Prospective Evaluation of a Commercial Dengue NS1 Antigen Rapid Diagnostic Test in New Caledonia
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting and Study Design
2.2. Laboratory Methods
2.2.1. Dengue NS1 Antigen RDT
2.2.2. Dengue RT-PCR and Virus Typing
2.3. Statistical Analysis
2.4. Ethical Statement
3. Results
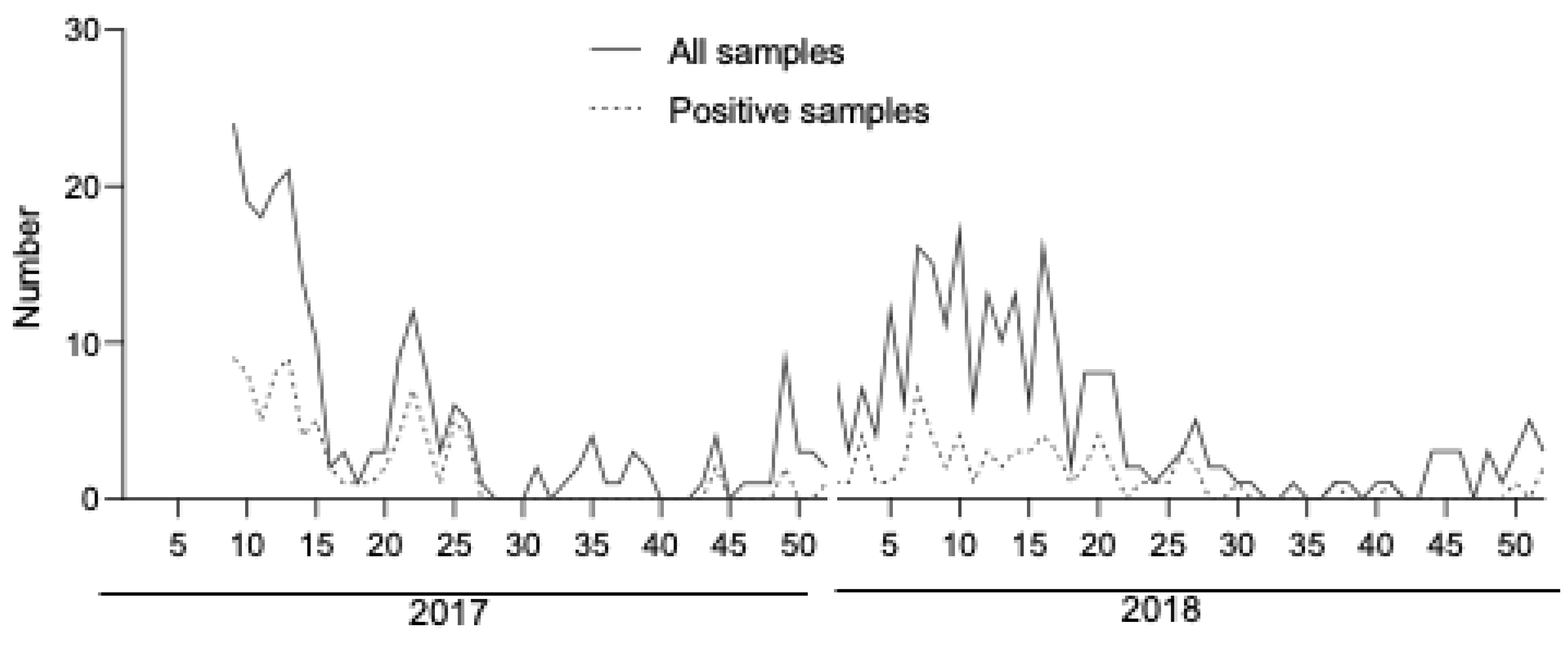
3.1. Patients and Samples
3.2. Overall RDT Performance
3.3. RDT Performance Varied by Time of Sampling and Dengue Serotype
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Dengue and Severe Dengue. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 11 September 2019).
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The Global Distribution and Burden of Dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Inizan, C.; Tarantola, A.; O’Connor, O.; Mangeas, M.; Pocquet, N.; Forfait, C.; Descloux, E.; Gourinat, A.-C.; Pfannstiel, A.; Klement-Frutos, E.; et al. Dengue in New Caledonia: Knowledge and Gaps. Trop. Med. Infect. Dis. 2019, 4, 95. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.A.; Depelsenaire, A.C.I.; Young, P.R. Clinical and Laboratory Diagnosis of Dengue Virus Infection. J. Infect. Dis. 2017, 215, S89–S95. [Google Scholar] [CrossRef] [PubMed]
- CDC. Dengue. Available online: https://www.cdc.gov/dengue/healthcare-providers/diagnosis.html (accessed on 11 September 2019).
- Peeling, R.W.; Artsob, H.; Pelegrino, J.L.; Buchy, P.; Cardosa, M.J.; Devi, S.; Enria, D.A.; Farrar, J.; Gubler, D.J.; Guzman, M.G.; et al. Evaluation of Diagnostic Tests: Dengue. Nat. Rev. Microbiol. 2010, 8, S30–S38. [Google Scholar] [CrossRef]
- Blacksell, S.D. Commercial Dengue Rapid Diagnostic Tests for Point-of-Care Application: Recent Evaluations and Future Needs? J. Biomed. Biotechnol. 2012, 2012, 151967. [Google Scholar] [CrossRef]
- Peeling, R.W.; Mabey, D. Point-of-Care Tests for Diagnosing Infections in the Developing World. Clin. Microbiol. Infect. 2010, 16, 1062–1069. [Google Scholar] [CrossRef]
- The Murtagh Group, LLC. Dengue Virus Infection Diagnostics Landscape. Available online: https://www.who.int/medical_devices/diagnostics/selection_in-vitro/selection_in-vitro-meetings/00022_01_Dengue_Dx_Landscape_2017.pdf. (accessed on 11 September 2019).
- Warrilow, D.; Northill, J.A.; Pyke, A.; Smith, G.A. Single Rapid TaqMan Fluorogenic Probe Based PCR Assay That Detects All Four Dengue Serotypes. J. Med. Virol. 2002, 66, 524–528. [Google Scholar] [CrossRef]
- Johnson, B.W.; Russell, B.J.; Lanciotti, R.S. Serotype-Specific Detection of Dengue Viruses in a Fourplex Real-Time Reverse Transcriptase PCR Assay. J. Clin. Microbiol. 2005, 43, 4977–4983. [Google Scholar] [CrossRef]
- CLSI. User Protocol for Evaluation of Qualitative Test Performance; Approved Guideline—Second Edition. CLSI document EP12-A2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Hunsperger, E.A.; Yoksan, S.; Buchy, P.; Nguyen, V.C.; Sekaran, S.D.; Enria, D.A.; Vazquez, S.; Cartozian, E.; Pelegrino, J.L.; Artsob, H.; et al. Evaluation of Commercially Available Diagnostic Tests for the Detection of Dengue Virus NS1 Antigen and Anti-Dengue Virus IgM Antibody. PLoS Negl. Trop. Dis. 2014, 8, e3171. [Google Scholar] [CrossRef]
- Hunsperger, E.A.; Sharp, T.M.; Lalita, P.; Tikomaidraubuta, K.; Cardoso, Y.R.; Naivalu, T.; Khan, A.S.; Marfel, M.; Hancock, W.T.; Tomashek, K.M.; et al. Use of a Rapid Test for Diagnosis of Dengue during Suspected Dengue Outbreaks in Resource-Limited Regions. J. Clin. Microbiol. 2016, 54, 2090–2095. [Google Scholar] [CrossRef]
- Santoso, M.S.; Yohan, B.; Denis, D.; Hayati, R.F.; Haryanto, S.; Trianty, L.; Noviyanti, R.; Hibberd, M.L.; Sasmono, R.T. Diagnostic Accuracy of 5 Different Brands of Dengue Virus Non-Structural Protein 1 (NS1) Antigen Rapid Diagnostic Tests (RDT) in Indonesia. Diagn. Microbiol. Infect. Dis. 2020, 98, 115116. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-T.; Dalipanda, T.; Jagilly, R.; Wang, Y.-H.; Lin, P.-C.; Tsai, C.-Y.; Lai, W.-T.; Tsai, J.-J. Comparison of Two Rapid Diagnostic Tests during a Large Dengue Virus Serotype 3 Outbreak in the Solomon Islands in 2013. PLoS ONE 2018, 13, e0202304. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-T.; Chen, C.-H.; Tsai, C.-Y.; Lin, P.-C.; Hsu, M.-C.; Huang, B.-Y.; Wang, Y.-H.; Tsai, J.-J. Evaluation of Rapid Diagnostic Tests to Detect Dengue Virus Infections in Taiwan. PLoS ONE 2020, 15, e0239710. [Google Scholar] [CrossRef] [PubMed]
- Shukla, M.K.; Singh, N.; Sharma, R.K.; Barde, P.V. Utility of Dengue NS1 Antigen Rapid Diagnostic Test for Use in Difficult to Reach Areas and Its Comparison with Dengue NS1 ELISA and QRT-PCR. J. Med. Virol. 2017, 89, 1146–1150. [Google Scholar] [CrossRef]
- Chong, Z.L.; Sekaran, S.D.; Soe, H.J.; Peramalah, D.; Rampal, S.; Ng, C.-W. Diagnostic Accuracy and Utility of Three Dengue Diagnostic Tests for the Diagnosis of Acute Dengue Infection in Malaysia. BMC Infect. Dis. 2020, 20, 210. [Google Scholar] [CrossRef]
- Wongsawat, E.; Suputtamongkol, Y.; Assanasaen, S.; Silpasakorn, S.; Avirutnan, P.; Puttikhunt, C.; Angkasekwinai, N.; Yamasmith, E.; Prommool, T.; Niwattayakul, K. Performance of a New Microfluidic Dengue NS1 Immuno-Magnetic Agglutination Assay for the Rapid Diagnosis of Dengue Infection in Adults. Am. J. Trop. Med. Hyg. 2021, 105, 771–776. [Google Scholar] [CrossRef]
- Jang, W.S.; Kwak, S.Y.; May, W.L.; Yang, D.J.; Nam, J.; Lim, C.S. Comparative Evaluation of Three Dengue Duo Rapid Test Kits to Detect NS1, IgM, and IgG Associated with Acute Dengue in Children in Myanmar. PLoS ONE 2019, 14, e0213451. [Google Scholar] [CrossRef]
- Kikuti, M.; Cruz, J.S.; Rodrigues, M.S.; Tavares, A.S.; Paploski, I.A.D.; Silva, M.M.O.; Santana, P.M.; Tauro, L.B.; Silva, G.A.O.F.; Campos, G.S.; et al. Accuracy of the SD BIOLINE Dengue Duo for Rapid Point-of-Care Diagnosis of Dengue. PLoS ONE 2019, 14, e0213301. [Google Scholar] [CrossRef]
- Kyaw, A.K.; Ngwe Tun, M.M.; Naing, S.T.; Htet, K.K.K.; Htwe, T.T.; Khaing, Y.Y.; Tu Mar, T.; Aung, T.; Win, K.N.; Tar, T.; et al. Evaluation of Commercially Available Three Dengue Rapid Diagnostic Test Kits for Diagnosis of Acute Dengue Virus Infection at the Point-of-Care Setting in Myanmar. J. Virol. Methods 2019, 273, 113724. [Google Scholar] [CrossRef]
- Bessoff, K.; Delorey, M.; Sun, W.; Hunsperger, E. Comparison of Two Commercially Available Dengue Virus (DENV) NS1 Capture Enzyme-Linked Immunosorbent Assays Using a Single Clinical Sample for Diagnosis of Acute DENV Infection. Clin. Vaccine Immunol. 2008, 15, 1513–1518. [Google Scholar] [CrossRef]
- Ramirez, A.H.; Moros, Z.; Comach, G.; Zambrano, J.; Bravo, L.; Pinto, B.; Vielma, S.; Cardier, J.; Liprandi, F. Evaluation of Dengue NS1 Antigen Detection Tests with Acute Sera from Patients Infected with Dengue Virus in Venezuela. Diagn. Microbiol. Infect. Dis. 2009, 65, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Teoh, B.-T.; Sam, S.-S.; Tan, K.-K.; Johari, J.; Abd-Jamil, J.; Hooi, P.-S.; AbuBakar, S. The Use of NS1 Rapid Diagnostic Test and QRT-PCR to Complement IgM ELISA for Improved Dengue Diagnosis from Single Specimen. Sci. Rep. 2016, 6, 27663. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Dauner, A.L.; Mitra, I.; Forshey, B.M.; Garcia, P.; Morrison, A.C.; Halsey, E.S.; Kochel, T.J.; Wu, S.-J.L. Evaluation of Dengue NS1 Antigen Rapid Tests and ELISA Kits Using Clinical Samples. PLoS ONE 2014, 9, e113411. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Number | Percentage |
|---|---|---|
| Time of sampling (n = 472) | ||
| 0–4 days | 411 | 87.1 |
| 5–7 days | 61 | 12.9 |
| RT-PCR result (n = 472) | ||
| Negative | 318 | 67.4 |
| Positive | 154 | 32.6 |
| Dengue virus serotype | ||
| DENV-1 | 103 | 66.9 |
| DENV-2 | 42 | 27.2 |
| DENV-3 | 6 | 3.9 |
| DENV-4 | 0 | 0 |
| Biosynex Dengue NS1 Ag RDT | Reference (RT-PCR) | |
|---|---|---|
| RDT Overall performance | Positive | Negative |
| Positive | 123 | 13 |
| Negative | 31 | 306 |
| Sensitivity (95% CI) | 79.9% (72.8–85.5%) | |
| Specificity (95% CI) | 96.2% (93.5–97.9%) | |
| Positive predictive value (95% CI) | 91.1% (85.0–95.0%) | |
| Negative predictive value (95% CI) | 90.8% (87.2–93.5%) | |
| RDT Performance by time of sampling | ||
| <5 days | ||
| Sensitivity (95% CI) | 80.7% (73.2–86.6%) | |
| Specificity (95% CI) | 98.2% (95.7–99.4%) | |
| ≥5 days | ||
| Sensitivity (95% CI) | 73.7% (50.9–88.6%) | |
| Specificity (95% CI) | 83.3% (69.1–92.0%) | |
| RDT Sensitivity by Dengue serotype | ||
| Dengue 1 Sensitivity (95% CI) | 92.2% (85.2–96.2%) | |
| Dengue 2 Sensitivity (95% CI) | 50.0% (35.5–64.5%) | |
| Dengue 3 Sensitivity (95% CI) | 83.3% (41.8–98.9%) | |
| Study | Country | Number of Samples | Assay Name (Details Below *) | Sensitivity | Specificity | Comments |
|---|---|---|---|---|---|---|
| [15] | Indonesia | 149 | Biosynex 1 | 80% | 100% | Samples were collected within 5 days post symptom onset. Significant number of DENV-1, 2, 3, and 4 positive samples. Sensitivity ranged from 44.4 to 100% across dengue serotypes for RDTs. All RDTs performed better for DENV-3. |
| CTK Biotech 2 | 71% | 100% | ||||
| Boson Biotech 3 | 73% | 100% | ||||
| Panbio 4 | 74% | 100% | ||||
| SD Bioline 5 | 73% | 100% | ||||
| This study | New caledonia | 471 | Biosynex 1 | 79.9% | 96.2% | Samples were collected within 7 days post symptom onset. Significant number of DENV-1 and 2 positive samples. The sensitivity was 92.2 and 50% for DENV-1 and DENV-2, respectively. |
| [17] | Taiwan | 173 | NS1 Ag Bio-Rad 6 | 85.3% | 94.6% | Samples were collected within 6 days post symptom onset. Significant number of DENV-1, 2, and 3 positive samples. Sensitivity ranged from 84 to 100% across dengue serotypes for RDTs. |
| CTK Biotech 2 | 89% | 73% | ||||
| SD Bioline 5 | 89.7% | 91.9% | ||||
| [16] | Solomon Islands | 412 | SD Bioline 5 | 90.9% | 100% | Samples collected within 6 days post symptom onset. Data were not available for sensitivity across dengue serotypes. |
| CTK Biotech 2 | 92.6% | 78.8% | ||||
| [18] | India | 253 | Dengue DAY 1 8 | 91.1 | 93.6 | Samples were collected within 5 days post symptom onset. Significant number of DENV-1, 2, and 3 positive samples. The sensitivity was higher than 90% for DENV-1, 2, and 3. |
| [19] | Malaysia | 490 | ViroTrack 7 | 62.3% | 95% | Samples were collected within 11 days post symptom onset. Significant number of DENV-1, 2, and 3 positive samples. Sensitivity ranged from 54.8 to 96.7% across dengue serotypes for RDTs. Both NS1 RDTs performed better for DENV-3 (sensitivity > 90%) than DENV-1 and 2. |
| SD Bioline 5 | 52.4% | 97.7% | ||||
| [20] | Thailand | 778(433 patients) | ViroTrack 7 | 85.5% | 97% | Samples collected within 5 days post symptom onset. Significant number of DENV-1, 2, 3, and 4 positive samples. Sensitivity ranged from 75 to 90% across dengue serotypes for both RDTs. |
| SD Bioline 5 | 78.9% | 99% | ||||
| [21] | Myanmar | 172 (children) | Humasis Dengue 9 | 63.3% | 100% | Samples collected within 3–7 days post symptom onset. Significant number of DENV-1 and 2 positive samples. Sensitivity from 52 to 88% across dengue serotypes for RDTs. |
| SD Bioline 5 | 48.6% | 100% | ||||
| CareUS Dengue 10 | 79.8% | 100% | ||||
| [22] | Brazil | 500 | SD Bioline 5 | 38.6% | 98.2% | The median duration of illness before sample collection was 2 (IQR: 2–4) days. Significant number of DENV-1, 2, and 4 positive samples. The sensitivity was 55.6%, 46%, and 61.2% for DENV-1, DENV-2 and DENV-4, respectively. (DENV typing not performed on all positive samples). |
| [23] | Myanmar | 202 | CareUS Dengue 10 | 72.1% | 87.1% | Samples were collected within 7 days post symptom onset. Data were not available for sensitivity across dengue serotypes. |
| Humasis Dengue 9 | 68.6% | 90.3% | ||||
| Wondfo Dengue 11 | 67.1% | 91.9% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alidjinou, E.K.; Tardieu, S.; Vrenken, I.; Hober, D.; Gourinat, A.-C. Prospective Evaluation of a Commercial Dengue NS1 Antigen Rapid Diagnostic Test in New Caledonia. Microorganisms 2022, 10, 346. https://doi.org/10.3390/microorganisms10020346
Alidjinou EK, Tardieu S, Vrenken I, Hober D, Gourinat A-C. Prospective Evaluation of a Commercial Dengue NS1 Antigen Rapid Diagnostic Test in New Caledonia. Microorganisms. 2022; 10(2):346. https://doi.org/10.3390/microorganisms10020346
Chicago/Turabian StyleAlidjinou, Enagnon Kazali, Sylvie Tardieu, Isabelle Vrenken, Didier Hober, and Ann-Claire Gourinat. 2022. "Prospective Evaluation of a Commercial Dengue NS1 Antigen Rapid Diagnostic Test in New Caledonia" Microorganisms 10, no. 2: 346. https://doi.org/10.3390/microorganisms10020346
APA StyleAlidjinou, E. K., Tardieu, S., Vrenken, I., Hober, D., & Gourinat, A.-C. (2022). Prospective Evaluation of a Commercial Dengue NS1 Antigen Rapid Diagnostic Test in New Caledonia. Microorganisms, 10(2), 346. https://doi.org/10.3390/microorganisms10020346







