Effects of Different pH Control Strategies on Microalgae Cultivation and Nutrient Removal from Anaerobic Digestion Effluent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgae Cultivation in AD Effluent
2.2. Physicochemical Analyses
2.3. DNA Extraction and Digital Polymeric Chain Reaction
2.4. High-Throughput Sequencing
3. Results and Discussion
3.1. Culture pH Profiles
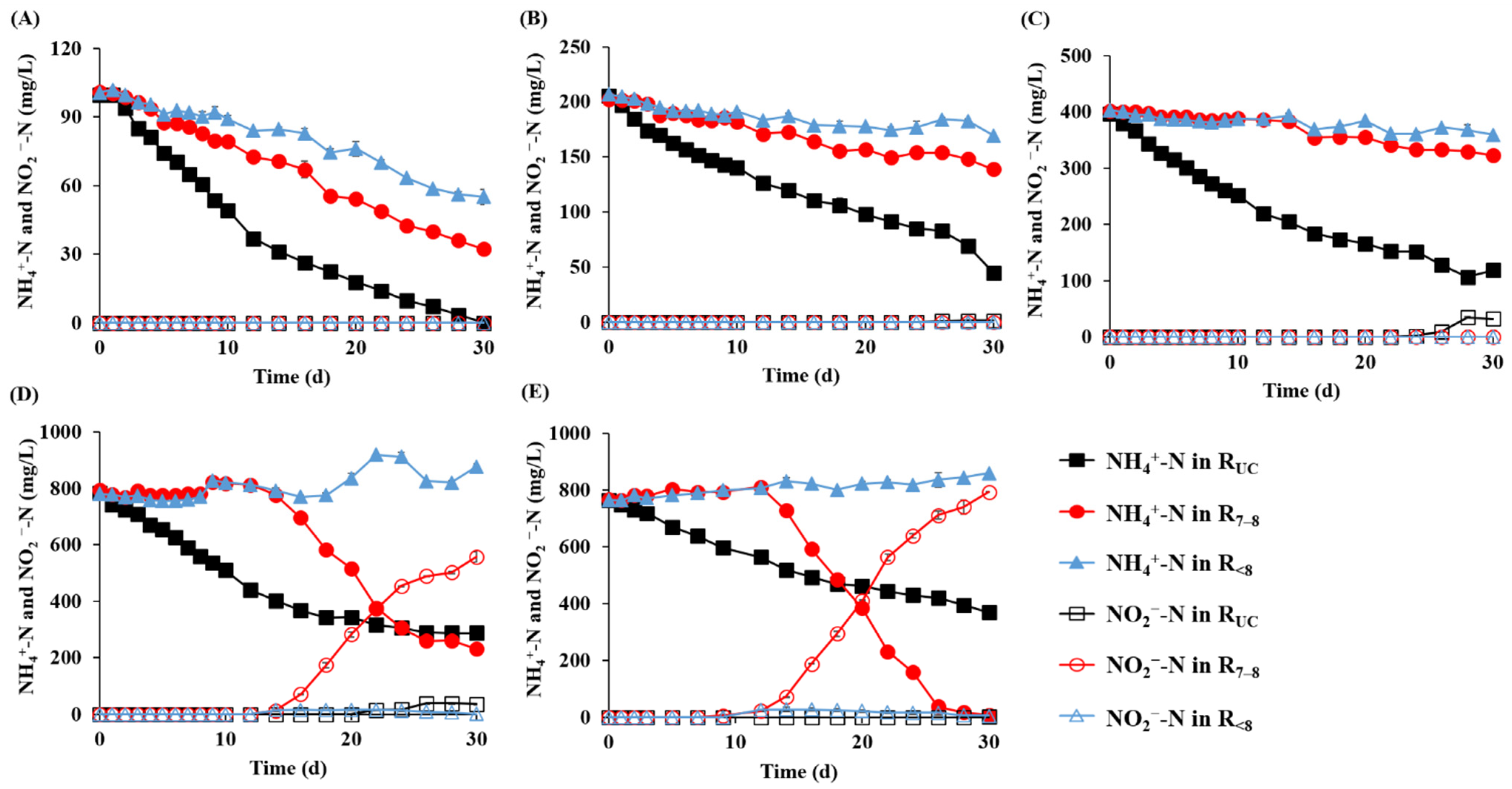
3.2. Microalgal Growth and Nutrient Removal
3.3. Biomass Production and Harvesting
3.4. Microbial Community Structure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chai, W.S.; Tan, W.G.; Halimatul Munawaroh, H.S.; Gupta, V.K.; Ho, S.H.; Show, P.L. Multifaceted roles of microalgae in the application of wastewater biotreatment: A review. Environ. Pollut. 2021, 269, 116236. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.; Bhatti, H.N.; Maqbool, M.; Iqbal, M. Microalgae biosorption, bioaccumulation and biodegradation efficiency for the remediation of wastewater and carbon dioxide mitigation: Prospects, challenges and opportunities. J. Water Process Eng. 2021, 41, 102009. [Google Scholar] [CrossRef]
- Li, K.; Liu, Q.; Fang, F.; Luo, R.H.; Lu, Q.; Zhou, W.G.; Huo, S.H.; Cheng, P.F.; Liu, J.Z.; Addy, M.; et al. Microalgae-based wastewater treatment for nutrients recovery: A review. Bioresour. Technol. 2019, 291, 121934. [Google Scholar] [CrossRef]
- Xia, A.; Murphy, J.D. Microalgal cultivation in treating liquid digestate from biogas systems. Trends Biotechnol. 2016, 34, 264–275. [Google Scholar] [CrossRef]
- Yu, H.; Kim, J.; Lee, C. Potential of mixed-culture microalgae enriched from aerobic and anaerobic sludges for nutrient removal and biomass production from anaerobic effluents. Bioresour. Technol. 2019, 280, 325–336. [Google Scholar] [CrossRef]
- Rahimi, S.; Modin, O.; Mijakovic, I. Technologies for biological removal and recovery of nitrogen from wastewater. Biotechnol. Adv. 2020, 43, 107570. [Google Scholar] [CrossRef]
- Stiles, W.A.V.; Styles, D.; Chapman, S.P.; Esteves, S.; Bywater, A.; Melville, L.; Silkina, A.; Lupatsch, I.; Grunewald, C.F.; Lovitt, R.; et al. Using microalgae in the circular economy to valorise anaerobic digestate: Challenges and opportunities. Bioresour. Technol. 2018, 267, 732–742. [Google Scholar] [CrossRef] [Green Version]
- Passos, F.; Uggetti, E.; Carrere, H.; Ferrer, I. Pretreatment of microalgae to improve biogas production: A review. Bioresour. Technol. 2014, 172, 403–412. [Google Scholar] [CrossRef]
- Li, K.Q.; Li, M.; He, Y.F.; Gu, X.Y.; Pang, K.; Ma, Y.P.; Lu, D.L. Effects of pH and nitrogen form on Nitzschia closterium growth by linking dynamic with enzyme activity. Chemosphere 2020, 249, 126154. [Google Scholar] [CrossRef]
- Gatamaneni, B.L.; Orsat, V.; Lefsrud, M. Factors affecting growth of various microalgal species. Environ. Eng. Sci. 2018, 35, 1037–1048. [Google Scholar] [CrossRef]
- Wang, J.; Curtis, W.R. Proton stoichiometric imbalance during algae photosynthetic growth on various nitrogen sources: Toward metabolic pH control. J. Appl. Phycol. 2016, 28, 43–52. [Google Scholar] [CrossRef]
- Ryu, B.G.; Kim, J.; Yoo, G.; Lim, J.T.; Kim, W.; Han, J.I.; Yang, J.W. Microalgae-mediated simultaneous treatment of toxic thiocyanate and production of biodiesel. Bioresour. Technol. 2014, 158, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Darpito, C.; Shin, W.S.; Jeon, S.; Lee, H.; Nam, K.; Kwon, J.H.; Yang, J.W. Cultivation of Chlorella protothecoides in anaerobically treated brewery wastewater for cost-effective biodiesel production. Bioprocess Biosyst. Eng. 2015, 38, 523–530. [Google Scholar] [CrossRef] [PubMed]
- APHA-AWWA-WEF. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Kim, J.; Lee, C. Response of a continuous biomethanation process to transient organic shock loads under controlled and uncontrolled pH conditions. Water Res. 2015, 73, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lee, C.; Kim, J.; Hwang, S. Group-specific primer and probe sets to detect methanogenic communities using quantitative real-time polymerase chain reaction. Biotechnol. Bioeng. 2005, 89, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Elwood, H.J.; Olsen, G.J.; Sogin, M.L. The small-subunit ribosomal RNA gene sequences from the hypotrichous ciliates Oxytricha nova and Stylonychia pustulata. Mol. Biol. Evol. 1985, 2, 399–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Lens, P.N.L.; Shi, W.X.; Zhang, R.J.; Zhang, Z.Q.; Guo, Y.; Bao, X.; Cui, F.Y. Enhancement of aerobic granulation and nutrient removal by an algal-bacterial consortium in a lab-scale photobioreactor. Chem. Eng. J. 2018, 334, 2373–2382. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahe, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [Green Version]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Chuka-Ogwude, D.; Ogbonna, J.; Moheimani, N.R. A review on microalgal culture to treat anaerobic digestate food waste effluent. Algal Res. 2020, 47, 101841. [Google Scholar] [CrossRef]
- Gonzalez, C.; Marciniak, J.; Villaverde, S.; Garcia-Encina, P.A.; Munoz, R. Microalgae-based processes for the biodegradation of pretreated piggery wastewaters. Appl. Microbiol. Biotechnol. 2008, 80, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, L.; Zhao, Q.; Wei, W.; Sun, Y. Improving high carbon dioxide tolerance and carbon dioxide fixation capability of Chlorella sp. by adaptive laboratory evolution. Bioresour. Technol. 2015, 185, 269–275. [Google Scholar] [CrossRef]
- Ronda, S.R.; Bokka, C.S.; Ketineni, C.; Rijal, B.; Allu, P.R. Aeration effect on Spirulina platensis growth and γ-Linolenic acid production. Braz. J. Microbiol. 2012, 43, 12–20. [Google Scholar] [CrossRef] [Green Version]
- Collos, Y.; Harrison, P.J. Acclimation and toxicity of high ammonium concentrations to unicellular algae. Mar. Pollut. Bull. 2014, 80, 8–23. [Google Scholar] [CrossRef]
- Racharaks, R.; Ge, X.M.; Li, Y.B. Cultivation of marine microalgae using shale gas flowback water and anaerobic digestion effluent as the cultivation medium. Bioresour. Technol. 2015, 191, 146–156. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Chen, X.; Liu, H.; Liu, H.; Fu, B. Improved volatile fatty acids anaerobic production from waste activated sludge by pH regulation: Alkaline or neutral pH? Waste Manag. 2016, 48, 397–403. [Google Scholar] [CrossRef]
- Wang, M.; Shi, L.D.; Wang, Y.; Hu, S.Q.; Tao, X.M.; Tian, G.M. The evolution of bacterial community structure and function in microalgal-bacterial consortia with inorganic nitrogen fluctuations in piggery digestate. J. Clean. Prod. 2021, 315, 128120. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Q. Microalgae-based nitrogen bioremediation. Algal Res. 2020, 46, 101775. [Google Scholar] [CrossRef]
- Perez, L.; Salgueiro, J.L.; Maceiras, R.; Cancela, A.; Sanchez, A. An effective method for harvesting of marine microalgae: pH induced flocculation. Biomass Bioenergy 2017, 97, 20–26. [Google Scholar] [CrossRef]
- Salim, S.; Kosterink, N.R.; Wacka, N.D.T.; Vermue, M.H.; Wijffels, R.H. Mechanism behind autoflocculation of unicellular green micro algae Ettlia texensis. J. Biotechnol. 2014, 174, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Kuo, E.W.; Nagarajan, D.; Ho, S.H.; Dong, C.D.; Lee, D.J.; Chang, J.S. Cultivating Chlorella sorokiniana AK-1 with swine wastewater for simultaneous wastewater treatment and algal biomass production. Bioresour. Technol. 2020, 302, 122814. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Zhang, C.; Zhang, Y.; Ho, S.H. Optimizing real swine wastewater treatment with maximum carbohydrate production by a newly isolated indigenous microalga Parachlorella kessleri QWY28. Bioresour. Technol. 2019, 289, 121702. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.H.; Choi, J.W.; Kang, Z.; Kim, B.H.; Oh, H.M.; Kim, H.S.; Ramanan, R. Microalgal diversity fosters stable biomass productivity in open ponds treating wastewater. Sci. Rep. 2017, 7, 1979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.D.; Zhao, J.L.; Feng, J.; Lv, J.P.; Liu, Q.; Nan, F.R.; Xie, T.; Xie, S.L. A Parachlorella kessleri (Trebouxiophyceae, Chlorophyta) strain tolerant to high concentration of calcium chloride. J. Eukaryot. Microbiol. 2021, 69, e12872. [Google Scholar] [CrossRef] [PubMed]
- Osundeko, O.; Davies, H.; Pittman, K. Oxidative stress-tolerant microalgae strains are highly efficient for biofuel feedstock production on wastewater. Biomass Bioenergy 2013, 56, 284–294. [Google Scholar] [CrossRef]
- Juarez, A.B.; Velez, C.G.; Iniguez, A.R.; Martinez, D.E.; Rodriguez, M.C.; Vigna, M.S.; de Molina, M.D.R. A Parachlorella kessleri (Trebouxiophyceae, Chlorophyta) strain from an extremely acidic geothermal pond in Argentina. Phycologia 2011, 50, 413–421. [Google Scholar] [CrossRef]
- Felfoldi, T.; Ramganesh, S.; Somogyi, B.; Krett, G.; Jurecska, L.; Szabo, A.; Voros, L.; Marialigeti, K.; Mathe, I. Winter planktonic microbial communities in highland Aquatic habitats. Geomicrobiol. J. 2016, 33, 494–504. [Google Scholar] [CrossRef] [Green Version]
- Shimura, H.; Itoh, K.; Sugiyama, A.; Ichijo, S.; Ichijo, M.; Furuya, F.; Nakamura, Y.; Kitahara, K.; Kobayashi, K.; Yukawa, Y.; et al. Absorption of radionuclides from the fukushima nuclear accident by a novel algal strain. PLoS ONE 2012, 7, e44200. [Google Scholar] [CrossRef]
- Kuramae, E.E.; Derksen, S.; Schlemper, T.R.; Dimitrov, M.R.; Costa, O.Y.A.; da Silveira, A.P.D. Sorghum growth promotion by Paraburkholderia tropica and Herbaspirillum frisingense: Putative mechanisms revealed by genomics and metagenomics. Microorganisms 2020, 8, 725. [Google Scholar] [CrossRef] [PubMed]
- Di Gregorio, L.; Congestri, R.; Tandoi, V.; Neu, T.R.; Rossetti, S.; Di Pippo, F. Biofilm diversity, structure and matrix seasonality in a full-scale cooling tower. Biofouling 2018, 34, 1093–1109. [Google Scholar] [CrossRef] [PubMed]
- Rambo, I.M.; Dombrowski, N.; Constant, L.; Erdner, D.; Baker, B.J. Metabolic relationships of uncultured bacteria associated with the microalgae Gambierdiscus. Environ. Microbiol. 2020, 22, 1764–1783. [Google Scholar] [CrossRef]
- Zhang, B.; Li, W.; Guo, Y.; Zhang, Z.Q.; Shi, W.X.; Cui, F.Y.; Lens, P.N.L.; Tay, J.H. Microalgal-bacterial consortia: From interspecies interactions to biotechnological applications. Renew. Sustain. Energy Rev. 2020, 118, 109563. [Google Scholar] [CrossRef]
- Ramanan, R.; Kim, B.H.; Cho, D.H.; Oh, H.M.; Kim, H.S. Algae-bacteria interactions: Evolution, ecology and emerging applications. Biotechnol. Adv. 2016, 34, 14–29. [Google Scholar] [CrossRef] [Green Version]
- Duncan, S.H.; Louis, P.; Thomson, J.M.; Flint, H.J. The role of pH in determining the species composition of the human colonic microbiota. Environ. Microbiol. 2009, 11, 2112–2122. [Google Scholar] [CrossRef]
- Goecke, F.; Thiel, V.; Wiese, J.; Labes, A.; Imhoff, J.F. Algae as an important environment for bacteria—Phylogenetic relationships among new bacterial species isolated from algae. Phycologia 2013, 52, 14–24. [Google Scholar] [CrossRef]
- Lage, O.M.; Bondoso, J. Planctomycetes diversity associated with macroalgae. FEMS Microbiol. Ecol. 2011, 78, 366–375. [Google Scholar] [CrossRef] [Green Version]
- Udaiyappan, A.F.M.; Abu Hasan, H.; Takriff, M.S.; Abdullah, S.R.S.; Maeda, T.; Mustapha, N.A.; Yasin, N.H.M.; Hakimi, N.I.N.M. Microalgae-bacteria interaction in palm oil mill effluent treatment. J. Water Process Eng. 2020, 35, 101203. [Google Scholar] [CrossRef]




| Culture or Sample | Total Carbon (mg/L) | Inorganic Carbon (mg/L) | Total Nitrogen (mg/L) | NH4+-N (mg/L) | pH |
|---|---|---|---|---|---|
| Microalgae inoculum | 44.2 ± 0.8 | 0.0 ± 0.0 | 16.8 ± 0.3 | 12.7 ± 0.0 | 2.8 ± 0.0 |
| Raw AD effluent | 1285.1 ± 23.9 | 1066.3 ± 8.1 | 1841.7 ± 31.0 | 1848.2 ± 0.0 | 8.7 ± 0.0 |
| 100-mg NH4+-N/L test cultures | 103.8 ± 2.5 | 73.5 ± 0.5 | 100.0 ± 0.2 | 100.3 ± 0.6 | 8.6 ± 0.1 |
| 200-mg NH4+-N/L test cultures | 189.5 ± 5.7 | 154.7 ± 1.7 | 202.1 ± 1.4 | 205.0 ± 2.3 | 8.5 ± 0.1 |
| 400-mg NH4+-N/L test cultures | 356.7 ± 9.4 | 267.8 ± 1.9 | 422.7 ± 3.2 | 400.6 ± 3.5 | 8.6 ± 0.0 |
| 800-mg NH4+-N/L test cultures | 613.7 ± 25.7 | 462.9 ± 1.0 | 817.0 ± 1.3 | 786.0 ± 6.8 | 8.6 ± 0.0 |
| Uninoculated control cultures a | 568.2 ± 17.4 | 442.4 ± 20.4 | 810.3 ± 3.2 | 765.1 ± 0.0 | 8.8 ± 0.0 |
| Culture | TN0 (mg/L) | TN30 (mg/L) | AV a (%) | AR b (mg/L) | AO c (mg/L) | BAU d (mg/L) |
|---|---|---|---|---|---|---|
| RUC-100 | 99.8 ± 4.2 | 70.3 ± 1.0 | 29.6 | 99.7 ± 0.0 | – e | 70.2 ± 4.4 |
| R7–8-100 | 100.1 ± 1.0 | 100.6 ± 0.2 | – | 68.6 ± 0.2 | – | 68.6 ± 0.2 |
| R<8-100 | 100.2 ± 1.4 | 109.2 ± 0.8 | – | 45.5 ± 3.2 | – | 45.5 ± 3.2 |
| RUC-200 | 202.0 ± 2.4 | 58.6 ± 4.8 | 71.0 | 160.8 ± 0.2 | 1.4 ± 0.0 | 16.0 ± 5.3 |
| R7–8-200 | 200.8 ± 3.4 | 191.4 ± 2.9 | 4.7 | 63.9 ± 1.0 | – | 63.9 ± 1.0 |
| R<8-200 | 203.6 ± 2.2 | 193.8 ± 8.7 | 4.8 | 37.6 ± 2.1 | – | 37.6 ± 2.1 |
| RUC-400 | 419.2 ± 5.7 | 160.1 ± 5.0 | 61.8 | 278.1 ± 1.0 | 33.0 ± 1.0 | – |
| R7–8-400 | 425.4 ± 3.8 | 414.5 ± 0.4 | 2.6 | 79.4 ± 0.5 | – | 68.5 ± 3.9 |
| R<8-400 | 423.6 ± 0.1 | 416.9 ± 5.4 | 1.6 | 43.4 ± 1.5 | – | 43.4 ± 1.5 |
| RUC-800 | 815.5 ± 6.2 | 353.4 ± 1.8 | 56.7 | 496.1 ± 0.9 | 37.9 ± 0.2 | – |
| R7–8-800 | 817.8 ± 5.7 | 791.5 ± 2.4 | 3.2 | 563.3 ± 1.9 | 567.1 ± 1.8 | – |
| R<8-800 | 817.8 ± 1.6 | 802.4 ± 0.5 | 1.9 | – | 3.0 ± 0.2 | – |
| RUC-C | 807.1 ± 0.2 | 348.6 ± 1.7 | 56.8 | 396.7 ± 0.6 | 1.9 ± 0.0 | – |
| R7–8-C | 813.4 ± 4.0 | 779.8 ± 1.2 | 4.1 | 757.9 ± 0.2 | 801.3 ± 0.6 | – |
| R<8-C | 810.5 ± 4.6 | 862.7 ± 3.2 | – | – | 7.1 ± 0.1 | – |
| Biomass Production | Biomass Yield | Preferentiality | |||
|---|---|---|---|---|---|
| PVSS (mg/L) a | PCht (mg/L) b | YVSS (g/g) c | YCht (g/g) d | YCht/YVSS | |
| RUC-100 | 1050.0 ± 0.0 | 44.8 ± 0.8 | 10.53 ± 0.00 | 0.45 ± 0.00 | 0.043 ± 0.000 |
| R7–8-100 | 1180.0 ± 28.3 | 49.9 ± 1.1 | 11.71 ± 0.01 | 0.50 ± 0.00 | 0.042 ± 0.000 |
| R<8-100 | 895.0 ± 21.2 | 42.0 ± 1.3 | 8.90 ± 0.00 | 0.42 ± 0.00 | 0.047 ± 0.000 |
| RUC-200 | 340.0 ± 0.0 | 10.4 ± 0.2 | 1.65 ± 0.00 | 0.05 ± 0.00 | 0.031 ± 0.000 |
| R7–8-200 | 1025.0 ± 25.5 | 44.1 ± 1.2 | 5.06 ± 0.00 | 0.22 ± 0.00 | 0.043 ± 0.000 |
| R<8-200 | 785.0 ± 35.4 | 36.6 ± 0.1 | 3.79 ± 0.01 | 0.18 ± 0.00 | 0.047 ± 0.000 |
| RUC-400 | – e | – | – | – | – |
| R7–8-400 | 720.0 ± 28.3 | 36.9 ± 1.2 | 1.79 ± 0.00 | 0.09 ± 0.00 | 0.051 ± 0.000 |
| R<8-400 | 525.0 ± 15.8 | 32.1 ± 0.6 | 1.30 ± 0.00 | 0.08 ± 0.00 | 0.061 ± 0.000 |
| RUC-800 | – | 1.7 ± 0.3 | – | 0.00 ± 0.00 | – |
| R7–8-800 | – | – | – | – | – |
| R<8-800 | – | 10.2 ± 0.7 | – | 0.01 ± 0.00 | – |
| RUC-C | – | – | – | – | – |
| R7–8-C | – | – | – | – | – |
| R<8-C | – | 15.1 ± 0.2 | – | 0.02 ± 0.00 | – |
| ASV | Taxonomy a | Ino | ADe | RUC- 100 | R7–8- 100 | R<8- 100 | RUC- 400 | R7–8- 400 | R<8- 400 | Closest Species (Nucleotide Accession Number) b | Sim (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| B1 | Longimonas | – c | – | 0.7 | 3.3 | – | 1.5 | 2.5 | – | Longimonas halophila (NR136497) | 86.6 |
| B2 | Dysgonomonas | 0.0 | 7.9 | – | – | – | 0.0 | – | 0.0 | Dysgonomonas capnocytophagoides (NR113133) | 91.7 |
| B3 | Alistipes | 0.0 | 17.7 | – | – | – | 0.0 | 0.0 | – | Alistipes communis (NR133025) | 85.9 |
| B4 | Edaphocola | 0.0 | 0.7 | – | 0.4 | – | 0.0 | 3.9 | – | Edaphocola flava (NR165730) | 93.7 |
| B5 | Terrimonas | – | – | 0.1 | 4.7 | 0.1 | – | 0.2 | – | Terrimonas rubra (NR109417) | 95.3 |
| B6 | Emticicia | – | – | – | 23.9 | 0.0 | 0.0 | – | – | Emticicia soli (NR157720) | 100.0 |
| B7 | Rhabdobacter | – | – | 0.5 | 1.9 | – | 1.2 | 5.6 | 0.0 | Rhabdobacter roseus (NR147750) | 93.4 |
| B8 | Aequorivita | 0.0 | 0.0 | 0.8 | – | – | 0.1 | 5.9 | 0.0 | Aequorivita viscosa (NR109011) | 99.6 |
| B9 | Leptolinea | 0.0 | 4.9 | – | – | 0.0 | 0.0 | 0.0 | 0.1 | Leptolinea tardivitalis (NR040971) | 91.3 |
| B10 | Limnoraphis | – | – | 0.1 | – | 0.2 | – | – | 7.0 | Limnoraphis robusta (NR118325) | 87.1 |
| B11 | Alicyclobacillus | – | – | – | – | – | – | – | 57.5 | Alicyclobacillus acidiphilus (NR028637) | 98.0 |
| B12 | Ercella | 0.0 | 3.4 | 0.0 | – | – | – | – | 0.6 | Ercella succinigenes (NR134026) | 86.6 |
| B13 | Syntrophomonas | – | 3.9 | 0.0 | – | – | – | – | – | Syntrophomonas bryantii (NR104881) | 97.2 |
| B14 | Fimbriiglobus | – | – | 0.0 | 14.9 | 0.0 | – | 0.1 | – | Fimbriiglobus ruber (NR148575) | 95.7 |
| B15 | Gemmata | 0.0 | – | – | 7.8 | – | 0.1 | – | – | Gemmata massiliana (NR148576) | 95.7 |
| B16 | Bremerella | – | – | 20.3 | 0.0 | – | 2.5 | 5.4 | 0.2 | Bremerella volcania (NR169319) | 92.5 |
| B17 | Brevundimonas | – | 0.0 | 1.3 | 0.1 | – | 1.2 | 3.3 | – | Brevundimonas subvibrioides (NR112028) | 100.0 |
| B18 | Caulobacter | – | – | 0.1 | 1.9 | – | 0.0 | 4.9 | – | Caulobacter daechungensis (NR118485) | 100.0 |
| B19 | Phenylobacterium | 0.2 | – | 1.5 | 0.0 | 6.4 | 0.0 | 0.0 | 0.9 | Phenylobacterium zucineum (NR074119) | 96.4 |
| B20 | Bradyrhizobium | 0.2 | – | 1.2 | 0.0 | 6.8 | 0.0 | 0.1 | 2.1 | Bradyrhizobium ganzhouense (NR133706) | 100.0 |
| B21 | Variibacter | 0.9 | – | 4.6 | 0.2 | 21 | 0.1 | 0.1 | 8.7 | Variibacter gotjawalensis (NR134225) | 96.0 |
| B22 | Devosia | – | – | 0.0 | 7.2 | – | – | 0.8 | – | Devosia humi (NR147759) | 100.0 |
| B23 | Oceaniglobus | – | – | – | 0.1 | – | 0.8 | 6.4 | – | Oceaniglobus indicus (NR159234) | 100.0 |
| B24 | Reyranella | – | – | 15.5 | 5.4 | – | 0.0 | 0.5 | – | Reyranella aquatilis (NR158037) | 100.0 |
| B25 | Acidisoma | 1.0 | – | – | – | 3.4 | – | – | – | Acidisoma tundrae (NR042705) | 98.8 |
| B26 | Cupriavidus | 0.3 | – | 2.0 | 0.0 | 12.8 | 0.0 | – | 2.1 | Cupriavidus metallidurans (NR074704) | 100.0 |
| B27 | Paraburkholderia | 37.7 | – | – | – | 1.2 | – | – | – | Paraburkholderia xenovorans (NR118083) | 100.0 |
| B28 | Ralstonia | 0.4 | – | 2.6 | 0.1 | 10.5 | 0.0 | – | 5.6 | Ralstonia pickettii (NR113352) | 100.0 |
| B29 | Janthinobacterium | – | – | 0.7 | – | 3.6 | – | – | 0.4 | Janthinobacterium lividum (NR164625) | 100.0 |
| B30 | Sandaracinus | – | – | – | 0.8 | 0.0 | 0.4 | 3.0 | – | Sandaracinus amylolyticus (NR118001) | 99.2 |
| B31 | Pseudomonas | 0.1 | – | 1.1 | 0.0 | 3.4 | – | – | 0.6 | Pseudomonas veronii (NR112075) | 100.0 |
| B32 | Dyella | 45.4 | – | 17.5 | – | 6.9 | – | – | 2.1 | Dyella flava (NR157998) | 99.2 |
| B33 | Luteimonas | – | – | – | – | 0.1 | – | 10.9 | 0.1 | Luteimonas abyssi (NR133773) | 100.0 |
| B34 | Pseudoxanthomonas | – | – | – | – | – | 16.7 | 1.2 | 0.0 | Pseudoxanthomonas daejeonensis (NR113984) | 95.7 |
| B35 | Thermovirga | 0.1 | 9.4 | 0.1 | – | 0.3 | 29.5 | 0.1 | – | Thermovirga lienii (NR043522) | 93.7 |
| B36 | Mesotoga | – | 4.5 | – | – | – | – | – | – | Mesotoga infera (NR118572) | 100.0 |
| B37 | Mesotoga | 0.1 | 3.9 | – | – | – | 0.0 | 0.0 | – | Mesotoga prima (NR102952) | 100.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Kim, J.; Rhee, C.; Shin, J.; Shin, S.G.; Lee, C. Effects of Different pH Control Strategies on Microalgae Cultivation and Nutrient Removal from Anaerobic Digestion Effluent. Microorganisms 2022, 10, 357. https://doi.org/10.3390/microorganisms10020357
Yu H, Kim J, Rhee C, Shin J, Shin SG, Lee C. Effects of Different pH Control Strategies on Microalgae Cultivation and Nutrient Removal from Anaerobic Digestion Effluent. Microorganisms. 2022; 10(2):357. https://doi.org/10.3390/microorganisms10020357
Chicago/Turabian StyleYu, Hyeonjung, Jaai Kim, Chaeyoung Rhee, Juhee Shin, Seung Gu Shin, and Changsoo Lee. 2022. "Effects of Different pH Control Strategies on Microalgae Cultivation and Nutrient Removal from Anaerobic Digestion Effluent" Microorganisms 10, no. 2: 357. https://doi.org/10.3390/microorganisms10020357
APA StyleYu, H., Kim, J., Rhee, C., Shin, J., Shin, S. G., & Lee, C. (2022). Effects of Different pH Control Strategies on Microalgae Cultivation and Nutrient Removal from Anaerobic Digestion Effluent. Microorganisms, 10(2), 357. https://doi.org/10.3390/microorganisms10020357








