Variable Legionella Response to Building Occupancy Patterns and Precautionary Flushing
Abstract
:1. Introduction
2. Materials and Methods
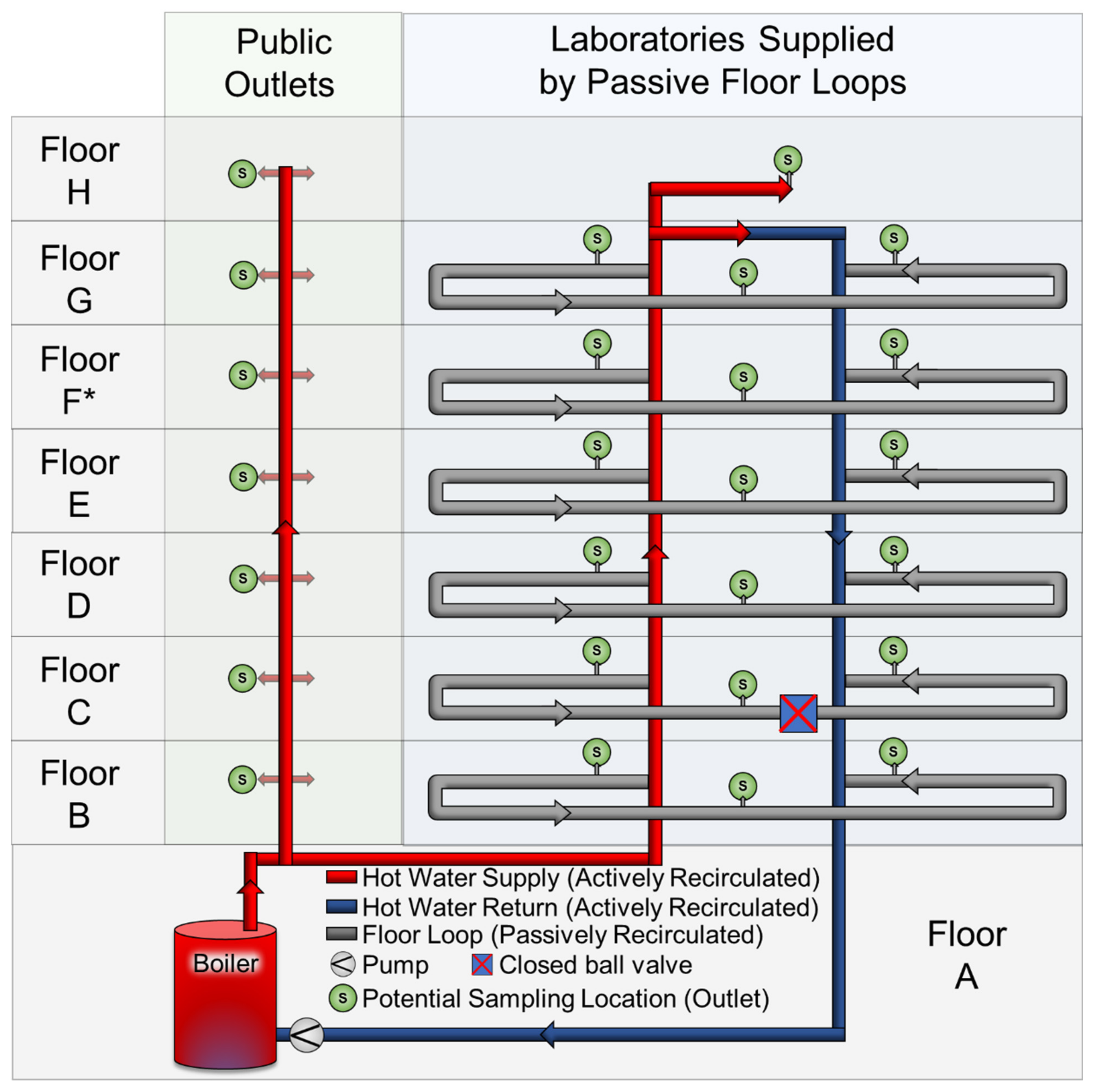
2.1. Site Description
2.2. Low Water Demand Case Study and Experiments
2.2.1. COVID Lockdown
2.2.2. Winter Holiday Breaks
2.2.3. Controlled Stagnation
2.3. Recommissioning Case Study and Experiments
2.3.1. Recommissioning Flushing
2.3.2. Boiler Turnover Flushing
2.4. Sample Processing and Analysis
2.4.1. Flow cytometry and Legionella culture
2.4.2. Sample Filtration and DNA Extraction
2.4.3. Digital Droplet PCR (ddPCR)
2.4.4. Water and Pipe Surface Temperature
2.5. Data Analysis
3. Results
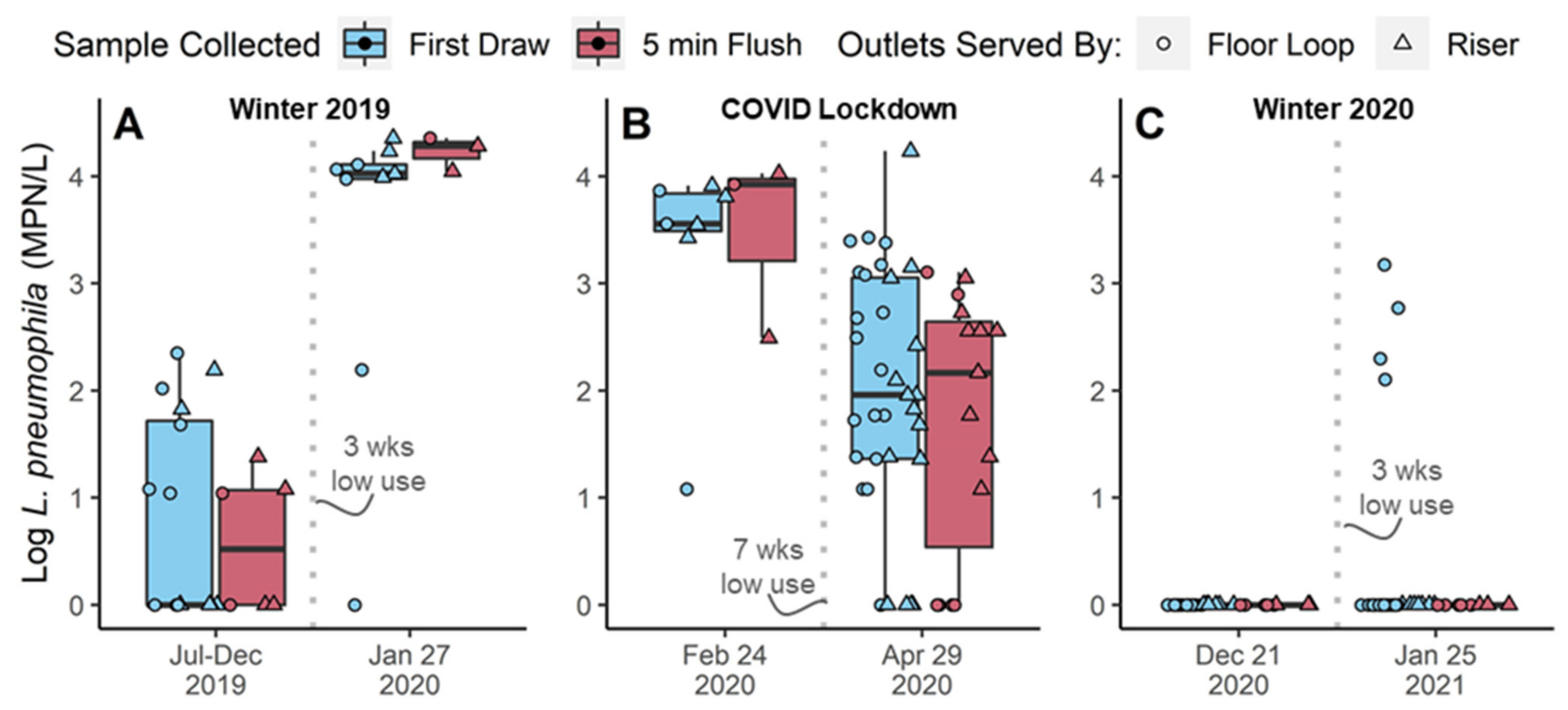
3.1. Low Baseline L. pneumophila Numbers during Normal Building Occupancy
3.2. Low Water Demand Case Study and Experiments
3.2.1. No Legionella Increase Observed after 7-Week COVID-Lockdown
3.2.2. Variable Legionella Response to Winter Holiday Breaks
3.2.3. No Culturable Legionella Detected during Controlled Stagnation
3.3. Pipe Surface Temperature Profiles
3.3.1. Thermal Loss in the Main Recirculation System Indicates Growth Potential
3.3.2. Pipe Temperature Profiles on Floors Indicate Variable Growth Potential
3.4. Recommissioning Case Study and Experiments
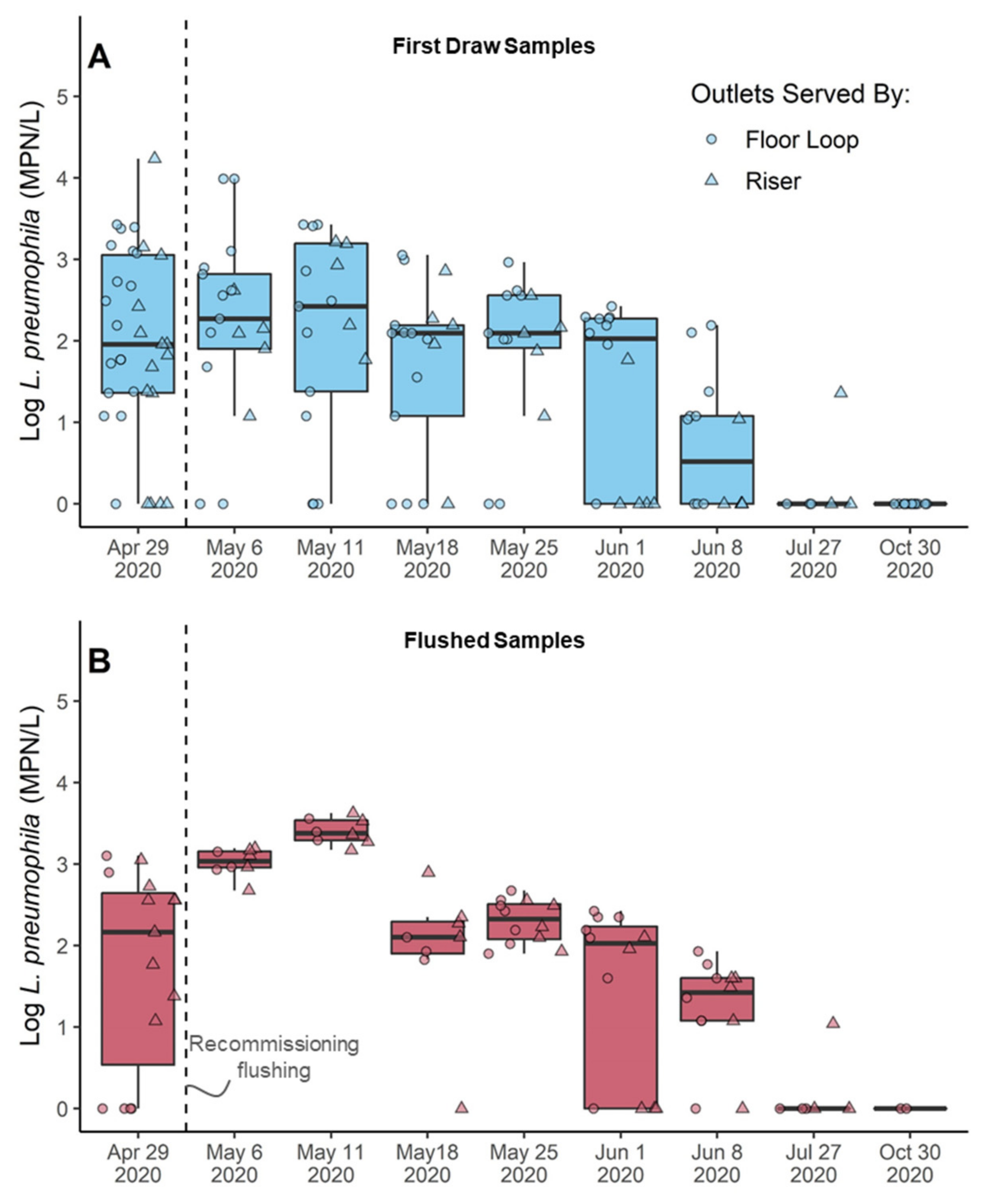
3.4.1. COVID-19 Recommissioning Flushing Temporarily Increased Legionella in the System
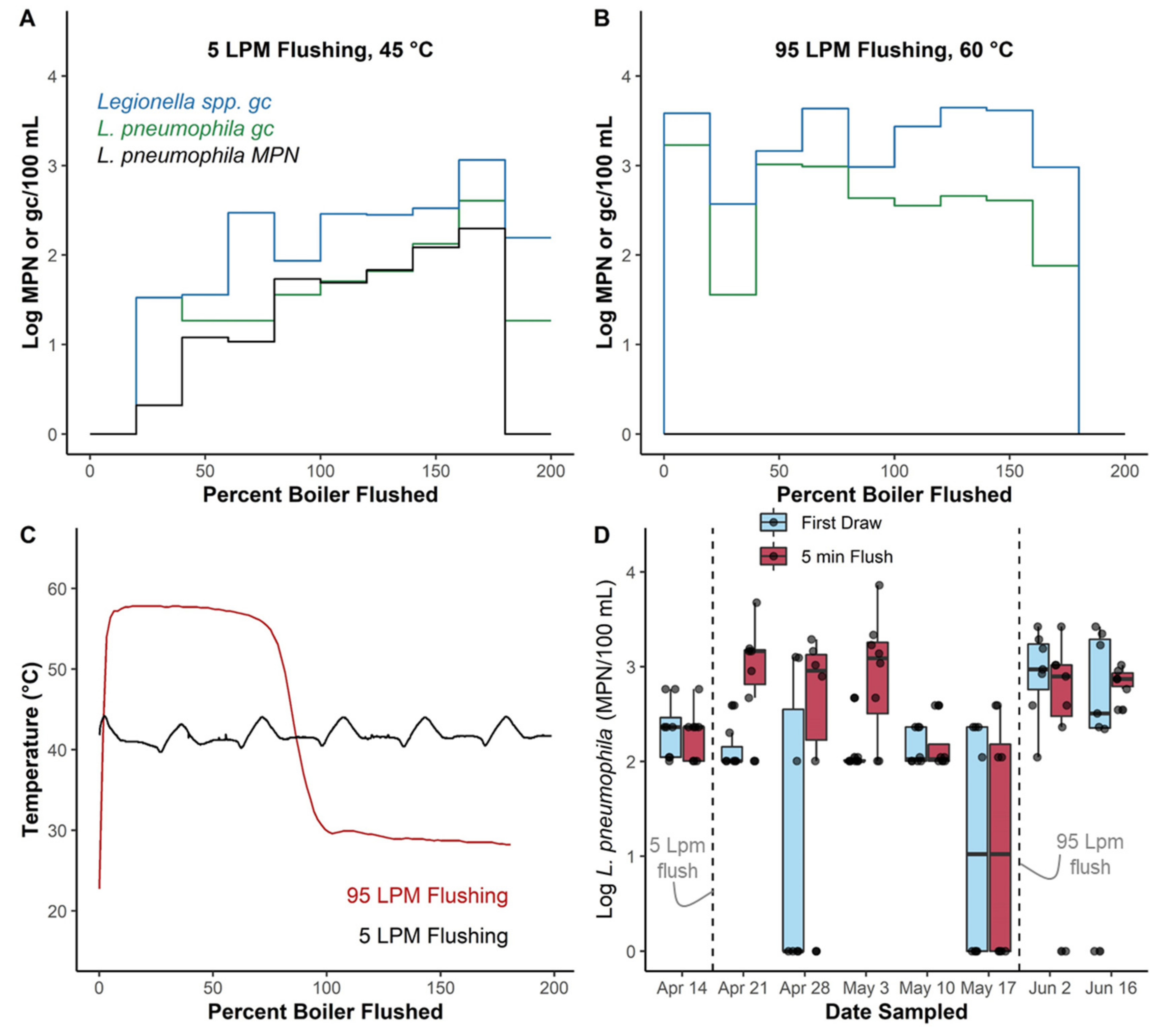
3.4.2. Thermal Barrier in Boiler Was Depleted during Recommissioning Flushing
3.4.3. Overall Bacteria Growth Responded to Changes in Water Demand
3.4.4. Intact Cells Trended Inversely with Legionella, but Not Significantly
3.5. Boiler Flushing Experiments Highlight Potential for Unintended Consequences of Some Flushing Practices
4. Discussion
4.1. Water Demand Alone Is Not an Adequate Predictor of Legionella
4.2. Consideration of Simultaneous Growth Mechanisms Sheds Light on Variable Response
4.3. Future Data Perspective
5. Conclusions
- Reduced water demand associated with low building occupancy does not always cause Legionella growth, even when the building has been historically colonized by Legionella;
- Reduced water demand coincides with myriad other reactions in building plumbing that have to be accounted for when defining or describing building plumbing stagnation. In this study temperature profiles associated with hot water recirculation and convective mixing, nutrient depletion, water use patterns at individual outlets, and external disturbances were hypothesized to contribute to increased Legionella occurrence;
- Some flushing practices have the potential to temporarily increase Legionella occurrence. In this study rapid boiler turnover, high shear sloughing of biofilm associated with flushing many outlets simultaneously, and rapid nutrient influx were hypothesized to contribute to increased Legionella occurrence;
- Pipe surface temperature loggers can offer a non-invasive alternative to in-line probes while still providing continuous performance metrics.
- Future published work should carefully consider and define stagnation, and how it relates to other phenomenon occurring in the systems being studied.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ciesielski, C.A.; Blaser, M.J.; Wang, W.L. Role of Stagnation and Obstruction of Water Flow in Isolation of Legionella Pneumophila from Hospital Plumbing. Appl. Environ. Microbiol. 1984, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Lin, Y.E.; Stout, J.E.; Hwang, C.C.; Vidic, R.D.; Yu, V.L. Effect of Flow Regimes on the Presence of Legionella within the Biofilm of a Model Plumbing System. J. Appl. Microbiol. 2006, 101, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, W.J.; Ji, P.; Pruden, A.; Edwards, M.A. Water Heater Temperature Set Point and Water Use Patterns Influence Legionella Pneumophila and Associated Microorganisms at the Tap. Microbiome 2015, 3, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhoads, W.J.; Pruden, A.; Edwards, M.A. Survey of Green Building Water Systems Reveals Elevated Water Age and Water Quality Concerns. Environ. Sci. Water Res. Technol. 2016, 2, 164–173. [Google Scholar] [CrossRef] [Green Version]
- Bédard, E.; Fey, S.; Charron, D.; Lalancette, C.; Cantin, P.; Dolcé, P.; Laferrière, C.; Déziel, E.; Prévost, M. Temperature Diagnostic to Identify High Risk Areas and Optimize Legionella Pneumophila Surveillance in Hot Water Distribution Systems. Water Res. 2015, 71, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Kool, J.; Bergmire-Sweat, D.; Butler, J.; Brown, E.; Peabody, D.; Massi, D.; Carpenter, J.; Pruckler, J.; Benson, R.; Fields, B. Hospital Characteristics Associated With Colonization of Water Systems by Legionella and Risk of Nosocomial Legionnaires’ Disease: A Cohort Study of 15 Hospitals. Infect. Control Hosp. Epidemiol. Off. J. Soc. Hosp. Epidemiol. Am. 1999, 20, 798–805. [Google Scholar] [CrossRef]
- Völker, S.; Schreiber, C.; Kistemann, T. Modelling Characteristics to Predict Legionella Contamination Risk—Surveillance of Drinking Water Plumbing Systems and Identification of Risk Areas. Int. J. Hyg. Environ. Health 2016, 219, 101–109. [Google Scholar] [CrossRef]
- Rhoads, W.J.; Hammes, F. Growth of Legionella during COVID-19 Lockdown Stagnation. Environ. Sci. Water Res. Technol. 2021, 7, 10–15. [Google Scholar] [CrossRef]
- Sidari III, F.P.; Stout, J.E.; Vanbriesen, J.M.; Bowman, A.M.; Grubb, D.; Neuner, A.; Wagener, M.M.; Yu, V.L. Keeping Legionella out of Water Systems. J. AWWA 2004, 96, 111–119. [Google Scholar] [CrossRef]
- Proctor, C.R.; Rhoads, W.J.; Keane, T.; Salehi, M.; Hamilton, K.; Pieper, K.J.; Cwiertny, D.M.; Prévost, M.; Whelton, A.J. Considerations for Large Building Water Quality after Extended Stagnation. AWWA Water Sci. 2020, 2, e1186. [Google Scholar] [CrossRef]
- CDC Reopening Buildings after Prolonged Shutdown or Reduced Operation. Available online: https://www.cdc.gov/nceh/ehs/water/legionella/building-water-system.html (accessed on 4 January 2022).
- US EPA. Information on Maintaining or Restoring Water Quality in Buildings with Low or No Use. Available online: https://www.epa.gov/coronavirus/information-maintaining-or-restoring-water-quality-buildings-low-or-no-use (accessed on 4 January 2022).
- Esgli Esgli Guidance for Managing Legionella in Dental Practices during the COVID-19 Pandemic. Available online: https://www.escmid.org/fileadmin/src/media/PDFs/3Research_Projects/ESGLI/ESGLI_GUIDANCE_FOR_MANAGING_LEGIONELLA_IN_DENTAL_WATER_SYSTEMS_DURING_THE_COVID-19_PANDEMIC_22042024_v01.01.pdf (accessed on 4 January 2022).
- Horberry, M. After Coronavirus, Office Workers Might Face Unexpected Health Threats The New York Times. 2020. Available online: https://www.nytimes.com/2020/05/20/health/coronavirus-legionnaires-offices.html (accessed on 2 February 2022).
- Morens, D.M.; Taubenberger, J.K.; Fauci, A.S. Predominant Role of Bacterial Pneumonia as a Cause of Death in Pandemic Influenza: Implications for Pandemic Influenza Preparedness. J. Infect. Dis. 2008, 198, 962–970. [Google Scholar] [CrossRef]
- Chertow, D.S.; Memoli, M.J. Bacterial Coinfection in Influenza: A Grand Rounds Review. JAMA 2013, 309, 275–282. [Google Scholar] [CrossRef]
- Donohue, M.J.; O’Connell, K.; Vesper, S.J.; Mistry, J.H.; King, D.; Kostich, M.; Pfaller, S. Widespread Molecular Detection of Legionella Pneumophila Serogroup 1 in Cold Water Taps across the United States. Environ. Sci. Technol. 2014, 48, 3145–3152. [Google Scholar] [CrossRef]
- Margot, C.; Rhoads, W.J.; Graf, N.; Rölli, F.; Hammes, F. Une étude de cas de contrôle des légionelles par la température. Aqua & Gas 31 April 2021. Available online: https://www.aquaetgas.ch/fr/eau/eau-potable-eau-souterraine/20210831_l%C3%A9gionelles-et-eau-potable-%C3%A9tude-de-cas/ (accessed on 2 February 2022).
- SVGW Sicherstellen der Hygiene in ungenutzten Trinkwasserinstallationen. Available online: https://www.aquaetgas.ch/svgw-news/wasser/20200423-faktenblatt-sicherstellen-hygiene/ (accessed on 4 January 2022).
- Sigrist, J.; Hammes, F. Determining the Total Cell Concentration (TCC) in Drinking Water with Flow Cytometry Protocol References. Available online: https://www.protocols.io/view/determining-the-total-cell-concentration-tcc-in-dr-bm38k8rw/references (accessed on 4 January 2022).
- IDEXX Legiolert. Available online: https://www.idexx.com/en/water/water-products-services/legiolert/ (accessed on 21 February 2022).
- Spies, K.; Pleischl, S.; Lange, B.; Langer, B.; Hübner, I.; Jurzik, L.; Luden, K.; Exner, M. Comparison of the Legiolert™/Quanti-Tray® MPN test for the enumeration of Legionella pneumophila from potable water samples with the German regulatory requirements methods ISO 11731-2 and ISO 11731. Int. J. Hyg. Environ. Health 2018, 221, 1047–1053. [Google Scholar] [CrossRef]
- Monteiro, S.N.; Robalo, A.M.; Santos, R.J. Evaluation of Legiolert™ for the Detection of Legionella pneumophila and Comparison with Spread-Plate Culture and qPCR Methods. Curr. Microbiol. 2021, 78, 1792–1797. [Google Scholar] [CrossRef]
- Scaturro, M.; Buffoni, M.; Girolamo, A.; Cristino, S.; Girolamini, L.; Mazzotta, M.; Ricci, M.L. Performance of Legiolert test vs. ISO 11731 to confirm Legionella pneumophila contamination in potable water samples. Pathogens 2020, 9, 690. [Google Scholar] [CrossRef]
- Petrisek, R.; Hall, J. Evaluation of a most probable number method for the enumeration of Legionella pneumophila from North American potable and nonpotable water samples. J. Water Health 2018, 16, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Checa, J.; Carbonell, I.; Manero, N.; Martí, I. Comparative study of Legiolert with ISO 11731-1998 standard method-conclusions from a Public Health Laboratory. J. Microbiol. Methods 2021, 186, 106242. [Google Scholar] [CrossRef]
- Boczek, L.A.; Tang, M.; Formal, C.; Lytle, D.; Ryu, H. Comparison of two culture methods for the enumeration of Legionella pneumophila from potable water samples. J. Water Health 2021, 19, 468–477. [Google Scholar] [CrossRef]
- Thurman, K.A.; Warner, A.K.; Cowart, K.C.; Benitez, A.J.; Winchell, J.M. Detection of Mycoplasma Pneumoniae, Chlamydia Pneumoniae, and Legionella Spp. in Clinical Specimens Using a Single-Tube Multiplex Real-Time PCR Assay. Diagn. Microbiol. Infect. Dis. 2011, 70, 1–9. [Google Scholar] [CrossRef]
- Benitez, A.J.; Winchell, J.M. Clinical Application of a Multiplex Real-Time PCR Assay for Simultaneous Detection of Legionella Species, Legionella Pneumophila, and Legionella Pneumophila Serogroup 1. J. Clin. Microbiol. 2013, 51, 348–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, S.; Jorgensen, F.; Willis, C.; Walker, J. Real-Time PCR to Supplement Gold-Standard Culture-Based Detection of Legionella in Environmental Samples. J. Appl. Microbiol. 2015, 119, 1158–1169. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Swanson, C.S.; Wang, L.; He, Q. Impact of Building Closures during the COVID-19 Pandemic on Legionella Infection Risks. Am. J. Infect. Control 2021, 49, 1564–1566. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Xian, X.; Bao, R.; Zhang, Y.; Feng, M.; Lin, W.; Yu, X. Recovery of Microbiological Quality of Long-Term Stagnant Tap Water in University Buildings during the COVID-19 Pandemic. Sci. Total Environ. 2022, 806, 150616. [Google Scholar] [CrossRef]
- De Giglio, O.; Diella, G.; Lopuzzo, M.; Triggiano, F.; Calia, C.; Pousis, C.; Fasano, F.; Caggiano, G.; Calabrese, G.; Rafaschieri, V.; et al. Impact of Lockdown on the Microbiological Status of the Hospital Water Network during COVID-19 Pandemic. Environ. Res. 2020, 191, 110231. [Google Scholar] [CrossRef]
- Sidari III, F.P.; Stout, J.E.; Duda, S.; Grubb, D.; Neuner, A. Maintaining Legionella Control in Building Water Systems. J. (Am. Water Work. Assoc.) 2014, 106, 24–32. [Google Scholar] [CrossRef]
- Kruse, E.-B.; Wehner, A.; Wisplinghoff, H. Prevalence and Distribution of Legionella Spp in Potable Water Systems in Germany, Risk Factors Associated with Contamination, and Effectiveness of Thermal Disinfection. Am. J. Infect. Control 2016, 44, 470–474. [Google Scholar] [CrossRef]
- Nisar, M.A.; Ross, K.E.; Brown, M.H.; Bentham, R.; Whiley, H. Legionella Pneumophila and Protozoan Hosts: Implications for the Control of Hospital and Potable Water Systems. Pathogens 2020, 9, 286. [Google Scholar] [CrossRef]
- Gavaldà, L.; Garcia-Nuñez, M.; Quero, S.; Gutierrez-Milla, C.; Sabrià, M. Role of Hot Water Temperature and Water System Use on Legionella Control in a Tertiary Hospital: An 8-Year Longitudinal Study. Water Res. 2019, 149, 460–466. [Google Scholar] [CrossRef]
- Boppe, I.; Bédard, E.; Taillandier, C.; Lecellier, D.; Nantel-Gauvin, M.-A.; Villion, M.; Laferrière, C.; Prévost, M. Investigative Approach to Improve Hot Water System Hydraulics through Temperature Monitoring to Reduce Building Environmental Quality Hazard Associated to Legionella. Build. Environ. 2016, 108, 230–239. [Google Scholar] [CrossRef] [Green Version]
- Schrammel, B.; Cervero-Aragó, S.; Dietersdorfer, E.; Walochnik, J.; Lück, C.; Sommer, R.; Kirschner, A. Differential Development of Legionella Sub-Populations during Short- and Long-Term Starvation. Water Res. 2018, 141, 417–427. [Google Scholar] [CrossRef]
- Bouyer, S.; Imbert, C.; Rodier, M.H.; Héchard, Y. Long-term Survival of Legionella Pneumophila Associated with Acanthamoeba Castellanii Vesicles. Environ. Microbiol. 2007, 9, 1341–1344. [Google Scholar] [CrossRef]
- Borella, P.; Montagna, M.T.; Stampi, S.; Stancanelli, G.; Romano-Spica, V.; Triassi, M.; Marchesi, I.; Bargellini, A.; Tatò, D.; Napoli, C.; et al. Legionella Contamination in Hot Water of Italian Hotels. Appl. Environ. Microbiol. 2005, 71, 5805–5813. [Google Scholar] [CrossRef] [Green Version]
- Dietersdorfer, E.; Kirschner, A.; Schrammel, B.; Ohradanova-Repic, A.; Stockinger, H.; Sommer, R.; Walochnik, J.; Cervero-Aragó, S. Starved Viable but Non-Culturable (VBNC) Legionella Strains Can Infect and Replicate in Amoebae and Human Macrophages. Water Res. 2018, 141, 428–438. [Google Scholar] [CrossRef]
- Ji, P.; Rhoads, W.J.; Edwards, M.A.; Pruden, A. Effect of Heat Shock on Hot Water Plumbing Microbiota and Legionella Pneumophila Control. Microbiome 2018, 6, 30. [Google Scholar] [CrossRef]
- Vervaeren, H.; Temmerman, R.; Devos, L.; Boon, N.; Verstraete, W. Introduction of a Boost of Legionella Pneumophila into a Stagnant-Water Model by Heat Treatment. FEMS Microbiol. Ecol. 2006, 58, 583–592. [Google Scholar] [CrossRef] [Green Version]
- Bridier, A.; Briandet, R.; Thomas, V.; Dubois-Brissonnet, F. Resistance of Bacterial Biofilms to Disinfectants: A Review. Biofouling 2011, 27, 1017–1032. [Google Scholar] [CrossRef]
- Wadowsky, R.M.; Yee, R.B.; Mezmar, L.; Wing, E.J.; Dowling, J.N. Hot Water Systems as Sources of Legionella Pneumophila in Hospital and Nonhospital Plumbing Fixtures. Appl. Environ. Microbiol. 1982, 43, 1104–1110. [Google Scholar] [CrossRef] [Green Version]
- Vickers, R.M.; Yu, V.L.; Hanna, S.S.; Muraca, P.; Diven, W.; Carmen, N.; Taylor, F.B. Determinants of Legionella Pneumophila Contamination of Water Distribution Systems: 15-Hospital Prospective Study. Infect. Control Hosp. Epidemiol. 1987, 8, 357–363. [Google Scholar] [CrossRef] [Green Version]
- Garrison, L.E.; Kunz, J.M.; Cooley, L.A.; Moore, M.R.; Lucas, C.; Schrag, S.; Sarisky, C.; Whitney, C.G. Vital Signs: Deficiencies in Environmental Control Identified in Outbreaks of Legionnaires’ Disease—North America, 2000–2014. Am. J. Transplant. 2016, 16, 3049–3058. [Google Scholar] [CrossRef] [Green Version]
- Rhoads, W.J.; Garner, E.; Ji, P.; Zhu, N.; Parks, J.; Schwake, D.O.; Pruden, A.; Edwards, M.A. Distribution System Operational Deficiencies Coincide with Reported Legionnaires’ Disease Clusters in Flint, Michigan. Environ. Sci. Technol. 2017, 51, 11986–11995. [Google Scholar] [CrossRef]
- Rhoads, W.J.; Keane, T.; Spencer, M.S.; Pruden, A.; Edwards, M.A. Did Municipal Water Distribution System Deficiencies Contribute to a Legionnaires’ Disease Outbreak in Quincy, IL? Environ. Sci. Technol. Lett. 2020, 7, 896–902. [Google Scholar] [CrossRef]
- Scanlon, M.M.; Gordon, J.L.; McCoy, W.F.; Cain, M.F. Water Management for Construction: Evidence for Risk Characterization in Community and Healthcare Settings: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 2168. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rhoads, W.J.; Sindelar, M.; Margot, C.; Graf, N.; Hammes, F. Variable Legionella Response to Building Occupancy Patterns and Precautionary Flushing. Microorganisms 2022, 10, 555. https://doi.org/10.3390/microorganisms10030555
Rhoads WJ, Sindelar M, Margot C, Graf N, Hammes F. Variable Legionella Response to Building Occupancy Patterns and Precautionary Flushing. Microorganisms. 2022; 10(3):555. https://doi.org/10.3390/microorganisms10030555
Chicago/Turabian StyleRhoads, William J., Meril Sindelar, Céline Margot, Nadine Graf, and Frederik Hammes. 2022. "Variable Legionella Response to Building Occupancy Patterns and Precautionary Flushing" Microorganisms 10, no. 3: 555. https://doi.org/10.3390/microorganisms10030555
APA StyleRhoads, W. J., Sindelar, M., Margot, C., Graf, N., & Hammes, F. (2022). Variable Legionella Response to Building Occupancy Patterns and Precautionary Flushing. Microorganisms, 10(3), 555. https://doi.org/10.3390/microorganisms10030555




