A Parallel Tracking of Salivary and Gut Microbiota Profiles Can Reveal Maturation and Interplay of Early Life Microbial Communities in Healthy Infants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects and Sample Collection
2.2. Fecal and Salivary Microbiota Profiles
2.3. Biocomputational and Statistical Analyses
3. Results
3.1. Microbiota Profiling
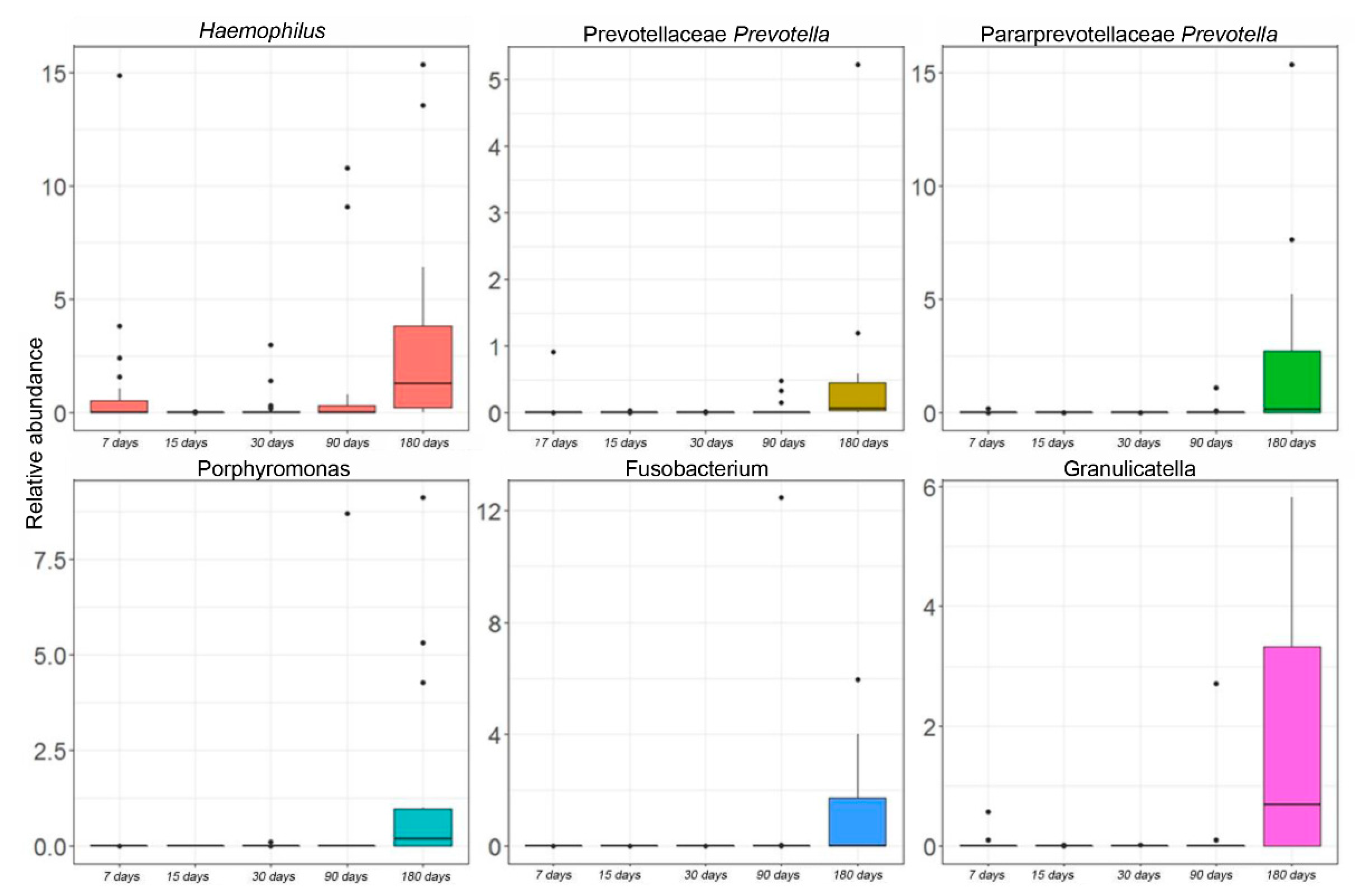
3.2. Salivary Microbiota Profiling
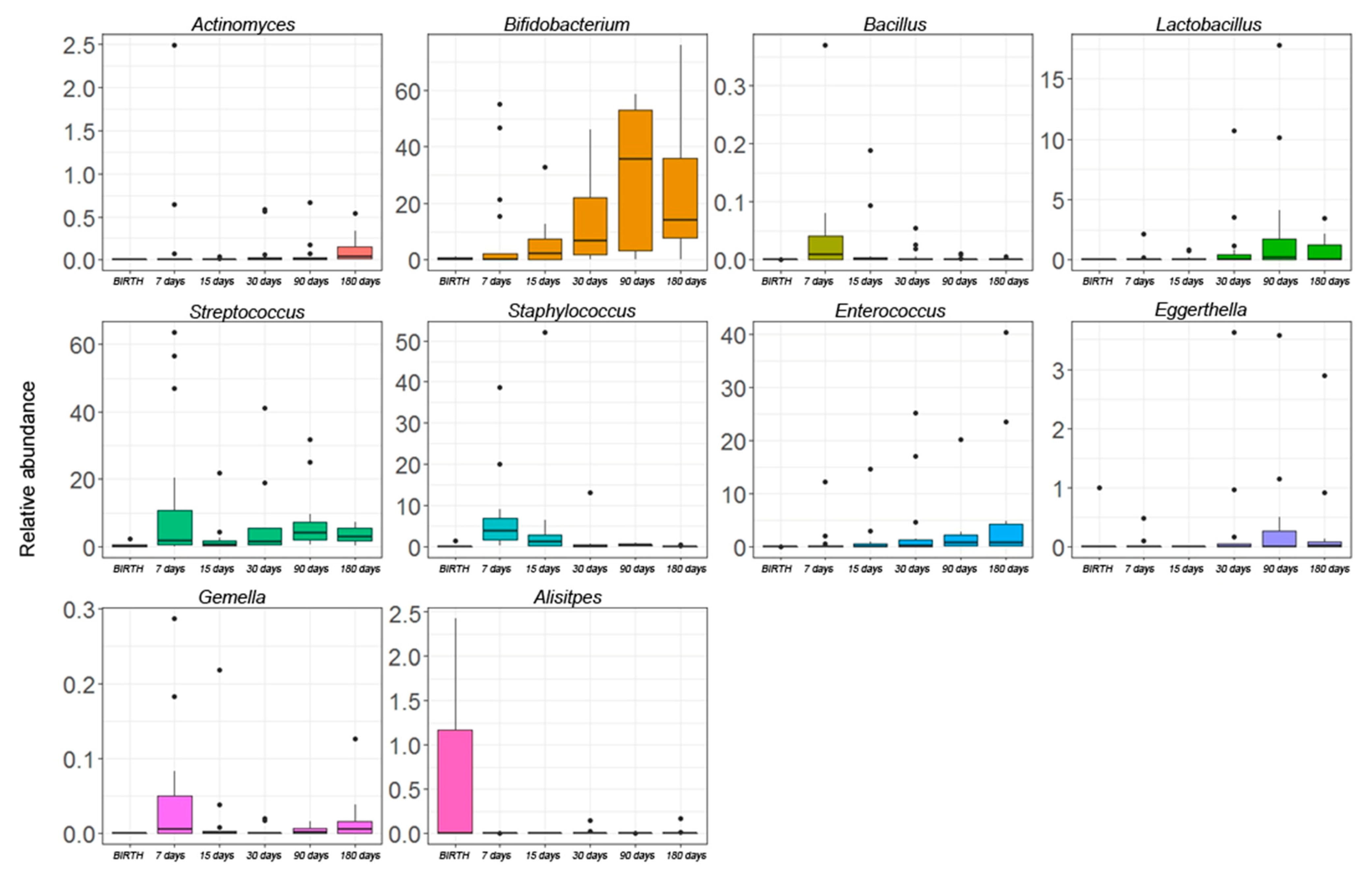
3.3. Gut Microbiota Profiling
3.4. OTUs at Genus Level Shared between Gut and Salivary Microbiota
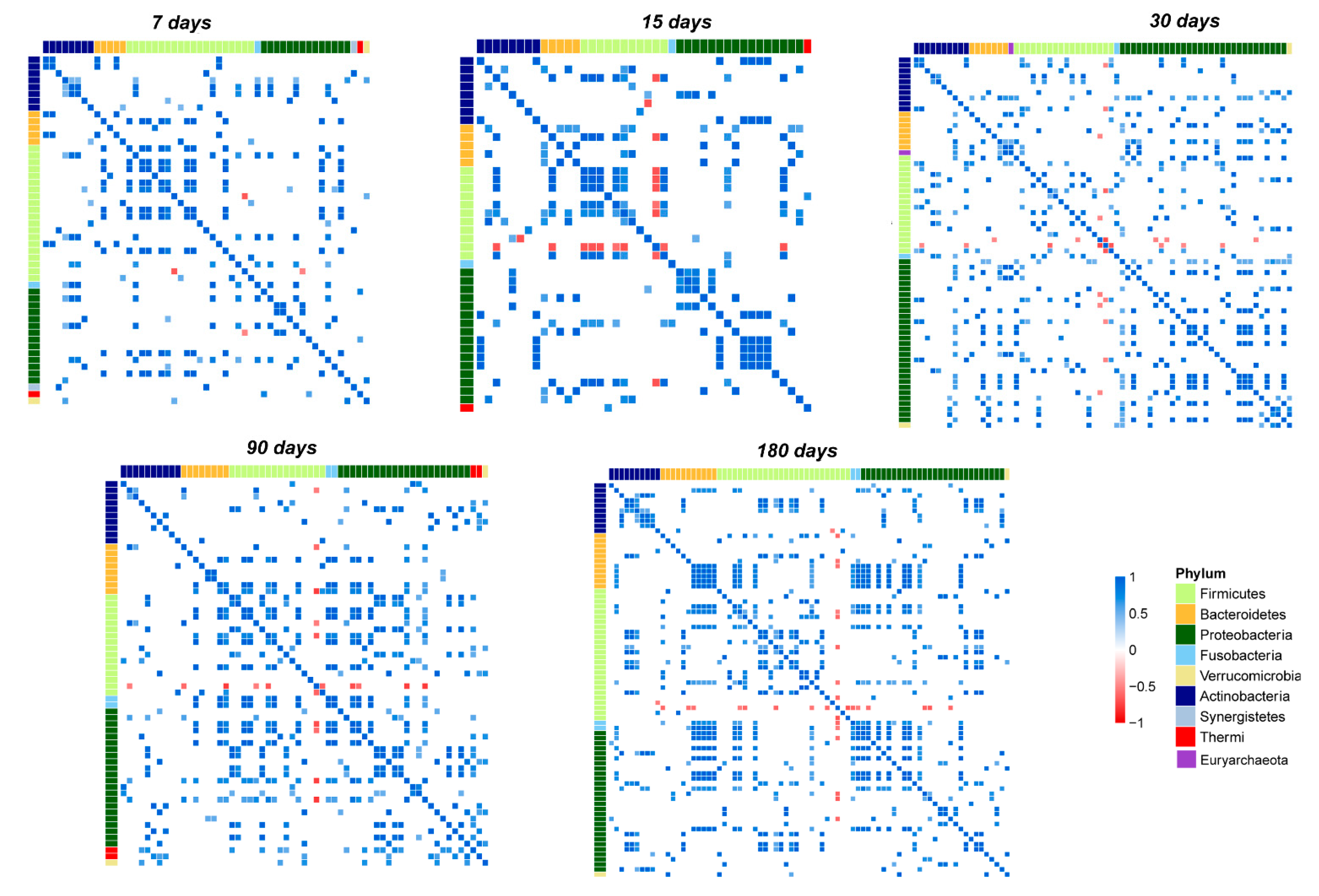
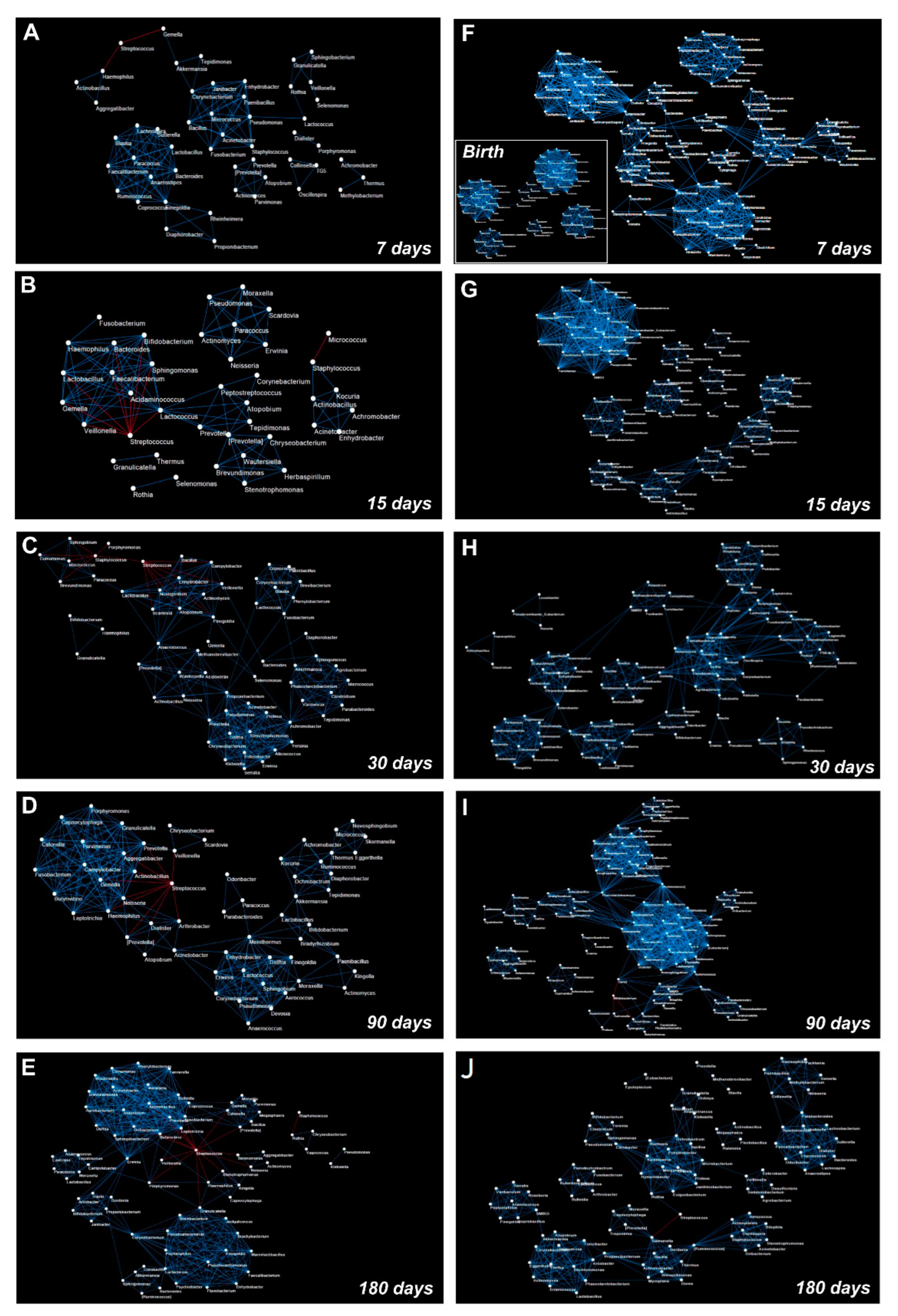
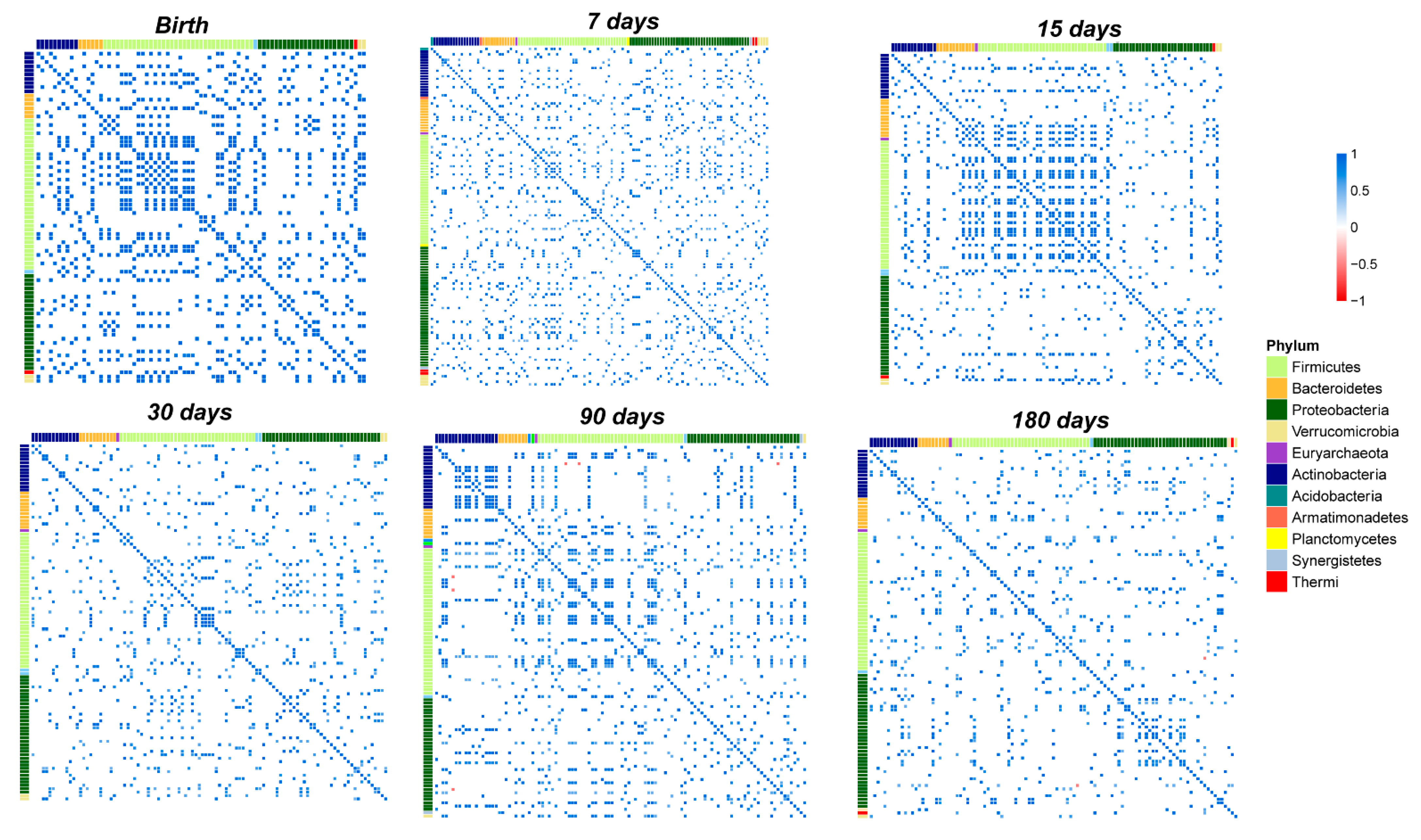
3.5. OTUs Correlations and Network Analysis in the Salivary Microbiota
3.6. OTUs Correlations and Network Analysis in the Gut Microbiota
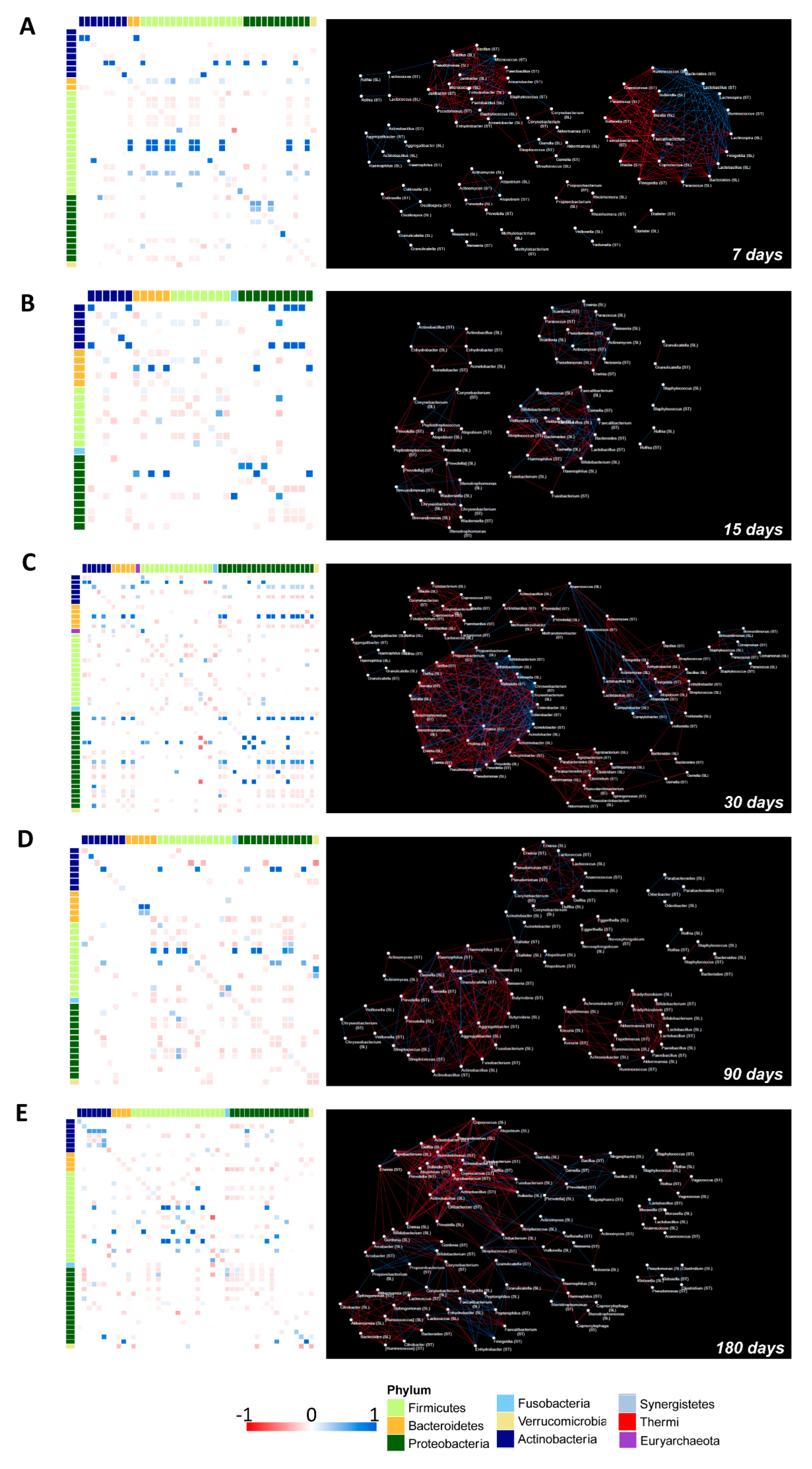
3.7. OTUs Correlations and Co-Occurrence Network Analysis between Salivary and Gut Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Penders, J.; Thijs, C.; Vink, C.; Stelma, F.F.; Snijders, B.; Kummeling, I.; van den Brandt, P.A.; Stobberingh, E.E. Factors Influencing the Composition of the Intestinal Microbiota in Early Infancy. Pediatrics 2006, 118, 511–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery Mode Shapes the Acquisition and Structure of the Initial Microbiota across Multiple Body Habitats in Newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bokulich, N.A.; Chung, J.; Battaglia, T.; Henderson, N.; Jay, M.; Li, H.; Lieber, A.D.; Wu, F.; Perez-Perez, G.I.; Chen, Y.; et al. Antibiotics, Birth Mode, and Diet Shape Microbiome Maturation during Early Life. Sci. Transl. Med. 2016, 8, 343ra82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagpal, R.; Tsuji, H.; Takahashi, T.; Nomoto, K.; Kawashima, K.; Nagata, S.; Yamashiro, Y. Ontogenesis of the Gut Microbiota Composition in Healthy, Full-Term, Vaginally Born and Breast-Fed Infants over the First 3 Years of Life: A Quantitative Bird’s-Eye View. Front. Microbiol. 2017, 8, 1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The Placenta Harbors a Unique Microbiome. Sci. Transl. Med. 2014, 6, 237ra65. [Google Scholar] [CrossRef] [Green Version]
- Collado, M.C.; Rautava, S.; Aakko, J.; Isolauri, E.; Salminen, S. Human Gut Colonisation May Be Initiated in Utero by Distinct Microbial Communities in the Placenta and Amniotic Fluid. Sci. Rep. 2016, 6, 23129. [Google Scholar] [CrossRef] [Green Version]
- Chu, D.M.; Ma, J.; Prince, A.L.; Antony, K.M.; Seferovic, M.D.; Aagaard, K.M. Maturation of the Infant Microbiome Community Structure and Function across Multiple Body Sites and in Relation to Mode of Delivery. Nat. Med. 2017, 23, 314–326. [Google Scholar] [CrossRef] [Green Version]
- Stinson, L.; Hallingström, M.; Barman, M.; Viklund, F.; Keelan, J.; Kacerovsky, M.; Payne, M.; Jacobsson, B. Comparison of Bacterial DNA Profiles in Mid-Trimester Amniotic Fluid Samples From Preterm and Term Deliveries. Front. Microbiol. 2020, 11, 415. [Google Scholar] [CrossRef] [Green Version]
- Perez-Muñoz, M.E.; Arrieta, M.-C.; Ramer-Tait, A.E.; Walter, J. A Critical Assessment of the “Sterile Womb” and “in Utero Colonization” Hypotheses: Implications for Research on the Pioneer Infant Microbiome. Microbiome 2017, 5, 48. [Google Scholar] [CrossRef]
- Gomez-Arango, L.F.; Barrett, H.L.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Nitert, M.D. Contributions of the Maternal Oral and Gut Microbiome to Placental Microbial Colonization in Overweight and Obese Pregnant Women. Sci. Rep. 2017, 7, 2860. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, X.; Ye, Y.; Wang, F.; Chen, F.; Zheng, C. The Role of Microbiota in Infant Health: From Early Life to Adulthood. Front. Immunol. 2021, 12, 708472. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, I.; Olejek, A.; Stencel-Gabriel, K.; Wielgoś, M. The Influence of Maternal Vaginal Flora on the Intestinal Colonization in Newborns and 3-Month-Old Infants. J. Matern. Fetal. Neonatal Med. 2018, 31, 1448–1453. [Google Scholar] [CrossRef] [PubMed]
- Socha-Banasiak, A.; Pawłowska, M.; Czkwianianc, E.; Pierzynowska, K. From Intrauterine to Extrauterine Life-The Role of Endogenous and Exogenous Factors in the Regulation of the Intestinal Microbiota Community and Gut Maturation in Early Life. Front. Nutr. 2021, 8, 696966. [Google Scholar] [CrossRef] [PubMed]
- Rautava, S.; Luoto, R.; Salminen, S.; Isolauri, E. Microbial Contact during Pregnancy, Intestinal Colonization and Human Disease. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 565–576. [Google Scholar] [CrossRef]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. MMBR 2017, 81, e00036-17. [Google Scholar] [CrossRef] [Green Version]
- Honda, K.; Littman, D.R. The Microbiota in Adaptive Immune Homeostasis and Disease. Nature 2016, 535, 75–84. [Google Scholar] [CrossRef]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef] [Green Version]
- Koenig, J.E.; Spor, A.; Scalfone, N.; Fricker, A.D.; Stombaugh, J.; Knight, R.; Angenent, L.T.; Ley, R.E. Succession of Microbial Consortia in the Developing Infant Gut Microbiome. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4578–4585. [Google Scholar] [CrossRef] [Green Version]
- Palmer, C.; Bik, E.M.; DiGiulio, D.B.; Relman, D.A.; Brown, P.O. Development of the Human Infant Intestinal Microbiota. PLoS Biol. 2007, 5, e177. [Google Scholar] [CrossRef] [Green Version]
- Hill, C.J.; Lynch, D.B.; Murphy, K.; Ulaszewska, M.; Jeffery, I.B.; O’Shea, C.A.; Watkins, C.; Dempsey, E.; Mattivi, F.; Tuohy, K.; et al. Evolution of Gut Microbiota Composition from Birth to 24 Weeks in the INFANTMET Cohort. Microbiome 2017, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Moore, R.E.; Townsend, S.D. Temporal Development of the Infant Gut Microbiome. Open Biol. 2019, 9, 190128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaan, A.M.M.; Kahharova, D.; Zaura, E. Acquisition and Establishment of the Oral Microbiota. Periodontology 2021, 86, 123–141. [Google Scholar] [CrossRef] [PubMed]
- Yang, I.; Hu, Y.-J.; Corwin, E.J.; Dunlop, A.L. Exploring the Maternal and Infant Oral Microbiomes: A Pilot Study. J. Perinat. Neonatal Nurs. 2020, 34, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Fiscella, K.A.; Gill, S.R. Oral Microbiome: Possible Harbinger for Children’s Health. Int. J. Oral Sci. 2020, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Mason, M.R.; Chambers, S.; Dabdoub, S.M.; Thikkurissy, S.; Kumar, P.S. Characterizing Oral Microbial Communities across Dentition States and Colonization Niches. Microbiome 2018, 6, 67. [Google Scholar] [CrossRef] [PubMed]
- Dzidic, M.; Collado, M.C.; Abrahamsson, T.; Artacho, A.; Stensson, M.; Jenmalm, M.C.; Mira, A. Oral Microbiome Development during Childhood: An Ecological Succession Influenced by Postnatal Factors and Associated with Tooth Decay. ISME J. 2018, 12, 2292–2306. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Haas, B.J.; Gevers, D.; Earl, A.M.; Feldgarden, M.; Ward, D.V.; Giannoukos, G.; Ciulla, D.; Tabbaa, D.; Highlander, S.K.; Sodergren, E.; et al. Chimeric 16S RRNA Sequence Formation and Detection in Sanger and 454-Pyrosequenced PCR Amplicons. Genome Res. 2011, 21, 494–504. [Google Scholar] [CrossRef] [Green Version]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a Chimera-Checked 16S RRNA Gene Database and Workbench Compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, S.; Xu, Z.Z.; Peddada, S.; Amir, A.; Bittinger, K.; Gonzalez, A.; Lozupone, C.; Zaneveld, J.R.; Vázquez-Baeza, Y.; Birmingham, A.; et al. Normalization and Microbial Differential Abundance Strategies Depend upon Data Characteristics. Microbiome 2017, 5, 27. [Google Scholar] [CrossRef] [Green Version]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [Green Version]
- Derrien, M.; Alvarez, A.-S.; de Vos, W.M. The Gut Microbiota in the First Decade of Life. Trends Microbiol. 2019, 27, 997–1010. [Google Scholar] [CrossRef] [Green Version]
- Del Chierico, F.; Vernocchi, P.; Petrucca, A.; Paci, P.; Fuentes, S.; Praticò, G.; Capuani, G.; Masotti, A.; Reddel, S.; Russo, A.; et al. Phylogenetic and Metabolic Tracking of Gut Microbiota during Perinatal Development. PLoS ONE 2015, 10, e0137347. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Ge, Y.; Cheng, L.; Zeng, B.; Yu, J.; Peng, X.; Zhao, J.; Li, W.; Ren, B.; Li, M.; et al. Oral Bacteria Colonize and Compete with Gut Microbiota in Gnotobiotic Mice. Int. J. Oral Sci. 2019, 11, 10. [Google Scholar] [CrossRef] [Green Version]
- Biagi, E.; Quercia, S.; Aceti, A.; Beghetti, I.; Rampelli, S.; Turroni, S.; Faldella, G.; Candela, M.; Brigidi, P.; Corvaglia, L. The Bacterial Ecosystem of Mother’s Milk and Infant’s Mouth and Gut. Front. Microbiol. 2017, 8, 1214. [Google Scholar] [CrossRef] [Green Version]
- Cephas, K.D.; Kim, J.; Mathai, R.A.; Barry, K.A.; Dowd, S.E.; Meline, B.S.; Swanson, K.S. Comparative Analysis of Salivary Bacterial Microbiome Diversity in Edentulous Infants and Their Mothers or Primary Care Givers Using Pyrosequencing. PLoS ONE 2011, 6, e23503. [Google Scholar] [CrossRef]
- Luo, A.H.; Yang, D.Q.; Xin, B.C.; Paster, B.J.; Qin, J. Microbial Profiles in Saliva from Children with and without Caries in Mixed Dentition. Oral Dis. 2012, 18, 595–601. [Google Scholar] [CrossRef]
- Boix-Amorós, A.; Collado, M.C.; Mira, A. Relationship between Milk Microbiota, Bacterial Load, Macronutrients, and Human Cells during Lactation. Front. Microbiol. 2016, 7, 492. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, J.M. The Origin of Human Milk Bacteria: Is There a Bacterial Entero-Mammary Pathway during Late Pregnancy and Lactation? Adv. Nutr. Bethesda Md 2014, 5, 779–784. [Google Scholar] [CrossRef] [Green Version]
- Jost, T.; Lacroix, C.; Braegger, C.; Chassard, C. Assessment of Bacterial Diversity in Breast Milk Using Culture-Dependent and Culture-Independent Approaches. Br. J. Nutr. 2013, 110, 1253–1262. [Google Scholar] [CrossRef] [Green Version]
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K.; et al. Association Between Breast Milk Bacterial Communities and Establishment and Development of the Infant Gut Microbiome. JAMA Pediatr. 2017, 171, 647–654. [Google Scholar] [CrossRef]
- Carlsson, J.; Grahnén, H.; Jonsson, G.; Wikner, S. Early Establishment of Streptococcus Salivarius in the Mouth of Infants. J. Dent. Res. 1970, 49, 415–418. [Google Scholar] [CrossRef]
- Cole, M.F.; Evans, M.; Fitzsimmons, S.; Johnson, J.; Pearce, C.; Sheridan, M.J.; Wientzen, R.; Bowden, G. Pioneer Oral Streptococci Produce Immunoglobulin A1 Protease. Infect. Immun. 1994, 62, 2165–2168. [Google Scholar] [CrossRef] [Green Version]
- Fernández, L.; Langa, S.; Martín, V.; Maldonado, A.; Jiménez, E.; Martín, R.; Rodríguez, J.M. The Human Milk Microbiota: Origin and Potential Roles in Health and Disease. Pharmacol. Res. 2013, 69, 1–10. [Google Scholar] [CrossRef]
- Ruiz, L.; Bacigalupe, R.; García-Carral, C.; Boix-Amoros, A.; Argüello, H.; Silva, C.B.; de los Angeles Checa, M.; Mira, A.; Rodríguez, J.M. Microbiota of Human Precolostrum and Its Potential Role as a Source of Bacteria to the Infant Mouth. Sci. Rep. 2019, 9, 8435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajilić-Stojanović, M.; de Vos, W.M. The First 1000 Cultured Species of the Human Gastrointestinal Microbiota. FEMS Microbiol. Rev. 2014, 38, 996–1047. [Google Scholar] [CrossRef]
- Gomez, A.; Nelson, K.E. The Oral Microbiome of Children: Development, Disease, and Implications Beyond Oral Health. Microb. Ecol. 2017, 73, 492–503. [Google Scholar] [CrossRef] [Green Version]
- Sprockett, D.; Fukami, T.; Relman, D.A. Role of Priority Effects in the Early-Life Assembly of the Gut Microbiota. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 197–205. [Google Scholar] [CrossRef]
- Cabrera-Rubio, R.; Collado, M.C.; Laitinen, K.; Salminen, S.; Isolauri, E.; Mira, A. The Human Milk Microbiome Changes over Lactation and Is Shaped by Maternal Weight and Mode of Delivery. Am. J. Clin. Nutr. 2012, 96, 544–551. [Google Scholar] [CrossRef] [Green Version]
- Jost, T.; Lacroix, C.; Braegger, C.P.; Rochat, F.; Chassard, C. Vertical Mother-Neonate Transfer of Maternal Gut Bacteria via Breastfeeding. Environ. Microbiol. 2014, 16, 2891–2904. [Google Scholar] [CrossRef] [PubMed]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the Normal Bacterial Flora of the Oral Cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adlerberth, I.; Wold, A. Establishment of the Gut Microbiota in Western Infants: Establishment of the Gut Microbiota in Western Infants. Acta Paediatr. 2009, 98, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Shamash, M.; Maurice, C.F. Phages in the Infant Gut: A Framework for Virome Development during Early Life. ISME J. 2021. [Google Scholar] [CrossRef]
- Matsuki, T.; Yahagi, K.; Mori, H.; Matsumoto, H.; Hara, T.; Tajima, S.; Ogawa, E.; Kodama, H.; Yamamoto, K.; Yamada, T.; et al. A Key Genetic Factor for Fucosyllactose Utilization Affects Infant Gut Microbiota Development. Nat. Commun. 2016, 7, 11939. [Google Scholar] [CrossRef]
- Schwab, C.; Gänzle, M. Lactic Acid Bacteria Fermentation of Human Milk Oligosaccharide Components, Human Milk Oligosaccharides and Galactooligosaccharides: LAB Fermentation of HMOs and GOSs. FEMS Microbiol. Lett. 2011, 315, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Le Doare, K.; Holder, B.; Bassett, A.; Pannaraj, P.S. Mother’s Milk: A Purposeful Contribution to the Development of the Infant Microbiota and Immunity. Front. Immunol. 2018, 9, 361. [Google Scholar] [CrossRef] [Green Version]
- Zoetendal, E.G.; Raes, J.; van den Bogert, B.; Arumugam, M.; Booijink, C.C.G.M.; Troost, F.J.; Bork, P.; Wels, M.; de Vos, W.M.; Kleerebezem, M. The Human Small Intestinal Microbiota Is Driven by Rapid Uptake and Conversion of Simple Carbohydrates. ISME J. 2012, 6, 1415–1426. [Google Scholar] [CrossRef]
- Wang, M.; Li, M.; Wu, S.; Lebrilla, C.B.; Chapkin, R.S.; Ivanov, I.; Donovan, S.M. Fecal Microbiota Composition of Breast-Fed Infants Is Correlated with Human Milk Oligosaccharides Consumed. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 825–833. [Google Scholar] [CrossRef] [Green Version]
- Conta, G.; Del Chierico, F.; Reddel, S.; Marini, F.; Sciubba, F.; Capuani, G.; Tomassini, A.; Di Cocco, M.E.; Laforgia, N.; Baldassarre, M.E.; et al. Longitudinal Multi-Omics Study of a Mother-Infant Dyad from Breastfeeding to Weaning: An Individualized Approach to Understand the Interactions Among Diet, Fecal Metabolome and Microbiota Composition. Front. Mol. Biosci. 2021, 8, 688440. [Google Scholar] [CrossRef]
- Murphy, K.; Curley, D.; O’Callaghan, T.F.; O’Shea, C.-A.; Dempsey, E.M.; O’Toole, P.W.; Ross, R.P.; Ryan, C.A.; Stanton, C. The Composition of Human Milk and Infant Faecal Microbiota Over the First Three Months of Life: A Pilot Study. Sci. Rep. 2017, 7, 40597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferretti, P.; Pasolli, E.; Tett, A.; Asnicar, F.; Gorfer, V.; Fedi, S.; Armanini, F.; Truong, D.T.; Manara, S.; Zolfo, M.; et al. Mother-to-Infant Microbial Transmission from Different Body Sites Shapes the Developing Infant Gut Microbiome. Cell Host Microbe 2018, 24, 133–145.e5. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Price, J.; Mahurkar, A.; Rahnavard, G.; Crabtree, J.; Orvis, J.; Hall, A.B.; Brady, A.; Creasy, H.H.; McCracken, C.; Giglio, M.G.; et al. Strains, Functions and Dynamics in the Expanded Human Microbiome Project. Nature 2017, 550, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Drell, T.; Štšepetova, J.; Simm, J.; Rull, K.; Aleksejeva, A.; Antson, A.; Tillmann, V.; Metsis, M.; Sepp, E.; Salumets, A.; et al. The Influence of Different Maternal Microbial Communities on the Development of Infant Gut and Oral Microbiota. Sci. Rep. 2017, 7, 9940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Rosa, P.S.; Warner, B.B.; Zhou, Y.; Weinstock, G.M.; Sodergren, E.; Hall-Moore, C.M.; Stevens, H.J.; Bennett, W.E.; Shaikh, N.; Linneman, L.A.; et al. Patterned Progression of Bacterial Populations in the Premature Infant Gut. Proc. Natl. Acad. Sci. USA 2014, 111, 12522–12527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyman, M.; Clerc, M.; van Houten, M.A.; Arp, K.; Chu, M.L.J.N.; Hasrat, R.; Sanders, E.A.M.; Bogaert, D. Microbial Community Networks across Body Sites Are Associated with Susceptibility to Respiratory Infections in Infants. Commun. Biol. 2021, 4, 1233. [Google Scholar] [CrossRef]


| Characteristics | Time Point | |||||
|---|---|---|---|---|---|---|
| Age (days) | 0 | 7 | 15 | 30 | 90 | 180 |
| Subjects (no.) | 5 | 19 | 15 | 18 | 15 | 15 |
| Saliva samples | 0 | 19 | 15 | 18 | 15 | 15 |
| Stool samples | 5 | 17 | 14 | 15 | 14 | 15 |
| Gestational age (mean, weeks) | 39.2 | 39.4 | 39.4 | 39.3 | 39.3 | 39.3 |
| Female/male | 1/4 | 9/10 | 5/10 | 10/8 | 8/7 | 9/6 |
| Birth weight (g) mean ± SEM a | 3528 ± 95 | 3418 ± 75 | 3506 ± 102 | 3267 ± 69 | 3350 ± 58 | 3221 ± 81 |
| Weight at the time of stool/saliva collection (g) mean ± SEM a | 3430 ± 100 | 3390 ± 82 | 3744 ± 76 | 4099 ± 118 | 5828 ± 134 | 6979 ± 172 |
| Weaning b | No | No | No | No | No | Yes (10/15) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reddel, S.; Pascucci, G.R.; Foligno, S.; Del Chierico, F.; Vernocchi, P.; Marzullo, A.; Pattumelli, M.G.; Palma, P.; Salvatori, G.; Putignani, L. A Parallel Tracking of Salivary and Gut Microbiota Profiles Can Reveal Maturation and Interplay of Early Life Microbial Communities in Healthy Infants. Microorganisms 2022, 10, 468. https://doi.org/10.3390/microorganisms10020468
Reddel S, Pascucci GR, Foligno S, Del Chierico F, Vernocchi P, Marzullo A, Pattumelli MG, Palma P, Salvatori G, Putignani L. A Parallel Tracking of Salivary and Gut Microbiota Profiles Can Reveal Maturation and Interplay of Early Life Microbial Communities in Healthy Infants. Microorganisms. 2022; 10(2):468. https://doi.org/10.3390/microorganisms10020468
Chicago/Turabian StyleReddel, Sofia, Giuseppe Rubens Pascucci, Silvia Foligno, Federica Del Chierico, Pamela Vernocchi, Alessandra Marzullo, Maria Grazia Pattumelli, Paolo Palma, Guglielmo Salvatori, and Lorenza Putignani. 2022. "A Parallel Tracking of Salivary and Gut Microbiota Profiles Can Reveal Maturation and Interplay of Early Life Microbial Communities in Healthy Infants" Microorganisms 10, no. 2: 468. https://doi.org/10.3390/microorganisms10020468
APA StyleReddel, S., Pascucci, G. R., Foligno, S., Del Chierico, F., Vernocchi, P., Marzullo, A., Pattumelli, M. G., Palma, P., Salvatori, G., & Putignani, L. (2022). A Parallel Tracking of Salivary and Gut Microbiota Profiles Can Reveal Maturation and Interplay of Early Life Microbial Communities in Healthy Infants. Microorganisms, 10(2), 468. https://doi.org/10.3390/microorganisms10020468







