Effect of Atmospheric Conditions on Pathogenic Phenotypes of Arcobacter butzleri
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Growth Conditions
2.2. Oxidative Stress
2.3. Acidic Stress Survival
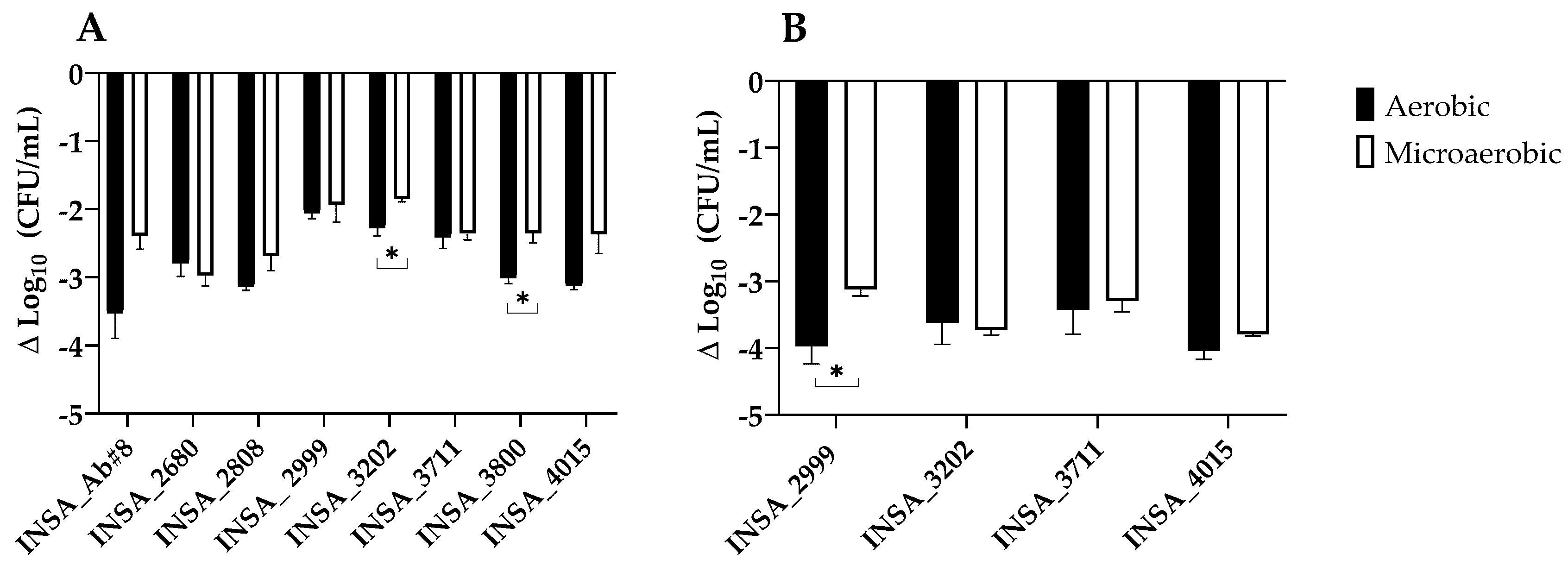
2.4. Serum Survival
2.5. Haemolytic Activity
2.6. Motility and flaA Relative Expression Assays
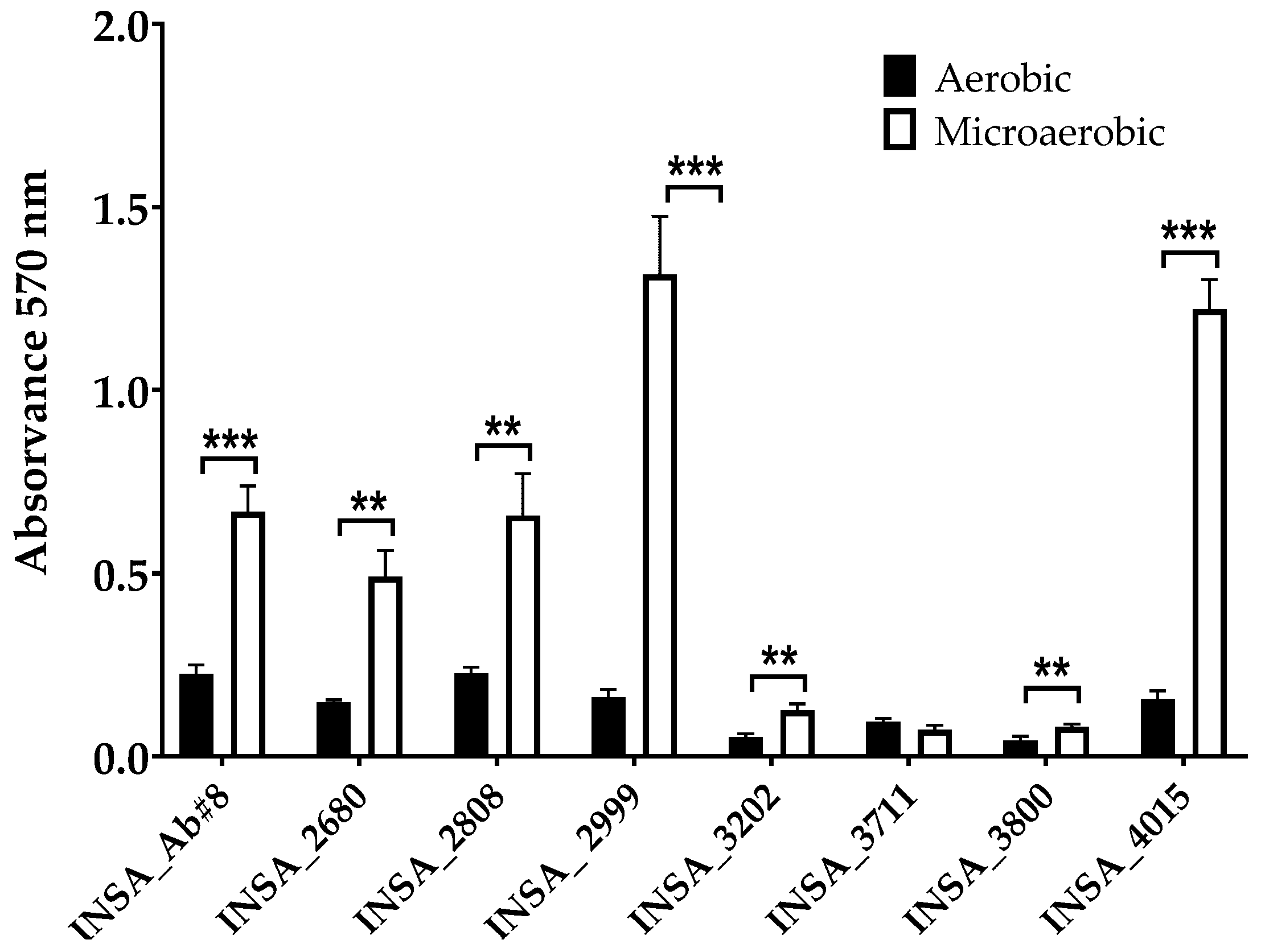
2.7. Biofilm Formation Ability
2.8. Adhesion and Invasion of Caco-2 Cells Line
2.9. Statistical Analysis
3. Results
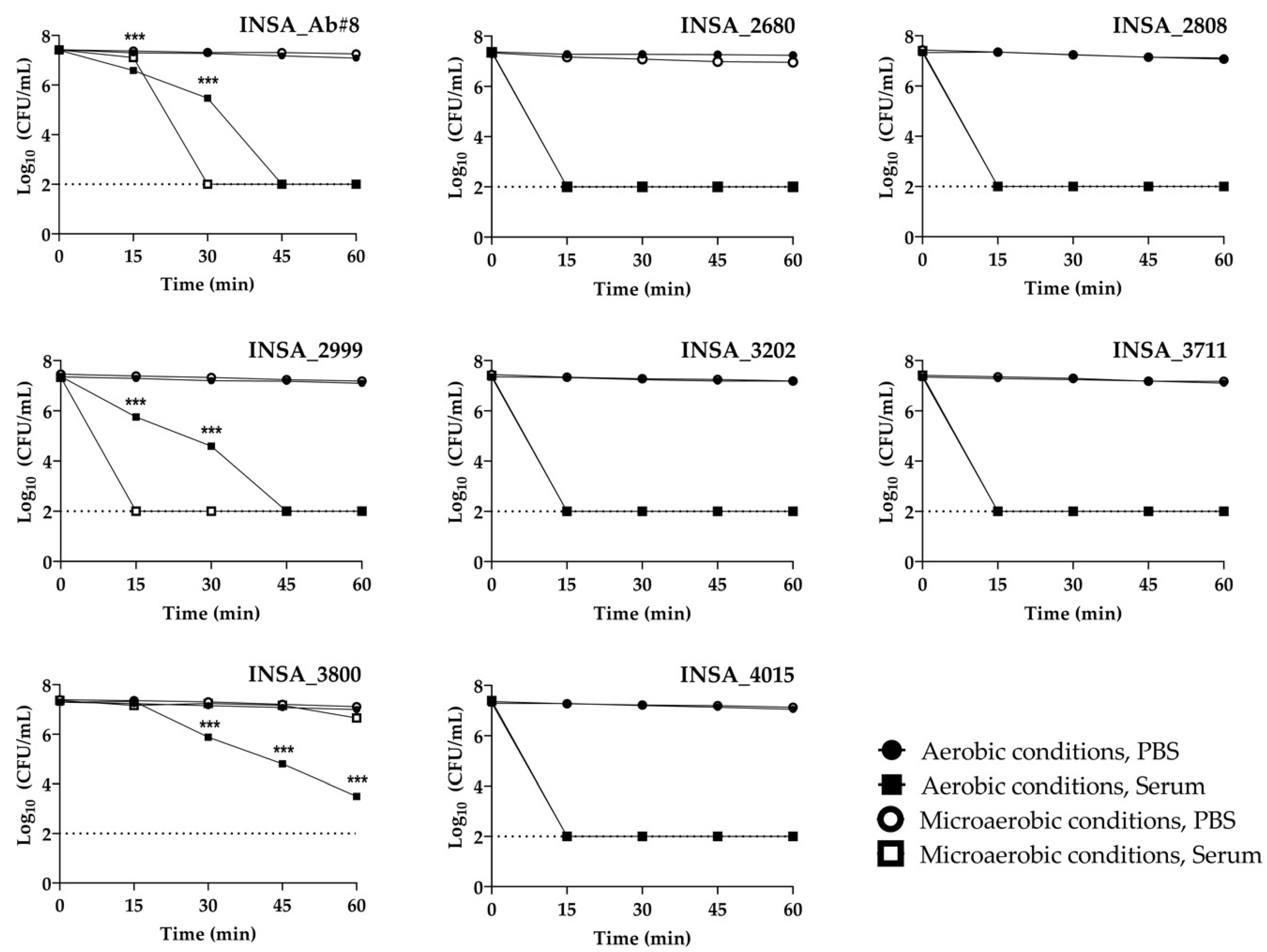
3.1. Effect of Atmospheric Conditions on Arcobacter butzleri in Host Conditions of Stress
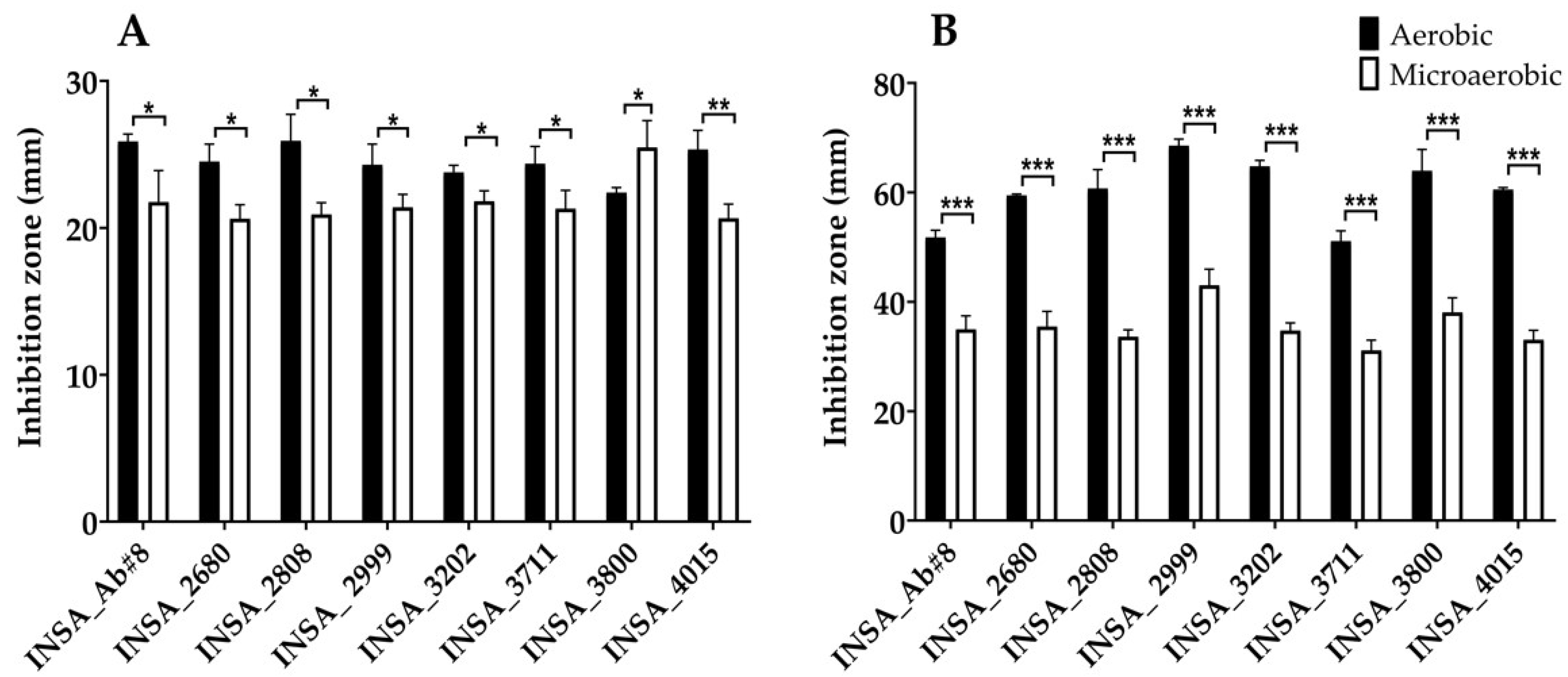
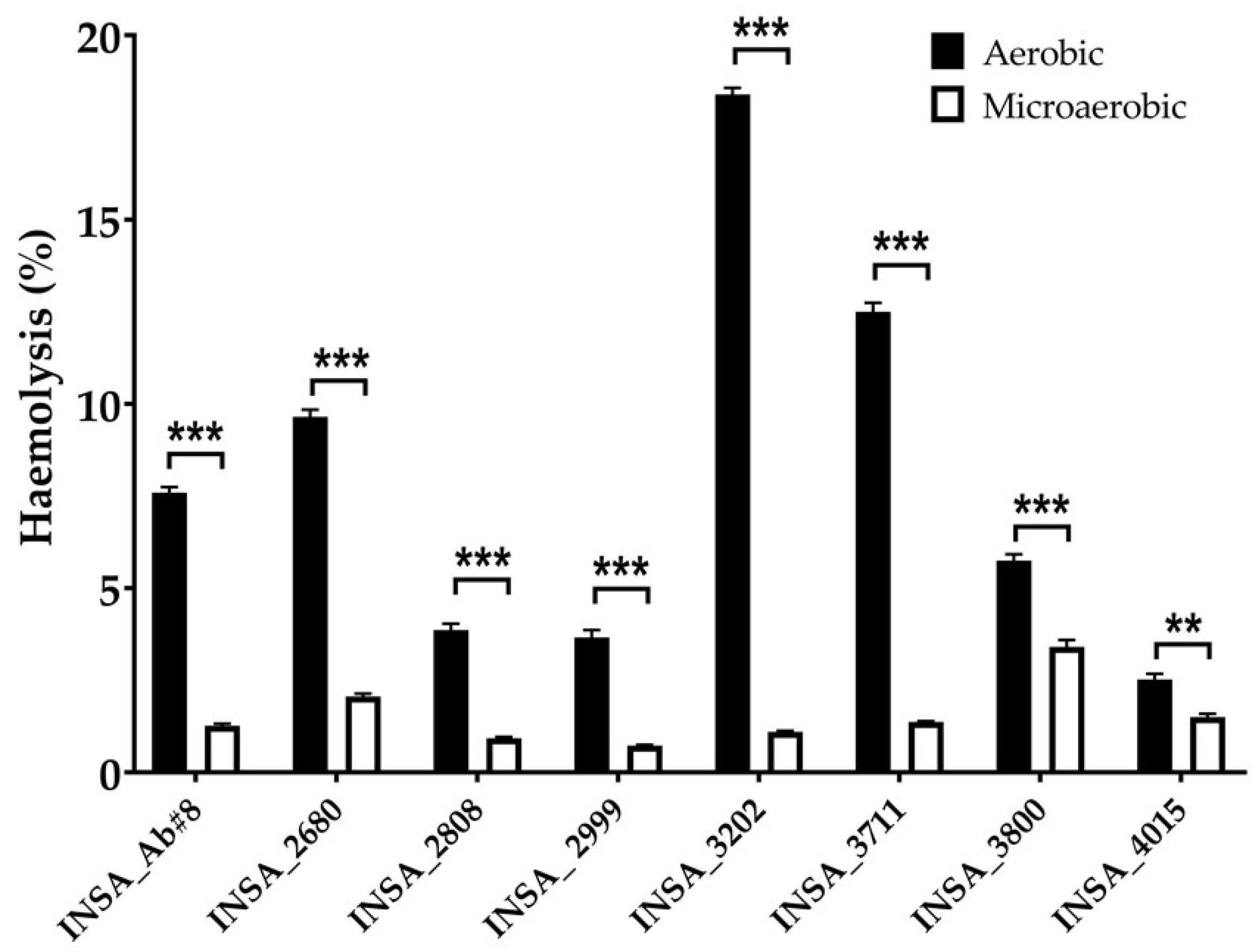
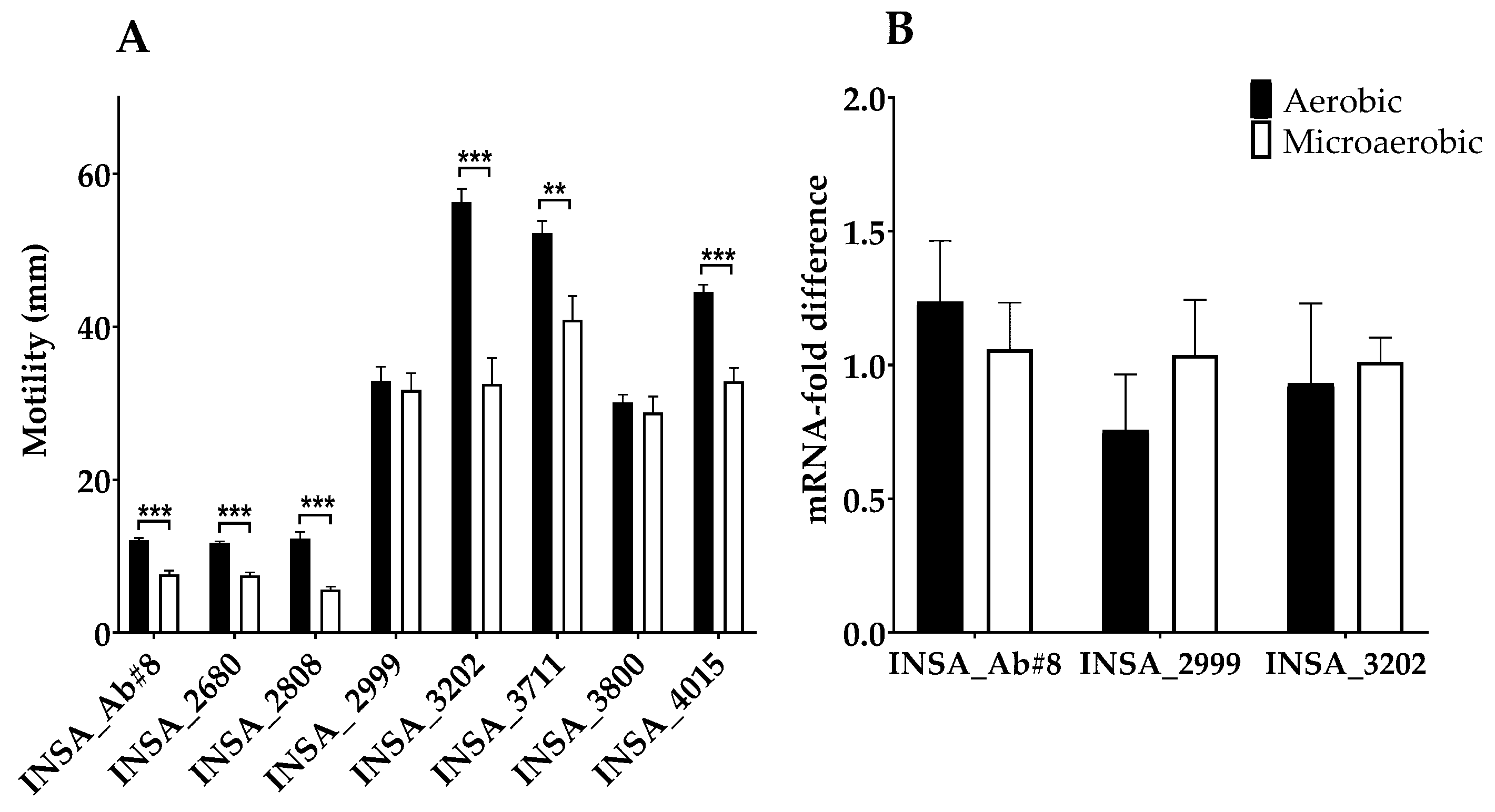
3.2. Effect of Atmospheric Conditions on Virulence Traits of Arcobacter butzleri
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pérez-Cataluña, A.; Salas-Massó, N.; Diéguez, A.L.; Balboa, S.; Lema, A.; Romalde, J.L.; Figueras, M.J. Revisiting the Taxonomy of the Genus Arcobacter: Getting Order from the Chaos. Front. Microbiol. 2018, 9, 2077. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, P.; Vancanneyt, M.; Pot, B.; Mels, L.; Hoste, B.; Dewettinck, D.; Vlaes, L.; van den Borre, C.; Higgins, R.; Hommez, J. Polyphasic Taxonomic Study of the Emended Genus Arcobacter with Arcobacter butzleri Comb. Nov. and Arcobacter skirrowii sp. Nov., an Aerotolerant Bacterium Isolated from Veterinary Specimens. Int. J. Syst. Bacteriol. 1992, 42, 344–356. [Google Scholar] [CrossRef] [PubMed][Green Version]
- de Blackburn, C.W.; McClure, P.J. 20—Campylobacter and Arcobacter. In Foodborne Pathogens, 2nd ed.; de Blackburn, C.W., McClure, P.J., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2009; pp. 718–762. ISBN 978-1-84569-362-6. [Google Scholar]
- Ferreira, S.; Oleastro, M.; Domingues, F. Current Insights on Arcobacter butzleri in Food Chain. Curr. Opin. Food Sci. 2019, 26, 9–17. [Google Scholar] [CrossRef]
- Collado, L.; Figueras, M.J. Taxonomy, Epidemiology, and Clinical Relevance of the Genus Arcobacter. Clin. Microbiol. Rev. 2011, 24, 174–192. [Google Scholar] [CrossRef]
- Collado, L.; Gutiérrez, M.; González, M.; Fernández, H. Assessment of the Prevalence and Diversity of Emergent Campylobacteria in Human Stool Samples Using a Combination of Traditional and Molecular Methods. Diagn. Microbiol. Infect. Dis. 2013, 75, 434–436. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.; Queiroz, J.A.; Oleastro, M.; Domingues, F.C. Genotypic and Phenotypic Features of Arcobacter butzleri Pathogenicity. Microb. Pathog. 2014, 76, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Van den Abeele, A.M.; Vogelaers, D.; Van Hende, J.; Houf, K. Prevalence of Arcobacter Species among Humans, Belgium, 2008–2013. Emerg. Infect. Dis. 2014, 20, 1731–1734. [Google Scholar] [CrossRef]
- Vandenberg, O.; Dediste, A.; Houf, K.; Ibekwem, S.; Souayah, H.; Cadranel, S.; Douat, N.; Zissis, G.; Butzler, J.P.; Vandamme, P. Arcobacter Species in Humans. Emerg. Infect. Dis. 2004, 10, 1863–1867. [Google Scholar] [CrossRef] [PubMed]
- International Commission on Microbiological Specifications for Foods. Microorganisms in Foods 7: Microbiological Testing in Food Safety Management, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 9783319684604. [Google Scholar]
- Ferreira, S.; Júlio, C.; Queiroz, J.A.; Domingues, F.C.; Oleastro, M. Molecular Diagnosis of Arcobacter and Campylobacter in Diarrhoeal Samples among Portuguese Patients. Diagn. Microbiol. Infect. Dis. 2014, 78, 220–225. [Google Scholar] [CrossRef]
- Ferreira, S.; Queiroz, J.A.; Oleastro, M.; Domingues, F.C. Insights in the Pathogenesis and Resistance of Arcobacter: A Review. Crit. Rev. Microbiol. 2016, 42, 364–383. [Google Scholar] [PubMed]
- Chieffi, D.; Fanelli, F.; Fusco, V. Arcobacter butzleri: Up-to-Date Taxonomy, Ecology, and Pathogenicity of an Emerging Pathogen. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2071–2109. [Google Scholar] [CrossRef] [PubMed]
- Bücker, R.; Troeger, H.; Kleer, J.; Fromm, M.; Schulzke, J.D. Arcobacter butzleri induces barrier dysfunction in intestinal HT-29/B6 cells. J. Infect. Dis. 2009, 200, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.; Butcher, J.; Stintzi, A. Stress Responses, Adaptation, and Virulence of Bacterial Pathogens During Host Gastrointestinal Colonization. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Chelliah, R.; Ramakrishnan, S.R.; Perumal, A.S.; Bang, W.-S.; Rubab, M.; Daliri, E.B.-M.; Barathikannan, K.; Elahi, F.; Park, E.; et al. Review on Stress Tolerance in Campylobacter jejuni. Front. Cell. Infect. Microbiol. 2021, 10, 596570. [Google Scholar] [CrossRef] [PubMed]
- Poole, K. Bacterial Stress Responses as Determinants of Antimicrobial Resistance. J. Antimicrob. Chemother. 2012, 67, 2069–2089. [Google Scholar] [CrossRef]
- Gundogdu, O.; da Silva, D.T.; Mohammad, B.; Elmi, A.; Wren, B.W.; van Vliet, A.H.M.; Dorrell, N. The Campylobacter jejuni Oxidative Stress Regulator RrpB Is Associated with a Genomic Hypervariable Region and Altered Oxidative Stress Resistance. Front. Microbiol. 2016, 7, 2117. [Google Scholar] [CrossRef]
- Kim, J.C.; Oh, E.; Hwang, S.; Ryu, S.; Jeon, B. Non-Selective Regulation of Peroxide and Superoxide Resistance Genes by PerR in Campylobacter jejuni. Front. Microbiol. 2015, 6, 1–7. [Google Scholar] [CrossRef]
- Oh, E.; McMullen, L.; Jeon, B. Impact of Oxidative Stress Defense on Bacterial Survival and Morphological Change in Campylobacter jejuni under Aerobic Conditions. Front. Microbiol. 2015, 6, 126. [Google Scholar] [CrossRef]
- Gundogdu, O.; da Silva, D.T.; Mohammad, B.; Elmi, A.; Mills, D.C.; Wren, B.W.; Dorrell, N. The Campylobacter jejuni MarR-like Transcriptional Regulators RrpA and RrpB Both Influence Bacterial Responses to Oxidative and Aerobic Stresses. Front. Microbiol. 2015, 6, 724. [Google Scholar] [CrossRef]
- Houf, K.; Tutenel, A.; Zutter, L.; Hoof, J.V.; Vandamme, P. Development of a Multiplex PCR Assay for the Simultaneous Detection and Identification of Arcobacter butzleri, Arcobacter cryaerophilus and Arcobacter skirrowii. FEMS Microbiol. Lett. 2000, 1, 89–94. [Google Scholar] [CrossRef]
- Kern, M.; Volz, J.; Simon, J. The Oxidative and Nitrosative Stress Defence Network of Wolinella succinogenes: Cytochrome c Nitrite Reductase Mediates the Stress Response to Nitrite, Nitric Oxide, Hhydroxylamine and Hydrogen Peroxide. Environ Microbiol. 2011, 13, 2478–2494. [Google Scholar] [CrossRef] [PubMed]
- Isohanni, P.; Huehn, S.; Aho, T.; Alter, T.; Lyhs, U. Heat Stress Adaptation Induces Cross-protection Against Lethal Acid Stress Conditions in Arcobacter butzleri but not in Campylobacter jejuni. Food Microbiol. 2013, 34, 431–435. [Google Scholar] [CrossRef]
- Mateus, C.; Nunes, A.R.; Oleastro, M.; Domingues, F.; Ferreira, S. RND Efflux Systems Contribute to Resistance and Virulence of Aliarcobacter butzleri. Antibiotics 2021, 10, 823. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.; Correia, D.R.; Oleastro, M.; Domingues, F.C. Arcobacter butzleri Ciprofloxacin Resistance: Point Mutations in DNA Gyrase A and Role on Fitness Cost. Microb. Drug Resist. 2018, 24, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Medina, G.; Neves, P.; Flores-Martin, S.; Manosalva, C.; Andaur, M.; Otth, C.; Lincopan, N.; Fernández, H. Transcriptional Analysis of Flagellar and Putative Virulence Genes of Arcobacter butzleri as an Endocytobiont of Acanthamoeba Castellanii. Arch. Microbiol. 2019, 201, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Muyzer, G.; De Waal, E.C.; Uitterlinden, A.G. Profiling of Complex Microbial Populations by Denaturing Gradient Gel Electrophoresis Analysis of Polymerase Chain Reaction-Amplified Genes Coding for 16S RRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [CrossRef]
- Comtois, S.L.; Gidley, M.D.; Kelly, D.J. Role of the Thioredoxin System and the Thiol-Peroxidases Tpx and Bcp in Mediating Resistance to Oxidative and Nitrosative Stress in Helicobacter pylori. Microbiology 2003, 149, 121–129. [Google Scholar] [CrossRef]
- Imlay, J.A. Cellular Defenses against Superoxide and Hydrogen Peroxide. Annu Rev Biochem. 2008, 77, 755–776. [Google Scholar] [CrossRef]
- Kim, J.C.; Oh, E.; Kim, J.; Jeon, B. Regulation of Oxidative Stress Resistance in Campylobacter jejuni, a Microaerophilic Foodborne Pathogen. Front. Microbiol. 2015, 6, 751. [Google Scholar] [CrossRef]
- Miller, W.G.; Parker, C.T.; Rubenfield, M.; Mendz, G.L.; Wösten, M.M.S.M.; Ussery, D.W.; Stolz, J.F.; Binnewies, T.T.; Hallin, P.F.; Wang, G.; et al. The Complete Genome Sequence and Analysis of the Epsilonproteobacterium Arcobacter Butzleri. PLoS ONE 2007, 2, e1358. [Google Scholar] [CrossRef]
- Atack, J.M.; Kelly, D.J. Contribution of the stereospecific methionine sulphoxide reductases MsrA and MsrB to oxidative and nitrosative stress resistance in the food-borne pathogen Campylobacter jejuni. Microbiology 2008, 154, 2219–2230. [Google Scholar] [CrossRef]
- Wainwright, L.M.; Elvers, K.T.; Park, S.F.; Poole, R.K. A truncated haemoglobin implicated in oxygen metabolism by the microaerophilic food-borne pathogen Campylobacter jejuni. Microbiology 2005, 151, 4079–4091. [Google Scholar] [CrossRef]
- Hill, C.; O’Driscoll, B.; Booth, I. Acid Adaptation and Food Poisoning Microorganisms. Int. J. Food Microbiol. 1995, 28, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Sahu, G.K.; Das, J. Stress Response in Pathogenic Bacteria. J. Biosci. 1996, 21, 149–160. [Google Scholar] [CrossRef]
- Dressman, J.B.; Berardi, R.R.; Dermentzoglou, L.C.; Russell, T.L.; Schmaltz, S.P.; Barnett, J.L.; Jarvenpaa, K.M. Upper Gastrointestinal (GI) PH in Young, Healthy Men and Women. Pharm. Res. 1990, 7, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Cervenka, L. Survival and Inactivation of Arcobacter spp., a Current Status and Future Prospect. Crit. Rev. Microbiol. 2007, 33, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.; Carroll, C.; Jordan, K.N. Induction of an Adaptive Tolerance Response in the Foodborne Pathogen, Campylobacter jejuni. FEMS Microbiol. Lett. 2003, 223, 89–93. [Google Scholar] [CrossRef][Green Version]
- Murphy, C.; Carroll, C.; Jordan, K.N. Environmental Survival Mechanisms of the Foodborne Pathogen Campylobacter jejuni. J. Appl. Microbiol. 2006, 100, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.; Otth, L.; Aron, R.; Fernández, H. Susceptibility of Arcobacter Butzleri to Human Blood Serum. Arq. Bras. Med. Veterinária e Zootec. 2010, 62, 232–235. [Google Scholar] [CrossRef][Green Version]
- Blaser, M.J.; Smith, P.F.; Hopkins, J.A.; Heinzer, I.; Bryner, J.H.; Wang, W.L.L. Pathogenesis of Campylobacter Fetus Infections: Serum Resistance Associated with High-Molecular-Weight Surface Proteins. J. Infect. Dis. 1987, 155, 696–706. [Google Scholar] [CrossRef]
- Blaser, M.J.; Perez, G.P.; Smith, P.F.; Patton, C.; Tenover, F.C.; Lastovica, A.J.; Wang, W.-I.L. Extraintestinal Campylobacter jejuni and Campylobacter coli Infections: Host Factors and Strain Characteristics. J. Infect. Dis. 1986, 153, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Keo, T.; Collins, J.; Kunwar, P.; Blaser, M.J.; Iovine, N.M. Campylobacter capsule and lipooligosaccharide confer resistance to serum and cationic antimicrobials. Virulence 2011, 2, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.W. Bactericidal and Bacteriolytic Activity of Serum against Gram-Negative Bacteria. Microbiol. Rev. 1983, 47, 46–83. [Google Scholar] [CrossRef] [PubMed]
- McManus, D.C.; David Josephy, P. Superoxide Dismutase Protects Escherichia Coli against Killing by Human Serum. Arch. Biochem. Biophys. 1995, 317, 57–61. [Google Scholar] [CrossRef]
- Ginsburg, I. Could Synergistic Interactions among Reactive Oxygen Species, Proteinases, Membrane-Perforating Enzymes, Hydrolases, Microbial Hemolysins and Cytokines Be the Main Cause of Tissue Damage in Infectious and Inflammatory Conditions? Med. Hypotheses 1998, 51, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, C.; Ray, S.; Chowdhury, R. Fine Tuning of Virulence Regulatory Pathways in Enteric Bacteria in Response to Varying Bile and Oxygen Concentrations in the Gastrointestinal Tract. Gut Pathog. 2014, 6, 1–6. [Google Scholar] [CrossRef][Green Version]
- Alm, R.A.; Guerry, P.; Trust, T.J. The Campylobacter Sigma 54 FlaB Flagellin Promoter Is Subject to Environmental Regulation. J. Bacteriol. 1993, 175, 4448–4455. [Google Scholar] [CrossRef]
- Ovesen, S.; Durack, J.; Kirk, K.F.; Nielsen, H.L.; Nielsen, H.; Lynch, S.V. Motility and Biofilm Formation of the Emerging Gastrointestinal Pathogen Campylobacter Concisus Differs under Microaerophilic and Anaerobic Environments. Gut Microbes 2019, 10, 34–44. [Google Scholar] [CrossRef]
- Marteyn, B.; Scorza, F.B.; Sansonetti, P.J.; Tang, C. Breathing Life into Pathogens: The Influence of Oxygen on Bacterial Virulence and Host Responses in the Gastrointestinal Tract. Cell. Microbiol. 2011, 13, 171–176. [Google Scholar] [CrossRef]
- Ho, H.T.K.; Lipman, L.J.A.; Wösten, M.M.S.M.; Van Asten, A.J.A.M.; Gaastra, W. Arcobacter spp. Possess Two Very Short Flagellins of Which FlaA Is Essential for Motility. FEMS Immunol. Med. Microbiol. 2008, 53, 85–95. [Google Scholar] [CrossRef]
- Magana, M.; Sereti, C.; Ioannidis, A.; Mitchell, C.A.; Ball, A.R.; Magiorkinis, E.; Chatzipanagiotou, S.; Hamblin, M.R.; Hadjifrangiskou, M.; Tegos, G.P. Options and Limitations in Clinical Investigation of Bacterial Biofilms. Clin. Microbiol. Rev. 2018, 31. [Google Scholar] [CrossRef] [PubMed]
- Adetunji, V.O.; Adedeji, A.O.; Kwaga, J. Assessment of the Contamination Potentials of Some Foodborne Bacteria in Biofilms for Food Products. Asian Pac. J. Trop. Med. 2014, 7, S232–S237. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Ferreira, S.; Fraqueza, M.J.; Queiroz, J.A.; Domingues, F.C.; Oleastro, M. Genetic Diversity, Antibiotic Resistance and Biofilm-Forming Ability of Arcobacter Butzleri Isolated from Poultry and Environment from a Portuguese Slaughterhouse. Int. J. Food Microbiol. 2013, 162, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Šilha, D.; Sirotková, S.; Švarcová, K.; Hofmeisterová, L.; Koryčanová, K.; Šilhová, L. Biofilm Formation Ability of Arcobacter-like and Campylobacter Strains under Different Conditions and on Food Processing Materials. Microorganisms 2021, 9, 2017. [Google Scholar] [CrossRef]
- Girbau, C.; Martinez-Malaxetxebarria, I.; Muruaga, G.; Carmona, S.; Alonso, R.; Fernandez-Astorga, A. Study of Biofilm Formation Ability of Foodborne Arcobacter Butzleri under Different Conditions. J. Food Prot. 2017, 80, 758–762. [Google Scholar] [CrossRef]
- Rather, M.A.; Gupta, K.; Mandal, M. Microbial Biofilm_ Formation, Architecture, Antibiotic Resistance, and Control Strategies.Pdf. Brazilian J. Microbiol. 2021, 52, 1701–1718. [Google Scholar] [CrossRef]
- Švarcová, K.; Hofmeisterová, L.; Švecová, B.; Šilha, D. In Vitro Activity of Water Extracts of Olive Oil against Planktonic Cells and Biofilm Formation of Arcobacter-like Species.Pdf. Molecules 2022, 9, 2017. [Google Scholar]
- Švarcová, K.; Pejchalová, M.; Šilha, D. The Effect of Antibiotics on Planktonic Cells and Biofilm Formation Ability of Collected Arcobacter-like Strains and Strains Isolated within the Czech Republic. Antibiotics 2022, 11, 87. [Google Scholar] [CrossRef]
- Chaban, B.; Hughes, H.V.; Beeby, M. The Flagellum in Bacterial Pathogens: For Motility and a Whole Lot More. Semin. Cell Dev. Biol. 2015, 46, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Pizarro-Cerdá, J.; Cossart, P. Bacterial Adhesion and Entry into Host Cells. Cell 2006, 124, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Cossart, P.; Sansonetti, P.J. Bacterial Invasion: The Paradigms of Enteroinvasive Pathogens. Science 2004, 304, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Schönknecht, A.; Alter, T.; Gölz, G. Detection of Arcobacter Species in Different Intestinal Compartments of Broiler Chicken during Slaughter and Processing. Microbiologyopen 2020, 9, e1106. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.T.K.; Lipman, L.J.A.; Hendriks, H.G.C.J.M.; Tooten, P.C.J.; Ultee, T.; Gaastra, W. Interaction of Arcobacter spp. with Human and Porcine Intestinal Epithelial Cells. FEMS Immunol. Med. Microbiol. 2007, 50, 51–58. [Google Scholar] [CrossRef]
- Buzzanca, D.; Botta, C.; Ferrocino, I.; Alessandria, V.; Houf, K.; Rantsiou, K. Functional pangenome analysis reveals high virulence plasticity of Aliarcobacter butzleri and affinity to human mucus. Genomics 2021, 113, 2065–2076. [Google Scholar] [CrossRef]
- Koolman, L.; Whyte, P.; Burgess, C.; Bolton, D. Virulence gene expression, adhesion and invasion of Campylobacter jejuni exposed to oxidative stress (H2O2). Int. J. Food Microbiol. 2016, 220, 33–38. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, R.; Mateus, C.; Domingues, F.; Bücker, R.; Oleastro, M.; Ferreira, S. Effect of Atmospheric Conditions on Pathogenic Phenotypes of Arcobacter butzleri. Microorganisms 2022, 10, 2409. https://doi.org/10.3390/microorganisms10122409
Martins R, Mateus C, Domingues F, Bücker R, Oleastro M, Ferreira S. Effect of Atmospheric Conditions on Pathogenic Phenotypes of Arcobacter butzleri. Microorganisms. 2022; 10(12):2409. https://doi.org/10.3390/microorganisms10122409
Chicago/Turabian StyleMartins, Rodrigo, Cristiana Mateus, Fernanda Domingues, Roland Bücker, Mónica Oleastro, and Susana Ferreira. 2022. "Effect of Atmospheric Conditions on Pathogenic Phenotypes of Arcobacter butzleri" Microorganisms 10, no. 12: 2409. https://doi.org/10.3390/microorganisms10122409
APA StyleMartins, R., Mateus, C., Domingues, F., Bücker, R., Oleastro, M., & Ferreira, S. (2022). Effect of Atmospheric Conditions on Pathogenic Phenotypes of Arcobacter butzleri. Microorganisms, 10(12), 2409. https://doi.org/10.3390/microorganisms10122409









