Continuous Culture of Auxenochlorella protothecoides on Biodiesel Derived Glycerol under Mixotrophic and Heterotrophic Conditions: Growth Parameters and Biochemical Composition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Algal Strain, Medium and Subculture Conditions
2.2. Continuous Culture Conditions
2.3. Analyses
2.4. Calculations
3. Results and Discussion
3.1. Growth Dynamic Parameters under Different Trophic Modes and Feed Glycerol Concentrations
3.2. Proteins, Lipids and Carbohydrates Production
3.2.1. Carbohydrates
3.2.2. Proteins
3.2.3. Lipids
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thompson, J.C.; He, B.B. Characterization of crude glycerol from biodiesel production from multiple feedstocks. Appl. Eng. Agric. 2006, 22, 261–265. [Google Scholar] [CrossRef]
- Yang, F.; Hanna, M.A.; Sun, R. Value-added uses for crude glycerol—A byproduct of biodiesel production. Biotechnol. Biofuels 2012, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-K. Microalgal mass culture systems and methods: Their limitation and potential. J. Appl. Phycol. 2001, 13, 307–315. [Google Scholar] [CrossRef]
- Darienko, T.; Pröschold, T. Genetic variability and taxonomic revision of the genus Auxenochlorella (Shihira et Krauss) Kalina et Puncocharova (Trebouxiophyceae, Chlorophyta). J. Phycol. 2015, 51, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Heredia-Arroyo, T.; Wei, W.; Hu, B. Oil Accumulation via Heterotrophic/Mixotrophic Chlorella protothecoides. Appl. Biochem. Biotechnol. 2010, 162, 1978–1995. [Google Scholar] [CrossRef] [PubMed]
- Krohn, B.J.; McNeff, C.V.; Yan, B.; Nowlan, D. Production of algae-based biodiesel using the continuous catalytic Mcgyan® process. Bioresour. Technol. 2011, 102, 94–100. [Google Scholar] [CrossRef]
- Xu, H.; Miao, X.; Wu, Q. High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J. Biotechnol. 2006, 126, 499–507. [Google Scholar] [CrossRef]
- Patel, A.; Matsakas, L.; Rova, U.; Christakopoulos, P. Heterotrophic cultivation of Auxenochlorella protothecoides using forest biomass as a feedstock for sustainable biodiesel production. Biotechnol. Biofuels 2018, 11, 1–16. [Google Scholar] [CrossRef]
- Del Río, E.; Armendáriz, A.; García-Gómez, E.; García-González, M.; Guerrero, M.G. Continuous culture methodology for the screening of microalgae for oil. J. Biotechnol. 2015, 195, 103–107. [Google Scholar] [CrossRef] [Green Version]
- Coelho, R.S.; Vidotti, A.D.S.; Reis, E.M.; Franco, T.T. High cell density cultures of microalgae under fed-batch and con-tinuous growth. Chem. Eng. Transact. 2014, 38, 313–318. [Google Scholar] [CrossRef]
- Wen, X.; Geng, Y.; Li, Y. Enhanced lipid production in Chlorella pyrenoidosa by continuous culture. Bioresour. Technol. 2014, 161, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, M.; Van Hille, R.P.; Harrison, S.T.L. Lipid productivity, settling potential and fatty acid profile of 11 microalgal species grown under nitrogen replete and limited conditions. J. Appl. Phycol. 2011, 24, 989–1001. [Google Scholar] [CrossRef]
- Bischoff, H.W.; Bold, H.C. Phycological Studies Some Algae from Enchanted Rock and Related Algae Species, 4th ed.; The University of Texas Press: Austin, TX, USA, 1963; Volume 6318, pp. 1–95. [Google Scholar]
- Markou, G.; Diamantis, A.; Korozi, E.; Tsagou, V.; Kefalogianni, I.; Chatzipavlidis, I. Effects of Monochromatic Illumination with LEDs Lights on the Growth and Photosynthetic Performance of Auxenochlorella protothecoides in Photo-and Mixo-trophic Conditions. Plants 2021, 10, 799. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, M.; Garcin, C.; van Hille, R.P.; Harrison, S.T. Interference by pigment in the estimation of microalgal biomass concentration by optical density. J. Microbiol. Methods 2011, 85, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Kochert, G. Carbohydrate determination by phenol-sulfuric acid method. In Handbook of Phycological Methods. Physiological and Biochemical Methods; Hellebust, J.A., Craige, J.S., Eds.; Cambridge University Press: London, UK, 1978; pp. 95–97. [Google Scholar]
- Izard, J.; Limberger, R.J. Rapid screening method for quantitation of bacterial cell lipids from whole cells. J. Microbiol. Methods 2003, 55, 411–418. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Kotzamanis, Y.; Tsironi, T.; Brezas, A.; Grigorakis, K.; Ilia, V.; Vatsos, I.; Romano, N.; Van Eys, J.; Kumar, V. High taurine supplementation in plant protein-based diets improves growth and organoleptic characteristics of European seabass (Dicentrarchus labrax). Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Monod, J. The growth of bacterial cultures. Annu. Rev. Microbiol. 1949, 3, 371–394. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.M.; Zhang, X.W.; Chen, F. Heterotrophic production of biomass and lutein by Chlorella protothecoides on various ni-trogen sources. Enzym. Microb. Technol. 2000, 27, 312–318. [Google Scholar] [CrossRef]
- Gonzalez-Bashan, L.E.; Lebsky, V.K.; Hernandez, J.P.; Bustillos, J.J.; Bashan, Y. Changes in the metabolism of the microalga Chlorella vulgaris when coimmobilized in alginate with the nitrogen-fixing Phyllobacterium myrsinacearum. Can. J. Mirobiol. 2000, 46, 653–659. [Google Scholar] [CrossRef]
- Illman, A.; Scragg, A.; Shales, S. Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzym. Microb. Technol. 2000, 27, 631–635. [Google Scholar] [CrossRef]
- Ethier, S.; Woisard, K.; Vaughan, D.; Wen, Z. Continuous culture of the microalgae Schizochytrium limacinum on biodiesel-derived crude glycerol for producing docosahexaenoic acid. Bioresour. Technol. 2011, 102, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Ezkauriatza, E.; Aguilar-Yáñez, J.M.; Ramírez-Medrano, A.; Alvarez, M. Production of probiotic biomass (Lactobacillus casei) in goat milk whey: Comparison of batch, continuous and fed-batch cultures. Bioresour. Technol. 2010, 101, 2837–2844. [Google Scholar] [CrossRef]
- Wen, Z.Y.; Chen, F. Continuous cultivation of the diatom Nitzschia laevis for eicosapentaenoic acid production: Physio-logical study and process optimization. Proc. Biochem. 2002, 37, 1447–1453. [Google Scholar] [CrossRef]
- Xiong, W.; Li, X.; Xiang, J.; Wu, Q. High-density fermentation of microalga Chlorella protothecoides in bioreactor for microbio-diesel production. Appl. Microbiol. Biotechnol. 2007, 78, 29–36. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, D.; Zhang, Y.; Chen, F. Glucose triggers cell structure changes and regulates astaxanthin biosynthesis in Chromochloris zofingiensis. Algal Res. 2019, 39, 101455. [Google Scholar] [CrossRef]
- Schulze, P.S.; Barreira, L.A.; Pereira, H.; Perales, J.A.; Varela, J.C. Light emitting diodes (LEDs) applied to microalgal production. Trends Biotechnol. 2014, 32, 422–430. [Google Scholar] [CrossRef]
- Ra, C.-H.; Kang, C.-H.; Jung, J.-H.; Jeong, G.-T.; Kim, S.-K. Effects of light-emitting diodes (LEDs) on the accumulation of lipid content using a two-phase culture process with three microalgae. Bioresour. Technol. 2016, 212, 254–261. [Google Scholar] [CrossRef]
- Xiao, Y.; Guo, J.; Zhu, H.; Muhammad, A.; Deng, H.; Hu, Z.; Wu, Q. Inhibition of glucose assimilation in Auxenochlorella protothecoides by light. Biotechnol. Biofuels 2020, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Haass, D.; Tanner, W. Regulation of hexose transport in Chlorella vulgaris: Characteristics of induction and turnover. Plant. Physiol. 1974, 53, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Pastore, M.; Santaeufemia, S.; Bertucco, A.; Sforza, E. Light intensity affects the mixotrophic carbon exploitation in Chlorella protothecoides: Consequences on microalgae-bacteria based wastewater treatment. Water Sci. Technol. 2018, 78, 1762–1771. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Quinn, A.H.; Sriram, G. Experimental evidence and isotopomer analysis of mixotrophic glucose metabolism in the marine diatom Phaeodactylum tricornutum. Microb. Cell Factories 2013, 12, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harwati, T.U.; Willke, T.; Vorlop, K.D. Characterization of the lipid accumulation in a tropical freshwater microalgae Chlorococcum sp. Bioresour. Technol. 2012, 121, 54–60. [Google Scholar] [CrossRef]
- Wilhelm, C.; Jakob, T. From photons to biomass and biofuels: Evaluation of different strategies for the improvement of algal biotechnology based on comparative energy balances. Appl. Microbiol. Biotechnol. 2011, 92, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Markou, G.; Angelidaki, I.; Georgakakis, D. Microalgal carbohydrates: An overview of the factors influencing carbohydrates production, and of main bioconversion technologies for production of biofuels. Appl. Microbiol. Biotechnol. 2012, 96, 631–645. [Google Scholar] [CrossRef]
- Bohutskyi, P.; Ketter, B.; Chow, S.; Adams, K.J.; Betenbaugh, M.J.; Allnutt, F.C.T.; Bouwer, E.J. Anaerobic digestion of li-pid-extracted Auxenochlorella protothecoides biomass for methane generation and nutrient recovery. Bioresour. Technol. 2015, 183, 229–239. [Google Scholar] [CrossRef] [Green Version]
- Canelli, G.; Neutsch, L.; Carpine, R.; Tevere, S.; Giuffrida, F.; Rohfritsch, Z.; Dionisi, F.; Bolten, C.J.; Mathys, A. Chlorella vulgaris in a heterotrophic bi-oprocess: Study of the lipid bioaccessibility and oxidative stability. Algal Res. 2020, 45, 101754. [Google Scholar] [CrossRef]
- D’Alesandro, E.B.; Antoniosi Filho, N.R. Concepts and studies on lipid and pigments of microalgae: A review. Renew. Sustain. Energy Rev. 2016, 58, 832–841. [Google Scholar] [CrossRef]
- García, J.R.; Fernández, F.A.; Sevilla, J.M.F. Development of a process for the production of l-amino-acids concentrates from microalgae by enzymatic hydrolysis. Bioresour. Technol. 2012, 112, 164–170. [Google Scholar] [CrossRef]
- Markou, G.; Kougia, E.; Kefalogianni, I.; Tsagou, V.; Arapoglou, D.; Chatzipavlidis, I. Effect of Glycerol Concentration and Light Intensity on Growth and Biochemical Composition of Arthrospira (Spirulina) Platensis: A Study in Semi-Continuous Mode with Non-Aseptic Conditions. Appl. Sci. 2019, 9, 4703. [Google Scholar] [CrossRef] [Green Version]
- Lane, C.D.; Coury, D.A.; Allnutt, F.C.T. Composition and potential products from Auxenochlorella protothecoides, Chlorella sorokiniana and Chlorella vulgaris. Ind. Biotechnol. 2017, 13, 270–276. [Google Scholar] [CrossRef]
- Bekirogullari, M.; Bekirogullari, M.; Torres, G.M.F.; Torres, G.M.F.; Pittman, J.K.; Pittman, J.K.; Theodoropoulos, C.; Theodoropoulos, C. Models of microalgal cultivation for added-value products-A review. Biotechnol. Adv. 2020, 44, 107609. [Google Scholar] [CrossRef]
- Gao, C.; Wang, Y.; Shen, Y.; Yan, D.; He, X.; Dai, J.; Wu, Q. Oil accumulation mechanisms of the oleaginous microalga Chlorella protothecoides revealed through its genome, transcriptomes, and proteomes. BMC Genom. 2014, 15, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Johns, M.R. Effect of C/N ratio and aeration on the fatty acid composition of heterotrophicChlorella sorokiniana. J. Appl. Phycol. 1991, 3, 203–209. [Google Scholar] [CrossRef]
- Safdar, W.; Shamoon, M.; Zan, X.; Haider, J.; Sharif, H.R.; Shoaib, M.; Song, Y. Growth kinetics, fatty acid composition and metabolic activity changes of Crypthecodinium cohnii under different nitrogen source and concentration. AMB Express 2017, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rismani-Yazdi, H.; Hampel, K.H.; Lane, C.D.; Kessler, B.A.; White, N.M.; Moats, K.M.; Allnutt, F.C.T. High-productivity lipid production using mixed trophic state cultivation of Auxenochlorella (Chlorella) protothecoides. Bioprocess Biosyst. Eng. 2014, 38, 639–650. [Google Scholar] [CrossRef] [PubMed]
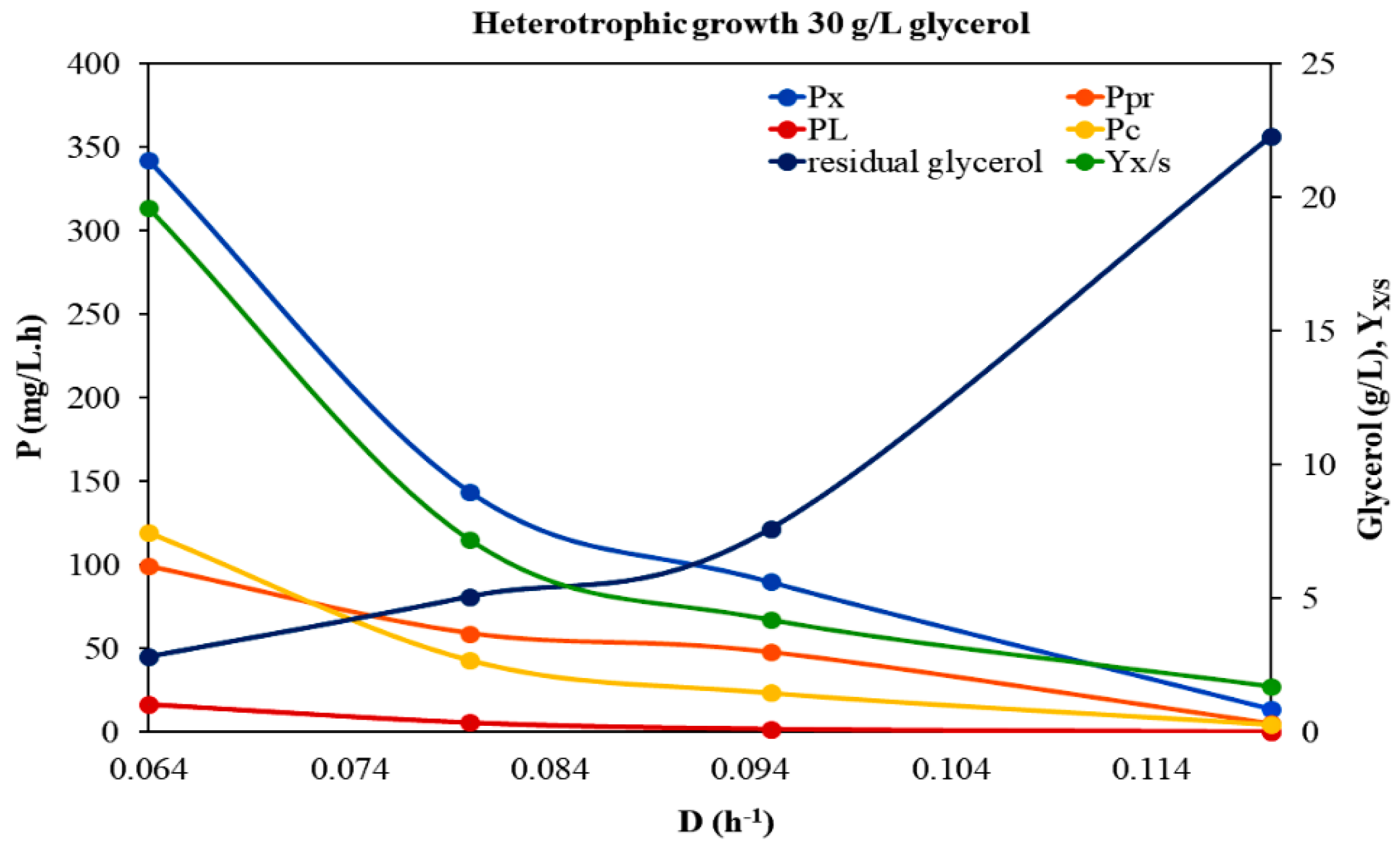

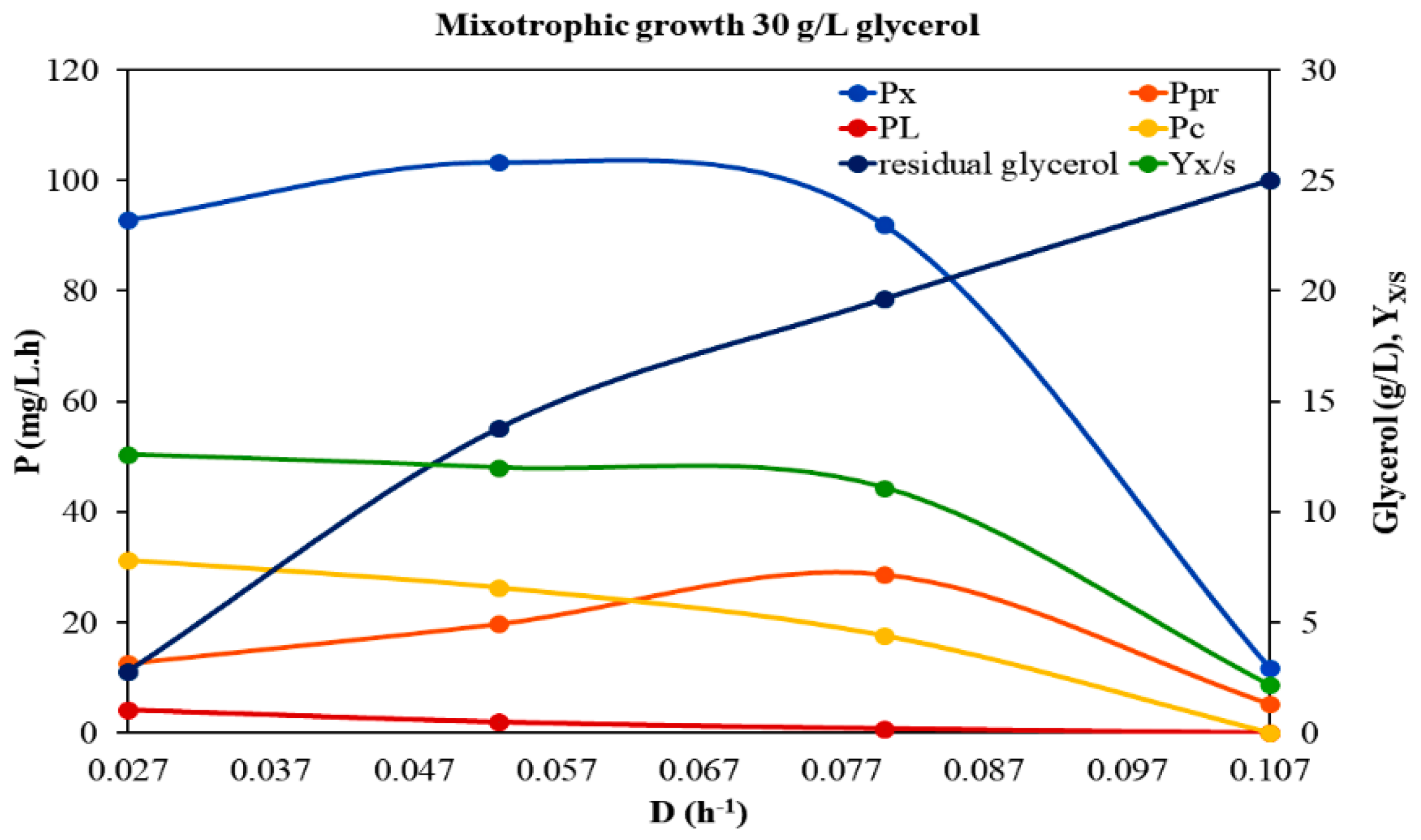
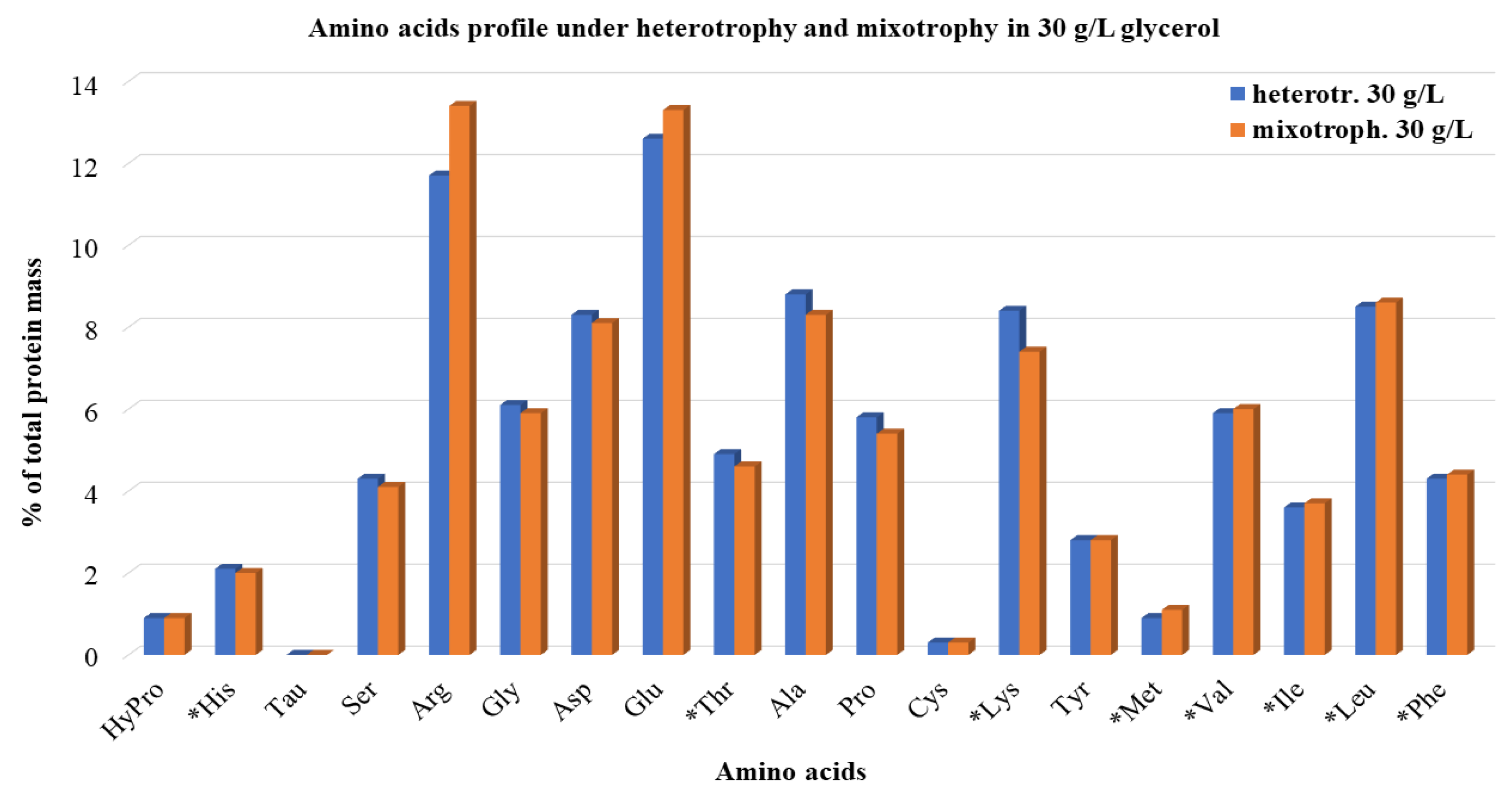
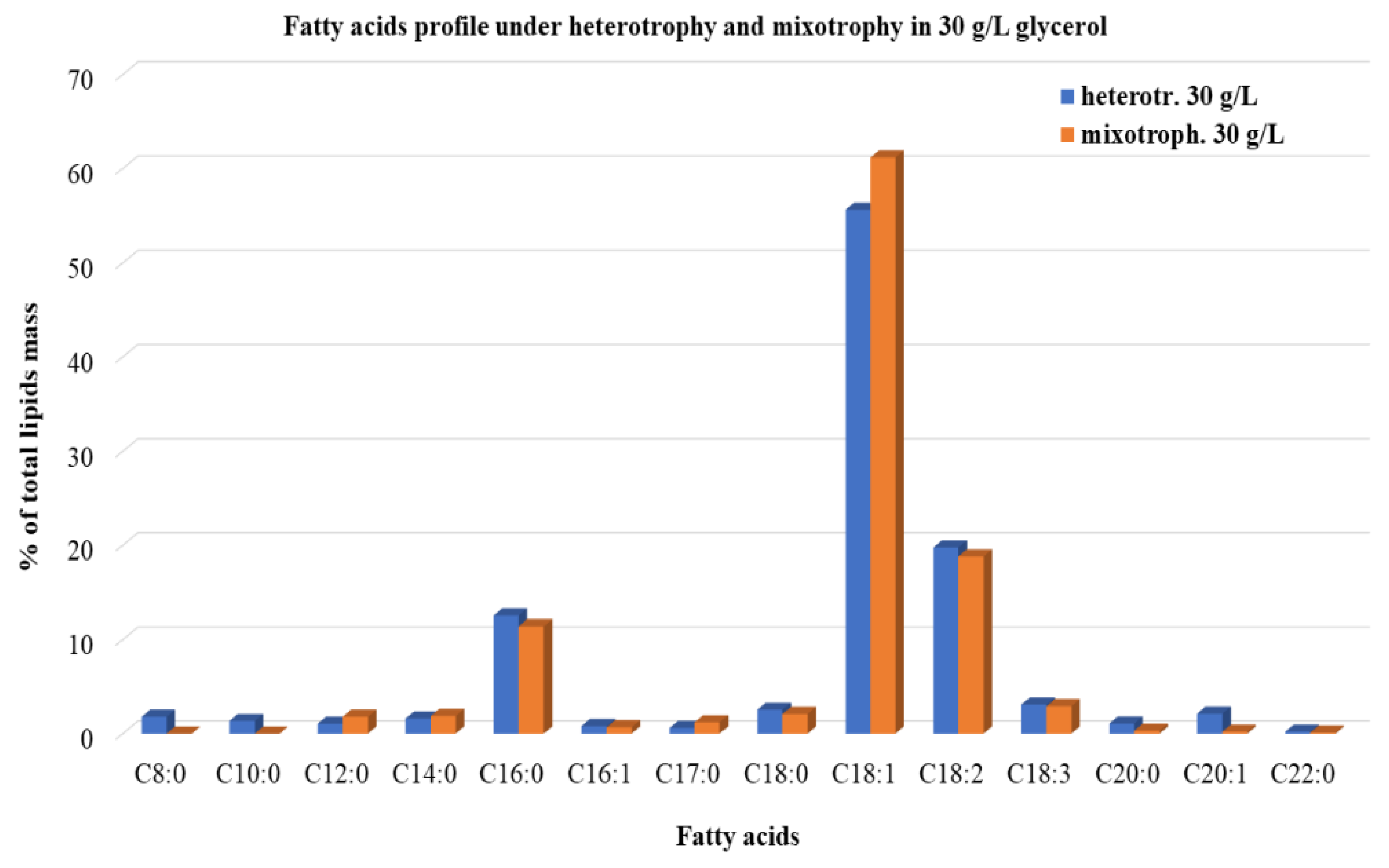
| Heterotrophic Growth 30 g/L Glycerol | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D (h−1) | Biomass (x) 1 (d.w. g/L) | Proteins (g/L) | Proteins 2 (% d.w.) | Lipids (g/L) | Lipids 2 (% d.w.) | Carbohydrates (g/L) | Carboh.2 (% d.w.) | Yx/S | Px (mg/L·h) | PPr (mg/L·h) | PL (mg/L·h) | Pc (mg/L·h) |
| 0.064 | 5.341 ± 0.689 | 1.549 ± 0.175 | 29 | 0.2560 ± 0.0478 | 4.8 | 1.8694 ± 0.137 | 35 | 0.196 | 342 | 99.14 | 16.38 | 119.64 |
| 0.080 | 1.800 ± 0.236 | 0.738 ± 0.159 | 41 | 0.0710 ± 0.0210 | 3.9 | 0.5400 ± 0.080 | 30 | 0.072 | 144 | 59.04 | 5.62 | 43.2 |
| 0.095 | 0.950 ± 0.122 | 0.500 ± 0.089 | 53 | 0.0193 ± 0.0023 | 2.0 | 0.2470 ± 0.061 | 26 | 0.042 | 90 | 47.7 | 1.80 | 23.47 |
| 0.120 | 0.130 ± 0.022 | 0.046 ± 0.013 | 35 | 0.0010 ± 0.0006 | 0.8 | 0.0377 ± 0.014 | 29 | 0.017 | 14 | 4.9 | 0.10 | 4.52 |
| Heterotrophic Growth 50 g/L Glycerol | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D (h−1) | Biomass (×) 1 (d.w. g/L) | Proteins (g/L) | Proteins 2 (% d.w.) | Lipids (g/L) | Lipids 2 (% d.w.) | Carbohydrates (g/L) | Carboh.2 (% d.w.) | Yx/S | Px (mg/L·h) | PPr (mg/L·h) | PL (mg/L·h) | Pc (mg/L·h) |
| 0.027 | 7.888 ± 1.072 | 1.36 ± 0.13 | 17.2 | 0.670 ± 0.088 | 3.5 | 3.518 ± 0.206 | 44.6 | 0.163 | 212.98 | 36.72 | 18.09 | 94.99 |
| 0.040 | 6.850 ± 1.168 | 1.31 ± 0.16 | 19.1 | 0.548 ± 0.077 | 4.5 | 2.925 ± 0.241 | 42.7 | 0.144 | 274.00 | 52.40 | 21.92 | 117.00 |
| 0.067 | 2.750 ± 0.923 | 0.72 ± 0.15 | 26.2 | 0.270 ± 0.042 | 9.8 | 1.015 ± 0.175 | 36.9 | 0.062 | 184.25 | 48.24 | 18.09 | 68.00 |
| 0.093 | 1.380 ± 0.259 | 0.47 ± 0.12 | 34.4 | 0.080 ± 0.016 | 5.8 | 0.520 ± 0.147 | 37.7 | 0.035 | 128.34 | 43.71 | 7.44 | 48.36 |
| 0.120 | 0.780 ± 0.085 | 0.31 ± 0.07 | 40.0 | 0.017 ± 0.005 | 2.2 | 0.229 ± 0.041 | 29.4 | 0.027 | 93.60 | 37.20 | 2.04 | 27.48 |
| 0.147 | 0.167 ± 0.020 | 0.06 ± 0.01 | 35.0 | 0.004 ± 0.001 | 2.4 | 0.050± 0.013 | 30.2 | 0.020 | 24.55 | 8.82 | 0.59 | 7.35 |
| Mixotrophic Growth 30 g/L glycerol | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D (h−1) | Biomass (×) 1 (d.w. g/L) | Proteins (g/L) | Proteins 2 (% d.w.) | Lipids (g/L) | Lipids 2 (% d.w.) | Carbohydrates (g/L) | Carboh 2 (% d.w.) | Yx/S | Px (mg/L·h) | PPr (mg/L·h) | PL (mg/L·h) | Pc (mg/L·h) |
| 0.027 | 3.44 ± 0.397 | 0.46440 ± 0.0352 | 13.50 | 0.15136 ± 0.0367 | 4.40 | 1.162 ± 0.158 | 33.78 | 0.126 | 92.88 | 12.54 | 4.09 | 31.37 |
| 0.053 | 1.95 ± 0.132 | 0.37226 ± 0.02665 | 19.09 | 0.03705 ± 0.01078 | 1.90 | 0.498 ± 0.173 | 25.54 | 0.120 | 103.35 | 19.73 | 1.96 | 26.39 |
| 0.080 | 1.15 ± 0.229 | 0.35892 ± 0.02692 | 31.21 | 0.00978 ± 0.00238 | 0.85 | 0.221 ± 0.033 | 19.26 | 0.111 | 92.00 | 28.71 | 0.78 | 17.68 |
| 0.107 | 0.11 ± 0.035 | 0.04890 ± 0.00593 | 44.45 | 0.00018 ± 0.00005 | 0.16 | 0 | 0 | 0.022 | 11.77 | 5.23 | 0.019 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korozi, E.; Tsagou, V.; Kefalogianni, I.; Markou, G.; Antonopoulos, D.; Chakalis, L.; Kotzamanis, Y.; Chatzipavlidis, I. Continuous Culture of Auxenochlorella protothecoides on Biodiesel Derived Glycerol under Mixotrophic and Heterotrophic Conditions: Growth Parameters and Biochemical Composition. Microorganisms 2022, 10, 541. https://doi.org/10.3390/microorganisms10030541
Korozi E, Tsagou V, Kefalogianni I, Markou G, Antonopoulos D, Chakalis L, Kotzamanis Y, Chatzipavlidis I. Continuous Culture of Auxenochlorella protothecoides on Biodiesel Derived Glycerol under Mixotrophic and Heterotrophic Conditions: Growth Parameters and Biochemical Composition. Microorganisms. 2022; 10(3):541. https://doi.org/10.3390/microorganisms10030541
Chicago/Turabian StyleKorozi, Evagelina, Vasiliki Tsagou, Io Kefalogianni, Giorgos Markou, Dimitris Antonopoulos, Lambis Chakalis, Yannis Kotzamanis, and Iordanis Chatzipavlidis. 2022. "Continuous Culture of Auxenochlorella protothecoides on Biodiesel Derived Glycerol under Mixotrophic and Heterotrophic Conditions: Growth Parameters and Biochemical Composition" Microorganisms 10, no. 3: 541. https://doi.org/10.3390/microorganisms10030541
APA StyleKorozi, E., Tsagou, V., Kefalogianni, I., Markou, G., Antonopoulos, D., Chakalis, L., Kotzamanis, Y., & Chatzipavlidis, I. (2022). Continuous Culture of Auxenochlorella protothecoides on Biodiesel Derived Glycerol under Mixotrophic and Heterotrophic Conditions: Growth Parameters and Biochemical Composition. Microorganisms, 10(3), 541. https://doi.org/10.3390/microorganisms10030541







