Flavonoids Are Intra- and Inter-Kingdom Modulator Signals
Abstract
1. Flavonoids: Plant Secondary Metabolites with a Broad and Diversified Spectrum of Activities across All Life Kingdoms
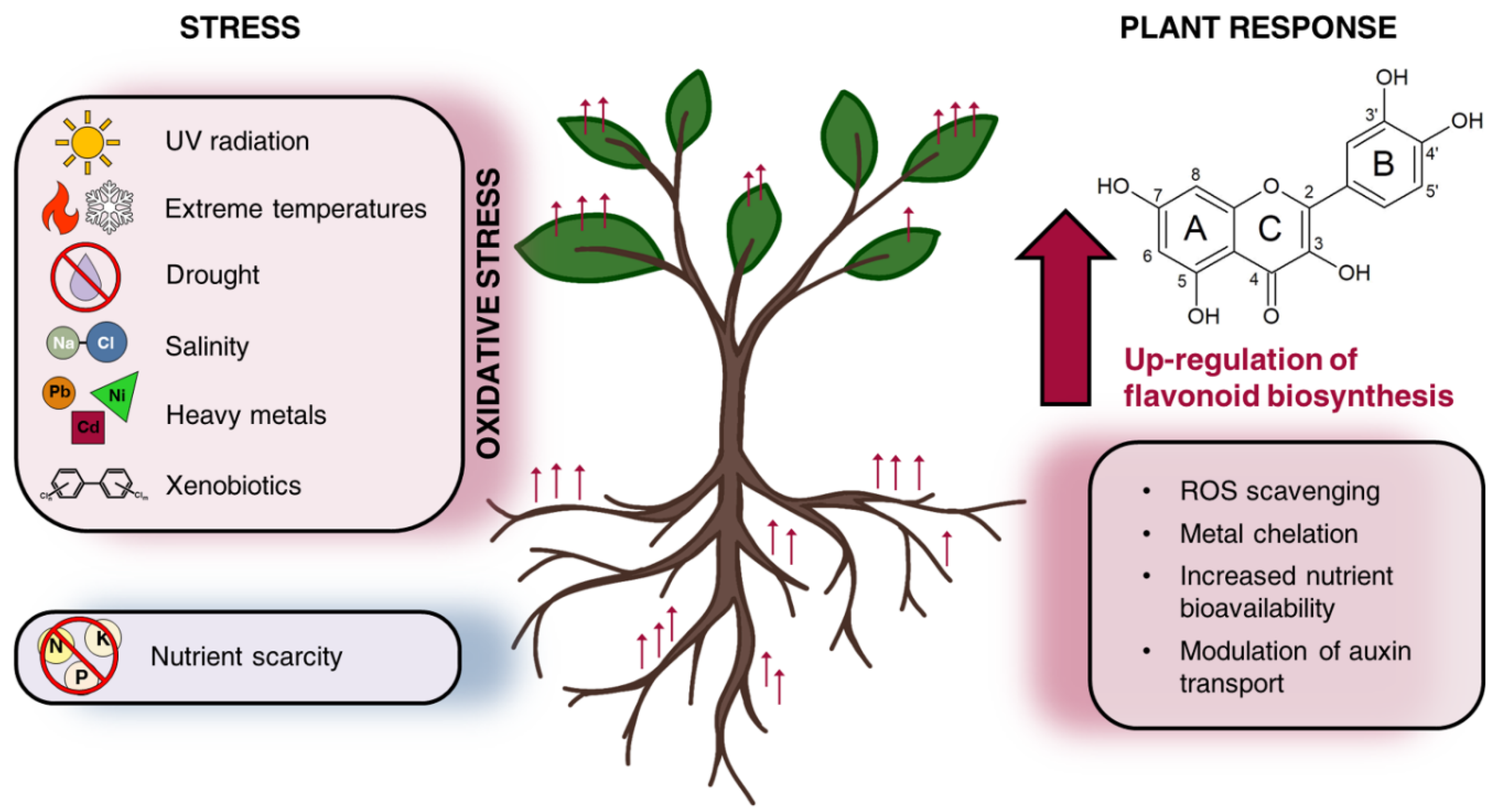
2. Flavonoids Tune Plant Physiology Response under Biotic and Abiotic Stress Conditions
3. Flavonoids Mediate Plant-Plant Interactions
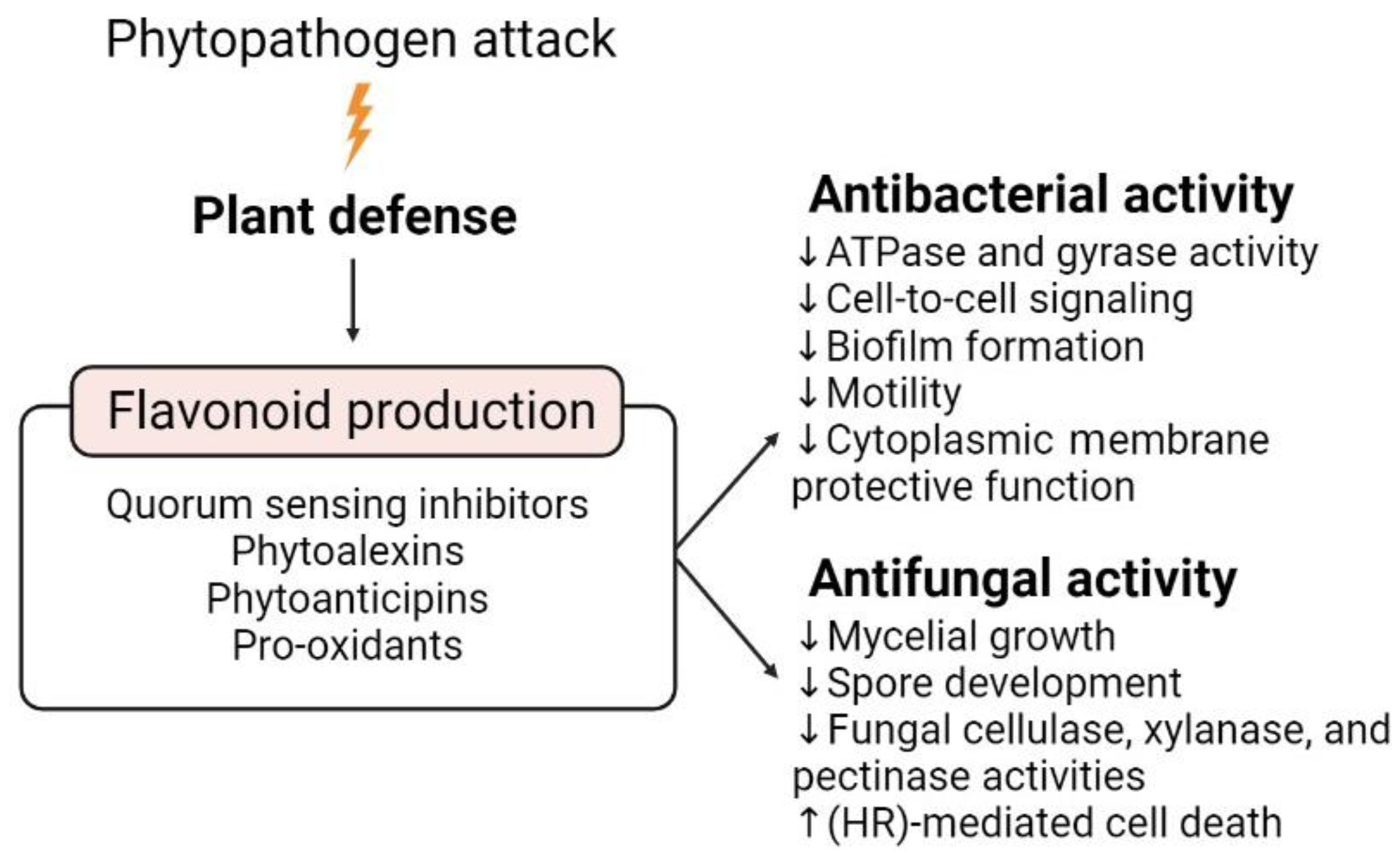
4. Flavonoids as Weapons against Phytopathogenic Attacks
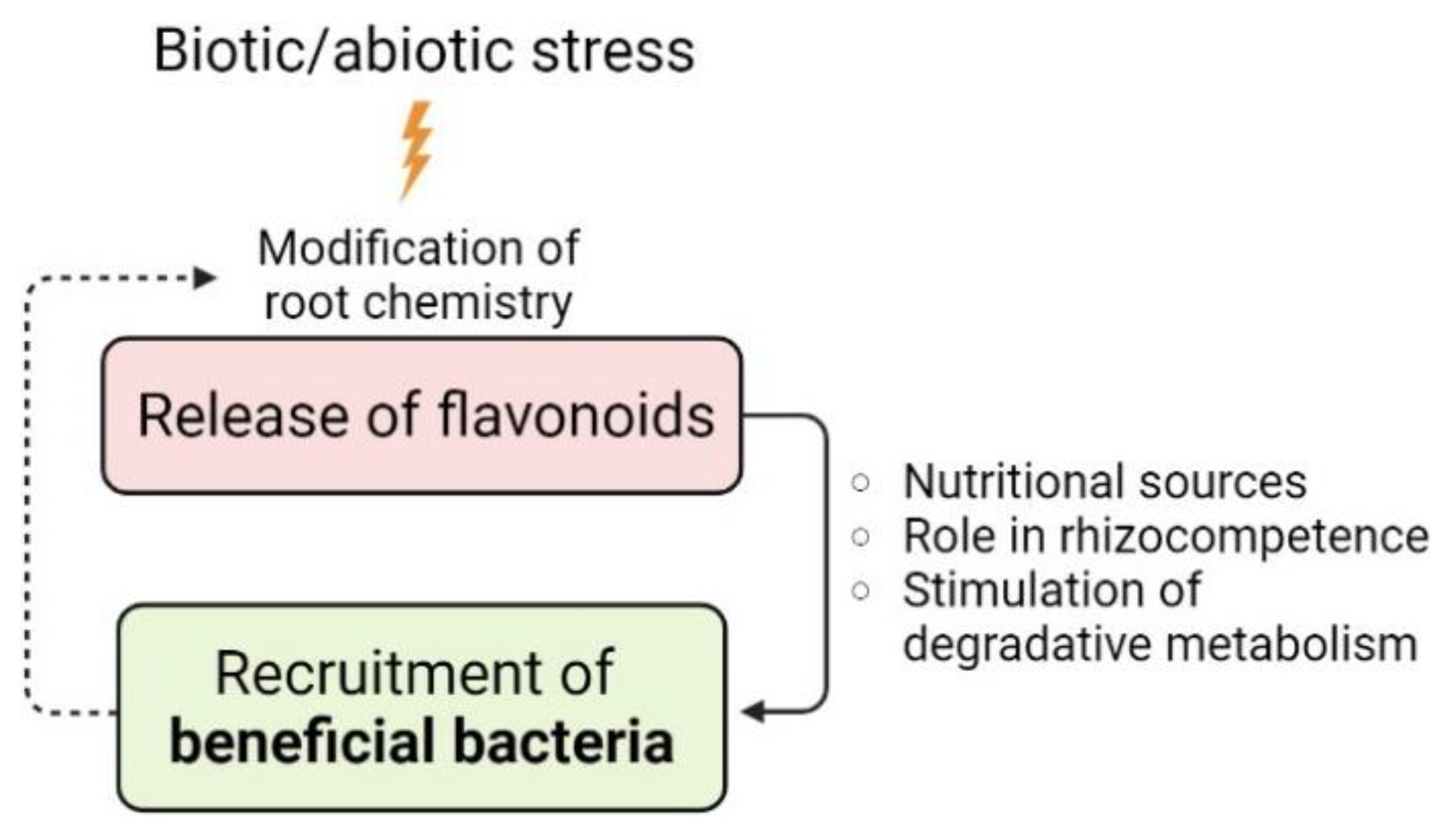
5. Flavonoid Exudation Affects the Structure and Function of Root-Associated Microbiomes
6. Flavonoids Modulate Behavior and Life History Traits of Insect Herbivores, Natural Enemies and Pollinators
7. Flavonoids as Nutraceuticals and Prebiotics for Human Health
8. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Sweetlove, L.J.; Fernie, A.R. The spatial organization of metabolism within the plant cell. Annu. Rev. Plant Biol. 2013, 64, 723–746. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, C.; Gallage, N.J.; Hansen, C.C.; Møller, B.L.; Laursen, T. Dynamic metabolic solutions to the sessile life style of plants. Nat. Prod. Rep. 2018, 35, 1140–1155. [Google Scholar] [CrossRef] [PubMed]
- Desmet, S.; Morreel, K.; Dauwe, R. Origin and function of structural diversity in the plant specialized metabolome. Plants 2021, 10, 2393. [Google Scholar] [CrossRef]
- Williams, C.A.; Grayer, R.J. Anthocyanins and other flavonoids. Nat. Prod. Rep. 2004, 21, 539. [Google Scholar] [CrossRef] [PubMed]
- Yonekura-Sakakibara, K.; Higashi, Y.; Nakabayashi, R. The origin and evolution of plant flavonoid metabolism. Front. Plant Sci. 2019, 10, 943. [Google Scholar] [CrossRef]
- Sugiyama, A.; Yazaki, K. Flavonoids in plant rhizospheres: Secretion, fate and their effects on biological communication. Plant Biotechnol. 2014, 31, 431–443. [Google Scholar] [CrossRef]
- Bowman, J.L.; Kohchi, T.; Yamato, K.T.; Jenkins, J.; Shu, S.; Ishizaki, K.; Yamaoka, S.; Nishihama, R.; Nakamura, Y.; Berger, F.; et al. Insights into land plant evolution garnered from the Marchantia polymorpha genome. Cell 2017, 171, 287–304.e15. [Google Scholar] [CrossRef]
- Santos, E.L.; Maia, B.H.L.N.S.; Ferriani, A.P.; Teixeira, S.D. Flavonoids: Classification, biosynthesis and chemical ecology. In Flavonoids-from Biosynthesis to Human Health; InTech: London, UK, 2017. [Google Scholar]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef]
- Mathesius, U. Flavonoid functions in plants and their interactions with other organisms. Plants 2018, 7, 30. [Google Scholar] [CrossRef]
- del Valle, J.C.; Buide, M.L.; Casimiro-Soriguer, I.; Whittall, J.B.; Narbona, E. On flavonoid accumulation in different plant parts: Variation patterns among individuals and populations in the shore campion (Silene littorea). Front. Plant Sci. 2015, 6, 939. [Google Scholar] [CrossRef]
- Taylor, L.P.; Grotewold, E. Flavonoids as developmental regulators. Curr. Opin. Plant Biol. 2005, 8, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, J.M.; Badri, D.V.; Bakker, M.G.; Sugiyama, A.; Manter, D.K.; Vivanco, J.M. Root exudation of phytochemicals in Arabidopsis follows specific patterns that are developmentally programmed and correlate with soil microbial functions. PLoS ONE 2013, 8, e55731. [Google Scholar] [CrossRef]
- Cetinkaya, H.; Kulak, M.; Karaman, M.; Karaman, H.S.; Kocer, F. Flavonoid accumulation behavior in response to the abiotic stress: Can a uniform mechanism be illustrated for all plants? In Flavonoids-from Biosynthesis to Human Health; InTech: London, UK, 2017. [Google Scholar]
- Dardanelli, M.S.; de Córdoba, F.J.F.; Estévez, J.; Contreras, R.; Cubo, M.T.; Rodríguez-Carvajal, M.Á.; Gil-Serrano, A.M.; López-Baena, F.J.; Bellogín, R.; Manyani, H.; et al. Changes in flavonoids secreted by Phaseolus vulgaris roots in the presence of salt and the plant growth-promoting rhizobacterium Chryseobacterium balustinum. Appl. Soil Ecol. 2012, 57, 31–38. [Google Scholar] [CrossRef]
- Li, B.; Fan, R.; Sun, G.; Sun, T.; Fan, Y.; Bai, S.; Guo, S.; Huang, S.; Liu, J.; Zhang, H.; et al. Flavonoids improve drought tolerance of maize seedlings by regulating the homeostasis of reactive oxygen species. Plant Soil 2021, 461, 389–405. [Google Scholar] [CrossRef]
- Su, X.; Wang, W.; Xia, T.; Gao, L.; Shen, G.; Pang, Y. Characterization of a heat responsive UDP: Flavonoid glucosyltransferase gene in tea plant (Camellia sinensis). PLoS ONE 2018, 13, e0207212. [Google Scholar] [CrossRef] [PubMed]
- Schulz, E.; Tohge, T.; Zuther, E.; Fernie, A.R.; Hincha, D.K. Flavonoids are determinants of freezing tolerance and cold acclimation in Arabidopsis thaliana. Sci. Rep. 2016, 6, 34027. [Google Scholar] [CrossRef]
- Gonzalez-Mendoza, D.; Mendez-Trujillo, V.; Grimaldo-Juarez, O.; Ceceña-Duran, C.; Tzintzun-Camacho, O.; Gutierrez-Miceli, F.; Sanchez-Viveros, G.; Aviles Marin, M. Changes of photochemical efficiency and epidermal polyphenols content of Prosopis glandulosa and Prosopis juliflora leaves exposed to cadmium and copper. Open Life Sci. 2017, 12, 373–378. [Google Scholar] [CrossRef]
- Ite, A.E.; Hanney, N.F.; Semple, K.T. The Effect of hydroxycinnamic acids on the microbial mineralisation of phenanthrene in soil. Int. J. Environ. Bioremediat. Biodegrad. 2015, 3, 40–47. [Google Scholar] [CrossRef]
- Liang, T.; Shi, C.; Peng, Y.; Tan, H.; Xin, P.; Yang, Y.; Wang, F.; Li, X.; Chu, J.; Huang, J.; et al. Brassinosteroid-activated BRI1-EMS-SUPPRESSOR 1 inhibits flavonoid biosynthesis and coordinates growth and UV-B stress responses in plants. Plant Cell 2020, 32, 3224–3239. [Google Scholar] [CrossRef]
- Vives-Peris, V.; de Ollas, C.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Root exudates: From plant to rhizosphere and beyond. Plant Cell Rep. 2020, 39, 3–17. [Google Scholar] [CrossRef]
- Weston, L.A.; Mathesius, U. Flavonoids: Their structure, biosynthesis and role in the rhizosphere, including allelopathy. J. Chem. Ecol. 2013, 39, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Badri, D.V.; Chaparro, J.M.; Zhang, R.; Shen, Q.; Vivanco, J.M. Application of natural blends of phytochemicals derived from the root exudates of Arabidopsis to the soil reveal that phenolic-related compounds predominantly modulate the soil microbiome. J. Biol. Chem. 2013, 288, 4502–4512. [Google Scholar] [CrossRef] [PubMed]
- Szoboszlay, M.; White-Monsant, A.; Moe, L.A. The effect of root exudate 7,4′-dihydroxyflavone and naringenin on soil bacterial community structure. PLoS ONE 2016, 11, e0146555. [Google Scholar] [CrossRef]
- Quilbé, J.; Montiel, J.; Arrighi, J.-F.; Stougaard, J. Molecular mechanisms of intercellular rhizobial infection: Novel findings of an ancient process. Front. Plant Sci. 2022, 13, 922982. [Google Scholar] [CrossRef]
- Hassan, S.; Mathesius, U. Flavonoids play multiple roles in symbiotic root-rhizosphere interactions. In Biological Nitrogen Fixation; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 499–510. [Google Scholar]
- Mathesius, U.; Watt, M. Rhizosphere signals for plant–microbe interactions: Implications for field-grown plants. In Progress in Botany 72; Lüttge, U.E., Beyschlag, W., Büdel, B., Francis, D., Eds.; Progress in Botany; Springer: Berlin/Heidelberg, Germany, 2011; Volume 72, pp. 125–161. ISBN 978-3-642-13144-8. [Google Scholar]
- Berrabah, F.; Salem, E.H.A.; Garmier, M.; Ratet, P. The multiple faces of the Medicago-Sinorhizobium symbiosis. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2018; Volume 1822, pp. 241–260. ISBN 9781493986330. [Google Scholar]
- Dong, Q.; Zhao, X.; Zhou, D.; Liu, Z.; Shi, X.; Yuan, Y.; Jia, P.; Liu, Y.; Song, P.; Wang, X.; et al. Maize and peanut intercropping improves the nitrogen accumulation and yield per plant of maize by promoting the secretion of flavonoids and abundance of Bradyrhizobium in rhizosphere. Front. Plant Sci. 2022, 13, 957336. [Google Scholar] [CrossRef]
- Berrabah, F.; Ratet, P.; Gourion, B. Legume nodules: Massive infection in the absence of defense induction. Mol. Plant-Microbe Interact. 2019, 32, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Larose, G.; Chênevert, R.; Moutoglis, P.; Gagné, S.; Piché, Y.; Vierheilig, H. Flavonoid levels in roots of Medicago sativa are modulated by the developmental stage of the symbiosis and the root colonizing arbuscular mycorrhizal fungus. J. Plant Physiol. 2002, 159, 1329–1339. [Google Scholar] [CrossRef]
- Cesco, S.; Neumann, G.; Tomasi, N.; Pinton, R.; Weisskopf, L. Release of plant-borne flavonoids into the rhizosphere and their role in plant nutrition. Plant Soil 2010, 329, 1–25. [Google Scholar] [CrossRef]
- Yu, P.; He, X.; Baer, M.; Beirinckx, S.; Tian, T.; Moya, Y.A.T.; Zhang, X.; Deichmann, M.; Frey, F.P.; Bresgen, V.; et al. Plant flavones enrich rhizosphere Oxalobacteraceae to improve maize performance under nitrogen deprivation. Nat. Plants 2021, 7, 481–499. [Google Scholar] [CrossRef]
- Narasimhan, K.; Basheer, C.; Bajic, V.B.; Swarup, S. Enhancement of plant-microbe interactions using a rhizosphere metabolomics-driven approach and its application in the removal of polychlorinated biphenyls. Plant Physiol. 2003, 132, 146–153. [Google Scholar] [CrossRef]
- Bag, S.; Mondal, A.; Majumder, A.; Mondal, S.K.; Banik, A. Flavonoid mediated selective cross-talk between plants and beneficial soil microbiome. Phytochem. Rev. 2022, 3, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Q.; Yan, X.; Zhang, M.Y.; Zhang, L.Q.; He, Y.X. Flavonoids repress the production of antifungal 2,4-DAPG but potentially facilitate root colonization of the rhizobacterium Pseudomonas fluorescens. Environ. Microbiol. 2020, 22, 5073–5089. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Montaño, F.; Alías-Villegas, C.; Bellogín, R.A.; del Cerro, P.; Espuny, M.R.; Jiménez-Guerrero, I.; López-Baena, F.J.; Ollero, F.J.; Cubo, T. Plant growth promotion in cereal and leguminous agricultural important plants: From microorganism capacities to crop production. Microbiol. Res. 2014, 169, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Vergani, L.; Mapelli, F.; Marasco, R.; Crotti, E.; Fusi, M.; Di Guardo, A.; Armiraglio, S.; Daffonchio, D.; Borin, S. Bacteria associated to plants naturally selected in a historical PCB polluted soil show potential to sustain natural attenuation. Front. Microbiol. 2017, 8, 1385. [Google Scholar] [CrossRef] [PubMed]
- Vergani, L.; Mapelli, F.; Zanardini, E.; Terzaghi, E.; Di Guardo, A.; Morosini, C.; Raspa, G.; Borin, S. Phyto-rhizoremediation of polychlorinated biphenyl contaminated soils: An outlook on plant-microbe beneficial interactions. Sci. Total Environ. 2017, 575, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Pei, R.; Liu, X.; Bolling, B. Flavonoids and gut health. Curr. Opin. Biotechnol. 2020, 61, 153–159. [Google Scholar] [CrossRef]
- Oteiza, P.I.; Fraga, C.G.; Mills, D.A.; Taft, D.H. Flavonoids and the gastrointestinal tract: Local and systemic effects. Mol. Asp. Med. 2018, 61, 41–49. [Google Scholar] [CrossRef]
- Xiao, L.; Sun, Y.; Tsao, R. Paradigm shift in phytochemicals research: Evolution from antioxidant capacity to anti-inflammatory effect and to roles in gut health and metabolic syndrome. J. Agric. Food Chem. 2022, 70, 8551–8568. [Google Scholar] [CrossRef]
- Kaushal, N.; Singh, M.; Singh Sangwan, R. Flavonoids: Food associations, therapeutic mechanisms, metabolism and nanoformulations. Food Res. Int. 2022, 157, 111442. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Salinity stress enhances color parameters, bioactive leaf pigments, vitamins, polyphenols, flavonoids and antioxidant activity in selected Amaranthus leafy vegetables. J. Sci. Food Agric. 2019, 99, 2275–2284. [Google Scholar] [CrossRef]
- Shojaie, B.; Mostajeran, A.; Ghannadian, M. Flavonoid dynamic responses to different drought conditions: Amount, type, and localization of flavonols in roots and shoots of Arabidopsis thaliana L. Turk. J. Biol. 2016, 40, 612–622. [Google Scholar] [CrossRef]
- Liu, S.; Fang, S.; Liu, C.; Zhao, L.; Cong, B.; Zhang, Z. Transcriptomics integrated with metabolomics reveal the effects of Ultraviolet-B radiation on flavonoid biosynthesis in antarctic moss. Front. Plant Sci. 2021, 12, 788377. [Google Scholar] [CrossRef] [PubMed]
- Alhaithloul, H.A.S.; Galal, F.H.; Seufi, A.M. Effect of extreme temperature changes on phenolic, flavonoid contents and antioxidant activity of tomato seedlings (Solanum lycopersicum L.). PeerJ 2021, 9, e11193. [Google Scholar] [CrossRef]
- Meng, L.; Yang, Y.; Ma, Z.; Jiang, J.; Zhang, X.; Chen, Z.; Cui, G.; Yin, X. Integrated physiological, transcriptomic and metabolomic analysis of the response of Trifolium pratense L. to Pb toxicity. J. Hazard. Mater. 2022, 436, 129128. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Smith, D.L. Flavonoids in agriculture: Chemistry and roles in, biotic and abiotic stress responses, and microbial associations. Agronomy 2020, 10, 1209. [Google Scholar] [CrossRef]
- Nascimento, L.B.D.S.; Tattini, M. Beyond photoprotection: The multifarious roles of flavonoids in plant terrestrialization. Int. J. Mol. Sci. 2022, 23, 5284. [Google Scholar] [CrossRef]
- Erb, M.; Kliebenstein, D.J. Plant secondary metabolites as defenses, regulators, and primary metabolites: The blurred functional trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef]
- Neilson, E.H.; Goodger, J.Q.D.; Woodrow, I.E.; Møller, B.L. Plant chemical defense: At what cost? Trends Plant Sci. 2013, 18, 250–258. [Google Scholar] [CrossRef]
- Agati, G.; Tattini, M. Multiple functional roles of flavonoids in photoprotection. New Phytol. 2010, 186, 786–793. [Google Scholar] [CrossRef]
- Brunetti, C.; Di Ferdinando, M.; Fini, A.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants and developmental regulators: Relative significance in plants and humans. Int. J. Mol. Sci. 2013, 14, 3540–3555. [Google Scholar] [CrossRef]
- Hernandez, I.; Alegre, L.; Munne-Bosch, S. Drought-induced changes in flavonoids and other low molecular weight antioxidants in Cistus clusii grown under mediterranean field conditions. Tree Physiol. 2004, 24, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V. Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ. Exp. Bot. 2015, 109, 212–228. [Google Scholar] [CrossRef]
- You, J.; Chan, Z. ROS Regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef] [PubMed]
- Fini, A.; Guidi, L.; Ferrini, F.; Brunetti, C.; Di Ferdinando, M.; Biricolti, S.; Pollastri, S.; Calamai, L.; Tattini, M. Drought stress has contrasting effects on antioxidant enzymes activity and phenylpropanoid biosynthesis in Fraxinus ornus leaves: An excess light stress affair? J. Plant Physiol. 2012, 169, 929–939. [Google Scholar] [CrossRef]
- Šudomová, M.; Berchová-Bímová, K.; Mazurakova, A.; Šamec, D.; Kubatka, P.; Hassan, S.T.S. Flavonoids target human herpesviruses that infect the nervous system: Mechanisms of action and therapeutic insights. Viruses 2022, 14, 592. [Google Scholar] [CrossRef]
- Csepregi, K.; Hideg, É. Phenolic compound diversity explored in the context of photo-oxidative stress protection. Phytochem. Anal. 2018, 29, 129–136. [Google Scholar] [CrossRef]
- Lü, J.-M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell. Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Shao, M.; Liu, W.; Zha, L.; Zhou, C.; Zhang, Y.; Li, B. Differential effects of high light duration on growth, nutritional quality, and oxidative stress of hydroponic lettuce under red and blue LED irradiation. Sci. Hortic. 2020, 268, 109366. [Google Scholar] [CrossRef]
- Di Ferdinando, M.; Brunetti, C.; Fini, A.; Tattini, M. Flavonoids as antioxidants in plants under abiotic stresses. In Abiotic Stress Responses in Plants: Metabolism, Productivity and Sustainability; Ahmad, P., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2012; pp. 159–179. ISBN 978-1-4614-0633-4. [Google Scholar]
- Tattini, M.; Galardi, C.; Pinelli, P.; Massai, R.; Remorini, D.; Agati, G. Differential accumulation of flavonoids and hydroxycinnamates in leaves of Ligustrum vulgare under excess light and drought stress. New Phytol. 2004, 163, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Righini, S.; Rodriguez, E.J.; Berosich, C.; Grotewold, E.; Casati, P.; Falcone Ferreyra, M.L. Apigenin produced by maize flavone synthase I and II protects plants against UV-B-induced damage. Plant. Cell Environ. 2019, 42, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Gai, Q.-Y.; Yao, L.-P.; Niu, L.-L.; Zang, Y.-P.; Fu, Y.-J. Ultraviolet radiation for flavonoid augmentation in Isatis tinctoria L. hairy root cultures mediated by oxidative stress and biosynthetic gene expression. Ind. Crops Prod. 2018, 118, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Mariz-Ponte, N.; Mendes, R.J.; Sario, S.; Ferreira de Oliveira, J.M.P.; Melo, P.; Santos, C. Tomato plants use non-enzymatic antioxidant pathways to cope with moderate UV-A/B irradiation: A contribution to the use of UV-A/B in horticulture. J. Plant Physiol. 2018, 221, 32–42. [Google Scholar] [CrossRef]
- Fini, A.; Brunetti, C.; Di Ferdinando, M.; Ferrini, F.; Tattini, M. Stress-induced flavonoid biosynthesis and the antioxidant machinery of plants. Plant Signal. Behav. 2011, 6, 709–711. [Google Scholar] [CrossRef]
- Martinez, V.; Mestre, T.C.; Rubio, F.; Girones-Vilaplana, A.; Moreno, D.A.; Mittler, R.; Rivero, R.M. Accumulation of flavonols over hydroxycinnamic acids favors oxidative damage protection under abiotic stress. Front. Plant Sci. 2016, 7, 838. [Google Scholar] [CrossRef]
- Agati, G.; Matteini, P.; Goti, A.; Tattini, M. Chloroplast-located flavonoids can scavenge singlet oxygen. New Phytol. 2007, 174, 77–89. [Google Scholar] [CrossRef]
- Ferreres, F.; Figueiredo, R.; Bettencourt, S.; Carqueijeiro, I.; Oliveira, J.; Gil-Izquierdo, A.; Pereira, D.M.; Valentão, P.; Andrade, P.B.; Duarte, P.; et al. Identification of phenolic compounds in isolated vacuoles of the medicinal plant Catharanthus roseus and their interaction with vacuolar class III peroxidase: An H2O2 affair? J. Exp. Bot. 2011, 62, 2841–2854. [Google Scholar] [CrossRef]
- Jan, R.; Khan, M.-A.; Asaf, S.; Lubna; Waqas, M.; Park, J.-R.; Asif, S.; Kim, N.; Lee, I.-J.; Kim, K.-M. Drought and UV radiation stress tolerance in rice is improved by overaccumulation of non-enzymatic antioxidant flavonoids. Antioxidants 2022, 11, 917. [Google Scholar] [CrossRef]
- Laoué, J.; Fernandez, C.; Ormeño, E. Plant flavonoids in mediterranean species: A focus on flavonols as protective metabolites under climate stress. Plants 2022, 11, 172. [Google Scholar] [CrossRef]
- Sardans, J.; Gargallo-Garriga, A.; Urban, O.; Klem, K.; Walker, T.W.N.; Holub, P.; Janssens, I.A.; Peñuelas, J. Ecometabolomics for a better understanding of plant responses and acclimation to abiotic factors linked to global change. Metabolites 2020, 10, 239. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Prasad, A.; Srivastava, D.; Niranjan, A.; Saxena, G.; Singh, S.S.; Misra, P.; Chakrabarty, D. Genotype-dependent and temperature-induced modulation of secondary metabolites, antioxidative defense and gene expression profile in Solanum viarum Dunal. Environ. Exp. Bot. 2022, 194, 104686. [Google Scholar] [CrossRef]
- Hodaei, M.; Rahimmalek, M.; Arzani, A.; Talebi, M. The effect of water stress on phytochemical accumulation, bioactive compounds and expression of key genes involved in flavonoid biosynthesis in Chrysanthemum morifolium L. Ind. Crops Prod. 2018, 120, 295–304. [Google Scholar] [CrossRef]
- Ma, D.; Sun, D.; Wang, C.; Li, Y.; Guo, T. Expression of flavonoid biosynthesis genes and accumulation of flavonoid in wheat leaves in response to drought stress. Plant Physiol. Biochem. 2014, 80, 60–66. [Google Scholar] [CrossRef]
- Jayaraman, K.; Venkat Raman, K.; Sevanthi, A.M.; Sivakumar, S.R.; Gayatri; Viswanathan, C.; Mohapatra, T.; Mandal, P.K. Stress-inducible expression of chalcone isomerase2 gene improves accumulation of flavonoids and imparts enhanced abiotic stress tolerance to rice. Environ. Exp. Bot. 2021, 190, 104582. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Sales, C.; Beltrán, J.; Gómez-Cadenas, A.; Arbona, V. Activation of secondary metabolism in citrus plants is associated to sensitivity to combined drought and high temperatures. Front. Plant Sci. 2017, 7, 1954. [Google Scholar] [CrossRef]
- Xu, N.; Liu, S.; Lu, Z.; Pang, S.; Wang, L.; Wang, L.; Li, W. Gene expression profiles and flavonoid accumulation during salt stress in Ginkgo biloba seedlings. Plants 2020, 9, 1162. [Google Scholar] [CrossRef]
- Jorge, T.F.; Tohge, T.; Wendenburg, R.; Ramalho, J.C.; Lidon, F.C.; Ribeiro-Barros, A.I.; Fernie, A.R.; António, C. Salt-stress secondary metabolite signatures involved in the ability of Casuarina glauca to mitigate oxidative stress. Environ. Exp. Bot. 2019, 166, 103808. [Google Scholar] [CrossRef]
- Brossa, R.; Casals, I.; Pintó-Marijuan, M.; Fleck, I. Leaf flavonoid content in Quercus ilex L. resprouts and its seasonal variation. Trees 2009, 23, 401–408. [Google Scholar] [CrossRef]
- Cloutier, M.; Chatterjee, D.; Elango, D.; Cui, J.; Bruns, M.A.; Chopra, S. Sorghum root flavonoid chemistry, cultivar, and frost stress effects on rhizosphere bacteria and fungi. Phytobiomes J. 2021, 5, 39–50. [Google Scholar] [CrossRef]
- Georgieva, K.; Mihailova, G.; Gigova, L.; Dagnon, S.; Simova-Stoilova, L.; Velitchkova, M. The role of antioxidant defense in freezing tolerance of resurrection plant Haberlea rhodopensis. Physiol. Mol. Biol. Plants 2021, 27, 1119–1133. [Google Scholar] [CrossRef] [PubMed]
- Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Polish J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Hassanein, R.A.; Hashem, H.A.; El-deep, M.H.; Shouman, A. Soil contamination with heavy metals and its effect on growth, yield and physiological responses of vegetable crop plants (turnip and lettuce). J. Stress Physiol. Biochem. 2013, 9, 145–162. [Google Scholar]
- Márquez-García, B.; Fernández-Recamales, M.Á.; Córdoba, F. Effects of cadmium on phenolic composition and antioxidant activities of Erica andevalensis. J. Bot. 2012, 2012, 1–6. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, Y.; Zhang, X.; Xie, G.; Qin, M. Copper stress-induced changes in biomass accumulation, antioxidant activity and flavonoid contents in Belamcanda chinensis calli. Plant Cell Tissue Organ Cult. 2020, 142, 299–311. [Google Scholar] [CrossRef]
- Peer, W.A.; Murphy, A.S. Flavonoids and auxin transport: Modulators or regulators? Trends Plant Sci. 2007, 12, 556–563. [Google Scholar] [CrossRef]
- Cesco, S.; Mimmo, T.; Tonon, G.; Tomasi, N.; Pinton, R.; Terzano, R.; Neumann, G.; Weisskopf, L.; Renella, G.; Landi, L.; et al. Plant-borne flavonoids released into the rhizosphere: Impact on soil bio-activities related to plant nutrition. A Review. Biol. Fertil. Soils 2012, 48, 123–149. [Google Scholar] [CrossRef]
- Besseau, S.; Hoffmann, L.; Geoffroy, P.; Lapierre, C.; Pollet, B.; Legrand, M. Flavonoid accumulation in Arabidopsis repressed in lignin synthesis affects auxin transport and plant growth. Plant Cell 2007, 19, 148–162. [Google Scholar] [CrossRef]
- Buer, C.S.; Kordbacheh, F.; Truong, T.T.; Hocart, C.H.; Djordjevic, M.A. Alteration of flavonoid accumulation patterns in transparent testa mutants disturbs auxin transport, gravity responses, and imparts long-term effects on root and shoot architecture. Planta 2013, 238, 171–189. [Google Scholar] [CrossRef]
- Gayomba, S.R.; Watkins, J.M.; Muday, G.K. Flavonols regulate plant growth and development through regulation of auxin transport and cellular redox status. In Recent Advances in Polyphenol Research; John Wiley & Sons, Ltd.: Chichester, UK, 2016; Volume 5, pp. 143–170. ISBN 9781118883303. [Google Scholar]
- Zhang, X.; Huang, X.; Li, Y.; Tao, F.; Zhao, Q.; Li, W. Polar auxin transport may be responsive to specific features of flavonoid structure. Phytochemistry 2021, 185, 112702. [Google Scholar] [CrossRef]
- Petrella, D.P.; Han, E.; Nangle, E.J.; Scheerens, J.C.; Gardner, D.S.; Blakeslee, J.J. Modulation of halotropic growth in rough bluegrass (Poa trivialis L.) by flavonoids and light. Environ. Exp. Bot. 2018, 153, 163–175. [Google Scholar] [CrossRef]
- Shi, J.; Zhou, H.; Liu, X.; Wang, N.; Xu, Q.; Yan, G. Correlation analysis of the transcriptome and metabolome reveals the role of the flavonoid biosynthesis pathway in regulating axillary buds in upland cotton (Gossypium hirsutum L.). Planta 2021, 254, 7. [Google Scholar] [CrossRef] [PubMed]
- Peer, W.A.; Bandyopadhyay, A.; Blakeslee, J.J.; Makam, S.N.; Chen, R.J.; Masson, P.H.; Murphy, A.S. Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with altered auxin transport in Arabidopsis thaliana. Plant Cell 2004, 16, 1898–1911. [Google Scholar] [CrossRef]
- Weisskopf, L.; Tomasi, N.; Santelia, D.; Martinoia, E.; Langlade, N.B.; Tabacchi, R.; Abou-Mansour, E. Isoflavonoid exudation from white lupin roots is influenced by phosphate supply, root type and cluster-root stage. New Phytol. 2006, 171, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Lateif, K.; Bogusz, D.; Hocher, V. The role of flavonoids in the establishment of plant roots endosymbioses with arbuscular mycorrhiza fungi, rhizobia and Frankia bacteria. Plant Signal. Behav. 2012, 7, 636–641. [Google Scholar] [CrossRef]
- Liu, C.-W.; Murray, J.D. The role of flavonoids in nodulation host-range specificity: An update. Plants 2016, 5, 33. [Google Scholar] [CrossRef]
- Løvdal, T.; Olsen, K.M.; Slimestad, R.; Verheul, M.; Lillo, C. Synergetic effects of nitrogen depletion, temperature, and light on the content of phenolic compounds and gene expression in leaves of tomato. Phytochemistry 2010, 71, 605–613. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, X.; Yang, T.; Su, Y.; Lin, S.; Zhang, S.; Zhang, Z. Nitrogen-regulated theanine and flavonoid biosynthesis in tea plant roots: Protein-level regulation revealed by multiomics analyses. J. Agric. Food Chem. 2021, 69, 10002–10016. [Google Scholar] [CrossRef]
- Valentinuzzi, F.; Venuti, S.; Pii, Y.; Marroni, F.; Cesco, S.; Hartmann, F.; Mimmo, T.; Morgante, M.; Pinton, R.; Tomasi, N.; et al. Common and specific responses to iron and phosphorus deficiencies in roots of apple tree (Malus × domestica). Plant Mol. Biol. 2019, 101, 129–148. [Google Scholar] [CrossRef]
- Šoln, K.; Klemenčič, M.; Koce, J.D. Plant cell responses to allelopathy: From oxidative stress to programmed cell death. Protoplasma 2022, 259, 1111–1124. [Google Scholar] [CrossRef]
- Gniazdowska, A.; Bogatek, R. Allelopathic interactions between plants. multi site action of allelochemicals. Acta Physiol. Plant. 2005, 27, 395–407. [Google Scholar] [CrossRef]
- Halarewicz, A.; Szumny, A.; Bączek, P. Effect of Prunus serotina Ehrh. volatile compounds on germination and seedling growth of Pinus sylvestris L. Forests 2021, 12, 846. [Google Scholar] [CrossRef]
- Šoln, K.; Horvat, M.; Iskra, J.; Dolenc Koce, J. Inhibitory effects of methanol extracts from Fallopia japonica and F. × bohemica rhizomes and selected phenolic compounds on radish germination and root growth. Chemoecology 2022, 32, 159–170. [Google Scholar] [CrossRef]
- Hegde, R.S.; Miller, D.A. Concentration dependency and stage of crop growth in alfalfa autotoxicity. Agron. J. 1992, 84, 940–946. [Google Scholar] [CrossRef]
- Jennings, J.A.; Nelson, C.J. Zone of autotoxic influence around established alfalfa plants. Agron. J. 2002, 94, 1104–1111. [Google Scholar] [CrossRef]
- Ghimire, B.K.; Ghimire, B.; Yu, C.Y.; Chung, I.-M. Allelopathic and autotoxic effects of Medicago sativa—Derived allelochemicals. Plants 2019, 8, 233. [Google Scholar] [CrossRef]
- Lal, R.; Kaur, A.; Kaur, S.; Batish, D.R.; Singh, H.P.; Sharma, M.; Kohli, R.K. Nature of phytotoxic interference of alien weed ‘Calyptocarpus vialis’ against some crop plants. Environ. Monit. Assess. 2021, 193, 334. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Wu, B.; Yu, Y.; Wang, S.; Wei, M.; Wang, C.; Du, D. The allelopathy of horseweed with different invasion degrees in three provinces along the Yangtze river in China. Physiol. Mol. Biol. Plants 2021, 27, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Vilà, M.; Beaury, E.M.; Blumenthal, D.M.; Bradley, B.A.; Early, R.; Laginhas, B.B.; Trillo, A.; Dukes, J.S.; Sorte, C.J.B.; Ibáñez, I. Understanding the combined impacts of weeds and climate change on crops. Environ. Res. Lett. 2021, 16, 034043. [Google Scholar] [CrossRef]
- Bodey, T.W.; Angulo, E.; Bang, A.; Bellard, C.; Fantle-Lepczyk, J.; Lenzner, B.; Turbelin, A.; Watari, Y.; Courchamp, F. Economic costs of protecting islands from invasive alien species. Conserv. Biol. 2022, 1–14. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Allelopathy and allelochemicals of Imperata cylindrica as an invasive plant species. Plants 2022, 11, 2551. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, K.; Walker, M.; Irwin, D.; Cohn, J.; Guida-English, S.M.; Garcia, L.; Pavlović, I.; Novák, O.; Tarkowská, D.; Strnad, M.; et al. The phytotoxin myrigalone A triggers a phased detoxification programme and inhibits Lepidium sativum seed germination via multiple mechanisms including interference with auxin homeostasis. Int. J. Mol. Sci. 2022, 23, 4618. [Google Scholar] [CrossRef] [PubMed]
- Mehal, K.K.; Kaur, A.; Singh, H.P.; Batish, D.R. Investigating the phytotoxic potential of Verbesina encelioides: Effect on growth and performance of co-occurring weed species. Protoplasma 2022, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bouhaouel, I.; Richard, G.; Fauconnier, M.-L.; Ongena, M.; Franzil, L.; Gfeller, A.; Slim Amara, H.; du Jardin, P. Identification of barley (Hordeum vulgare L. subsp. vulgare) root exudates allelochemicals, their autoallelopathic activity and against Bromus diandrus Roth. germination. Agronomy 2019, 9, 345. [Google Scholar] [CrossRef]
- Borda, V.; Reinhart, K.O.; Ortega, M.G.; Burni, M.; Urcelay, C. Roots of invasive woody plants produce more diverse flavonoids than non-invasive taxa, a global analysis. Biol. Invasions 2022, 24, 2757–2768. [Google Scholar] [CrossRef]
- Farooq, N.; Abbas, T.; Tanveer, A.; Jabran, K. Allelopathy for weed management. In Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2020; pp. 505–519. ISBN 9783319963976. [Google Scholar]
- Saludes-Zanfaño, M.I.; Vivar-Quintana, A.M.; Morales-Corts, M.R. Pistacia root and leaf extracts as potential bioherbicides. Plants 2022, 11, 916. [Google Scholar] [CrossRef]
- Lupini, A.; Araniti, F.; Sunseri, F.; Abenavoli, M.R. Coumarin interacts with auxin polar transport to modify root system architecture in Arabidopsis thaliana. Plant Growth Regul. 2014, 74, 23–31. [Google Scholar] [CrossRef]
- Anwar, S.; Naseem, S.; Karimi, S.; Asi, M.R.; Akrem, A.; Ali, Z. Bioherbicidal activity and metabolic profiling of potent allelopathic plant fractions against major weeds of wheat—Way forward to lower the risk of synthetic herbicides. Front. Plant Sci. 2021, 12, 333. [Google Scholar] [CrossRef]
- Khan, Z.; Midega, C.A.O.; Hooper, A.; Pickett, J. Push-pull: Chemical ecology-based integrated pest management technology. J. Chem. Ecol. 2016, 42, 689–697. [Google Scholar] [CrossRef]
- Hooper, A.M.; Tsanuo, M.K.; Chamberlain, K.; Tittcomb, K.; Scholes, J.; Hassanali, A.; Khan, Z.R.; Pickett, J.A. Isoschaftoside, a C-glycosylflavonoid from Desmodium uncinatum root exudate, is an allelochemical against the development of Striga. Phytochemistry 2010, 71, 904–908. [Google Scholar] [CrossRef]
- Guchu, S.M.; Yenesew, A.; Tsanuo, M.K.; Gikonyo, N.K.; Pickett, J.A.; Hooper, A.M.; Hassanali, A. C-methylated and C-prenylated isoflavonoids from root extract of Desmodium uncinatum. Phytochemistry 2007, 68, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Tesio, F.; Vidotto, F.; Ferrero, A. Allelopathic persistence of Helianthus tuberosus L. residues in the soil. Sci. Hortic. 2012, 135, 98–105. [Google Scholar] [CrossRef]
- Scavo, A.; Abbate, C.; Mauromicale, G. Plant allelochemicals: Agronomic, nutritional and ecological relevance in the soil system. Plant Soil 2019, 442, 23–48. [Google Scholar] [CrossRef]
- Carlsen, S.C.K.; Pedersen, H.A.; Spliid, N.H.; Fomsgaard, I.S. Fate in soil of flavonoids released from white clover (Trifolium repens L.). Appl. Environ. Soil Sci. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Kong, C.H.; Zhao, H.; Xu, X.H.; Wang, P.; Gu, Y. Activity and allelopathy of soil of flavone O-glycosides from rice. J. Agric. Food Chem. 2007, 55, 6007–6012. [Google Scholar] [CrossRef] [PubMed]
- Šoln, K.; Dolenc Koce, J. Allelopathic root inhibition and its mechanisms. Allelopath. J. 2021, 52, 181–198. [Google Scholar] [CrossRef]
- Zhang, H.; Rutherford, S.; Qi, S.; Huang, P.; Dai, Z.; Du, D. Transcriptome profiling of Arabidopsis thaliana roots in response to allelopathic effects of Conyza canadensis. Ecotoxicology 2022, 31, 53–63. [Google Scholar] [CrossRef]
- Bais, H.P.; Vepachedu, R.; Gilroy, S.; Callaway, R.M.; Vivanco, J.M. Allelopathy and exotic plant invasion: From molecules and genes to species interactions. Science 2003, 301, 1377–1380. [Google Scholar] [CrossRef]
- Bais, H.P.; Kaushik, S. Catechin secretion & phytotoxicity. Commun. Integr. Biol. 2010, 3, 468–470. [Google Scholar] [CrossRef]
- Berlanga-Clavero, M.V.; Molina-Santiago, C.; Vicente, A.; Romero, D. More than words: The chemistry behind the interactions in the plant holobiont. Environ. Microbiol. 2020, 22, 4532–4544. [Google Scholar] [CrossRef]
- Vandenkoornhuyse, P.; Quaiser, A.; Duhamel, M.; Le Van, A.; Dufresne, A. The importance of the microbiome of the plant holobiont. New Phytol. 2015, 206, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Olanrewaju, O.S.; Ayangbenro, A.S.; Glick, B.R.; Babalola, O.O. Plant Health: Feedback effect of root exudates-rhizobiome interactions. Appl. Microbiol. Biotechnol. 2019, 103, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Deryabin, D.; Galadzhieva, A.; Kosyan, D.; Duskaev, G. Plant-derived inhibitors of AHL-mediated quorum sensing in bacteria: Modes of action. Int. J. Mol. Sci. 2019, 20, 5588. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Gao, J.; Ma, A.; Fu, S.; Zhuang, G. Bioactive molecules in soil ecosystems: Masters of the underground. Int. J. Mol. Sci. 2013, 14, 8841–8868. [Google Scholar] [CrossRef]
- Päsold, S.; Siegel, I.; Seidel, C.; Ludwig-Müller, J. Flavonoid accumulation in Arabidopsis thaliana root galls caused by the obligate biotrophic pathogen Plasmodiophora brassicae. Mol. Plant Pathol. 2010, 11, 545–562. [Google Scholar] [CrossRef]
- Truchado, P.; Larrosa, M.; Castro-Ibáñez, I.; Allende, A. Plant food extracts and phytochemicals: Their role as quorum sensing inhibitors. Trends Food Sci. Technol. 2015, 43, 189–204. [Google Scholar] [CrossRef]
- Vikram, A.; Jayaprakasha, G.K.; Jesudhasan, P.R.; Pillai, S.D.; Patil, B.S. Suppression of bacterial cell–cell signalling, biofilm formation and type III secretion system by citrus flavonoids. J. Appl. Microbiol. 2010, 109, 515–527. [Google Scholar] [CrossRef]
- Attila, C.; Ueda, A.; Cirillo, S.L.G.; Cirillo, J.D.; Chen, W.; Wood, T.K. Pseudomonas aeruginosa PAO1 virulence factors and poplar tree response in the rhizosphere. Microb. Biotechnol. 2008, 1, 17–29. [Google Scholar] [CrossRef]
- Vandeputte, O.M.; Kiendrebeogo, M.; Rasamiravaka, T.; Stévigny, C.; Duez, P.; Rajaonson, S.; Diallo, B.; Mol, A.; Baucher, M.; El Jaziri, M. The flavanone naringenin reduces the production of quorum sensing-controlled virulence factors in Pseudomonas aeruginosa PAO1. Microbiology 2011, 157, 2120–2132. [Google Scholar] [CrossRef]
- Ouyang, J.; Sun, F.; Feng, W.; Sun, Y.; Qiu, X.; Xiong, L.; Liu, Y.; Chen, Y. Quercetin is an effective inhibitor of quorum sensing, biofilm formation and virulence factors in Pseudomonas aeruginosa. J. Appl. Microbiol. 2016, 120, 966–974. [Google Scholar] [CrossRef]
- Paczkowski, J.E.; Mukherjee, S.; McCready, A.R.; Cong, J.-P.; Aquino, C.J.; Kim, H.; Henke, B.R.; Smith, C.D.; Bassler, B.L. Flavonoids suppress Pseudomonas aeruginosa virulence through allosteric inhibition of quorum-sensing receptors. J. Biol. Chem. 2017, 292, 4064–4076. [Google Scholar] [CrossRef]
- Castellanos, L.; Naranjo-Gaybor, S.J.; Forero, A.M.; Morales, G.; Wilson, E.G.; Ramos, F.A.; Choi, Y.H. Metabolic fingerprinting of banana passion fruits and its correlation with quorum quenching activity. Phytochemistry 2020, 172, 112272. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Kim, J.; Kim, S.; Kang, Y.; Nagamatsu, T.; Hwang, I. Toxoflavin produced by Burkholderia glumae causing rice grain rot is responsible for inducing bacterial wilt in many field crops. Plant Dis. 2003, 87, 890–895. [Google Scholar] [CrossRef]
- Choi, O.; Lee, Y.; Han, I.; Kim, H.; Goo, E.; Kim, J.; Hwang, I. A simple and sensitive biosensor strain for detecting toxoflavin using β-galactosidase activity. Biosens. Bioelectron. 2013, 50, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Do, H.T.T.; Nguyen, T.H.; Nghiem, T.D.; Nguyen, H.T.; Choi, G.J.; Ho, C.T.; Le Dang, Q. Phytochemical constituents and extracts of the roots of Scutellaria baicalensis exhibit in vitro and in vivo control efficacy against various phytopathogenic microorganisms. S. Afr. J. Bot. 2021, 142, 1–11. [Google Scholar] [CrossRef]
- Ali, K.; Maltese, F.; Figueiredo, A.; Rex, M.; Fortes, A.M.; Zyprian, E.; Pais, M.S.; Verpoorte, R.; Choi, Y.H. Alterations in grapevine leaf metabolism upon inoculation with Plasmopara viticola in different time-points. Plant Sci. 2012, 191–192, 100–107. [Google Scholar] [CrossRef]
- Tao, S.; Zhang, S.; Tsao, R.; Charles, M.T.; Yang, R.; Khanizadeh, S. In vitro antifungal activity and mode of action of selected polyphenolic antioxidants on Botrytis cinerea. Arch. Phytopathol. Plant Prot. 2010, 43, 1564–1578. [Google Scholar] [CrossRef]
- De Coninck, B.; Timmermans, P.; Vos, C.; Cammue, B.P.A.; Kazan, K. What lies beneath: Belowground defense strategies in plants. Trends Plant Sci. 2015, 20, 91–101. [Google Scholar] [CrossRef]
- Baetz, U.; Martinoia, E. Root Exudates: The hidden part of plant defense. Trends Plant Sci. 2014, 19, 90–98. [Google Scholar] [CrossRef]
- Hassan, S.; Mathesius, U. The role of flavonoids in root-rhizosphere signalling: Opportunities and challenges for improving plant-microbe interactions. J. Exp. Bot. 2012, 63, 3429–3444. [Google Scholar] [CrossRef]
- Guenoune, D.; Galili, S.; Phillips, D.A.; Volpin, H.; Chet, I.; Okon, Y.; Kapulnik, Y. The defense response elicited by the pathogen Rhizoctonia solani is suppressed by colonization of the AM-fungus Glomus intraradices. Plant Sci. 2001, 160, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; VanEtten, H.D. Introduction of plant and fungal genes into pea (Pisum sativum L.) hairy roots reduces their ability to produce pisatin and affects their response to a fungal pathogen. Mol. Plant-Microbe Interact. 2004, 17, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Albanchez, E.; Gradillas, A.; García, A.; García-Villaraco, A.; Gutierrez-Mañero, F.J.; Ramos-Solano, B. Elicitation with Bacillus QV15 reveals a pivotal role of F3H on flavonoid metabolism improving adaptation to biotic stress in blackberry. PLoS ONE 2020, 15, e0232626. [Google Scholar] [CrossRef]
- Algar, E.; Gutierrez-Mañero, F.J.; Garcia-Villaraco, A.; García-Seco, D.; Lucas, J.A.; Ramos-Solano, B. The Role of isoflavone metabolism in plant protection depends on the rhizobacterial MAMP that triggers systemic resistance against Xanthomonas axonopodis pv. glycines in Glycine max (L.) Merr. cv. Osumi. Plant Physiol. Biochem. 2014, 82, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Kalli, S.; Araya-Cloutier, C.; Lin, Y.; de Bruijn, W.J.C.; Chapman, J.; Vincken, J.-P. Enhanced biosynthesis of the natural antimicrobial glyceollins in soybean seedlings by priming and elicitation. Food Chem. 2020, 317, 126389. [Google Scholar] [CrossRef]
- Tebayashi, S.; Ishihara, A.; Iwamura, H. Elicitor-induced changes in isoflavonoid metabolism in red clover roots. J. Exp. Bot. 2001, 52, 681–689. [Google Scholar] [CrossRef]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Jia, Z.; Zou, B.; Wang, X.; Qiu, J.; Ma, H.; Gou, Z.; Song, S.; Dong, H. Quercetin-induced H2O2 mediates the pathogen resistance against Pseudomonas syringae pv. tomato DC3000 in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2010, 396, 522–527. [Google Scholar] [CrossRef]
- An, J.; Kim, S.H.; Bahk, S.; Vuong, U.T.; Nguyen, N.T.; Do, H.L.; Kim, S.H.; Chung, W.S. Naringenin induces pathogen resistance against Pseudomonas syringae through the activation of NPR1 in Arabidopsis. Front. Plant Sci. 2021, 12, 853. [Google Scholar] [CrossRef]
- Chamam, A.; Wisniewski-Dyé, F.; Comte, G.; Bertrand, C.; Prigent-Combaret, C. Differential responses of Oryza sativa secondary metabolism to biotic interactions with cooperative, commensal and phytopathogenic bacteria. Planta 2015, 242, 1439–1452. [Google Scholar] [CrossRef]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.-L.; Touraine, B.; Moënne-Loccoz, Y.; Muller, D.; Legendre, L.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Plant growth-promoting rhizobacteria and root system functioning. Front. Plant Sci. 2013, 4, 356. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, F.; Marasco, R.; Rolli, E.; Barbato, M.; Cherif, H.; Guesmi, A.; Ouzari, I.; Daffonchio, D.; Borin, S. Potential for plant growth promotion of rhizobacteria associated with Salicornia growing in tunisian hypersaline soils. BioMed Res. Int. 2013, 2013, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Marasco, R.; Rolli, E.; Vigani, G.; Borin, S.; Sorlini, C.; Ouzari, H.; Zocchi, G.; Daffonchio, D. Are drought-resistance promoting bacteria cross-compatible with different plant models? Plant Signal. Behav. 2013, 8, e26741. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Ahmad, I.; Khan, M.S. Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol. Res. 2008, 163, 173–181. [Google Scholar] [CrossRef]
- Etesami, H.; Jeong, B.R.; Glick, B.R. Contribution of arbuscular mycorrhizal fungi, phosphate–solubilizing bacteria, and silicon to P uptake by plant. Front. Plant Sci. 2021, 12, 699618. [Google Scholar] [CrossRef]
- Frey-Klett, P.; Garbaye, J.; Tarkka, M. The mycorrhiza helper bacteria revisited. New Phytol. 2007, 176, 22–36. [Google Scholar] [CrossRef]
- Vierheilig, H. Regulatory mechanisms during the plant-arbuscular mycorrhizal fungus interaction. Can. J. Bot. 2004, 82, 1166–1176. [Google Scholar] [CrossRef]
- Rolli, E.; Marasco, R.; Vigani, G.; Ettoumi, B.; Mapelli, F.; Deangelis, M.L.; Gandolfi, C.; Casati, E.; Previtali, F.; Gerbino, R.; et al. Improved plant resistance to drought is promoted by the root-associated microbiome as a water stress-dependent trait. Environ. Microbiol. 2015, 17, 316–331. [Google Scholar] [CrossRef]
- Zboralski, A.; Filion, M. Genetic factors involved in rhizosphere colonization by phytobeneficial Pseudomonas spp. Comput. Struct. Biotechnol. J. 2020, 18, 3539–3554. [Google Scholar] [CrossRef]
- Bhattacharyya, C.; Bakshi, U.; Mallick, I.; Mukherji, S.; Bera, B.; Ghosh, A. Genome-guided insights into the plant growth promotion capabilities of the physiologically versatile Bacillus aryabhattai strain AB211. Front. Microbiol. 2017, 8, 411. [Google Scholar] [CrossRef]
- Romano, I.; Ventorino, V.; Pepe, O. Effectiveness of plant beneficial microbes: Overview of the methodological approaches for the assessment of root colonization and persistence. Front. Plant Sci. 2020, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.J.; Morris, P.; Hooker, J.E. Perception and modification of plant flavonoid signals by rhizosphere microorganisms. Environ. Microbiol. 2006, 8, 1867–1880. [Google Scholar] [CrossRef]
- Tian, Y.; Amand, S.; Buisson, D.; Kunz, C.; Hachette, F.; Dupont, J.; Nay, B.; Prado, S. The Fungal leaf endophyte paraconiothyrium variabile specifically metabolizes the host-plant metabolome for its own benefit. Phytochemistry 2014, 108, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Pillai, B.V.S.; Swarup, S. Elucidation of the flavonoid catabolism pathway in Pseudomonas putida PML2 by comparative metabolic profiling. Appl. Environ. Microbiol. 2002, 68, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Kim, H.R.; Rahmatallah, Y.; Wiggins, G.; Yang, Q.; Singh, R.; Glazko, G.; Mukherjee, A. RNA-Seq reveals differentially expressed genes in rice (Oryza sativa) roots during interactions with plant-growth promoting bacteria, Azospirillum brasilense. PLoS ONE 2019, 14, e0217309. [Google Scholar] [CrossRef]
- Feng, H.; Fu, R.; Hou, X.; Lv, Y.; Zhang, N.; Liu, Y.; Xu, Z.; Miao, Y.; Krell, T.; Shen, Q.; et al. Chemotaxis of beneficial rhizobacteria to root exudates: The first step towards root–microbe rhizosphere interactions. Int. J. Mol. Sci. 2021, 22, 6655. [Google Scholar] [CrossRef]
- He, D.; Singh, S.K.; Peng, L.; Kaushal, R.; Vílchez, J.I.; Shao, C.; Wu, X.; Zheng, S.; Morcillo, R.J.L.; Paré, P.W.; et al. Flavonoid-attracted Aeromonas sp. from the Arabidopsis root microbiome enhances plant dehydration resistance. ISME J. 2022, 16, 2622–2632. [Google Scholar] [CrossRef]
- Singh, A.; Gupta, R.; Pandey, R. Rice seed priming with picomolar rutin enhances rhizospheric Bacillus subtilis CIM colonization and plant growth. PLoS ONE 2016, 11, e0146013. [Google Scholar] [CrossRef]
- Yan, D.; Tajima, H.; Cline, L.C.; Fong, R.Y.; Ottaviani, J.I.; Shapiro, H.; Blumwald, E. Genetic modification of flavone biosynthesis in rice enhances biofilm formation of soil diazotrophic bacteria and biological nitrogen fixation. Plant Biotechnol. J. 2022, 20, 2135–2148. [Google Scholar] [CrossRef]
- Kushwaha, A.; Hans, N.; Giri, B.S.; Rene, E.R.; Rani, R. Uncovering the phytochemicals of root exudates and extracts of lead (Pb) tolerant Chrysopogon zizanioides (L.) Roberty in response to lead contamination and their effect on the chemotactic behavior of rhizospheric bacteria. Environ. Sci. Pollut. Res. 2022, 29, 44998–45012. [Google Scholar] [CrossRef]
- Toussaint, J.P.; Pham, T.T.M.; Barriault, D.; Sylvestre, M. Plant exudates promote PCB degradation by a rhodococcal rhizobacteria. Appl. Microbiol. Biotechnol. 2012, 95, 1589–1603. [Google Scholar] [CrossRef] [PubMed]
- Tadra-Sfeir, M.Z.; Faoro, H.; Camilios-Neto, D.; Brusamarello-Santos, L.; Balsanelli, E.; Weiss, V.; Baura, V.A.; Wassem, R.; Cruz, L.M.; De Oliveira Pedrosa, F.; et al. Genome wide transcriptional profiling of Herbaspirillum seropedicae SmR1 grown in the presence of naringenin. Front. Microbiol. 2015, 6, 491. [Google Scholar] [CrossRef] [PubMed]
- Gough, C.; Galera, C.; Vasse, J.; Webster, G.; Cocking, E.C.; Dénarié, J. Specific flavonoids promote intercellular root colonization of Arabidopsis thaliana by Azorhizobium caulinodans ORS571. Mol. Plant-Microbe Interact. 1997, 10, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Gupta, K. The Flavonoid naringenin enhances intercellular colonization of rice roots by Azorhizobium caulinodans. Biol. Fertil. Soils 2003, 38, 119–123. [Google Scholar] [CrossRef]
- Arunachalam, M.; Mohan, N.; Sugadev, R.; Chellappan, P.; Mahadevan, A. Degradation of (+)-catechin by Acinetobacter calcoaceticus MTC 127. Biochim. Biophys. Acta-Gen. Subj. 2003, 1621, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Leigh, M.B.; Fletcher, J.S.; Fu, X.; Schmitz, F.J. Root turnover: An important source of microbial substrates in rhizosphere remediation of recalcitrant contaminants. Environ. Sci. Technol. 2002, 36, 1579–1583. [Google Scholar] [CrossRef] [PubMed]
- Pino, N.J.; Muñera, L.M.; Peñuela, G.A. Root exudates and plant secondary metabolites of different plants enhance polychlorinated biphenyl degradation by rhizobacteria. Bioremediat. J. 2016, 20, 108–116. [Google Scholar] [CrossRef]
- Witzel, K.; Strehmel, N.; Baldermann, S.; Neugart, S.; Becker, Y.; Becker, M.; Berger, B.; Scheel, D.; Grosch, R.; Schreiner, M.; et al. Arabidopsis thaliana root and root exudate metabolism is altered by the growth-promoting bacterium Kosakonia radicincitans DSM 16656T. Plant Soil 2017, 419, 557–573. [Google Scholar] [CrossRef]
- Singh, A.; Jain, A.; Sarma, B.K.; Upadhyay, R.S.; Singh, H.B. Rhizosphere microbes facilitate redox homeostasis in Cicer arietinum against biotic stress. Ann. Appl. Biol. 2013, 163, 33–46. [Google Scholar] [CrossRef]
- Ali, B.; Wang, X.; Saleem, M.H.; Sumaira; Hafeez, A.; Afridi, M.S.; Khan, S.; Zaib-Un-Nisa; Ullah, I.; Do Amaral Júnior, A.T.; et al. PGPR-mediated salt tolerance in maize by modulating plant physiology, antioxidant defense, compatible solutes accumulation and bio-surfactant producing genes. Plants 2022, 11, 345. [Google Scholar] [CrossRef]
- Ali, B.; Wang, X.; Saleem, M.H.; Azeem, M.A.; Afridi, M.S.; Nadeem, M.; Ghazal, M.; Batool, T.; Qayyum, A.; Alatawi, A.; et al. Bacillus mycoides PM35 reinforces photosynthetic efficiency, antioxidant defense, expression of stress-responsive genes, and ameliorates the effects of salinity stress in maize. Life 2022, 12, 219. [Google Scholar] [CrossRef] [PubMed]
- Asghari, B.; Khademian, R.; Sedaghati, B. Plant growth promoting rhizobacteria (PGPR) confer drought resistance and stimulate biosynthesis of secondary metabolites in pennyroyal (Mentha pulegium L.) under water shortage condition. Sci. Hortic. 2020, 263, 109132. [Google Scholar] [CrossRef]
- Rizaludin, M.S.; Stopnisek, N.; Raaijmakers, J.M.; Garbeva, P. The chemistry of stress: Understanding the ‘cry for help’ of plant roots. Metabolites 2021, 11, 357. [Google Scholar] [CrossRef]
- Rolfe, S.A.; Griffiths, J.; Ton, J. Crying out for help with root exudates: Adaptive mechanisms by which stressed plants assemble health-promoting soil microbiomes. Curr. Opin. Microbiol. 2019, 49, 73–82. [Google Scholar] [CrossRef]
- Schwitzguébel, J.-P. Phytoremediation of soils contaminated by organic compounds: Hype, hope and facts. J. Soils Sediments 2017, 17, 1492–1502. [Google Scholar] [CrossRef]
- Rolli, E.; Vergani, L.; Ghitti, E.; Patania, G.; Mapelli, F.; Borin, S. ‘Cry-for-help’ in contaminated soil: A dialogue among plants and soil microbiome to survive in hostile conditions. Environ. Microbiol. 2021, 23, 5690–5703. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Using soil bacteria to facilitate phytoremediation. Biotechnol. Adv. 2010, 28, 367–374. [Google Scholar] [CrossRef]
- Rohrbacher, F.; St-Arnaud, M. Root exudation: The ecological driver of hydrocarbon rhizoremediation. Agronomy 2016, 6, 19. [Google Scholar] [CrossRef]
- Passatore, L.; Rossetti, S.; Juwarkar, A.A.; Massacci, A. Phytoremediation and bioremediation of polychlorinated biphenyls (PCBs): State of knowledge and research perspectives. J. Hazard. Mater. 2014, 278, 189–202. [Google Scholar] [CrossRef]
- Sylvestre, M. Prospects for using combined engineered bacterial enzymes and plant systems to rhizoremediate polychlorinated biphenyls. Environ. Microbiol. 2013, 15, 907–915. [Google Scholar] [CrossRef]
- Musilova, L.; Ridl, J.; Polivkova, M.; Macek, T.; Uhlik, O. Effects of secondary plant metabolites on microbial populations: Changes in community structure and metabolic activity in contaminated environments. Int. J. Mol. Sci. 2016, 17, 1205. [Google Scholar] [CrossRef]
- Van Aken, B.; Correa, P.A.; Schnoor, J.L. Phytoremediation of polychlorinated biphenyls: New trends and promises. Environ. Sci. Technol. 2010, 44, 2767–2776. [Google Scholar] [CrossRef] [PubMed]
- Zubrova, A.; Michalikova, K.; Semerad, J.; Strejcek, M.; Cajthaml, T.; Suman, J.; Uhlik, O. Biphenyl 2,3-dioxygenase in Pseudomonas alcaliphila JAB1 is both induced by phenolics and monoterpenes and involved in their transformation. Front. Microbiol. 2021, 12, 657311. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Inoue, R.; Kimura, N.; Furukawa, K. Versatile transcription of biphenyl catabolic bph operon in Pseudomonas pseudoalcaligenes KF707. J. Biol. Chem. 2000, 275, 31016–31023. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Darias, J.; García, V.; Rico-Jiménez, M.; Corral-Lugo, A.; Krell, T. Identification and characterization of bacterial chemoreceptors using quantitative capillary and gradient plate chemotaxis assays. Bio-Protocol 2016, 6, e1789. [Google Scholar] [CrossRef]
- Terzaghi, E.; Vergani, L.; Mapelli, F.; Borin, S.; Raspa, G.; Zanardini, E.; Morosini, C.; Anelli, S.; Nastasio, P.; Sale, V.M.; et al. Rhizoremediation of weathered PCBs in a heavily contaminated agricultural soil: Results of a biostimulation trial in semi field conditions. Sci. Total Environ. 2019, 686, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.T.M.; Rodriguez, N.J.P.; Hijri, M.; Sylvestre, M. Optimizing polychlorinated biphenyl degradation by flavonoid-induced cells of the rhizobacterium Rhodococcus erythropolis U23A. PLoS ONE 2015, 10, e0126033. [Google Scholar] [CrossRef]
- Uhlik, O.; Musilova, L.; Ridl, J.; Hroudova, M.; Vlcek, C.; Koubek, J.; Holeckova, M.; Mackova, M.; Macek, T. Plant secondary metabolite-induced shifts in bacterial community structure and degradative ability in contaminated soil. Appl. Microbiol. Biotechnol. 2013, 97, 9245–9256. [Google Scholar] [CrossRef]
- Ely, C.S.; Smets, B.F. Bacteria from wheat and cucurbit plant roots metabolize PAHs and aromatic root exudates: Implications for rhizodegradation. Int. J. Phytoremediat. 2017, 19, 877–883. [Google Scholar] [CrossRef]
- Segura, A.; Ramos, J.L. Plant–bacteria interactions in the removal of pollutants. Curr. Opin. Biotechnol. 2013, 24, 467–473. [Google Scholar] [CrossRef]
- Sugiyama, A. Flavonoids and saponins in plant rhizospheres: Roles, dynamics, and the potential for agriculture. Biosci. Biotechnol. Biochem. 2021, 85, 1919–1931. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, M.S.J. Flavonoid–insect interactions: Recent advances in our knowledge. Phytochemistry 2003, 64, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, M.S.J. Importance of flavonoids in insect–plant interactions: Feeding and oviposition. Phytochemistry 2001, 56, 245–252. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef]
- Liao, L.-H.; Wu, W.-Y.; Berenbaum, M.R. Behavioral responses of honey bees (Apis mellifera) to natural and synthetic xenobiotics in food. Sci. Rep. 2017, 7, 15924. [Google Scholar] [CrossRef]
- Hoballah, M.E.; Gübitz, T.; Stuurman, J.; Broger, L.; Barone, M.; Mandel, T.; Dell’Olivo, A.; Arnold, M.; Kuhlemeier, C. Single gene–mediated shift in pollinator attraction in Petunia. Plant Cell 2007, 19, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kaur, I.; Kariyat, R. The multifunctional roles of polyphenols in plant-herbivore interactions. Int. J. Mol. Sci. 2021, 22, 1442. [Google Scholar] [CrossRef]
- Yao, Q.; Peng, Z.; Tong, H.; Yang, F.; Xing, G.; Wang, L.; Zheng, J.; Zhang, Y.; Su, Q. Tomato plant flavonoids increase whitefly resistance and reduce spread of Tomato yellow leaf curl virus. J. Econ. Entomol. 2019, 112, 2790–2796. [Google Scholar] [CrossRef] [PubMed]
- Du, S.S.; Zhang, H.M.; Bai, C.Q.; Wang, C.F.; Liu, Q.Z.; Liu, Z.L.; Wang, Y.Y.; Deng, Z.W. Nematocidal flavone-C-glycosides against the root-knot nematode (Meloidogyne incognita) from Arisaema erubescens tubers. Molecules 2011, 16, 5079–5086. [Google Scholar] [CrossRef]
- Diaz Napal, G.N.; Palacios, S.M. Bioinsecticidal effect of the flavonoids pinocembrin and quercetin against Spodoptera frugiperda. J. Pest Sci. 2015, 88, 629–635. [Google Scholar] [CrossRef]
- Schnarr, L.; Segatto, M.L.; Olsson, O.; Zuin, V.G.; Kümmerer, K. Flavonoids as biopesticides—Systematic assessment of sources, structures, activities and environmental fate. Sci. Total Environ. 2022, 824, 153781. [Google Scholar] [CrossRef] [PubMed]
- Riddick, E.W. Potential of quercetin to reduce herbivory without disrupting natural enemies and pollinators. Agriculture 2021, 11, 476. [Google Scholar] [CrossRef]
- Liao, L.H.; Wu, W.Y.; Berenbaum, M.R. Impacts of dietary phytochemicals in the presence and absence of pesticides on longevity of honey bees (Apis mellifera). Insects 2017, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Schuler, M.A.; Berenbaum, M.R. Honey constituents up-regulate detoxification and immunity genes in the western honey bee Apis mellifera. Proc. Natl. Acad. Sci. USA 2013, 110, 8842–8846. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, X.; Shen, H.; Xue, H.; Tian, T.; Zhang, Q.; Hu, J.; Tong, H.; Zhang, Y.; Su, Q. Flavonoid-producing tomato plants have a direct negative effect on the zoophytophagous biological control agent Orius sauteri. Insect Sci. 2022, 1–12. [Google Scholar] [CrossRef]
- Diaz Napal, G.N.; Defagó, M.T.; Valladares, G.R.; Palacios, S.M. Response of Epilachna paenulata to two flavonoids, pinocembrin and quercetin, in a comparative study. J. Chem. Ecol. 2010, 36, 898–904. [Google Scholar] [CrossRef]
- Haribal, M.; Renwick, J.A.A. Identification and distribution of oviposition stimulants for monarch butterflies in hosts and nonhosts. J. Chem. Ecol. 1998, 24, 891–904. [Google Scholar] [CrossRef]
- Riddick, E.W.; Wu, Z.; Eller, F.J.; Berhow, M.A. Do Bioflavonoids in Juniperus virginiana heartwood stimulate oviposition in the ladybird Coleomegilla maculata? Int. J. Insect Sci. 2018, 10, 1–13. [Google Scholar] [CrossRef]
- Riddick, E.; Wu, Z.; Eller, F.; Berhow, M. Utilization of quercetin as an oviposition stimulant by lab-cultured Coleomegilla maculata in the presence of conspecifics and a tissue substrate. Insects 2018, 9, 77. [Google Scholar] [CrossRef]
- Salunke, B.K.; Kotkar, H.M.; Mendki, P.S.; Upasani, S.M.; Maheshwari, V.L. Efficacy of flavonoids in controlling Callosobruchus chinensis (L.) (Coleoptera: Bruchidae), a post-harvest pest of grain legumes. Crop Prot. 2005, 24, 888–893. [Google Scholar] [CrossRef]
- Sharma, R.; Sohal, S.K. Oviposition response of melon fruit fly, Bactrocera cucurbitae (Coquillett) to different phenolic compounds. J. Biopestic. 2016, 9, 46–51. [Google Scholar]
- Su, Q.; Chen, G.; Mescher, M.C.; Peng, Z.; Xie, W.; Wang, S.; Wu, Q.; Liu, J.; Li, C.; Wang, W.; et al. Whitefly aggregation on tomato is mediated by feeding-induced changes in plant metabolites that influence the behaviour and performance of conspecifics. Funct. Ecol. 2018, 32, 1180–1193. [Google Scholar] [CrossRef]
- Tapas, A.; Sakarkar, D.; Kakde, R. Flavonoids as nutraceuticals: A review. Trop. J. Pharm. Res. 2008, 7, 1089–1099. [Google Scholar] [CrossRef]
- Wink, M. Current understanding of modes of action of multicomponent bioactive phytochemicals: Potential for nutraceuticals and antimicrobials. Annu. Rev. Food Sci. Technol. 2022, 13, 337–359. [Google Scholar] [CrossRef] [PubMed]
- de Arruda Nascimento, E.; de Lima Coutinho, L.; da Silva, C.J.; de Lima, V.L.A.G.; dos Santos Aguiar, J. In vitro anticancer properties of anthocyanins: A systematic review. Biochim. Biophys. Acta-Rev. Cancer 2022, 1877, 188748. [Google Scholar] [CrossRef]
- Fabbrini, M.; D’Amico, F.; Barone, M.; Conti, G.; Mengoli, M.; Brigidi, P.; Turroni, S. Polyphenol and tannin nutraceuticals and their metabolites: How the human gut microbiota influences their properties. Biomolecules 2022, 12, 875. [Google Scholar] [CrossRef]
- Vamanu, E. Polyphenolic nutraceuticals to combat oxidative stress through microbiota modulation. Front. Pharmacol. 2019, 10, 492. [Google Scholar] [CrossRef]
- Ruskovska, T.; Budić-Leto, I.; Corral-Jara, K.F.; Ajdžanović, V.; Arola-Arnal, A.; Bravo, F.I.; Deligiannidou, G.-E.; Havlik, J.; Janeva, M.; Kistanova, E.; et al. Systematic bioinformatic analyses of nutrigenomic modifications by polyphenols associated with cardiometabolic health in humans—Evidence from targeted nutrigenomic studies. Nutrients 2021, 13, 2326. [Google Scholar] [CrossRef]
- Shanmugavadivu, A.; Balagangadharan, K.; Selvamurugan, N. Angiogenic and osteogenic effects of flavonoids in bone regeneration. Biotechnol. Bioeng. 2022, 119, 2313–2330. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.; Shin, D.W. The molecular mechanism of polyphenols with anti-aging activity in aged human dermal fibroblasts. Molecules 2022, 27, 4351. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, H.; Liu, W.; Li, C. Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. J. Zhejiang Univ. Sci. B 2012, 13, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Wilmsen, P.K.; Spada, D.S.; Salvador, M. Antioxidant activity of the flavonoid hesperidin in chemical and biological systems. J. Agric. Food Chem. 2005, 53, 4757–4761. [Google Scholar] [CrossRef]
- Cavia-Saiz, M.; Busto, M.D.; Pilar-Izquierdo, M.C.; Ortega, N.; Perez-Mateos, M.; Muñiz, P. Antioxidant properties, radical scavenging activity and biomolecule protection capacity of flavonoid naringenin and its glycoside naringin: A comparative study. J. Sci. Food Agric. 2010, 90, 1238–1244. [Google Scholar] [CrossRef]
- Jagetia, G.C.; Reddy, T.K. Alleviation of iron induced oxidative stress by the grape fruit flavanone naringin in vitro. Chem. Biol. Interact. 2011, 190, 121–128. [Google Scholar] [CrossRef]
- Allaqaband, S.; Dar, A.H.; Patel, U.; Kumar, N.; Nayik, G.A.; Khan, S.A.; Ansari, M.J.; Alabdallah, N.M.; Kumar, P.; Pandey, V.K.; et al. Utilization of fruit seed-based bioactive compounds for formulating the nutraceuticals and functional food: A review. Front. Nutr. 2022, 9, 1–13. [Google Scholar] [CrossRef]
- Ferreres, F.; Taveira, M.; Pereira, D.M.; Valentão, P.; Andrade, P.B. Tomato (Lycopersicon esculentum) seeds: New flavonols and cytotoxic effect. J. Agric. Food Chem. 2010, 58, 2854–2861. [Google Scholar] [CrossRef]
- Navarro-González, I.; García-Valverde, V.; García-Alonso, J.; Periago, M.J. Chemical profile, functional and antioxidant properties of tomato peel fiber. Food Res. Int. 2011, 44, 1528–1535. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, X.; Zhang, Y.; Ma, Y.; Li, L.; Li, D.; Zhang, L.; Zhang, Z. Comprehensive study of the in vivo and in vitro metabolism of dietary isoflavone biochanin A based on UHPLC-Q-TOF-MS/MS. J. Agric. Food Chem. 2019, 67, 12481–12495. [Google Scholar] [CrossRef]
- Nakamura, K.; Zhu, S.; Komatsu, K.; Hattori, M.; Iwashima, M. Deglycosylation of the isoflavone C-glucoside puerarin by a combination of two recombinant bacterial enzymes and 3-oxo-glucose. Appl. Environ. Microbiol. 2020, 86, e00607-20. [Google Scholar] [CrossRef] [PubMed]
- Van Duynhoven, J.; Vaughan, E.E.; Jacobs, D.M.; Kemperman, R.A.; van Velzen, E.J.J.; Gross, G.; Roger, L.C.; Possemiers, S.; Smilde, A.K.; Doré, J.; et al. Metabolic fate of polyphenols in the human superorganism. Proc. Natl. Acad. Sci. USA 2011, 108, 4531–4538. [Google Scholar] [CrossRef]
- Morais, C.A.; de Rosso, V.V.; Estadella, D.; Pisani, L.P. Anthocyanins as inflammatory modulators and the role of the gut microbiota. J. Nutr. Biochem. 2016, 33, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mithul Aravind, S.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of dietary polyphenols on gut microbiota, their metabolites and health benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef] [PubMed]
- Osonga, F.J.; Akgul, A.; Miller, R.M.; Eshun, G.B.; Yazgan, I.; Akgul, A.; Sadik, O.A. Antimicrobial activity of a new class of phosphorylated and modified flavonoids. ACS Omega 2019, 4, 12865–12871. [Google Scholar] [CrossRef]
- Duda-Chodak, A. The inhibitory effect of polyphenols and the gut microbiota. J. Physiol. Pharmacol. 2012, 63, 497–503. [Google Scholar]
- Molinero, N.; Conti, E.; Walker, A.W.; Margolles, A.; Duncan, S.H.; Delgado, S. Survival strategies and metabolic interactions between Ruminococcus gauvreauii and Ruminococcoides bili, isolated from human bile. Microbiol. Spectr. 2022, 10, e02776-21. [Google Scholar] [CrossRef]
- Toya, T.; Corban, M.T.; Marrietta, E.; Horwath, I.E.; Lerman, L.O.; Murray, J.A.; Lerman, A. coronary artery disease is associated with an altered gut microbiome composition. PLoS ONE 2020, 15, e0227147. [Google Scholar] [CrossRef]
- Gwiazdowska, D.; Juś, K.; Jasnowska-Małecka, J.; Kluczyńska, K. The impact of polyphenols on Bifidobacterium growth. Acta Biochim. Pol. 2015, 62, 895–901. [Google Scholar] [CrossRef]
- Grosso, G.; Godos, J.; Lamuela-Raventos, R.; Ray, S.; Micek, A.; Pajak, A.; Sciacca, S.; D’Orazio, N.; Del Rio, D.; Galvano, F. A comprehensive meta-analysis on dietary flavonoid and lignan intake and cancer risk: Level of evidence and limitations. Mol. Nutr. Food Res. 2017, 61, 1600930. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.; Jaggers, G.K.; Verstraeten, S.V.; Erlejman, A.G.; Fraga, C.G.; Oteiza, P.I. Large procyanidins prevent bile-acid-induced oxidant production and membrane-initiated ERK1/2, P38, and Akt activation in Caco-2 cells. Free Radic. Biol. Med. 2012, 52, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-S.; Chong, Y.; Kim, M.K. Myricetin: Biological activity related to human health. Appl. Biol. Chem. 2016, 59, 259–269. [Google Scholar] [CrossRef]
- Javed, Z.; Khan, K.; Herrera-Bravo, J.; Naeem, S.; Iqbal, M.J.; Raza, Q.; Sadia, H.; Raza, S.; Bhinder, M.; Calina, D.; et al. Myricetin: Targeting signaling networks in cancer and its implication in chemotherapy. Cancer Cell Int. 2022, 22, 239. [Google Scholar] [CrossRef]
- Ha, T.K.; Jung, I.; Kim, M.E.; Bae, S.K.; Lee, J.S. Anti-cancer activity of myricetin against human papillary thyroid cancer cells involves mitochondrial dysfunction–mediated apoptosis. Biomed. Pharmacother. 2017, 91, 378–384. [Google Scholar] [CrossRef]
- Knickle, A.; Fernando, W.; Greenshields, A.L.; Rupasinghe, H.P.V.; Hoskin, D.W. Myricetin-induced apoptosis of triple-negative breast cancer cells is mediated by the iron-dependent generation of reactive oxygen species from hydrogen peroxide. Food Chem. Toxicol. 2018, 118, 154–167. [Google Scholar] [CrossRef]
- Motallebi, M.; Bhia, M.; Rajani, H.F.; Bhia, I.; Tabarraei, H.; Mohammadkhani, N.; Pereira-Silva, M.; Kasaii, M.S.; Nouri-Majd, S.; Mueller, A.-L.; et al. Naringenin: A potential flavonoid phytochemical for cancer therapy. Life Sci. 2022, 305, 120752. [Google Scholar] [CrossRef]
- Jafari, A.; Esmaeilzadeh, Z.; Khezri, M.R.; Ghasemnejad-Berenji, H.; Pashapour, S.; Sadeghpour, S.; Ghasemnejad-Berenji, M. An overview of possible pivotal mechanisms of genistein as a potential phytochemical against SARS-CoV-2 infection: A hypothesis. J. Food Biochem. 2022, 46, e14345. [Google Scholar] [CrossRef]
- Kim, T.I.; Kwon, E.B.; Oh, Y.C.; Go, Y.; Choi, J.G. Mori ramulus and its major component morusin inhibit Herpes simplex virus type 1 replication and the virus-induced reactive oxygen species. Am. J. Chin. Med. 2021, 49, 163–179. [Google Scholar] [CrossRef]
- Čulenová, M.; Sychrová, A.; Hassan, S.T.S.; Berchová-Bímová, K.; Svobodová, P.; Helclová, A.; Michnová, H.; Hošek, J.; Vasilev, H.; Suchý, P.; et al. Multiple in vitro biological effects of phenolic compounds from Morus alba root bark. J. Ethnopharmacol. 2020, 248, 112296. [Google Scholar] [CrossRef]
- Balentine, D.A.; Dwyer, J.T.; Erdman, J.W.; Ferruzzi, M.G.; Gaine, P.C.; Harnly, J.M.; Kwik-Uribe, C.L. Recommendations on reporting requirements for flavonoids in research. Am. J. Clin. Nutr. 2015, 101, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, R.; Mills, C.; Istas, G.; Heiss, C.; Rodriguez-Mateos, A. Absorption, metabolism and excretion of cranberry (poly)phenols in humans: A dose response study and assessment of inter-individual variability. Nutrients 2017, 9, 268. [Google Scholar] [CrossRef] [PubMed]
- Fiamegos, Y.C.; Nanos, C.G.; Vervoort, J.; Stalikas, C.D. Analytical procedure for the in-vial derivatization—Extraction of phenolic acids and flavonoids in methanolic and aqueous plant extracts followed by gas chromatography with mass-selective detection. J. Chromatogr. A 2004, 1041, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shen, X. Preparation and application of molecularly imprinted polymers for flavonoids: Review and perspective. Molecules 2022, 27, 7355. [Google Scholar] [CrossRef] [PubMed]
- Le, H.-T.-T.; Chau, Q.-C.; Duong, T.-H.; Tran, Q.-T.-P.; Pham, N.-K.-T.; Nguyen, T.-H.-T.; Nguyen, N.-H.; Sichaem, J. new derivatives of lupeol and their biological activity. Molbank 2021, 2021, M1306. [Google Scholar] [CrossRef]
- Hussain, N.; Kakoti, B.B.; Rudrapal, M.; Sarwa, K.K.; Celik, I.; Attah, E.I.; Khairnar, S.J.; Bhattacharya, S.; Sahoo, R.K.; Walode, S.G. Bioactive antidiabetic flavonoids from the stem bark of Cordia dichotoma Forst.: Identification, docking and ADMET studies. Molbank 2021, 2021, M1234. [Google Scholar] [CrossRef]
- Hofmann, J.; Spatz, P.; Walther, R.; Gutmann, M.; Maurice, T.; Decker, M. Synthesis and biological evaluation of flavonoid-cinnamic acid amide hybrids with distinct activity against neurodegeneration in vitro and in vivo. Chem.–A Eur. J. 2022, 28, e202200786. [Google Scholar] [CrossRef]
- Isika, D.K.; Özkömeç, F.N.; Çeşme, M.; Sadik, O.A. Synthesis, biological and computational studies of flavonoid acetamide derivatives. RSC Adv. 2022, 12, 10037–10050. [Google Scholar] [CrossRef]
- Liu, Z.Q. What about the progress in the synthesis of flavonoid from 2020? Eur. J. Med. Chem. 2022, 243, 114671. [Google Scholar] [CrossRef]
- Bilia, A.R.; Isacchi, B.; Righeschi, C.; Guccione, C.; Bergonzi, M.C. Flavonoids loaded in nanocarriers: An opportunity to increase oral bioavailability and bioefficacy. Food Nutr. Sci. 2014, 05, 1212–1327. [Google Scholar] [CrossRef]
- Wadhwa, R.; Paudel, K.R.; Chin, L.H.; Hon, C.M.; Madheswaran, T.; Gupta, G.; Panneerselvam, J.; Lakshmi, T.; Singh, S.K.; Gulati, M.; et al. Anti-inflammatory and anticancer activities of naringenin-loaded liquid crystalline nanoparticles in vitro. J. Food Biochem. 2021, 45, e13572. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, S.; Shanmugam, K. Synthesis and characterization of naringenin-loaded chitosan-dextran sulfate nanocarrier. J. Pharm. Innov. 2021, 16, 269–278. [Google Scholar] [CrossRef]
- Huey, R.B. Plants versus animals: Do they deal with stress in different ways? Integr. Comp. Biol. 2002, 42, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Mittler, R. Reactive oxygen species-dependent wound responses in animals and plants. Free Radic. Biol. Med. 2012, 53, 2269–2276. [Google Scholar] [CrossRef]
- Brunetti, C.; Fini, A.; Sebastiani, F.; Gori, A.; Tattini, M. Modulation of phytohormone signaling: A primary function of flavonoids in plant–environment interactions. Front. Plant Sci. 2018, 9, 1042. [Google Scholar] [CrossRef]
- Krautkramer, K.A.; Fan, J.; Bäckhed, F. Gut microbial metabolites as multi-kingdom intermediates. Nat. Rev. Microbiol. 2021, 19, 77–94. [Google Scholar] [CrossRef]
- Hickman, D.T.; Rasmussen, A.; Ritz, K.; Birkett, M.A.; Neve, P. Review: Allelochemicals as multi-kingdom plant defence compounds: Towards an integrated approach. Pest Manag. Sci. 2021, 77, 1121–1131. [Google Scholar] [CrossRef]
- Jańczak-Pieniążek, M.; Migut, D.; Piechowiak, T.; Buczek, J.; Balawejder, M. The effect of exogenous application of quercetin derivative solutions on the course of physiological and biochemical processes in wheat seedlings. Int. J. Mol. Sci. 2021, 22, 6882. [Google Scholar] [CrossRef]
- Aslam, M.A.; Ahmed, S.; Saleem, M.; Shah, A.A.; Shah, A.N.; Tanveer, M.; Ali, H.M.; Ghareeb, R.Y.; Hasan, M.E.; Khan, J. Quercetin ameliorates chromium toxicity through improvement in photosynthetic activity, antioxidative defense system; and suppressed oxidative stress in Trigonella corniculata L. Front. Plant Sci. 2022, 13, 956249. [Google Scholar] [CrossRef]
- Sun, M.; Li, L.; Wang, C.; Wang, L.; Lu, D.; Shen, D.; Wang, J.; Jiang, C.; Cheng, L.; Pan, X.; et al. Naringenin confers defence against Phytophthora nicotianae through antimicrobial activity and induction of pathogen resistance in tobacco. Mol. Plant Pathol. 2022, 23, 1737–1750. [Google Scholar] [CrossRef]
- Wang, J.; Hao, K.; Yu, F.; Shen, L.; Wang, F.; Yang, J.; Su, C. Field application of nanoliposomes delivered quercetin by inhibiting specific Hsp70 Gene expression against plant virus disease. J. Nanobiotechnol. 2022, 20, 1–16. [Google Scholar] [CrossRef]
- Del Valle, I.; Webster, T.M.; Cheng, H.-Y.; Thies, J.E.; Kessler, A.; Miller, M.K.; Ball, Z.T.; MacKenzie, K.R.; Masiello, C.A.; Silberg, J.J.; et al. Soil organic matter attenuates the efficacy of flavonoid-based plant-microbe communication. Sci. Adv. 2020, 6, eaax8254. [Google Scholar] [CrossRef] [PubMed]
- Keiluweit, M.; Nico, P.; Harmon, M.E.; Mao, J.; Pett-Ridge, J.; Kleber, M. Long-term litter decomposition controlled by manganese redox cycling. Proc. Natl. Acad. Sci. USA 2015, 112, E5253–E5260. [Google Scholar] [CrossRef] [PubMed]
- Marin, A.M.; de la Torre, J.; Ricardo Marques Oliveira, A.; Barison, A.; Satie Chubatsu, L.; Adele Monteiro, R.; de Oliveira Pedrosa, F.; Maltempi de Souza, E.; Wassem, R.; Duque, E.; et al. Genetic and functional characterization of a novel meta-pathway for degradation of naringenin in Herbaspirillum seropedicae SmR1. Environ. Microbiol. 2016, 18, 4653–4661. [Google Scholar] [CrossRef] [PubMed]
- Leoni, F.; Hazrati, H.; Fomsgaard, I.S.; Moonen, A.-C.; Kudsk, P. Determination of the effect of co-cultivation on the production and root exudation of flavonoids in four legume species using LC–MS/MS analysis. J. Agric. Food Chem. 2021, 69, 9208–9219. [Google Scholar] [CrossRef] [PubMed]


| Bacteria | Rhizocompetence Traits | Flavonoids | Concentration | Main Effects | Reference |
|---|---|---|---|---|---|
| Aeromonas sp. H1 |
| Naringenin, kaempferol, quercetin | 100 µM |
| [185] |
| Gluconacetobacter diazotrophicus |
| Apigenin | 20–100 µM |
| [187] |
| Bacillus licheniformis, Bacillus subtilis, Acinetobacter junii Pb1 |
| Chrysopogon zizanioides (L.) Roberty-exuded flavonoids | n.d. * |
| [188] |
| Rhodococcus erythropolis U23A |
| Concentrated A. thaliana root exudates containing flavonoids | n.d. * (flavanone approx. 0.5–1 mM) |
| [189] |
| Pseudomonas fluorescens 2P24 |
| Apigenin, phloretin | 100 µM |
| [37] |
| Herbaspirillum seropedicae SmR1 |
| Naringenin | 100 µM |
| [190] |
| Bacillus subtilis CIM |
| Rutin | 1 pM |
| [186] |
| Azorhizobium caulinodans ORS571, Herbaspirillum seropedicae |
| Naringenin, daidzein | 50 µM |
| [191] |
| Azorhizobium caulinodans, Azospirillum brasilense |
| Naringenin | 50 µM |
| [192] |
| Pseudomonas putida PML2 |
| Naringenin, quercetin | 10 mM |
| [182] |
| Acinetobacter calcoaceticus MTC 127 |
| (+)-Catechin | 3 mM |
| [193] |
| Paraburkholderia xenovorans LB400 |
| Morusin, morusinol, kuwanon C | 100 µg/mL |
| [194] |
| Rhizobacteria consortium (Pseudomonas sp. + Stenotrophomonas sp.) |
| Flavone, flavanone, isoflavone, 7-hydroxyflavanone, 7-hydroxyflavone, 6-hydroxyflavone | 200 µM |
| [195] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghitti, E.; Rolli, E.; Crotti, E.; Borin, S. Flavonoids Are Intra- and Inter-Kingdom Modulator Signals. Microorganisms 2022, 10, 2479. https://doi.org/10.3390/microorganisms10122479
Ghitti E, Rolli E, Crotti E, Borin S. Flavonoids Are Intra- and Inter-Kingdom Modulator Signals. Microorganisms. 2022; 10(12):2479. https://doi.org/10.3390/microorganisms10122479
Chicago/Turabian StyleGhitti, Elisa, Eleonora Rolli, Elena Crotti, and Sara Borin. 2022. "Flavonoids Are Intra- and Inter-Kingdom Modulator Signals" Microorganisms 10, no. 12: 2479. https://doi.org/10.3390/microorganisms10122479
APA StyleGhitti, E., Rolli, E., Crotti, E., & Borin, S. (2022). Flavonoids Are Intra- and Inter-Kingdom Modulator Signals. Microorganisms, 10(12), 2479. https://doi.org/10.3390/microorganisms10122479





