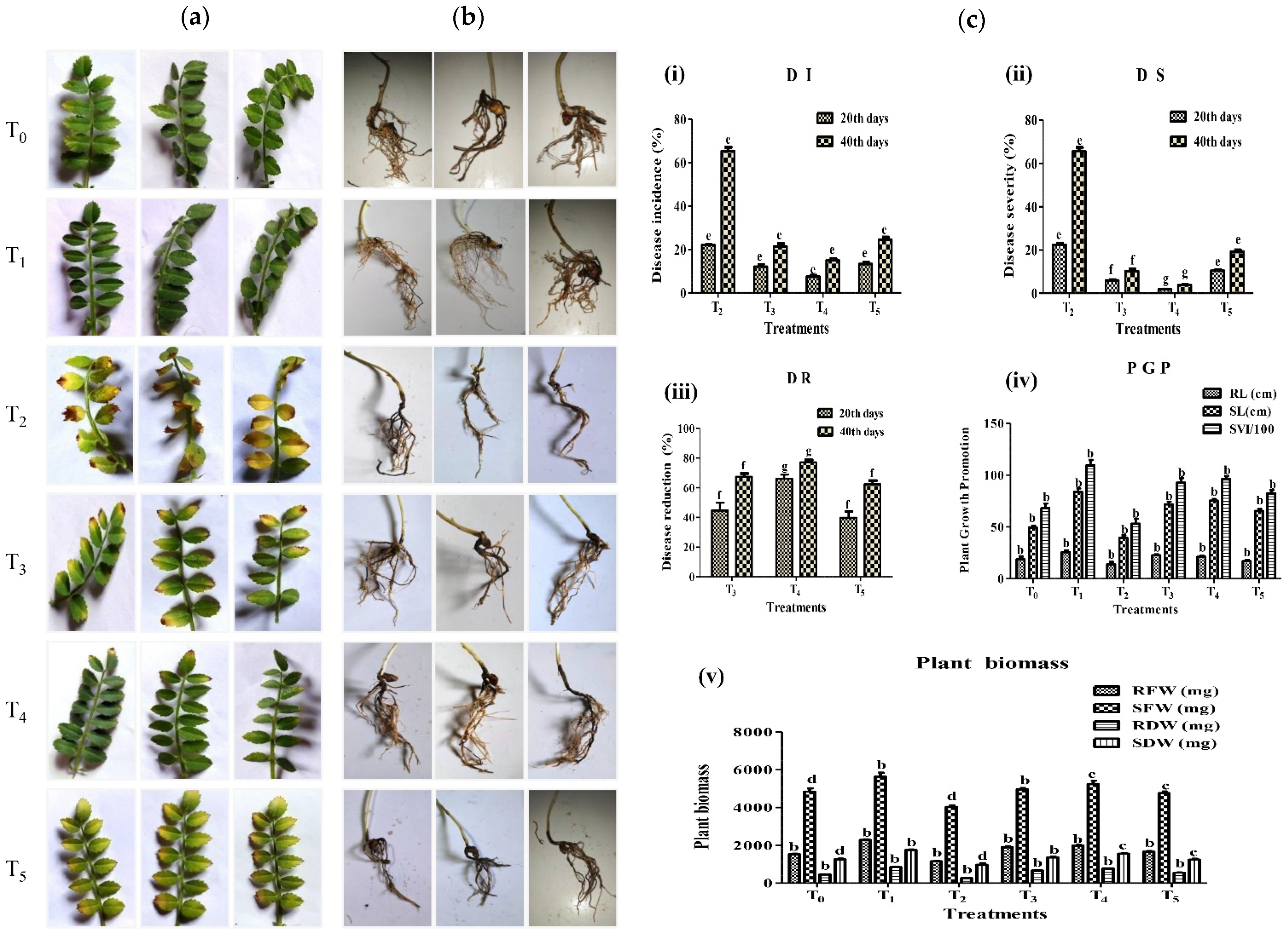
2.7. Control of Fusarium Wilt of Chickpea by Strain A7 in Pot Assay Experiment
2.8. Detection of Antifungal Antibiotic Genes Present in A7 by PCR Amplification
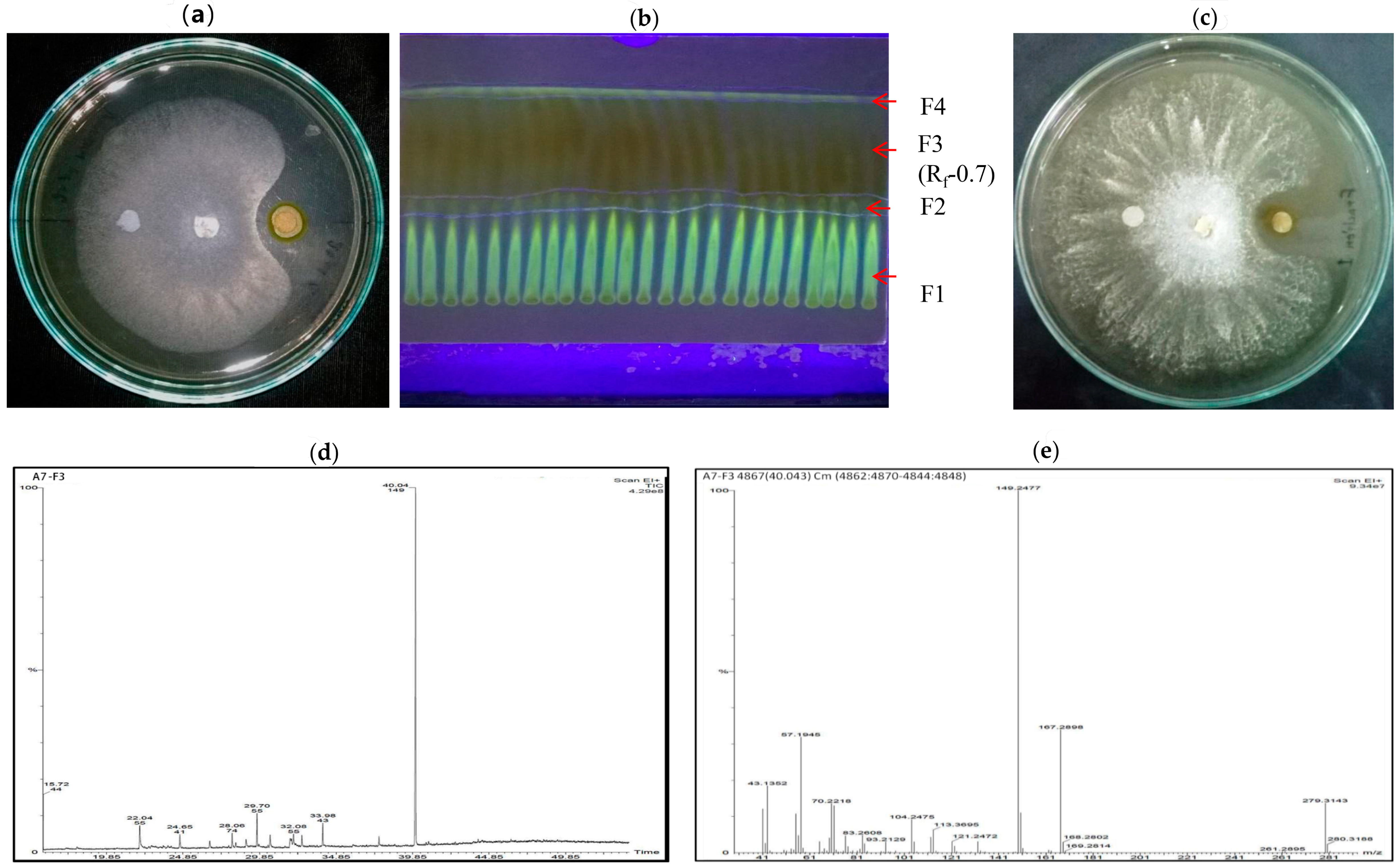
2.9. Characterization and Analysis of Bioactive Natural Compounds Extract Present in A7 by TLC, HPLC, and GC-MS
2.10. Statistical Analysis
3. Results
3.1. Isolation of the Strain and Its Morphological and Biochemical Characterization
3.2. A7 Was Identified as Pseudomonas Aeruginosa Using Molecular Tools
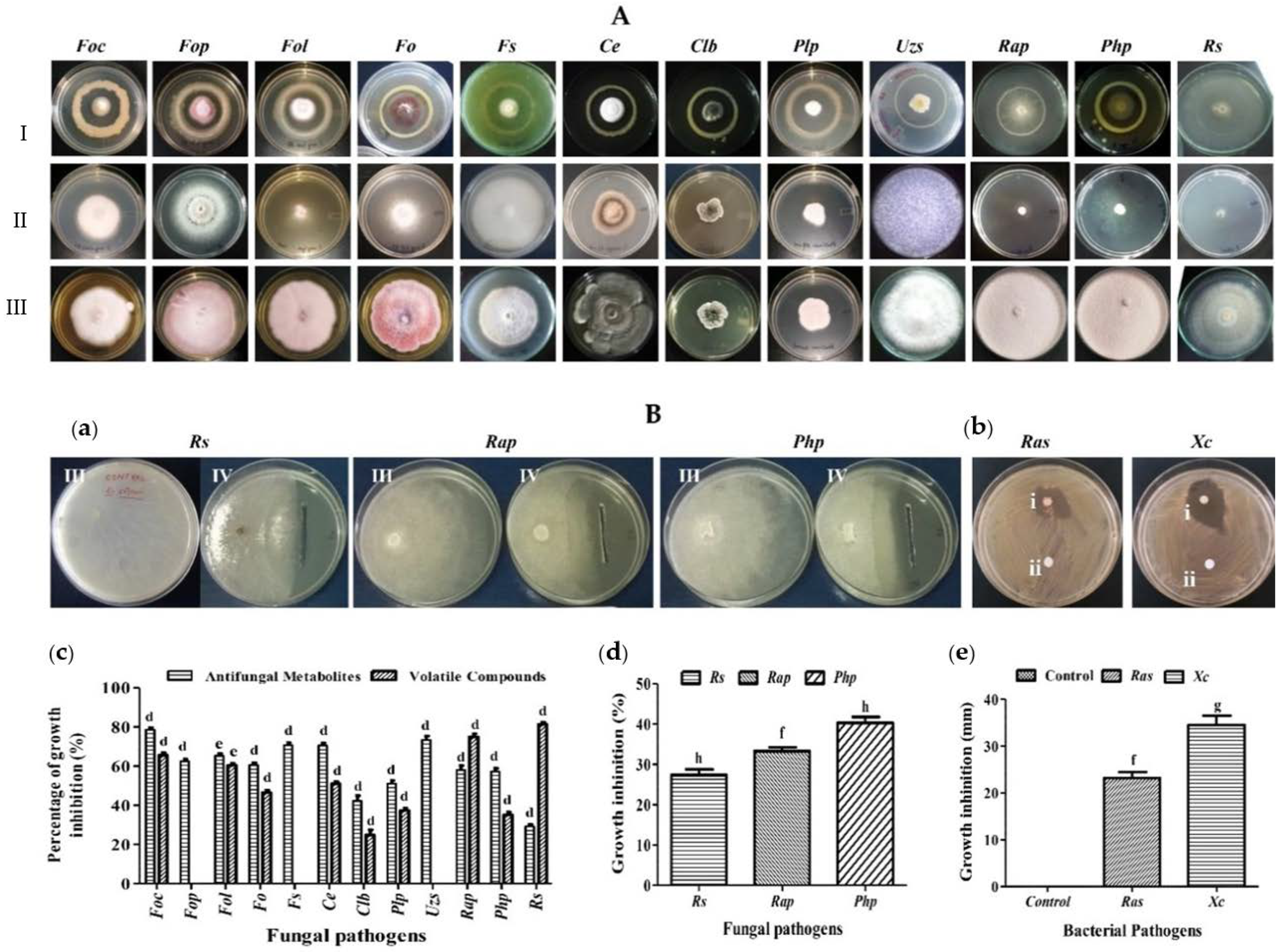
3.3. A7 Showed Bioactivity against Different Phytopathogens
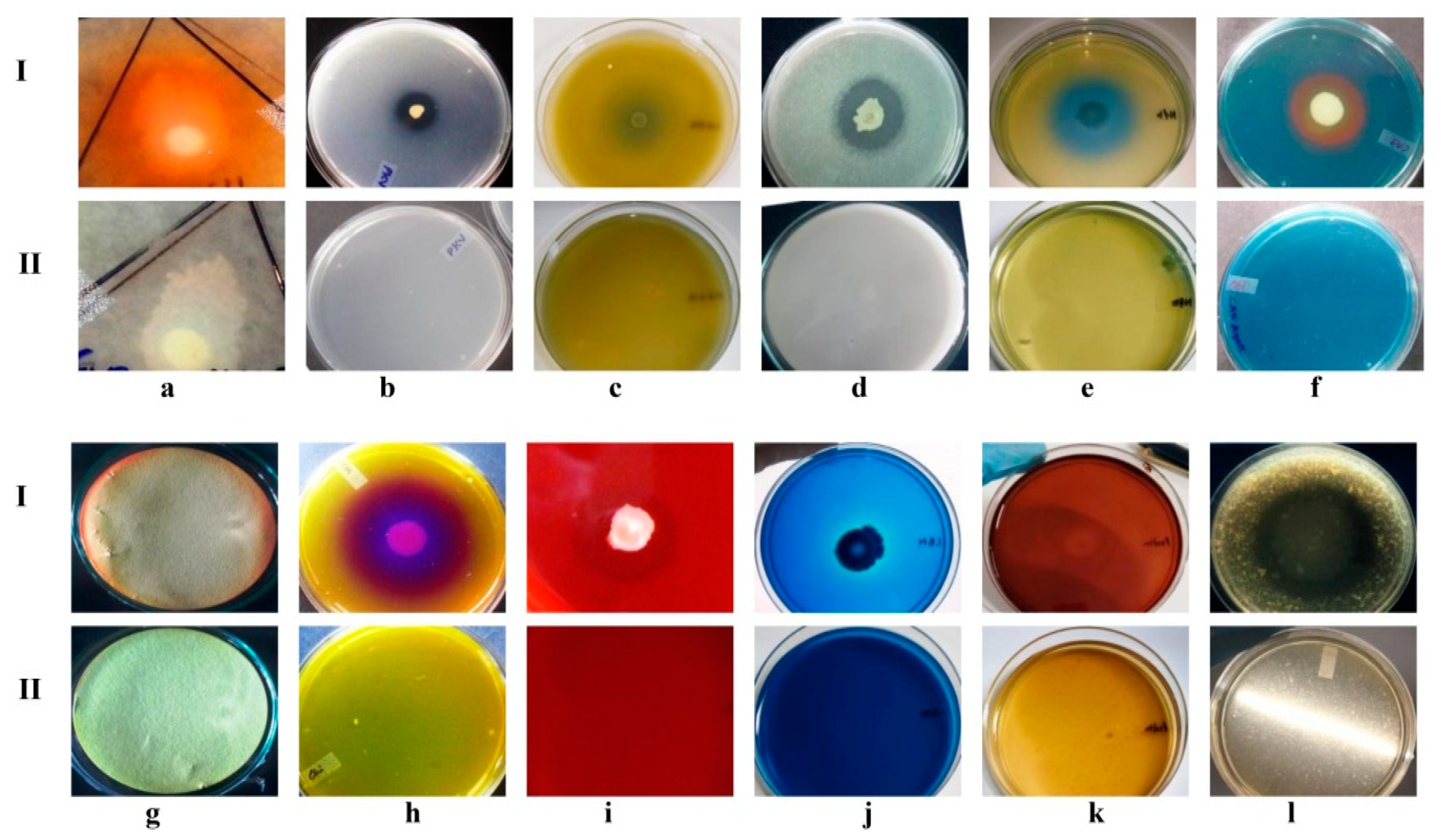
3.4. A7 Is Endowed with Multiple Plant Growth-Promoting Traits
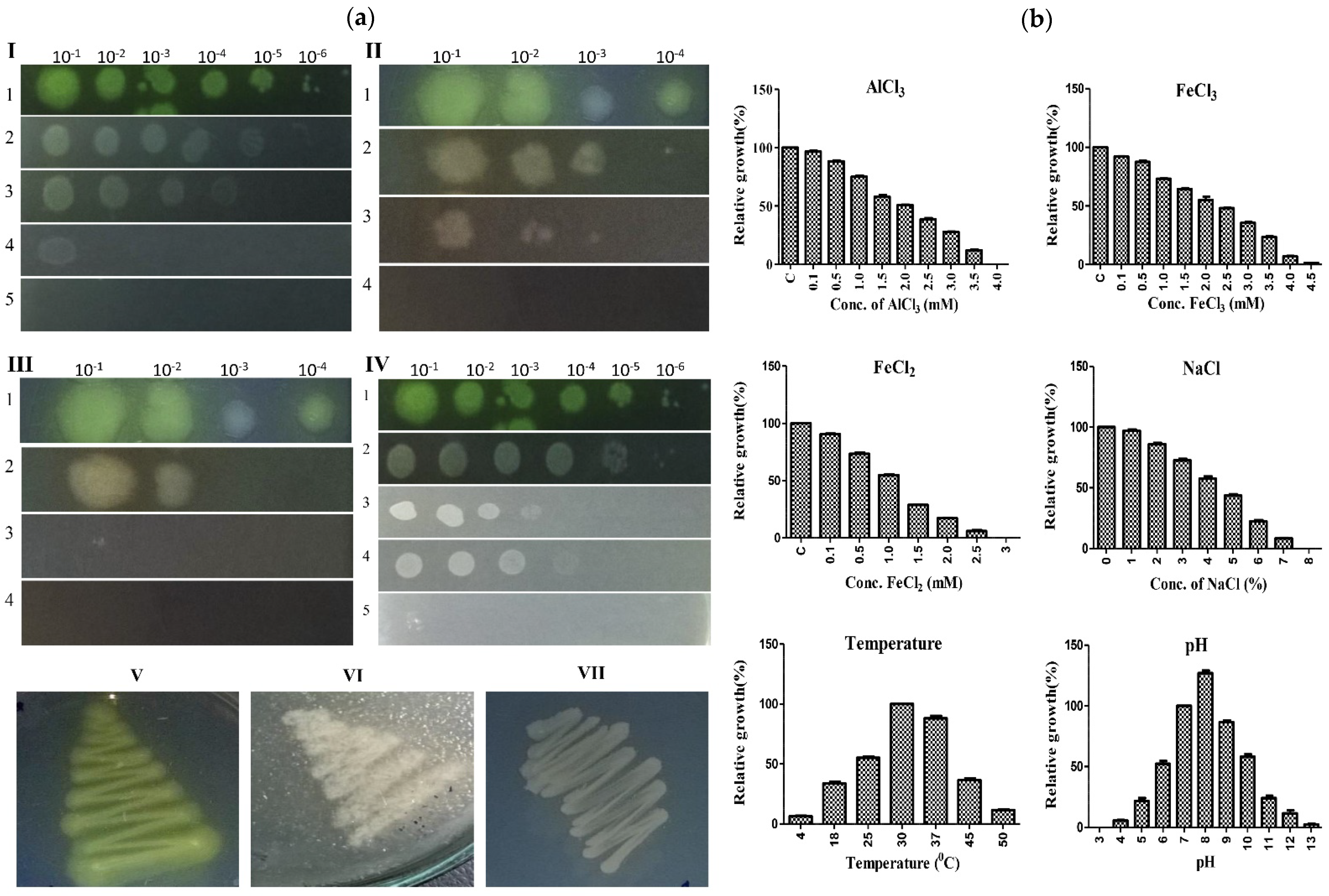
3.5. A7 Is Tolerant to Concentrations of Aluminum and Other Stress Conditions
3.6. In Vitro Protective Effect of A7 against Aluminum Toxicity in Chickpea
3.7. A7 Was a Potential Biocontrol Candidate against Fusarium Wilt of Chickpea
3.8. A7 Genome Had Antibiotics, DAPG, HCN, and PCN Biosynthesis Capabilities
3.9. Characterization and Analysis of Bioactive Natural Compounds in A7 through TLC, HPLC, and GC-MS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van der Maesen, L. Origin, history and taxonomy of chickpea. In The Chickpea; 1987; pp. 11–34. Available online: https://edepot.wur.nl/304694 (accessed on 20 October 2021).
- Merga, B.; Haji, J. Economic importance of chickpea: Production, value, and world trade. Cogent Food Agric. 2019, 5, 1615718. [Google Scholar] [CrossRef]
- Jukanti, A.; Gaur, P.; Gowda, C.; Chibbar, R. Nutritional quality and health benefits of chickpea (Cicer arietinum L.): A review. Br. J. Nutr. 2012, 108, S11–S26. [Google Scholar] [CrossRef] [PubMed]
- Solh, M.; Halila, H.; Hernandez-Bravo, G.; Malik, B.; Mihov, M.; Sadri, B. Biotic and abiotic stresses constraining the productivity of cool season food legumes in different farming systems: Specific examples. In Expanding the Production and Use of Cool Season Food Legumes; Springer: Berlin/Heidelberg, Germany, 1994; pp. 219–230. [Google Scholar]
- Haware, M.P.; Nene, Y.L. Symptomless carriers of the chickpea wilt Fusarium. Plant Dis. 1982, 66, 250–251. [Google Scholar] [CrossRef]
- Jendoubi, W.; Bouhadida, M.; Boukteb, A.; Béji, M.; Kharrat, M. Fusarium Wilt Affecting Chickpea Crop. Agriculture 2017, 7, 23. [Google Scholar] [CrossRef]
- Srivastava, A.; Dixit, G.P.; Singh, N.P.; Saxena, D.R.; Saabale, P.R.; Raghuvanshi, K.S.; Anandani, V.P. Evaluation of chickpea (Cicer arietinum L.) accessions for Fusarium wilt resistance. J. Environ. Biol. 2021, 42, 82–89. [Google Scholar] [CrossRef]
- Dubey, S.C.; Suresh, M.; Singh, B. Evaluation of Trichoderma species against Fusarium oxysporum f. sp. ciceris for integrated management of chickpea wilt. Biol. Control 2007, 40, 118–127. [Google Scholar] [CrossRef]
- Jiménez-Díaz, R.M.; Castillo, P.; del Mar Jiménez-Gasco, M.; Landa, B.B.; Navas-Cortés, J.A. Fusarium wilt of chickpeas: Biology, ecology and management. Crop Prot. 2015, 73, 16–27. [Google Scholar] [CrossRef]
- Pande, S.; Kishore, G.K.; Upadhyaya, H.D.; Rao, J.N. Identification of Sources of Multiple Disease Resistance in Mini-core Collection of Chickpea. Plant Dis. 2006, 90, 1214–1218. [Google Scholar] [CrossRef]
- Chobe, D.R.; Om, G.; Maruti, P. Radiation induced mutation for resistance against races/pathotypes of Fusarium oxysporum f. sp. ciceris in chickpea (Cicer arietinum L.). Indian Phytopathol. 2016, 69, 699–701. [Google Scholar]
- Sharma, M.; Babu, T.K.; Gaur, P.; Ghosh, R.; Rameshwar, T.; Chaudhary, R.; Upadhyay, J.; Gupta, O.; Saxena, D.; Kaur, L. Identification and multi-environment validation of resistance to Fusarium oxysporum f. sp. ciceris in chickpea. Field Crops Res. 2012, 135, 82–88. [Google Scholar] [CrossRef]
- Bhar, A.; Jain, A.; Das, S. Soil pathogen, Fusarium oxysporum induced wilt disease inchickpea: A review on its dynamicity and possible control strategies. Proc. Indian Natl. Sci. Acad. 2021, 87, 260–274. [Google Scholar] [CrossRef]
- Kumar, B.D. Fusarial wilt suppression and crop improvement through two rhizobacterial strains in chick pea growing in soils infested with Fusarium oxysporum f. sp. ciceris. Biol. Fertil. Soils 1999, 29, 87–91. [Google Scholar] [CrossRef]
- Landa, B.B.; Navas-Cortés, J.A.; Jiménez-Díaz, R.M. Integrated management of Fusarium wilt of chickpea with sowing date, host resistance, and biological control. Phytopathology 2004, 94, 946–960. [Google Scholar] [CrossRef] [PubMed]
- He, D.-C.; He, M.-H.; Amalin, D.M.; Liu, W.; Alvindia, D.G.; Zhan, J. Biological Control of Plant Diseases: An Evolutionary and Eco-Economic Consideration. Pathogens 2021, 10, 1311. [Google Scholar] [CrossRef]
- Compant, S.; Duffy, B.; Nowak, J.; Clément, C.; Barka, E.A. Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef]
- Thambugala, K.M.; Daranagama, D.A.; Phillips, A.J.; Kannangara, S.D.; Promputtha, I. Fungi vs. fungi in biocontrol: An overview of fungal antagonists applied against fungal plant pathogens. Front. Cell. Infect. Microbiol. 2020, 10, 10. [Google Scholar] [CrossRef]
- Almeida, F.; Rodrigues, M.L.; Coelho, C. The still underestimated problem of fungal diseases worldwide. Front. Microbiol. 2019, 10, 214. [Google Scholar] [CrossRef]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant Growth Promoting Rhizobacteria (PGPR) as Green Bioinoculants: Recent Developments, Constraints, and Prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Oleńska, E.; Małek, W.; Wójcik, M.; Swiecicka, I.; Thijs, S.; Vangronsveld, J. Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: A methodical review. Sci. Total Environ. 2020, 743, 140682. [Google Scholar] [CrossRef]
- Jiao, X.; Takishita, Y.; Zhou, G.; Smith, D.L. Plant Associated Rhizobacteria for Biocontrol and Plant Growth Enhancement. Front. Plant Sci. 2021, 12, 634796. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef] [PubMed]
- Pereg, L.; McMillan, M. Scoping the potential uses of beneficial microorganisms for increasing productivity in cotton cropping systems. Soil Biol. Biochem. 2015, 80, 349–358. [Google Scholar] [CrossRef]
- Anjaiah, V.; Cornelis, P.; Koedam, N. Effect of genotype and root colonization in biological control of fusarium wilts in pigeonpea and chickpea by Pseudomonas aeruginosa PNA1. Can. J. Microbiol. 2003, 49, 85–91. [Google Scholar] [CrossRef]
- Moradi, H.; Bahramnejad, B.; Amini, J.; Siosemardeh, A.; Haji-Allahverdipoor, K. Suppression of chickpea (‘Cicer arietinum’ L.)’Fusariums’ wilt by ‘Bacillus subtillis’ and ‘Trichoderma harzianum’. Plant Omics 2012, 5, 68–74. [Google Scholar]
- Cappellari, L.d.R.; Chiappero, J.; Palermo, T.B.; Giordano, W.; Banchio, E. Volatile Organic Compounds from Rhizobacteria Increase the Biosynthesis of Secondary Metabolites and Improve the Antioxidant Status in Mentha piperita L. Grown under Salt Stress. Agronomy 2020, 10, 1094. [Google Scholar] [CrossRef]
- Farh, M.E.-A.; Kim, Y.-J.; Sukweenadhi, J.; Singh, P.; Yang, D.-C. Aluminium resistant, plant growth promoting bacteria induce overexpression of Aluminium stress related genes in Arabidopsis thaliana and increase the ginseng tolerance against Aluminium stress. Microbiol. Res. 2017, 200, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Appanna, V.D.; St. Pierre, M. Influence of phosphate on aluminum tolerance in Pseudomonas fluorescens. FEMS Microbiol. Lett. 1994, 124, 327–332. [Google Scholar] [CrossRef]
- Maheshwari, D.K.; Dheeman, S. Field Crops: Sustainable Management by PGPR; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Qessaoui, R.; Bouharroud, R.; Furze, J.N.; El Aalaoui, M.; Akroud, H.; Amarraque, A.; Van Vaerenbergh, J.; Tahzima, R.; Mayad, E.H.; Chebli, B. Applications of New Rhizobacteria Pseudomonas Isolates in Agroecology via Fundamental Processes Complementing Plant Growth. Sci. Rep. 2019, 9, 12832. [Google Scholar] [CrossRef]
- Niu, X.; Song, L.; Xiao, Y.; Ge, W.; Niu, X.; Song, L.; Xiao, Y.; Ge, W. Drought-tolerant plant growth-promoting rhizobacteria associated with foxtail millet in a semi-arid agroecosystem and their potential in alleviating drought stress. Front. Microbiol. 2018, 8, 2580. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef]
- David, B.V.; Chandrasehar, G.; Selvam, P.N. Pseudomonas fluorescens: A Plant-Growth-Promoting Rhizobacterium (PGPR) with Potential Role in Biocontrol of Pests of Crops. In Crop Improvement Through Microbial Biotechnology; Prasad, R., Gill, S.S., Tuteja, N., Eds.; International Institute of Biotechnology and Toxicology (IIBAT): Padappai, India, 2018; pp. 221–243. [Google Scholar]
- Ram Kumar, A.; Selvaraj, S.; Selvam, K.A.; Devanathan, J. Isolation and characterization of drought stress tolerant plant growth promoting rhizobacter from chilli crop. Bull. Sci. Res. 2020, 3, 1–12. [Google Scholar] [CrossRef]
- Awasthi, J.P.; Saha, B.; Regon, P.; Sahoo, S.; Chowra, U.; Pradhan, A.; Roy, A.; Panda, S.K. Morpho-physiological analysis of tolerance to aluminum toxicity in rice varieties of North East India. PLoS ONE 2017, 12, e0176357. [Google Scholar] [CrossRef]
- Rahman, M.A.; Lee, S.-H.; Ji, H.C.; Kabir, A.H.; Jones, C.S.; Lee, K.-W. Importance of Mineral Nutrition for Mitigating Aluminum Toxicity in Plants on Acidic Soils: Current Status and Opportunities. Int. J. Mol. Sci. 2018, 19, 3073. [Google Scholar] [CrossRef] [PubMed]
- Greger, J. Aluminum metabolism. Annu. Rev. Nutr. 1993, 13, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Mora, M.; Alfaro, M.; Jarvis, S.; Demanet, R.; Cartes, P. Soil aluminium availability in And isolates of southern Chile and its effect on forage production and animal metabolism. Soil Use Manag. 2006, 22, 95–101. [Google Scholar] [CrossRef]
- Pellet, D.M.; Papernik, L.A.; Kochian, L.V. Multiple aluminum-resistance mechanisms in wheat (roles of root apical phosphate and malate exudation). Plant Physiol. 1996, 112, 591–597. [Google Scholar] [CrossRef]
- Silambarasan, S.; Logeswari, P.; Cornejo, P.; Kannan, V.R. Role of plant growth–promoting rhizobacterial consortium in improving the Vigna radiata growth and alleviation of aluminum and drought stresses. Environ. Sci. Pollut. Res. 2019, 26, 27647–27659. [Google Scholar] [CrossRef]
- Sukweenadhi, J.; Kim, Y.J.; Choi, E.S.; Koh, S.C.; Lee, S.W.; Kim, Y.J.; Yang, D.C. Paenibacillus yonginensis DCY84T induces changes in Arabidopsis thaliana gene expression against aluminum, drought, and salt stress. Microbiol. Res. 2015, 172, 7–15. [Google Scholar] [CrossRef]
- Labanca, E.R.G.; Andrade, S.A.L.; Kuramae, E.E.; Silveira, A.P.D. The modulation of sugarcane growth and nutritional profile under aluminum stress is dependent on beneficial endophytic bacteria and plantlet origin. Appl. Soil Ecol. 2020, 156, 103715. [Google Scholar] [CrossRef]
- Zerrouk, I.Z.; Rahmoune, B.; Khelifi, L.; Mounir, K.; Baluska, F.; Ludwig-Müller, J. Algerian Sahara PGPR confers maize root tolerance to salt and aluminum toxicity via ACC deaminase and IAA. Acta Physiol. Plant. 2019, 41, 91. [Google Scholar] [CrossRef]
- Tirry, N.; Joutey, N.T.; Sayel, H.; Kouchou, A.; Bahafid, W.; Asri, M.; El Ghachtouli, N. Screening of plant growth promoting traits in heavy metals resistant bacteria: Prospects in phytoremediation. J. Genet. Eng. Biotechnol. 2018, 16, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Brígido, C.; Menéndez, E.; Paço, A.; Glick, B.R.; Belo, A.; Félix, M.R.; Oliveira, S.; Carvalho, M. Mediterranean Native Leguminous Plants: A Reservoir of Endophytic Bacteria with Potential to Enhance Chickpea Growth under Stress Conditions. Microorganisms 2019, 7, 392. [Google Scholar] [CrossRef] [PubMed]
- Abd-Alla, M.H.; Nafady, N.A.; Bashandy, S.R.; Hassan, A.A. Mitigation of effect of salt stress on the nodulation, nitrogen fixation and growth of chickpea (Cicer arietinum L.) by triple microbial inoculation. Rhizosphere 2019, 10, 100148. [Google Scholar] [CrossRef]
- King, E.O.; Ward, M.K.; Raney, D.E. Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 1954, 44, 301–307. [Google Scholar] [PubMed]
- Opelt, K.; Berg, G. Diversity and antagonistic potential of bacteria associated with bryophytes from nutrient-poor habitats of the Baltic Sea Coast. Appl. Environ. Microbiol. 2004, 70, 6569–6579. [Google Scholar] [CrossRef]
- Somasegaran, P.; Hoben, H.J. Quantifying the growth of rhizobia. In Handbook for Rhizobia; Springer: Berlin/Heidelberg, Germany, 1994; pp. 47–57. [Google Scholar]
- Bergey, D.H. Bergey’s Manual of Determinative Bacteriology. Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1994. [Google Scholar]
- Maniatis, T.; Fritsch, E.; Sambrook, J. Molecular Cloning: A Laboratory Manual 3; Cold Spring Harbor Laboratory: New York, NY, USA, 1982. [Google Scholar]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef]
- Russell, D.W.; Sambrook, J. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Cold: New York, NY, USA, 2001; Volume 1. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Adetuyi, F.; Cartwright, D. Studies of the antagonistic activities of bacteria endemic to cereal seeds. II. Quantification of antimycotic activity. Ann. Appl. Biol. 1985, 107, 33–43. [Google Scholar] [CrossRef]
- Stracquadanio, C.; Quiles, J.M.; Meca, G.; Cacciola, S.O. Antifungal activity of bioactive metabolites produced by Trichoderma asperellum and Trichoderma atroviride in liquid medium. J. Fungi 2020, 6, 263. [Google Scholar] [CrossRef]
- Rahmansyah, M. Endophytic fungi isolated from mangrove plant and have antagonism role against fusarium wilt. J. Agric. Biol. Sci. 2013, 8, 251–257. [Google Scholar]
- Heatley, N.G. A method for the assay of penicillin. Biochem. J. 1944, 38, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.M.; Kim, J.-S.; Kim, H.-S.; Kim, Y.-Y.; Oh, J.-K.; Jung, H.-W.; Park, D.-S.; Bae, K.-H. Lactobacillus reuteri AN417 cell-free culture supernatant as a novel antibacterial agent targeting oral pathogenic bacteria. Sci. Rep. 2021, 11, 1631. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Bric, J.M.; Bostock, R.M.; Silverstone, S.E. Rapid in situ assay for indoleacetic acid production by bacteria immobilized on a nitrocellulose membrane. Appl. Environ. Microbiol. 1991, 57, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Gaur, A. Physiological Functions of Phosphate Solubilizing Micro-Organisms. In Phosphate Solubilizing Micro-organisms as Biofertilizers; Omega Scientific Publishers: New Delhi, India, 1990; pp. 16–72. [Google Scholar]
- King, E.J. The colorimetric determination of phosphorus. Biochem. J. 1932, 26, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Meena, V.; Maurya, B.; Verma, J.; Aeron, A.; Kumar, A.; Kim, K.; Bajpai, V.K. Potassium solubilizing rhizobacteria (KSR): Isolation, identification, and K-release dynamics from waste mica. Ecol. Eng. 2015, 81, 340–347. [Google Scholar] [CrossRef]
- Rajawat, M.V.S.; Singh, S.; Tyagi, S.P.; Saxena, A.K. A modified plate assay for rapid screening of potassium-solubilizing bacteria. Pedosphere 2016, 26, 768–773. [Google Scholar] [CrossRef]
- Khanghahi, M.Y.; Pirdashti, H.; Rahimian, H.; Nematzadeh, G.; Sepanlou, M.G. Potassium solubilising bacteria (KSB) isolated from rice paddy soil: From isolation, identification to K use efficiency. Symbiosis 2018, 76, 13–23. [Google Scholar] [CrossRef]
- Rokhbakhsh-Zamin, F.; Sachdev, D.; Kazemi-Pour, N.; Engineer, A.; Pardesi, K.R.; Zinjarde, S.; Dhakephalkar, P.K.; Chopade, B.A. Characterization of plant-growth-promoting traits of Acinetobacter species isolated from rhizosphere of Pennisetum glaucum. J. Microbiol. Biotechnol. 2011, 21, 556–566. [Google Scholar] [CrossRef]
- Day, J.; Döbereiner, J. Physiological aspects of N2-fixation by a Spirillum from Digitaria roots. Soil Biol. Biochem. 1976, 8, 45–50. [Google Scholar] [CrossRef]
- Cappuccino, J.G.; Sherman, N. Microbiology: A Laboratory Manual; Pearson: Upper Saddle River, NJ, USA, 2005. [Google Scholar]
- Schwyn, B.; Neilands, J. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Lorck, H. Production of hydrocyanic acid by bacteria. Physiol. Plant. 1948, 1, 142–146. [Google Scholar] [CrossRef]
- Agrawal, T.; Kotasthane, A.S. Chitinolytic assay of indigenous Trichoderma isolates collected from different geographical locations of Chhattisgarh in Central India. SpringerPlus 2012, 1, 73. [Google Scholar] [CrossRef] [PubMed]
- Cloete, K.J.; Valentine, A.J.; Stander, M.A.; Blomerus, L.M.; Botha, A. Evidence of symbiosis between the soil yeast Cryptococcus laurentii and a sclerophyllous medicinal shrub, Agathosma betulina (Berg.) Pillans. Microb. Ecol. 2009, 57, 624–632. [Google Scholar] [CrossRef]
- Gupta, P.; Samant, K.; Sahu, A. Isolation of Cellulose-Degrading Bacteria and Determination of Their Cellulolytic Potential. Int. J. Microbiol. 2012, 2012, 578925. [Google Scholar] [CrossRef]
- Lu, W.-J.; Wang, H.-T.; Nie, Y.-F.; Wang, Z.-C.; Huang, D.-Y.; Qiu, X.-Y.; Chen, J.-C. Effect of inoculating flower stalks and vegetable waste with ligno-cellulolytic microorganisms on the composting process. J. Environ. Sci. Health Part B 2004, 39, 871–887. [Google Scholar] [CrossRef]
- Hendricks, C.W.; Doyle, J.D.; Hugley, B. A new solid medium for enumerating cellulose-utilizing bacteria in soil. Appl. Environ. Microbiol. 1995, 61, 2016–2019. [Google Scholar] [CrossRef]
- Bandounas, L.; Wierckx, N.J.; de Winde, J.H.; Ruijssenaars, H.J. Isolation and characterization of novel bacterial strains exhibiting ligninolytic potential. BMC Biotechnol. 2011, 11, 94. [Google Scholar] [CrossRef]
- Mercimek Takcı, H.A.; Turkmen, F.U. Extracellular pectinase production and purification from a newly isolated Bacillus subtilis strain. Int. J. Food Prop. 2016, 19, 2443–2450. [Google Scholar] [CrossRef]
- Thomas, P.; Sekhar, A.C.; Upreti, R.; Mujawar, M.M.; Pasha, S.S. Optimization of single plate-serial dilution spotting (SP-SDS) with sample anchoring as an assured method for bacterial and yeast cfu enumeration and single colony isolation from diverse samples. Biotechnol. Rep. 2015, 8, 45–55. [Google Scholar] [CrossRef]
- Tank, N.; Saraf, M. Salinity-resistant plant growth promoting rhizobacteria ameliorates sodium chloride stress on tomato plants. J. Plant Interact. 2010, 5, 51–58. [Google Scholar] [CrossRef]
- Miller, T.L.; Wolin, M. A serum bottle modification of the Hungate technique for cultivating obligate anaerobes. Appl. Microbiol. 1974, 27, 985–987. [Google Scholar] [CrossRef] [PubMed]
- Woolfrey, B.F.; Gresser-Burns, M.E.; Lally, R.T. Effect of temperature on inoculum as a potential source of error in agar dilution plate count bactericidal measurements. Antimicrob. Agents Chemother. 1988, 32, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, B.; Sharma, G.; Paul, A. Isolation and characterization of heavy metal resistant bacteria from Barak River contaminated with pulp paper mill effluent, South Assam. Bull. Environ. Contam. Toxicol. 2012, 89, 263–268. [Google Scholar] [CrossRef]
- Abdul-Baki, A.A.; Anderson, J.D. Vigor Determination in Soybean Seed by Multiple Criteria1. Crop Sci. 1973, 13, 630–633. [Google Scholar] [CrossRef]
- Dubey, S.C.; Singh, V.; Priyanka, K.; Upadhyay, B.K.; Singh, B. Combined application of fungal and bacterial bio-agents, together with fungicide and Mesorhizobium for integrated management of Fusarium wilt of chickpea. Entomophaga 2015, 60, 413–424. [Google Scholar] [CrossRef]
- Idris, H.A.; Labuschagne, N.; Korsten, L. Screening rhizobacteria for biological control of Fusarium root and crown rot of sorghum in Ethiopia. Biol. Control 2007, 40, 97–106. [Google Scholar] [CrossRef]
- Zaim, S.; Bekkar, A.A.; Belabid, L. Efficacy of Bacillus subtilis and Trichoderma harzianum combination on chickpea Fusarium wilt caused by F. oxysporum f. sp. ciceris. Arch. Phytopathol. Plant Prot. 2018, 51, 217–226. [Google Scholar] [CrossRef]
- Landa, B.B.; Hervás, A.; Bettiol, W.; Jiménez-Díaz, R.M. Antagonistic activity of Bacteria from the chickpea rhizosphere against Fusarium Oxysporum f. sp. ciceris. Phytoparasitica 1997, 25, 305–318. [Google Scholar] [CrossRef]
- Sharma, K.D.; Chen, W.; Muehlbauer, F.J. Genetics of chickpea resistance to five races of Fusarium wilt and a concise set of race differentials for Fusarium oxysporum f. sp. ciceris. Plant Dis. 2005, 89, 385–390. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Weller, D.M.; Thomashow, L.S. Frequency of antibiotic-producing Pseudomonas spp. in natural environments. Appl. Environ. Microbiol. 1997, 63, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Ramette, A.; Frapolli, M.; Défago, G.; Moënne-Loccoz, Y. Phylogeny of HCN synthase-encoding hcnBC genes in biocontrol fluorescent pseudomonads and its relationship with host plant species and HCN synthesis ability. Mol. Plant-Microbe Interact. 2003, 16, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Mavrodi, D.V.; Bonsall, R.F.; Delaney, S.M.; Soule, M.J.; Phillips, G.; Thomashow, L.S. Functional analysis of genes for biosynthesis of pyocyanin and phenazine-1-carboxamide from Pseudomonas aeruginosa PAO1. J. Bacteriol. 2001, 183, 6454–6465. [Google Scholar] [CrossRef] [PubMed]
- de Souza, J.T.; Weller, D.M.; Raaijmakers, J.M. Frequency, Diversity, and Activity of 2,4-Diacetylphloroglucinol-Producing Fluorescent Pseudomonas spp. in Dutch Take-all Decline Soils. Phytopathology 2003, 93, 54–63. [Google Scholar] [CrossRef]
- Calderón, C.E.; Pérez-García, A.; de Vicente, A.; Cazorla, F.M. The dar genes of Pseudomonas chlororaphis PCL1606 are crucial for biocontrol activity via production of the antifungal compound 2-hexyl, 5-propyl resorcinol. Mol. Plant-Microbe Interact. 2013, 26, 554–565. [Google Scholar] [CrossRef]
- Gurusiddaiah, S.; David, R.G.; Kennedy, A.C.; Ogg, A.G., Jr. Isolation and Characterization of Metabolites from Pseudomonas fluorescens-D7 for Control of Downy Brome (Bromus tectorum). Weed Sci. 1994, 42, 492–501. [Google Scholar] [CrossRef]
- Wang, J.; Yue, Y.-D.; Tang, F.; Sun, J. TLC screening for antioxidant activity of extracts from fifteen bamboo species and identification of antioxidant flavone glycosides from leaves of Bambusa textilis McClure. Molecules 2012, 17, 12297–12311. [Google Scholar] [CrossRef]
- Pyka, A. Detection Progress of Selected Drugs in TLC. BioMed Res. Int. 2014, 2014, 732078. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, D.; Li, C.; Sun, Q.; Li, L.; Liu, F.; Shen, Q.; Shen, B. Isolation and characterization of Pseudomonas brassicacearum J12 as an antagonist against Ralstonia solanacearum and identification of its antimicrobial components. Microbiol. Res. 2012, 167, 388–394. [Google Scholar] [CrossRef]
- Karthikeyan, S.C.; Velmurugan, S.; Donio, M.B.S.; Michaelbabu, M.; Citarasu, T. Studies on the antimicrobial potential and structural characterization of fatty acids extracted from Sydney rock oyster Saccostrea glomerata. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 332. [Google Scholar] [CrossRef]
- Kumar, V.; Bhatnagar, A.; Srivastava, J. Antibacterial activity of crude extracts of Spirulina platensis and its structural elucidation of bioactive compound. J. Med. Plants Res. 2011, 5, 7043–7048. [Google Scholar]
- Shaaban, M.T.; Ghaly, M.F.; Fahmi, S.M. Antibacterial activities of hexadecanoic acid methyl ester and green-synthesized silver nanoparticles against multidrug-resistant bacteria. J. Basic Microbiol. 2021, 61, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Pu, Z.-H.; Zhang, Y.-Q.; Yin, Z.-Q.; Jiao, X.; Jia, R.-Y.; Yang, L.; Fan, Y. Antibacterial activity of 9-octadecanoic acid-hexadecanoic acid-tetrahydrofuran-3,4-diyl ester from neem oil. Agric. Sci. China 2010, 9, 1236–1240. [Google Scholar] [CrossRef]
- Rahman, M.; Anwar, M.N. Fungitoxic and cytotoxic activity of a novel compound 1,2-benzenedicarboxylic acid, diisooctyl ester of Plumbago zeylanica linn. Asian J. Microbiol. Biotechnol. Environ. Sci. 2006, 8, 461–464. [Google Scholar]
- Ramette, A.; Frapolli, M.; Fischer-Le Saux, M.; Gruffaz, C.; Meyer, J.-M.; Défago, G.; Sutra, L.; Moënne-Loccoz, Y. Pseudomonas protegens sp. nov., widespread plant-protecting bacteria producing the biocontrol compounds 2,4-diacetylphloroglucinol and pyoluteorin. Syst. Appl. Microbiol. 2011, 34, 180–188. [Google Scholar] [CrossRef]
- Ryan, R.P.; Germaine, K.; Franks, A.; Ryan, D.J.; Dowling, D.N. Bacterial endophytes: Recent developments and applications. FEMS Microbiol. Lett. 2008, 278, 1–9. [Google Scholar] [CrossRef]
- Sarwar, M.; Kremer, R.J. Enhanced suppression of plant growth through production of L-tryptophan-derived compounds by deleterious rhizobacteria. Plant Soil 1995, 172, 261–269. [Google Scholar] [CrossRef]
- Shao, J.; Xu, Z.; Zhang, N.; Shen, Q.; Zhang, R. Contribution of indole-3-acetic acid in the plant growth promotion by the rhizospheric strain Bacillus amyloliquefaciens SQR9. Biol. Fertil. Soils 2015, 51, 321–330. [Google Scholar] [CrossRef]
- Patten, C.L.; Glick, B.R. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl. Environ. Microbiol. 2002, 68, 3795–3801. [Google Scholar] [CrossRef]
- Boubekri, K.; Soumare, A.; Mardad, I.; Lyamlouli, K.; Hafidi, M.; Ouhdouch, Y.; Kouisni, L. The Screening of potassium-and phosphate-solubilizing actinobacteria and the assessment of their ability to promote wheat growth parameters. Microorganisms 2021, 9, 470. [Google Scholar] [CrossRef]
- Richardson, A.E.; Simpson, R.J. Soil microorganisms mediating phosphorus availability update on microbial phosphorus. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Majeed, A.; Abbasi, M.K.; Hameed, S.; Yasmin, S.; Hanif, M.K.; Naqqash, T.; Imran, A. Pseudomonas sp. AF-54 containing multiple plant beneficial traits acts as growth enhancer of Helianthus annuus L. under reduced fertilizer input. Microbiol. Res. 2018, 216, 56–69. [Google Scholar] [CrossRef] [PubMed]
- de Werra, P.; Péchy-Tarr, M.; Keel, C.; Maurhofer, M. Role of gluconic acid production in the regulation of biocontrol traits of Pseudomonas fluorescens CHA0. Appl. Environ. Microbiol. 2009, 75, 4162–4174. [Google Scholar] [CrossRef] [PubMed]
- Elhaissoufi, W.; Khourchi, S.; Ibnyasser, A.; Ghoulam, C.; Rchiad, Z.; Zeroual, Y.; Lyamlouli, K.; Bargaz, A. Phosphate solubilizing rhizobacteria could have a stronger influence on wheat root traits and aboveground physiology than rhizosphere P solubilization. Front. Plant Sci. 2020, 11, 979. [Google Scholar] [CrossRef]
- Islam, S.; Akanda, A.M.; Prova, A.; Islam, M.T.; Hossain, M.M. Isolation and Identification of Plant Growth Promoting Rhizobacteria from Cucumber Rhizosphere and Their Effect on Plant Growth Promotion and Disease Suppression. Front. Microbiol. 2016, 6, 1360. [Google Scholar] [CrossRef]
- Etesami, H.; Emami, S.; Alikhani, H. Potassium solubilizing bacteria (KSB): Mechanisms, promotion of plant growth, and future prospects-A review. J. Soil Sci. Plant Nutr. 2017, 17. [Google Scholar] [CrossRef]
- Sattar, A.; Naveed, M.; Ali, M.; Zahir, Z.A.; Nadeem, S.M.; Yaseen, M.; Meena, V.S.; Farooq, M.; Singh, R.; Rahman, M. Perspectives of potassium solubilizing microbes in sustainable food production system: A review. Appl. Soil Ecol. 2019, 133, 146–159. [Google Scholar] [CrossRef]
- Sun, F.; Ou, Q.; Wang, N.; xuan Guo, Z.; Ou, Y.; Li, N.; Peng, C. Isolation and identification of potassium-solubilizing bacteria from Mikania micrantha rhizospheric soil and their effect on M. micrantha plants. Glob. Ecol. Conserv. 2020, 23, e01141. [Google Scholar] [CrossRef]
- Bagyalakshmi, B.; Ponmurugan, P.; Balamurugan, A. Potassium solubilization, plant growth promoting substances by potassium solubilizing bacteria (KSB) from southern Indian Tea plantation soil. Biocatal. Agric. Biotechnol. 2017, 12, 116–124. [Google Scholar] [CrossRef]
- Dong, X.; Lv, L.; Wang, W.; Liu, Y.; Yin, C.; Xu, Q.; Yan, H.; Fu, J.; Liu, X. Differences in Distribution of Potassium-Solubilizing Bacteria in Forest and Plantation Soils in Myanmar. Int. J. Environ. Res. Public Health 2019, 16, 700. [Google Scholar] [CrossRef]
- Zhang, C.; Kong, F. Isolation and identification of potassium-solubilizing bacteria from tobacco rhizospheric soil and their effect on tobacco plants. Appl. Soil Ecol. 2014, 82, 18–25. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, X.; Chen, W.; Huang, Q. Isolation and identification of three potassium-solubilizing bacteria from rape rhizospheric soil and their effects on ryegrass. Geomicrobiol. J. 2017, 34, 873–880. [Google Scholar] [CrossRef]
- Navarro-Noya, Y.E.; Hernández-Mendoza, E.; Morales-Jiménez, J.; Jan-Roblero, J.; Martínez-Romero, E.; Hernández-Rodríguez, C. Isolation and characterization of nitrogen fixing heterotrophic bacteria from the rhizosphere of pioneer plants growing on mine tailings. Appl. Soil Ecol. 2012, 62, 52–60. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, J.; Dou, Y.; Chen, M.; Ping, S.; Peng, J.; Lu, W.; Zhang, W.; Yao, Z.; Li, H. Nitrogen fixation island and rhizosphere competence traits in the genome of root-associated Pseudomonas stutzeri A1501. Proc. Natl. Acad. Sci. USA 2008, 105, 7564–7569. [Google Scholar] [CrossRef]
- Boddey, R.M.; Dobereiner, J. Nitrogen fixation associated with grasses and cereals: Recent results and perspectives for future research. Plant Soil 1988, 108, 53–65. [Google Scholar] [CrossRef]
- Zakry, F.; Halimi, M.; Rahim, K.A.; Osumanu, H.; Wong, S.; Franklin, R.; Stephen, L.; Make, J. Isolation and plant growth-promoting properties of rhizobacterial diazotrophs from pepper vine (Piper nigrum L.). Malays. Appl. Biol. 2010, 39, 41–45. [Google Scholar]
- Vargas, L.; de Carvalho, T.L.G.; Ferreira, P.C.G.; Baldani, V.L.D.; Baldani, J.I.; Hemerly, A.S. Early responses of rice (Oryza sativa L.) seedlings to inoculation with beneficial diazotrophic bacteria are dependent on plant and bacterial genotypes. Plant Soil 2012, 356, 127–137. [Google Scholar] [CrossRef]
- Kotasthane, A.S.; Agrawal, T.; Zaidi, N.W.; Singh, U. Identification of siderophore producing and cynogenic fluorescent Pseudomonas and a simple confrontation assay to identify potential bio-control agent for collar rot of chickpea. 3 Biotech 2017, 7, 137. [Google Scholar] [CrossRef]
- Ahmed, E.; Holmström, S.J.M. Siderophores in environmental research: Roles and applications. Microb. Biotechnol. 2014, 7, 196–208. [Google Scholar] [CrossRef]
- Rajkumar, M.; Ae, N.; Prasad, M.N.V.; Freitas, H. Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol. 2010, 28, 142–149. [Google Scholar] [CrossRef]
- Rout, G.R.; Sahoo, S. Role Of Iron In Plant Growth And Metabolism. Rev. Agric. Sci. 2015, 3, 1–24. [Google Scholar] [CrossRef]
- Haas, D.; Keel, C. Regulation of antibiotic production in root-colonizing Pseudomonas spp. and relevance for biological control of plant disease. Annu. Rev. Phytopathol. 2003, 41, 117–153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, Y.; Harvey, P.R.; Ren, Y.; Zhang, G.; Zhou, H.; Yang, H. Enhancing plant disease suppression by Burkholderia vietnamiensis through chromosomal integration of Bacillus subtilis chitinase gene chi113. Biotechnol. Lett. 2012, 34, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Martínez-Hidalgo, P.; Ice, T.A.; Maymon, M.; Humm, E.A.; Nejat, N.; Sanders, E.R.; Kaplan, D.; Hirsch, A.M. Antifungal Activity of Bacillus Species Against Fusarium and Analysis of the Potential Mechanisms Used in Biocontrol. Front. Microbiol. 2018, 9, 2363. [Google Scholar] [CrossRef]
- Zarei, M.; Aminzadeh, S.; Zolgharnein, H.; Safahieh, A.; Daliri, M.; Noghabi, K.A.; Ghoroghi, A.; Motallebi, A. Characterization of a chitinase with antifungal activity from a native Serratia marcescens B4A. Braz. J. Microbiol. 2011, 42, 1017–1029. [Google Scholar] [CrossRef]
- Ji, Z.-L.; Peng, S.; Chen, L.-L.; Liu, Y.; Yan, C.; Zhu, F. Identification and characterization of a serine proteases from Bacillus licheniformis W10: A potential antifungal agent. Int. J. Biol. Macromol. 2020, 145, 594–603. [Google Scholar] [CrossRef]
- Bugg, T.D.; Ahmad, M.; Hardiman, E.M.; Singh, R. The emerging role for bacteria in lignin degradation and bio-product formation. Curr. Opin. Biotechnol. 2011, 22, 394–400. [Google Scholar] [CrossRef]
- Blumer, C.; Haas, D. Mechanism, regulation, and ecological role of bacterial cyanide biosynthesis. Arch. Microbiol. 2000, 173, 170–177. [Google Scholar] [CrossRef]
- Nandi, M.; Selin, C.; Brawerman, G.; Fernando, W.D.; de Kievit, T. Hydrogen cyanide, which contributes to Pseudomonas chlororaphis strain PA23 biocontrol, is upregulated in the presence of glycine. Biol. Control 2017, 108, 47–54. [Google Scholar] [CrossRef]
- Ramette, A.; Moënne-Loccoz, Y.; Défago, G. Genetic diversity and biocontrol potential of fluorescent pseudomonads producing phloroglucinols and hydrogen cyanide from Swiss soils naturally suppressive or conducive to Thielaviopsis basicola mediated black root rot of tobacco. FEMS Microbiol. Ecol. 2006, 55, 369–381. [Google Scholar] [CrossRef]
- Kumar, A.; Bahadur, I.; Maurya, B.; Raghuwanshi, R.; Meena, V.; Singh, D.; Dixit, J. Does a plant growth-promoting rhizobacteria enhance agricultural sustainability? J. Pure Appl. Microbiol. 2015, 9, 715–724. [Google Scholar]
- Siddiqui, I.A.; Shaukat, S.S.; Sheikh, I.H.; Khan, A. Role of cyanide production by Pseudomonas fluorescens CHA0 in the suppression of root-knot nematode, Meloidogyne javanica in tomato. World J. Microbiol. Biotechnol. 2006, 22, 641–650. [Google Scholar] [CrossRef]
- Gamez, R.M.; Rodríguez, F.; Vidal, N.M.; Ramirez, S.; Alvarez, R.V.; Landsman, D.; Mariño-Ramírez, L. Banana (Musa acuminata) transcriptome profiling in response to rhizobacteria: Bacillus amyloliquefaciens Bs006 and Pseudomonas fluorescens Ps006. BMC Genom. 2019, 20, 378. [Google Scholar] [CrossRef] [PubMed]
- Sarki, M.; Panhwar, Q.; Ali, A.; Jamali, M.; Rajpar, I.; Depar, N. Assessment of Potential Bacterial Isolates for Enhancing Plant Nutrient Uptake and Growth of Wheat. Malays. J. Soil Sci. 2021, 25, 107–124. [Google Scholar]
- Bojórquez-Quintal, E.; Escalante-Magaña, C.; Echevarría-Machado, I.; Martínez-Estévez, M. Aluminum, a Friend or Foe of Higher Plants in Acid Soils. Front. Plant Sci. 2017, 8, 1767. [Google Scholar] [CrossRef] [PubMed]
- Kochian, L.V.; Piñeros, M.A.; Liu, J.; Magalhaes, J.V. Plant Adaptation to Acid Soils: The Molecular Basis for Crop Aluminum Resistance. Annu. Rev. Plant Biol. 2015, 66, 571–598. [Google Scholar] [CrossRef]
- Zaidi, S.; Usmani, S.; Singh, B.R.; Musarrat, J. Significance of Bacillus subtilis strain SJ-101 as a bioinoculant for concurrent plant growth promotion and nickel accumulation in Brassica juncea. Chemosphere 2006, 64, 991–997. [Google Scholar] [CrossRef]
- Heera, S.; Rajor, A. Bacterial Treatment and Metal Characterization of Biomedical Waste Ash. J. Waste Manag. 2014, 2014, 956316. [Google Scholar] [CrossRef]
- Joseph, B.; Ranjan Patra, R.; Lawrence, R. Characterization of plant growth promoting rhizobacteria associated with chickpea (Cicer arietinum L.). Int. J. Plant Prod. 2012, 1, 141–152. [Google Scholar] [CrossRef]
- Schmidt, C.; Agostini, F.; Leifert, C.; Killham, K.; Mullins, C. Influence of soil temperature and matric potential on sugar beet seedling colonization and suppression of Pythium damping-off by the antagonistic bacteria Pseudomonas fluorescens and Bacillus subtilis. Phytopathology 2004, 94, 351–363. [Google Scholar] [CrossRef][Green Version]
- Upasani, M.L.; Limaye, B.M.; Gurjar, G.S.; Kasibhatla, S.M.; Joshi, R.R.; Kadoo, N.Y.; Gupta, V.S. Chickpea-Fusarium oxysporum interaction transcriptome reveals differential modulation of plant defense strategies. Sci. Rep. 2017, 7, 7746. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Diaz, R.; Trapero-Casas, A.; De La Colina, J.C. Races of Fusarium oxysporum f. sp. ciceri infecting chickpeas in southern Spain. In Vascular Wilt Diseases of Plants; Springer: Berlin/Heidelberg, Germany, 1989; pp. 515–520. [Google Scholar]
- Bubici, G.; Kaushal, M.; Prigigallo, M.I.; Gómez-Lama Cabanás, C.; Mercado-Blanco, J. Biological control agents against Fusarium wilt of banana. Front. Microbiol. 2019, 10, 616. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.R.; Mane, S. Effect of Different Organic Amendments on Fusarium oxysporum f. sp ciceri Causing Chick Pea Wilt. 2014. Available online: https://pesquisa.bvsalud.org/portal/resource/pt/sea-168214 (accessed on 22 November 2021).
- Verma, J.P.; Yadav, J.; Tiwari, K.N.; Jaiswal, D.K. Evaluation of plant growth promoting activities of microbial strains and their effect on growth and yield of chickpea (Cicer arietinum L.) in India. Soil Biol. Biochem. 2014, 70, 33–37. [Google Scholar] [CrossRef]
- Jousset, A.; Lara, E.; Wall, L.G.; Valverde, C. Secondary metabolites help biocontrol strain Pseudomonas fluorescens CHA0 to escape protozoan grazing. Appl. Environ. Microbiol. 2006, 72, 7083–7090. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yan, H.; Gu, B. Competitive complexation of metal ions with humic substances. Chemosphere 2005, 58, 1327–1337. [Google Scholar] [CrossRef]
- Casares, D.; Escribá, P.V.; Rosselló, C.A. Membrane lipid composition: Effect on membrane and organelle structure, function and compartmentalization and therapeutic avenues. Int. J. Mol. Sci. 2019, 20, 2167. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X.; Zhang, K.; Li, G. Bioactive constituents from the bacteirium Alcaligenes faecalis YMF 3.175. Appl. Biochem. Microbiol. 2015, 51, 52–57. [Google Scholar] [CrossRef]
- Al-Marzoqi, A.H.; Hameed, I.H.; Idan, S.A. Analysis of bioactive chemical components of two medicinal plants (Coriandrum sativum and Melia azedarach) leaves using gas chromatography-mass spectrometry (GC-MS). Afr. J. Biotechnol. 2015, 14, 2812–2830. [Google Scholar]
- Johannes, E.; Litaay, M. The Bioactivity Of Hexadecanoic Acid Compound Isolated From Hydroid Aglaophenia cupressina Lamoureoux As Antibacterial Agent Against Salmonella typhi. Int. J. Biol. Med. Res. 2016, 7, 5469–5472. [Google Scholar]
- Zakaria, N.A.; Ibrahim, D.; Shaida, S.F.; Supardy, N.A. Phytochemical composition and antibacterial potential of hexane extract from Malaysian red algae, Acanthophora spicifera (Vahl) Borgesen. World Appl. Sci. J. 2011, 15, 496–501. [Google Scholar]
- Jain, R.; Pandey, A. A phenazine-1-carboxylic acid producing polyextremophilic Pseudomonas chlororaphis (MCC2693) strain, isolated from mountain ecosystem, possesses biocontrol and plant growth promotion abilities. Microbiol. Res. 2016, 190, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Radhapriya, P.; Ramachandran, A.; Anandham, R.; Mahalingam, S. Pseudomonas aeruginosa RRALC3 enhances the biomass, nutrient and carbon contents of Pongamia pinnata seedlings in degraded forest soil. PLoS ONE 2015, 10, e0139881. [Google Scholar] [CrossRef] [PubMed]
- Durairaj, K.; Velmurugan, P.; Park, J.-H.; Chang, W.-S.; Park, Y.-J.; Senthilkumar, P.; Choi, K.-M.; Lee, J.-H.; Oh, B.-T. Potential for plant biocontrol activity of isolated Pseudomonas aeruginosa and Bacillus stratosphericus strains against bacterial pathogens acting through both induced plant resistance and direct antagonism. FEMS Microbiol. Lett. 2017, 364. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Buch, A. Pseudomonas aeruginosa Predominates as Multifaceted Rhizospheric Bacteria with Combined Abilities of P-solubilization and Biocontrol. J. Pure Appl. Microbiol. 2019, 13, 319–328. [Google Scholar] [CrossRef]
- Chopra, A.; Bobate, S.; Rahi, P.; Banpurkar, A.; Mazumder, P.B.; Satpute, S. Pseudomonas aeruginosa RTE4: A Tea Rhizobacterium With Potential for Plant Growth Promotion and Biosurfactant Production. Front. Bioeng. Biotechnol. 2020, 8, 861. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, X.; Cai, Z.; Guo, L.; Chen, X.; Chen, X.; Liu, J.; Feng, M.; Qiu, Y.; Zhang, Y.; et al. A Biocontrol Strain of Pseudomonas aeruginosa CQ-40 Promote Growth and Control Botrytis cinerea in Tomato. Pathogens 2020, 10, 22. [Google Scholar] [CrossRef]
- Ghadamgahi, F.; Tarighi, S.; Taheri, P.; Saripella, G.V.; Anzalone, A.; Kalyandurg, P.B.; Catara, V.; Ortiz, R.; Vetukuri, R.R. Plant Growth-Promoting Activity of Pseudomonas aeruginosa FG106 and Its Ability to Act as a Biocontrol Agent against Potato, Tomato and Taro Pathogens. Biology 2022, 11, 140. [Google Scholar] [CrossRef]
- Hu, S.; Wang, X.; Sun, W.; Wang, L.; Li, W. In Vitro Study of Biocontrol Potential of Rhizospheric Pseudomonas aeruginosa against Pathogenic Fungi of Saffron (Crocus sativus L.). Pathogens 2021, 10, 1423. [Google Scholar] [CrossRef]
- Berg, G.; Eberl, L.; Hartmann, A. The rhizosphere as a reservoir for opportunistic human pathogenic bacteria. Environ. Microbiol. 2005, 7, 1673–1685. [Google Scholar] [CrossRef]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef]
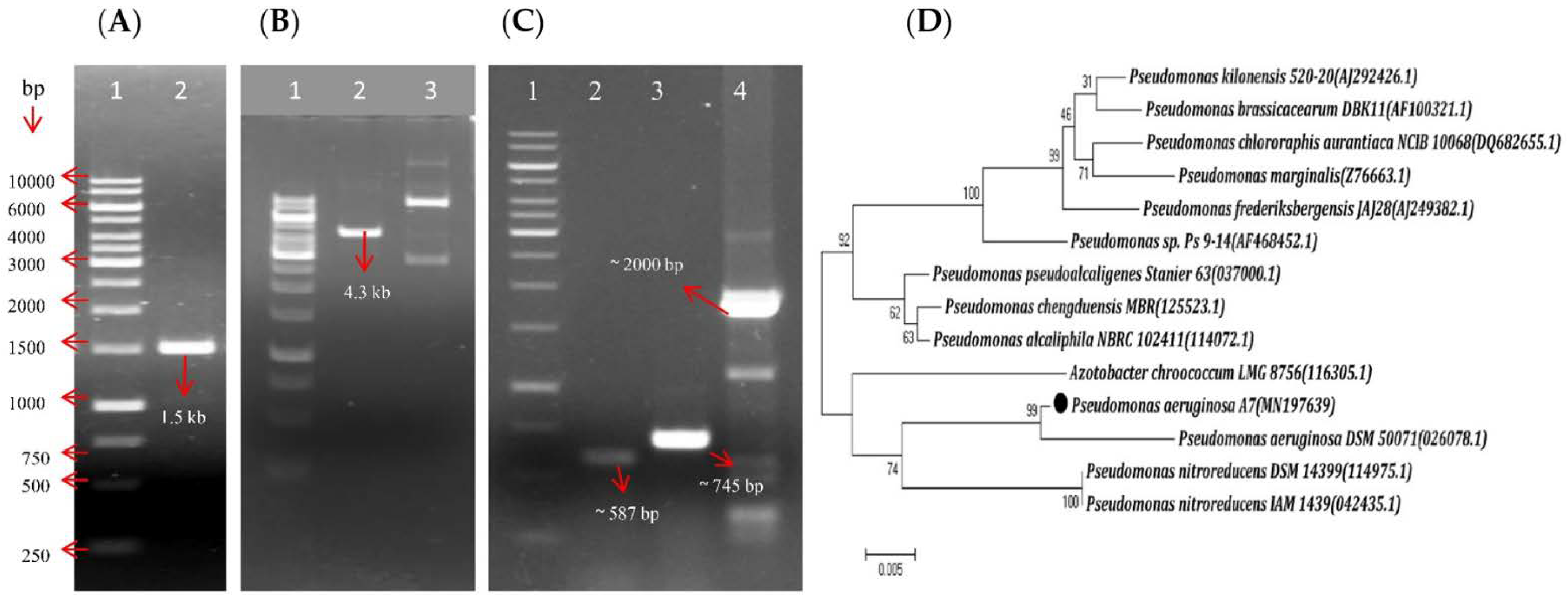
| Literature Name | Primer Sequence | Primer Characters | PCR Condition (°C) | Ref. |
|---|---|---|---|---|
| fD1 | F-5′-GAGTTTGATCCTGGCTCA-3′ | 16S rDNA | ID 98 °C for 30 s, FD 98 °C for 10 s, An 50 °C for 30 s, | [53] |
| rP2 | R-5′-ACGGCTACCTTGTTACGACTT-3′ | Ex 72 °C at 1.30 min, FEx 72 °C for 7 min. | ||
| Phl2a | F-5′-GAGGACGTCGAAGACCACCA-3′ | 2,4-DAPG | ID 94 °C for 1.30 min, FD 94 °C for 35 s, An 53 °C for 30 s, | [53] |
| Phl2b | R-5′-ACCGCAGCATCGTGTATGAG-3′ | Ex 72 °C for 45 s, FEx 72 °C for 10 min. | ||
| Aca | F-5′-ACTGCCAGGGGCGGATGTGC-3′ | HCN | ID 94 °C for 2.30 min, FD 94 °C for 30 s, An 63 °C for 30 s, | [91,92] |
| Acb | R-5′-ACGATGTGCTCGGCGTAC-3′ | Ex 72 °C for 60 s, FEx 72 °C for 10 min. | ||
| PCA2a | F-5′-TTGCCAAGCCTCGCTCCAAC-3′ | PCA | ID 94 °C for 2 min, FD 94 °C for 60 s, An 55 °C for 45 s, | [91] |
| PCA3b | R-5′-CCGCGTTGTTCCTCGTTCAT-3′ | Ex 72 °C for 60 s, FEx 72 °C for 10 min. | ||
| PhzH-up | F-5′-CGCACGGATCCTTTCAGAATGTTC-3′ | PCN | ID 98 °C for 30 s, FD 98 °C for 10 s, An 55 °C for 30 s, | [93] |
| PhzH-low | R-5′-GCCACGCCAAGCTTCACGCTCA-3′ | Ex 70 °C for 7 min, FEx 70 °C for 10 min. | ||
| PLTC1 | F-5′-AACAGATCGCCCCGGTACAGAACG-3′ | PLT | ID 95 °C for 2 min, FD 95 °C for 2 min, An 57 °C for 1 min, | [94] |
| PLTC2 | R-5′-AGGCCCGGACACTCAAGAAACTCG-3′ | Ex 72 °C for 1 min, FEx 72 °C for 7 min. | ||
| PRND1 | F-5′-GGGGCGGGCCGTGGTGATGGA-3′ | PRN | ID 95 °C for 2 min, FD 95 °C for 1 min, An 68 °C for 1 min, | [94] |
| PRND2 | R-5′-YCCCGCSGCCTGYCTGGTCTG-3′ | Ex 72 °C for 1 min, FEx 72 °C for 7 min. | ||
| darAF | F-5′-ATCGTCAATGATCTCTGGC-3′ | HPR, | ID 94 °C for 2 min, FD 94 °C for 1 min, An 50 °C for 1 min, | [95] |
| darAR | R-5′-TTATCCACTGCCTCTCCC-3′ | DARA, | Ex 72 °C for 1 min, FEx 72 °C for 10 min. | |
| darBF | F-5′-GATACTCAGCGAGCGTGC-3′ | HPR, | ID 94 °C for 2 min, FD 94 °C for 1 min, An 50 °C for 1 min, | |
| darBR | R-5′-CACCAGGTTATGGCGTCAG-3′ | DARB | Ex 72 °C for 1 min, FEx 72 °C for 10 min. |
| Morphological Characters | Response | ||
|---|---|---|---|
| Shape | Round | ||
| Gram reaction | Negative, rod shaped | ||
| Color | Green pigmented | ||
| Biochemical | Carbohydrate utilization | ||
| ONGP production | − | Arabinose | + |
| Lysine Utilization | + | Xylose | + |
| Ornithine utilization | − | Adonitol | − |
| Urease production | − | Rhamnose | − |
| Phenylalanine Deamination | − | Cellobiose | − |
| Nitrate reduction | + | Melibiose | + |
| H2S production | − | Saccharose | − |
| Citrate utilization | + | Raffinose | − |
| Voges Proskauer’s test | − | Trehalose | + |
| Methyl red test | − | Glucose | + |
| Indole production | − | Lactose | − |
| Malonate utilization | + | ||
| Esculin hydrolysis | − | ||
| Oxidase production | + | ||
| Fungal Pathogens | PGI (%) a | ||||
|---|---|---|---|---|---|
| Plant Fungal Pathogens | Source of Collection | Diseased Caused | ITCC No | Antifungal Metabolites | Volatile Compounds |
| Fusarium oxysporum f.sp. ciceris (Foc) | Chick pea | Fusarium wilt | 3636 | 78.59 ± 1.1 d | 65.51 ± 1.3 d |
| Fusarium oxysporum f. sp. pisi (Fop) | Pea | Wilt | 4814 | 62.51 ± 1.0 d | N/D c |
| Fusarium oxysporum f. sp. lycopersici (Sacc.) (Fol) | Tomato | Wilt | 1322 | 65.26 ± 1.2 e | 60.37 ± 1.0 e |
| Fusarium oxysporum (Fo) | Maize Stalk | Wilt | 7093 | 60.56 ± 1.2 d | 46.44 ± 1.3 d |
| Fusarium solani (Fs) | Paddy soil | Wilt | 6953 | 70.66 ± 1.2 d | N/D c |
| Rhizoctonia solani (Rs) | Cucumber | Collar rot | AAU | 29.20 ± 1.0 d | 81.25 ± 1.1 d |
| Curvularia eragrostidis (Ce) | Musa | Leaf spot | 5540 | 70.56 ± 1.3 d | 51.00 ± 1.3 d |
| Curvularia lunata (Wakker) Boedijn var. (Clb) | Rice | Leaf spot | 5193 | 42.24 ± 2.6 d | 24.88 ± 2.5 d |
| Rosellinia arcuata Petch (Rap) | Tea bush | Root rot | 4143 | 57.95 ± 2.3 d | 74.99 ± 1.5 d |
| Poria hypobrunnea Petch (Php) | Tea bush | Stem canker | 4141 | 57.26 ± 1.5 d | 35.14 ± 1.4 d |
| Phellinus lamaensis (Murrill) Pat.(Plp) | Tea bush | Brown root rot | 4140 | 51.01 ± 1.7 d | 37.36 ± 1.2 d |
| Ustulina zonata (Lev.) Sacco (Uzs) | Tea bush | Charcoal stump rot | 4144 | 73.34 ± 2.0 d | N/D c |
| Plant fungal and bacterial pathogens | PGI (%) b | ||||
| Rhizoctonia solani (Rs) | Cucumber | Collar rot | AAU | 27.33 ± 1.4 h | |
| Rosellinia arcuata Petch (Rap) | Tea bush | Root rot | 4143 | 33.33 ± 0.8 f | |
| Poria hypobrunnea Petch (Php) | Tea bush | Stem canker | 4141 | 40.33 ± 1.4 h | |
| Ralstonia solanacearum (Ras) | - | Brown rot | AAU | 23.17 ± 1.3 mm f | |
| Xanthomonas campestris (Xc) | - | Black rot | AAU | 34.50 ± 2.0 mm g | |
| Direct and Indirect PGP Traits of A7 | Response | Indole-3-Acetic ACID Production | ||
|---|---|---|---|---|
| Time(h) | With Tryptophan (µg/mL) | Without Tryptophan (µg/mL) | ||
| Phosphate solubilization | 146.00 ± 0.5 µg/mL | 48 | 3.30 ± 0.3 d | 1.30 ± 0.3 d |
| Potassium solubilization | 33% (SE) a | 96 | 6.50 ± 0.3 c | 3.60 ± 0.1 c |
| ZnO solubilization | 29% (SE) a | 144 | 5.40 ± 0.4 c | 2.50 ± 0.0 c |
| Nitrogen Fixation | 52.00 ± 0.05 mm | 196 | 3.10 ± 0.1 e | 2.40 ± 0.2 e |
| Siderophores production | 45.00 ± 0.05 mm | |||
| HCN production | + | Quantitative production of Indole-3-acetic acid at 48 h interval incubation period. Statistical differences were analyzed by two-way analysis of variances (ANOVA). | ||
| Chitinase production | 58.00 ± 0.05 mm | |||
| Cellulase production | 2.4 (HC) b | |||
| Lignin degradation | + | |||
| Pectinase production | 2 (HC) b | |||
| Proteases production | 1.3 (HC) b | |||
| Polyamine production | - | |||
| Amylase production | + | |||
| Xylanase production | - | |||
| Catalase production | + | |||
| AlCl3 | FeCl3 | FeCl2 | NaCl | Temperature | pH | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Conc. (mM) | RG (%) a | Conc. (mM) | RG (%) a | Conc. (mM) | RG (%) a | Conc. (%) | RG (%) a | Temp (°C) | RG (%) a | pH | RG (%) a |
| C | 100.00 ± 0.0 | C | 100.00 ± 0.0 | C | 100.00 ± 0.0 | C | 100.00 ± 0.0 | 4 | 6.35 ± 0.4 | 3 | 0.00 ± 0.0 |
| 0.1 | 96.55 ± 0.7 | 0.1 | 92.03 ± 0.4 | 0.1 | 90.54 ± 0.5 | 1 | 96.98 ± 1.0 | 18 | 33.66 ± 1.4 | 4 | 5.50 ± 0.5 |
| 0.5 | 88.25 ± 0.8 | 0.5 | 87.50 ± 1.0 | 0.5 | 73.38 ± 1.1 | 2 | 85.93 ± 1.0 | 25 | 55.22 ± 1.0 | 5 | 21.59 ± 2.4 |
| 1.0 | 75.00 ± 1.1 | 1.0 | 73.20 ± 0.3 | 1.0 | 54.75 ± 0.6 | 3 | 72.53 ± 1.2 | 30(C) | 100.00 ± 0.0 | 6 | 52.31 ± 2.3 |
| 1.5 | 57.81 ± 1.6 | 1.5 | 64.34 ± 0.8 | 1.5 | 28.78 ± 0.1 | 4 | 57.44 ± 1.8 | 37 | 88.00 ± 2.0 | 7(C) | 100.00 ± 0.0 |
| 2.0 | 50.74 ± 0.3 | 2.0 | 55.07 ± 2.7 | 2.0 | 17.21 ± 0.3 | 5 | 43.51 ± 1.1 | 45 | 36.50 ± 1.5 | 8 | 126.97 ± 2.0 |
| 2.5 | 38.70 ± 0.8 | 2.5 | 48.19 ± 0.4 | 2.5 | 6.08 ± 0.7 | 6 | 22.33 ± 1.0 | 50 | 11.56 ± 0.4 | 9 | 86.50 ± 1.5 |
| 3.0 | 27.63 ± 0.4 | 3.0 | 35.70 ± 0.8 | 3 | 0.00 ± 0.0 | 7 | 8.31 ± 0.3 | 10 | 58.00 ± 2.0 | ||
| 3.5 | 11.94 ± 0.7 | 3.5 | 23.62 ± 0.6 | 8 | 0.00 ± 0.0 | 11 | 24.00 ± 2.0 | ||||
| 4.0 | 0.00 ± 0.0 | 4.0 | 6.93 ± 0.5 | 12 | 11.50 ± 2.5 | ||||||
| 4.5 | 1.36 ± 0.0 | 13 | 2.40 ± 0.5 | ||||||||
| Inhibitory Concentration (IC) (mM) | |||||||||||
| Metal | IC20 b | IC50 c | Metal | IC20 b | IC50 c | Metal | IC20 b | IC50 c | |||
| AlCl3 | 0.76 ± 0.03 | 1.9 ± 0.0 | FeCl3 | 1.00 ± 0.04 | 2.50 ± 0.02 | FeCl2 | 0.47 ± 0.05 | 1.18 ± 0.08 | |||
| Time | Treatment | RL (mm) | SL (mm) | RFW (mg) | SFW (mg) | RDW (mg) | SDW (mg) | SVI a | RRL (%) b |
|---|---|---|---|---|---|---|---|---|---|
| 12 h | T0 | 80 ± 2.0 c | 50 ± 2.9 c | 256 ± 3.0 c | 307 ± 3.6 c | 26 ± 0.8 c | 29 ± 0.8 c | 1290 ± 1.0 c | 100 ± 0.0 f |
| T1 | 76 ± 1.5 c | 50 ± 1.2 c | 227 ± 3.9 c | 292 ± 1.2 d | 25 ± 0.8 c | 27 ± 0.6 c | 1240 ± 2.0 c | 95 ± 1.0 d | |
| T2 | 88 ± 1.4 c | 55 ± 1.4 c | 316 ± 3.4 c | 365 ± 2.6 c | 34 ± 0.5 c | 42 ± 0.8 c | 1445 ± 1.5 c | 100 ± 0.0 f | |
| T3 | 86 ± 1.8 c | 54 ± 0.8 c | 294 ± 2.3 c | 342 ± 1.2 c | 30 ± 0.5 c | 37 ± 0.8 c | 1400 ± 1.0 c | 97 ± 0.3 f | |
| 24 h | T0 | 71 ± 1.4 c | 52 ± 2.6 c | 254 ± 2.0 c | 334 ± 2.6 c | 32 ± 1.2 c | 54 ± 2.3 c | 1210 ± 1.0 c | 100 ± 0.0 f |
| T1 | 58 ± 2.6 f | 47 ± 0.5 c | 224 ± 2.0 c | 313 ± 1.7 f | 24 ± 1.5 c | 36 ± 0.3 c | 1077 ± 1.7 c | 82 ± 3.0 c | |
| T2 | 89 ± 1.4 c | 67 ± 1.0 c | 346 ± 3.1 c | 431 ± 1.4 c | 39 ± 0.5 c | 60 ± 0.3 c | 1580 ± 2.0 c | 100 ± 0.0 f | |
| T3 | 79 ± 2.3 c | 64 ± 0.8 c | 315 ± 2.7 c | 401 ± 2.4 c | 36 ± 3.3 c | 57 ± 0.8 c | 1445 ± 1.5 c | 89 ± 1.1 c | |
| 36 h | T0 | 53 ± 1.1 f | 60 ± 2.4 c | 231 ± 1.7 c | 420 ± 1.2 c | 18 ± 0.4 f | 42 ± 3.3 c | 1060 ± 1.0 c | 100 ± 0.0 f |
| T1 | 47 ± 1.2 e | 42 ± 1.0 d | 215 ± 2.9 c | 242 ± 1.2 e | 16 ± 3.2 f | 27 ± 3.3 c | 825 ± 1.5 d | 88 ± 1.8 c | |
| T2 | 78 ± 3.1c | 73 ± 1.2c | 382 ± 3.8 c | 565 ± 7.7 c | 40 ± 0.8 c | 70 ± 1.2 c | 1530 ± 1.0 c | 100 ± 0.0 f | |
| T3 | 78 ± 3.1 c | 73 ± 1.2 c | 381 ± 3.8 c | 565 ± 7.7 c | 39 ± 0.8 f | 70 ± 1.2 c | 1530 ± 1.0 c | 100 ± 0.0 c | |
| 48 h | T0 | 42 ± 1.7 c | 49 ± 2.5 c | 213 ± 1.5 c | 231 ± 1.2 f | 15 ± 0.9 f | 36 ± 0.3 c | 961.± 1.6 c | 100 ± 0.0 f |
| T1 | 40 ± 1.2 c | 38 ± 0.8 f | 123 ± 1.7 c | 143 ± 1.8 c | 13 ± 0.8 d | 27 ± 0.0 c | 815 ± 0.5 f | 91 ± 1.5 c | |
| T2 | 72 ± 1.4 c | 65 ± 1.7 c | 234 ± 2.0 c | 292 ± 1.4 c | 20 ± 0.8 c | 46 ± 0.5 c | 1400 ± 1.0 c | 100 ± 0.0 f | |
| T3 | 70 ± 0.6 c | 61 ± 1.2 c | 225 ± 2.9 c | 247 ± 4.7 c | 16 ± 0.5 f | 45 ± 0.8 c | 1345 ± 1.5 c | 97 ± 0.3 f |
| After 20th Days of Inoculation | After 40th Days of Inoculation | |||||
|---|---|---|---|---|---|---|
| Treatments | DI (%) a | DS (%) b | DR (%) c | DI (%) a | DS (%) b | DR (%) c |
| T2 | 22.03 ± 0.4 e | 22.43 ± 0.8 e | N/D d | 65.43 ± 1.7 e | 65.54 ± 1.7 e | N/D d |
| T3 | 12.12 ± 1.0 e | 5.95 ± 0.5 f | 44.70 ± 5.2 f | 21.37 ± 1.3 e | 10.29 ± 0.8 f | 67.23 ± 2.3 f |
| T4 | 7.4 ± 0.6 e | 1.85 ± 0.1 g | 66.01 ± 2.9 g | 14.99 ± 0.6 e | 3.92 ± 0.1 g | 77.00 ± 1.5 g |
| T5 | 13.20 ± 1.0 e | 10.48 ± 0.5 e | 39.73 ± 1.6 f | 24.56 ± 1.0 e | 19.38 ± 0.6 e | 62.26 ± 2.5 f |
| Plant Growth Promotion | Plant Biomass | ||||||
|---|---|---|---|---|---|---|---|
| T | RL (cm) | SL (cm) | SVI/100 a | RFW (mg) | SFW (mg) | RDW (mg) | SDW(mg) |
| T0 | 18.66 ± 2.6 b | 49.33 ± 2.0 b | 68.00 ± 4.6 b | 1530.00 ± 26.4 d | 4840.00 ± 173.8 d | 456.00 ± 14.0 d | 1263.33 ± 31.7 d |
| T1 | 25.66 ± 1.7 b | 83.66 ± 3.4 b | 109.33 ± 5.2 b | 2280.00 ± 43.5 a | 5633.33 ± 210.7 a | 853.33 ± 26.0 a | 1756.66 ± 29.6 a |
| T2 | 14.00 ± 2.3 b | 39.33 ± 2.6 b | 53.00 ± 4.9 b | 1157.00 ± 29.8 d | 4030.00 ± 88.8 d | 270.00 ± 17.3 d | 966.66 ± 88.1 d |
| T3 | 22.66 ± 1.7 b | 71.66 ± 2.6 b | 92.66 ± 4.3 b | 1903.33 ± 53.6 b | 4963.33 ± 68.3 b | 665.66 ± 11.0 b | 1363.33 ± 31.7 b |
| T4 | 21.00 ± 1.1 b | 75.33 ± 1.4 b | 96.33 ± 2.6 b | 1984.66 ± 45.1 c | 5240.00 ± 183.3 c | 762.00 ± 14.7 c | 1556.66 ± 29.6 c |
| T5 | 17.00 ± 1.1 b | 65.33 ± 2.0 b | 82.33 ± 3.1 b | 1663.00 ± 31.6 c | 4763.33 ± 87.6 c | 555.66 ± 12.8 c | 1240.00 ± 50.3 c |
| RT | Major Compounds | MM | MF | Activity | Ref. |
|---|---|---|---|---|---|
| 22.04 | Oleic acid | 282 | C18H32O2 | Unknown | |
| 4-fluoro-1-methyl-5-carboxylic acid | 172 | C7H9FN2O2 | Unknown | ||
| 2-tridecenoic acid | 212 | C7H9FN2O2 | Unknown | ||
| 6,10-dimethyl-4-undecanol | 200 | C13H28O | Unknown | ||
| 24.65 | 1-pentadecene | 200 | C15H30 | Unknown | |
| 1-tridecene | 182 | C13H26 | Unknown | ||
| 10-heneicosene (C, T) | 294 | C21H42 | Unknown | ||
| 1-hexadecene | 224 | C16H32 | antibacterial activity | [101] | |
| 28.06 | Tridecanoic acid, 12-methyl-ester | 242 | C15H30O2 | Unknown | |
| Hexadecanoic acid, methyl ester | 270 | C17H34O2 | antioxidant, antibacterial | [102] | |
| Tridecanoic acid, methyl ester | 228 | C14H28O2 | Unknown | ||
| Hexacosanoic acid, methyl ester | 410 | C27H54O2 | Unknown | ||
| 29.70 | 5-Eicosene | 280 | C20H40 | antimicrobial, anticancer | [101] |
| E-15-heptadecenal | 252 | C17H32O | antibacterial activity | ||
| Cycloeicosane | 280 | C20H40 | antimicrobial, anticancer | ||
| 1-heptadecene | 238 | C17H34 | Unknown | ||
| 32.08 | 9-octadecenoic acid | 296 | C19H36O2 | antibacterial activity | [103] |
| 12-octadecenoic acid | 296 | C18H34O2 | Unknown | ||
| Trans-13-octadecenoic acid | 238 | C18H34O2 | Unknown | ||
| 40.04 | 1,2-benzenedicarboxylic acid mono (2-ethylhexyl) ester | 278 | C16H22O4 | antimicrobial, anticancer | [100] |
| 6-ethyloct-3-yl 2-ethylhexyl ester | 418 | C26H42O4 | Unknown | ||
| 1,2-benzenedicarboxylic acid diisooctyl ester, Bis (2-ethylhexyl) phthalate | 390 | C24H38O4 | antifungal | [104] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mozumder, A.B.; Chanda, K.; Chorei, R.; Prasad, H.K. An Evaluation of Aluminum Tolerant Pseudomonas aeruginosa A7 for In Vivo Suppression of Fusarium Wilt of Chickpea Caused by Fusarium oxysporum f. sp. ciceris and Growth Promotion of Chickpea. Microorganisms 2022, 10, 568. https://doi.org/10.3390/microorganisms10030568
Mozumder AB, Chanda K, Chorei R, Prasad HK. An Evaluation of Aluminum Tolerant Pseudomonas aeruginosa A7 for In Vivo Suppression of Fusarium Wilt of Chickpea Caused by Fusarium oxysporum f. sp. ciceris and Growth Promotion of Chickpea. Microorganisms. 2022; 10(3):568. https://doi.org/10.3390/microorganisms10030568
Chicago/Turabian StyleMozumder, Atifa Begum, Kakoli Chanda, Ringhoilal Chorei, and Himanshu Kishore Prasad. 2022. "An Evaluation of Aluminum Tolerant Pseudomonas aeruginosa A7 for In Vivo Suppression of Fusarium Wilt of Chickpea Caused by Fusarium oxysporum f. sp. ciceris and Growth Promotion of Chickpea" Microorganisms 10, no. 3: 568. https://doi.org/10.3390/microorganisms10030568
APA StyleMozumder, A. B., Chanda, K., Chorei, R., & Prasad, H. K. (2022). An Evaluation of Aluminum Tolerant Pseudomonas aeruginosa A7 for In Vivo Suppression of Fusarium Wilt of Chickpea Caused by Fusarium oxysporum f. sp. ciceris and Growth Promotion of Chickpea. Microorganisms, 10(3), 568. https://doi.org/10.3390/microorganisms10030568






