Nine-Strain Bacterial Synbiotic Improves Crying and Lowers Fecal Calprotectin in Colicky Babies—An Open-Label Randomized Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
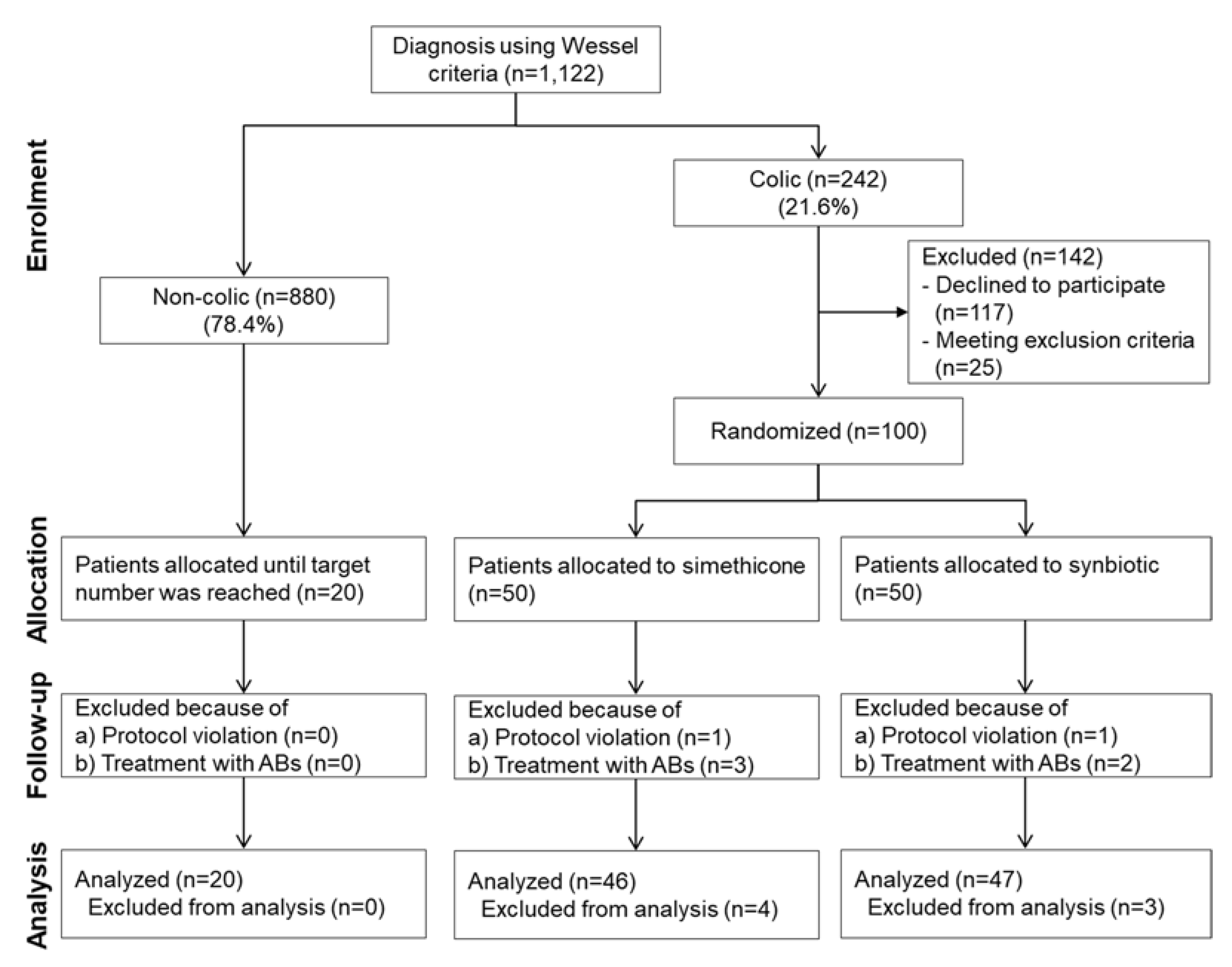
3.1. Patient Flow, Study Progress and Baseline Characteristics of Patient Groups
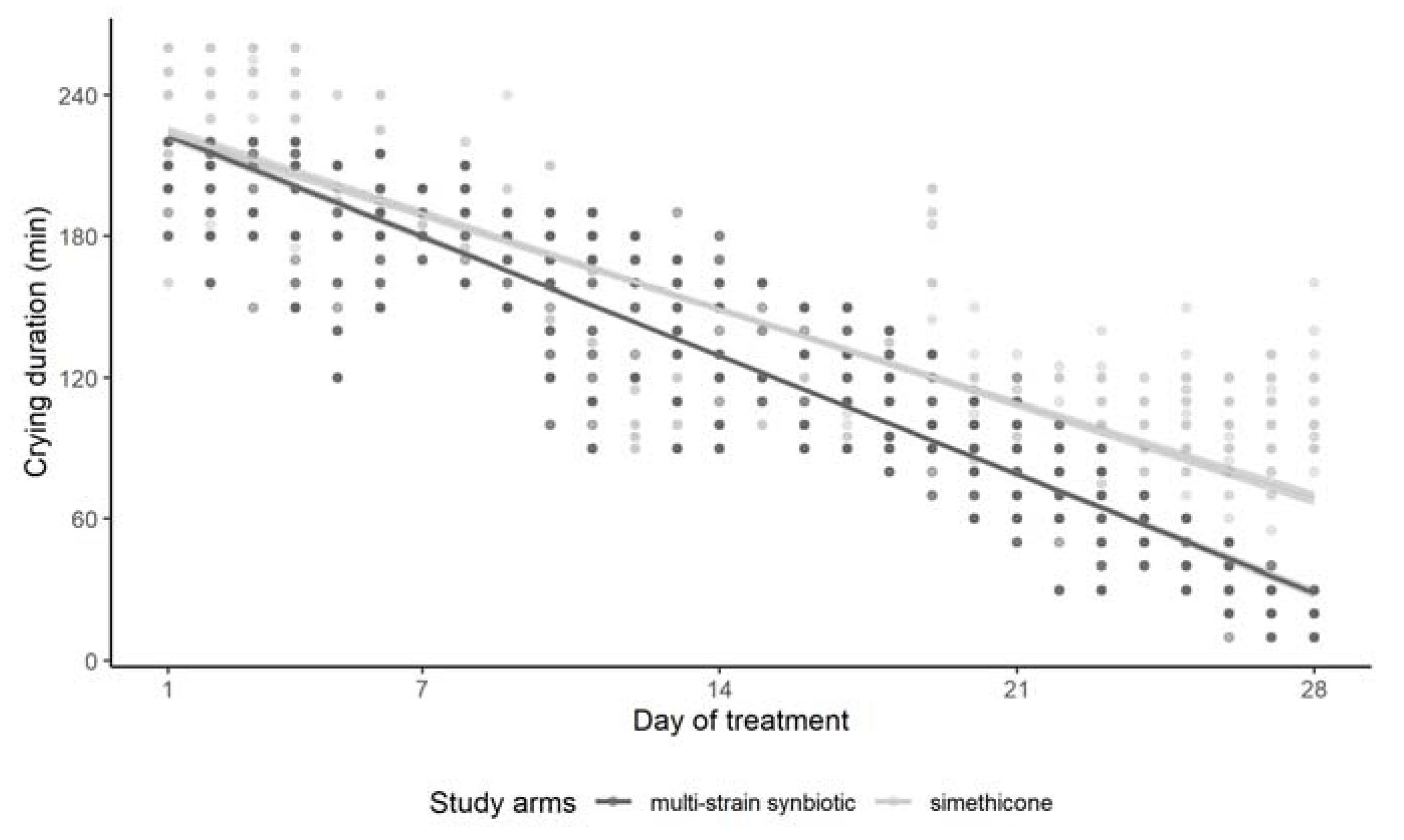
3.2. Effect of Simethicone and of the Multi-Strain Synbiotic on Crying Duration
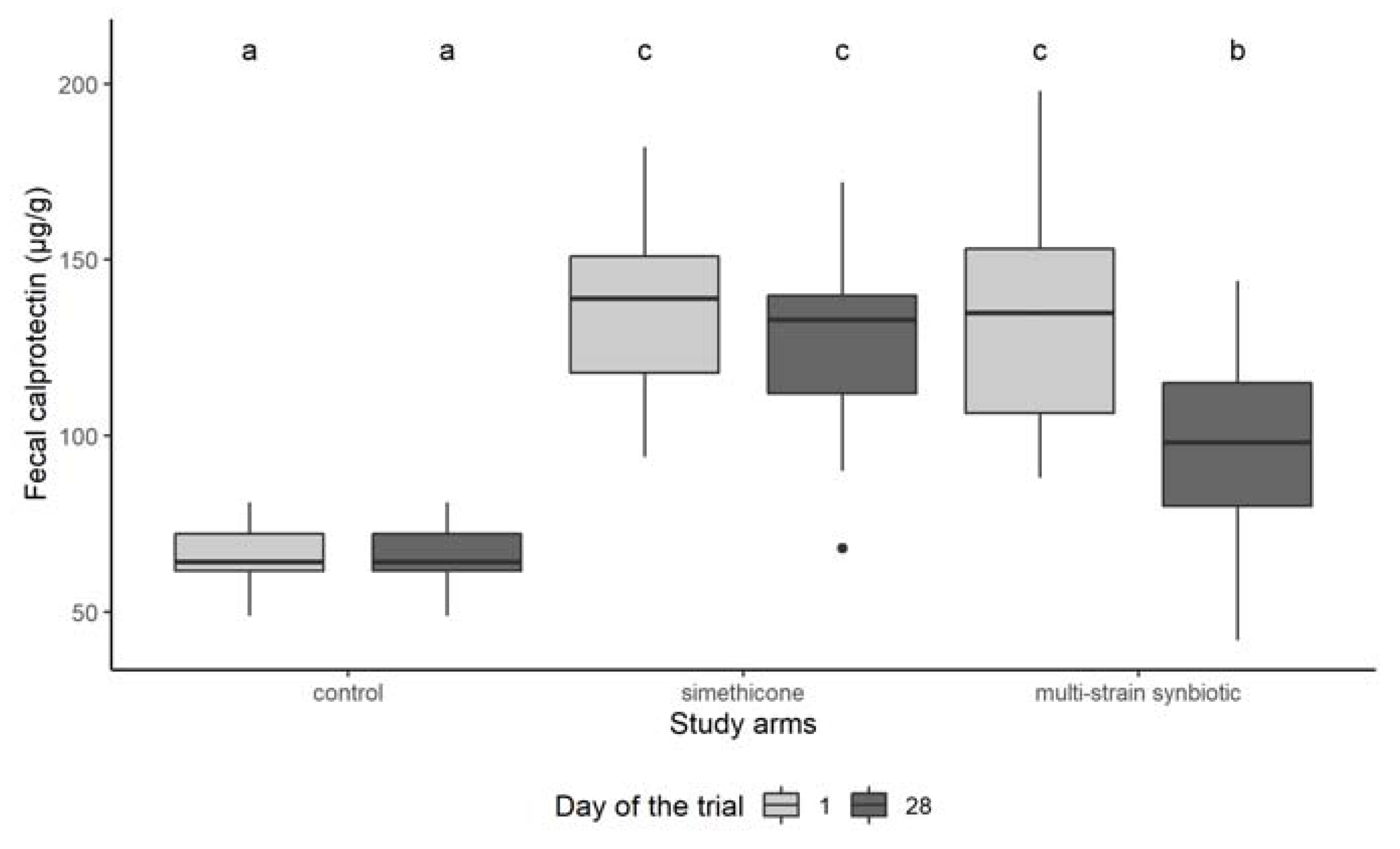
3.3. Fecal Calprotectin
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benninga, M.A.; Faure, C.; Hyman, P.E.; St James Roberts, I.; Schechter, N.L.; Nurko, S. Childhood Functional Gastrointestinal Disorders: Neonate/Toddler. Gastroenterology 2016, 150, 1443–1455.e2. [Google Scholar] [CrossRef]
- Wessel, M.A.; Jackson, E.B.; Harris, G.S.; Detwiler, A.C. Paroxysmal fussing in infancy, sometimes called colic. Pediatrics 1954, 14, 421–435. [Google Scholar] [CrossRef]
- Sung, V. Infantile colic. Aust. Prescr. 2018, 41, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Lucassen, P.; Assendelft, W.; van Eijk, J.T.M.; Gubbels, J.; Douwes, A.; van Geldrop, W.J. Systematic review of the occurrence of infantile colic in the community. Arch. Dis. Child. 2001, 84, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Lucassen, P. Colic in infants. BMJ Clin. Evid. 2010, 2010, 0309. [Google Scholar] [PubMed]
- Vik, T.; Grote, V.; Escribano, J.; Socha, J.; Verduci, E.; Fritsch, M.; Carlier, C.; von Kries, R.; Koletzko, B.; for the European Childhood Obesity Trial Study Group. Infantile colic, prolonged crying and maternal postnatal depression. Acta Paediatr. 2009, 98, 1344–1348. [Google Scholar] [CrossRef]
- Howard, C.R.; Lanphear, N.; Lanphear, B.P.; Eberly, S.; Lawrence, R.A. Parental Responses to Infant Crying and Colic: The Effect on Breastfeeding Duration. Breastfeed. Med. 2006, 1, 146–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barr, R.G. Preventing abusive head trauma resulting from a failure of normal interaction between infants and their caregivers. Proc. Natl. Acad. Sci. USA 2012, 109, 17294–17301. [Google Scholar] [CrossRef] [Green Version]
- Anabrees, J.; Indrio, F.; Paes, B.; AlFaleh, K. Probiotics for infantile colic: A systematic review. BMC Pediatr. 2013, 13, 186. [Google Scholar] [CrossRef] [Green Version]
- Sarasu, J.M.; Narang, M.; Shah, D. Infantile Colic: An Update. Indian Pediatr. 2018, 55, 979–987. [Google Scholar] [CrossRef]
- De Weerth, C.; Fuentes, S.; de Voss, W.M. Crying in infants. On the possible role of intestinal microbiota in the development of colic. Gut Microbes 2013, 4, 416–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellwood, J.; Draper-Rodi, J.; Carnes, D. Comparison of common interventions for the treatment of infantile colic: A systematic review of reviews and guidelines. BMJ Open 2020, 10, e035405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dryl, R.; Szajewska, H. Probiotics for management of infantile colic: A systematic review of randomized controlled trials. Arch. Med. Sci. 2018, 14, 1137–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreck Bird, A.; Gregory, P.J.; Jalloh, M.A.; Risoldi Cochrane, Z.; Hein, D.J. Probiotics for the Treatment of Infantile Colic: A Systematic Review. J. Pharm. Pract. 2017, 30, 366–374. [Google Scholar] [CrossRef]
- Tintore, M.; Colome, G.; Santas, J.; Espadaler, J. Gut Microbiota Dysbiosis and Role of Probiotics in Infant Colic. Arch. Clin. Microbiol. 2017, 8, 56. [Google Scholar]
- Pärtty, A.; Lehtonen, L.; Kalliomäki, M.; Salminen, S.; Isolauri, E. Probiotic Lactobacillus rhamnosus GG therapy and microbiological programming in infantile colic: A randomized, controlled trial. Pediatr. Res. 2015, 78, 470–475. [Google Scholar] [CrossRef] [Green Version]
- Fatheree, N.Y.; Liu, Y.; Ferris, M.; Van Arsdall, M.; McMurtry, V.; Zozaya, M.; Cai, C.; Rahbar, M.H.; Hessabi, M.; Vu, T.; et al. Hypoallergenic formula with Lactobacillus rhamnosus GG for babies with colic: A pilot study of recruitment, retention, and fecal biomarkers. World J. Gastrointest. Pathophysiol. 2016, 7, 160–170. [Google Scholar] [CrossRef]
- Kianifar, H.; Ahanchian, H.; Grover, Z.; Jafari, S.; Noorbakhsh, Z.; Khakshour, A.; Sedaghat, M.; Kiani, M. Synbiotic in the management of infantile colic: A randomised controlled trial. J. Paediatr. Child. Health 2014, 50, 801–805. [Google Scholar] [CrossRef]
- Piątek, J.; Bernatek, M.; Krauss, H.; Wojciechowska, M.; Chęcińska-Maciejewska, Z.; Kaczmarek, P.; Sommermeyer, H. Effects of a nine-strain bacterial synbiotic compared to simethicone in colicky babies—An open-label randomised study. Benef Microbes. 2021, 12, 249–257. [Google Scholar] [CrossRef]
- Savino, F.; Garro, M.; Montanari, P.; Galliano, I.; Bergallo, M. Crying Time and RORγ/FOXP3 Expression in Lactobacillus reuteri DSM17938-Treated Infants with Colic: A Randomized Trial. J. Pediatr. 2018, 192, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Pathirana, W.G.W.; Chubb, S.P.; Gillett, M.J.; Vasikaran, S.D. Faecal Calprotectin. Clin. Biochem. Rev. 2018, 39, 77–90. [Google Scholar]
- Li, F.; Ma, J.; Geng, S.; Wang, J.; Liu, J.; Zhang, J.; Sheng, X. Fecal calprotectin concentrations in healthy children aged 1–18 months. PLoS ONE 2015, 10, e0119574. [Google Scholar] [CrossRef] [Green Version]
- Sommermeyer, H.; Krauss, H.; Chęcińska-Maciejewska, Z.; Pszczola, M.; Piątek, J. Infantile Colic-The Perspective of German and Polish Pediatricians in 2020. Int. J. Environ. Res. Public Health 2020, 17, 7011. [Google Scholar] [CrossRef]
- Piatek, J.; Krauss, H.; Ciechelska-Rybarczyk, A.; Bernatek, M.; Wojtyla-Buciora, P.; Sommermeyer, H. In-Vitro Growth Inhibition of Bacterial Pathogens by Probiotics and a Synbiotic: Product Composition Matters. Int. J. Environ. Res. Public Health 2020, 17, 3332. [Google Scholar] [CrossRef] [PubMed]
- Team, R.C. R: A Language and Environment for Statistical Computing. In R Foundation for Statistical Computing; R Software: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 3 January 2022).
- Kassambra, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests. In R Foundation for Statistical Computing; R Software: Vienna, Austria, 2021; Available online: https://CRAN.R-project.org/package=rstatix/ (accessed on 3 January 2022).
- Lenth, R.V. Emmeans: Estimated Marginal Means, aka Least-Squares Means. In R Foundation for Statistical Computing; R Software: Vienna, Austria, 2022; Available online: https://CRAN.R-project.org/package=emmeans/ (accessed on 3 January 2022).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Meier, R.; Steuerwald, M. Review of the therapeutic use of simethicone in gastroenterology. Schweiz. Z. Für Ganzheitsmed. 2007, 19, 380–387. [Google Scholar] [CrossRef]
- Hizli, S.; Can, D.; Kiliç, I.; Örün, E.; Tunç, T.; Özkan, H. Diagnosis and Treatment Approaches in Infantile Colic (IC): Results of a Survey Among Paediatricians in Turkey. Front. Pediatr. 2021, 9, 1543. [Google Scholar] [CrossRef] [PubMed]
- Barr, R.G.; Kramer, M.S.; Boisjoly, C.; McVey-White, L.; Plesset, I.B. Parental diary of infant cry and fuss behaviour. Arch. Dis. Child. 1988, 63, 380–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, A.A.; Shiffman, S.; Schwartz, J.E.; Broderick, J.E.; Hufford, M.R. Patient compliance with paper and electronic diaries. Control. Clin. Trials 2003, 24, 182–199. [Google Scholar] [CrossRef]
- Lam, J.; Barr, R.G.; Catherine, N.; Tsui, H.; Hahnhaussen, C.L.; Pauwels, J.; Brant, R. Electronic and paper diary recording of infant and caregiver behaviors. J. Dev. Behav. Pediatr. 2010, 31, 685–693. [Google Scholar] [CrossRef]
| Control (n = 20) | Simethicone (n = 46) | Multi-Strain Synbiotic (n = 47) | ||
|---|---|---|---|---|
| Basic Characteristics | Gender 1 (female/male) | 9/11 a | 18/28 a | 24/23 a |
| Delivery 1 (normal/caesarean) | 15/5 a | 28/18 a | 29/18 a | |
| Feeding 1 (breast/formula/mixed) | 13/5/2 a | 25/17/4 a | 32/10/5 a | |
| Birthtime in pregnancy 2 (week) | 39.00 a ± 2.83 a | 39.48 a ± 2.21 a | 39.85 a ± 2.11 a | |
| Birthweight 2 (g) | 3365.0 a ± 417.4 | 3383.5 a ± 289.0 | 3501.9 a ± 372.0 | |
| Age at enrollment 2 (days) | 33.15 a ± 4.44 | 32.41 a ± 5.48 | 31.41 a ± 6.46 | |
| Crying Behavior | Crying days * last 3 weeks before treatment 2 | NA | 14.3 a ± 1.19 | 14.5 a ± 2.18 |
| Avg. crying duration (min/day) last 3 weeks before treatment 2 | 93.10 A ± 25.85 | 218.04 B ± 20.51 | 221.70 B ± 24.08 | |
| Avg. crying phases/day last 3 weeks before treatment 2 | 4.1 A ± 0.85 | 5.98 B ± 0.95 | 5.89 B ± 1.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernatek, M.; Piątek, J.; Pszczola, M.; Krauss, H.; Antczak, J.; Maciukajć, P.; Sommermeyer, H. Nine-Strain Bacterial Synbiotic Improves Crying and Lowers Fecal Calprotectin in Colicky Babies—An Open-Label Randomized Study. Microorganisms 2022, 10, 430. https://doi.org/10.3390/microorganisms10020430
Bernatek M, Piątek J, Pszczola M, Krauss H, Antczak J, Maciukajć P, Sommermeyer H. Nine-Strain Bacterial Synbiotic Improves Crying and Lowers Fecal Calprotectin in Colicky Babies—An Open-Label Randomized Study. Microorganisms. 2022; 10(2):430. https://doi.org/10.3390/microorganisms10020430
Chicago/Turabian StyleBernatek, Malgorzata, Jacek Piątek, Marcin Pszczola, Hanna Krauss, Janina Antczak, Paweł Maciukajć, and Henning Sommermeyer. 2022. "Nine-Strain Bacterial Synbiotic Improves Crying and Lowers Fecal Calprotectin in Colicky Babies—An Open-Label Randomized Study" Microorganisms 10, no. 2: 430. https://doi.org/10.3390/microorganisms10020430
APA StyleBernatek, M., Piątek, J., Pszczola, M., Krauss, H., Antczak, J., Maciukajć, P., & Sommermeyer, H. (2022). Nine-Strain Bacterial Synbiotic Improves Crying and Lowers Fecal Calprotectin in Colicky Babies—An Open-Label Randomized Study. Microorganisms, 10(2), 430. https://doi.org/10.3390/microorganisms10020430








