Virulence Factors in Colorectal Cancer Metagenomes and Association of Microbial Siderophores with Advanced Stages
Abstract
1. Introduction
2. Materials and Methods
2.1. Published Fecal Metagenomes
2.2. Detection of Virulence Factors (VFs)
2.3. Statistical Analysis
2.4. Functional Profiling of VFs, Pathway Analysis, and Association of VF’s Functions with Cancer Progression
3. Results
3.1. Published Fecal Metagenomes
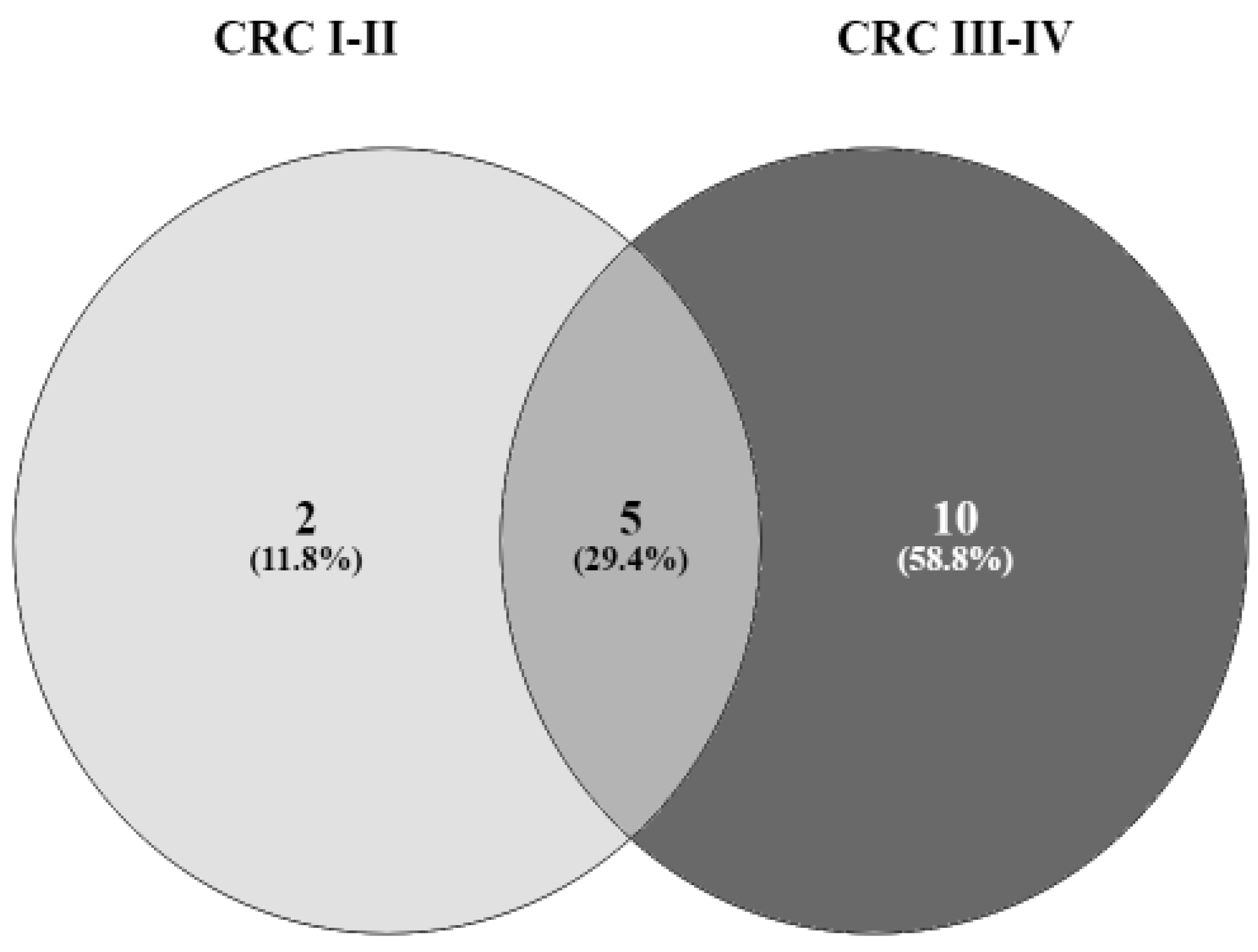
3.2. VFs as Markers of Cancer Progression: Bivariate Analysis
3.3. Multivariate Analysis
3.4. Functional Annotation of VFs
- -
- Cell adhesion in large adenoma, small adenoma, CRC I -II, and CRC III-IV;
- -
- Metabolic process and biosynthetic process in large adenoma, CRC I -II, and CRC III-IV;
- -
- DNA recombination, DNA integration, regulator activity, and cellular response to DNA damage stimulus in large adenoma and CRC III-IV;
- -
- Binding activity, the regulation of transcription, porin activity, transporters, and signal transduction in CRC I -II and CRC III-IV;
- -
- Pilus assembly in CRC I -II;
- -
- Response to an antibiotics, biofilm development, NAD metabolic process, viral entry into the host cell, homeostasis process, oxidoreductase activity, detection of virus, signaling receptor activity, mechanosensory behavior, ligase activity, protein phosphopantetheinylation, and host cell recognition only in CRC III-IV.
3.5. Pathway annotation of VFs
- -
- Pathways that include targeted VFs detected in all CRC stages: O-antigen nucleotide sugar biosynthesis, metabolic pathways, biosynthesis of secondary metabolites, biosynthesis of nucleotide sugars, biosynthesis of siderophore group nonribosomal peptides;
- -
- Pathways that include targeted VFs detected in CRC I-II: streptomycin biosynthesis and two-component system;
- -
- Pathways that include targeted VFs detected in CRC III-IV: ABC transporters, ubiquinone and terpenoid-quinone biosynthesis, a bacterial secretion system, pentose and glucuronate interconversions, ascorbate and aldarate metabolism, amino sugar and nucleotide sugar metabolism, the biosynthesis of cofactors, galactose metabolism, starch and sucrose metabolism, and microbial metabolism in diverse environments.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- International Agency for Research on Cancer. Colorectal Cancer. 2020. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/10_8_9-Colorectum-fact-sheet.pdf (accessed on 1 February 2022).
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Mármol, I.; Sánchez-De-Diego, C.; Pradilla Dieste, A.; Cerrada, E.; Rodriguez Yoldi, M. Colorectal carcinoma: A general overview and future perspectives in colorectal cancer. Int. J. Mol. Sci. 2017, 1, 197. [Google Scholar] [CrossRef] [PubMed]
- Al-Sohaily, S.; Biankin, A.; Leong, R.; Kohonen-Corish, M.; Warusavitarne, J. Molecular pathways in colorectal cancer. J. Gastroenterol. Hepatol. 2012, 27, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Kheirelseid, E.A.; Miller, N.; Kerin, M. Molecular biology of colorectal cancer: Review of the literature. Am. J. Mol. Biol. 2013, 3, 30688. [Google Scholar] [CrossRef]
- Amersi, F.; Agustin, M.; Ko, C.Y. Colorectal cancer: Epidemiology, risk factors, and health services. Clin. Colon Rectal Surg. 2005, 18, 133–140. [Google Scholar] [CrossRef]
- Giovannucci, E.; Rimm, E.B.; Stampfer, M.J.; Colditz, G.; Ascherio, A.; Willett, W.C. Intake of fat, meat, and fiber in relation to risk of colon cancer in men. Cancer Res. 1994, 54, 2390–2397. [Google Scholar]
- Jochem, C.; Leitzmann, M. Obesity and colorectal cancer. Obes. Cancer 2016, 208, 17–41. [Google Scholar] [CrossRef]
- Tayyem, R.F.; Shehadeh, I.N.; AbuMweis, S.S.; Bawadi, H.A.; Hammad, S.S.; Bani-Hani, K.E.; Al-Jaberi, T.M.; Alnusair, M.M. Physical inactivity, water intake and constipation as risk factors for colorectal cancer among adults in Jordan. APJCP 2013, 14, 5207–5212. [Google Scholar] [CrossRef]
- Liang, P.S.; Chen, T.Y.; Giovannucci, E. Cigarette smoking and colorectal cancer incidence and mortality: Systematic review and meta-analysis. IJC 2009, 124, 2406–2415. [Google Scholar] [CrossRef]
- Cheng, Y.; Ling, Z.; Li, L. The intestinal microbiota and colorectal cancer. Front. Immunol. 2020, 11, 615056. [Google Scholar] [CrossRef]
- Margulis, L. Words as battle cries: Symbiogenesis and the new field of endocytobiology. Bioscience 1990, 40, 673–677. [Google Scholar] [CrossRef]
- Cason, C.; D’Accolti, M.; Soffritti, I.; Mazzacane, S.; Comar, M.; Caselli, E. NGS and PCR technologies in monitoring the hospital microbiome and its drug resistance. Front. Microbiol. 2022, 13, 969863. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, J.; Yu, J.; Yao, Z.; Sun, L.; Shen, Y.; Jin, Q. VFDB: A reference database for bacterial virulence factors. Nucleic Acids Res. 2005, 33 (Suppl. S1), D325–D328. [Google Scholar] [CrossRef] [PubMed]
- Sun, J. Impact of bacterial infection and intestinal microbiome on colorectal cancer development. Chin. Med. J. 2022, 135, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Baron, S. Medical Microbiology; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 2013, 14, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Shin, J.; Zhang, G.; Cohen, M.; Franco, A.; Sears, C.L. The Bacteroides fragilis toxin binds to a specific intestinal epithelial cell receptor. Infect. Immun. 2006, 74, 5382–5390. [Google Scholar] [CrossRef]
- Fiorentini, C.; Carlini, F.; Germinario, E.A.P.; Maroccia, Z.; Travaglione, S.; Fabbri, A. Gut microbiota and Colon Cancer: A role for bacterial protein toxins? Int. J. Mol. Sci. 2020, 21, 6201. [Google Scholar] [CrossRef]
- Doye, A.; Mettouchi, A.; Bossis, G.; Clément, R.; Buisson-Touati, C.; Flatau, G.; Gagnoux, L.; Piechaczyk, M.; Boquet, P.; Lemichez, E. CNF1 exploits the ubiquitin-proteasome machinery to restrict Rho GTPase activation for bacterial host cell invasion. Cell 2002, 111, 553–564. [Google Scholar] [CrossRef]
- El-Aouar Filho, R.A.; Nicolas, A.; Castro, T.L.d.; Deplanche, M.; Azevedo, V.A.d.; Goossens, P.L.; Taieb, F.; Lina, G.; le Loir, Y.; Berkova, N. Heterogeneous family of cyclomodulins: Smart weapons that allow bacteria to hijack the eukaryotic cell cycle and promote infections. Front. Cell. Infect. 2017, 7, 208. [Google Scholar] [CrossRef]
- Zeller, G.; Tap, J.; Voigt, A.Y.; Sunagawa, S.; Kultima, J.R.; Costea, P.I.; Amiot, A.; Böhm, J.; Brunetti, F.; Habermann, N.; et al. Potential of fecal microbiota for early-stage detection of colorectal cancer. Mol. Syst. Biol. 2014, 10, 766. [Google Scholar] [CrossRef]
- Davis, J.J.; Wattam, A.R.; Aziz, R.K.; Brettin, T.; Butler, R.; Butler, R.M.; Stevens, R. The PATRIC Bioinformatics Resource Center: Expanding data and analysis capabilities. Nucleic Acids Res. 2020, 48, D606–D612. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Liao, C.; Taylor, B.P.; Fontana, E.; Amoretti, L.A.; Wright, R.J.; Xavier, J.B. A compilation of fecal microbiome shotgun metagenomics from hematopoietic cell transplantation patients. Sci. Data 2022, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Paczosa, M.K.; Mecsas, J. Klebsiella pneumoniae: Going on the offense with a strong defense. MMBR 2016, 80, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Zois, C.E.; Harris, A.L. Glycogen metabolism has a key role in the cancer microenvironment and provides new targets for cancer therapy. J. Mol. Med. 2016, 94, 137–154. [Google Scholar] [CrossRef]
- Pannen, D.; Fabisch, M.; Gausling, L.; Schnetz, K. Interaction of the RcsB response regulator with auxiliary transcription regulators in Escherichia coli. JBC 2016, 291, 2357–2370. [Google Scholar] [CrossRef]
- Bronowski, C.; Smith, S.L.; Yokota, K.; Corkill, J.E.; Martin, H.M.; Campbell, B.J.; Rhodes, J.M.; Hart, C.A.; Winstanley, C. A subset of mucosa-associated Escherichia coli isolates from patients with colon cancer, but not Crohn’s disease, share pathogenicity islands with urinary pathogenic E. coli. Microbiology 2008, 154, 571–583. [Google Scholar] [CrossRef]
- Saha, S.; Yao, S.; Elakad, O.; Lois, A.; Henric-Petri, H.; Buentzel, J.; Hinterthaner, M.; Danner, B.C.; Ströbel, P.; Emmert, A.; et al. UDP-glucose 6-dehydrogenase expression as a predictor of survival in patients with pulmonary adenocarcinoma. IJS Oncol. 2020, 5, e85. [Google Scholar] [CrossRef]
- Pasi, S.; Kant, R.; Surolia, A. Toll/interleukin-1 receptor domain derived from TcpC (TIR-TcpC) ameliorates experimental autoimmune arthritis by down-modulating Th17 cell response. JBC 2016, 291, 12358–12369. [Google Scholar] [CrossRef]
- Marsden, A.E.; Intile, P.J.; Schulmeyer, K.H.; Simmons-Patterson, E.R.; Urbanowski, M.L.; Wolfgang, M.C.; Yahr, T.L. Vfr directly activates exsA transcription to regulate expression of the Pseudomonas aeruginosa type III secretion system. J. Bacteriol. Res. 2016, 198, 1442–1450. [Google Scholar] [CrossRef]
- Bulir, D.C.; Waltho, D.A.; Stone, C.B.; Mwawasi, K.A.; Nelson, J.C.; Mahony, J.B. Chlamydia pneumoniae CopD translocator protein plays a critical role in type III secretion (T3S) and infection. PLoS ONE 2014, 9, e99315. [Google Scholar] [CrossRef]
- Wilson, M.M.; Anderson, D.E.; Bernstein, H.D. Analysis of the outer membrane proteome and secretome of Bacteroides fragilis reveals a multiplicity of secretion mechanisms. PLoS ONE 2015, 10, e0117732. [Google Scholar] [CrossRef]
- Allali, I.; Boukhatem, N.; Bouguenouch, L.; Hardi, H.; Boudouaya, H.A.; Cadenas, M.B.; Ouldim, K.; Amzazi, S.; Azcarate-Peril, M.A.; Ghazal, H. Gut microbiome of Moroccan colorectal cancer patients. Med. Microbiol. 2018, 207, 211–225. [Google Scholar] [CrossRef]
- Saha, P.; Yeoh, B.S.; Xiao, X.; Golonka, R.M.; Abokor, A.A.; Wenceslau, C.F.; Shah, Y.M.; Joe, B.; Vijay-Kumar, M. Enterobactin induces the chemokine, interleukin-8, from intestinal epithelia by chelating intracellular iron. Gut Microbes 2020, 12, 1841548. [Google Scholar] [CrossRef]
- Galardini, M.; Clermont, O.; Baron, A.; Busby, B.; Dion, S.; Schubert, S.; Beltrao, P.; Denamur, E. Major role of iron uptake systems in the intrinsic extra-intestinal virulence of the genus Escherichia revealed by a genome-wide association study. PLoS Genet. 2020, 16, e1009065. [Google Scholar] [CrossRef]
- Basak, D.; Uddin, M.N.; Hancock, J. The role of oxidative stress and its counteractive utility in colorectal cancer (CRC). Cancers 2020, 12, 3336. [Google Scholar] [CrossRef]
- Allsopp, L.P.; Bernal, P.; Nolan, L.M.; Filloux, A. Causalities of war: The connection between type VI secretion system and microbiota. Cell. Microbiol. 2020, 22, e13153. [Google Scholar] [CrossRef]
- Fabbri, A.; Travaglione, S.; Rosadi, F.; Ballan, G.; Maroccia, Z.; Giambenedetti, M.; Guidotti, M.; Ødum, N.; Krejsgaard, T.; Fiorentini, C. The Escherichia coli protein toxin cytotoxic necrotizing factor 1 induces epithelial mesenchymal transition. Cell. Microbiol. 2020, 22, e13138. [Google Scholar] [CrossRef]
- Bernacki, R.J.; Niedbala, M.J.; Korytnyk, W. Glycosidases in cancer and invasion. Cancer Metastasis Rev. 1985, 4, 81–101. [Google Scholar] [CrossRef]
- Oliero, M.; Calvé, A.; Fragoso, G.; Cuisiniere, T.; Hajjar, R.; Dobrindt, U.; Santos, M.M. Oligosaccharides increase the genotoxic effect of colibactin produced by pks+ Escherichia coli strains. BMC Cancer 2021, 21, 1–10. [Google Scholar] [CrossRef]
- Dalmasso, G.; Cougnoux, A.; Delmas, J.; Darfeuille-Michaud, A.; Bonnet, R. The bacterial genotoxin colibactin promotes colon tumor growth by modifying the tumor microenvironment. Gut Microbes 2014, 5, 675–680. [Google Scholar] [CrossRef]
- Jamet, A.; Dervyn, R.; Lapaque, N.; Bugli, F.; Perez-Cortez, N.G.; Blottière, H.M.; Twizere, J.-C.; Sanguinetti, M.; Posteraro, B.; Serror, P.; et al. The Enterococcus faecalis virulence factor ElrA interacts with the human Four-and-a-Half LIM Domains Protein 2. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Liu, C.; Li, Z.; Ding, J.; Zhen, H.; Fang, M.; Nie, C. Species-Level Analysis of the Human Gut Microbiome Shows Antibiotic Resistance Genes Associated with Colorectal Cancer. Front. Microbiol. 2021, 12, 765291. [Google Scholar] [CrossRef] [PubMed]
- Dejea, C.M.; Wick, E.C.; Hechenbleikner, E.M.; White, J.R.; Welch, J.L.M.; Rossetti, B.J.; Peterson, S.N.; Snesrud, E.C.; Borisy, G.G.; Lazarev, M.; et al. Microbiota organization is a distinct feature of proximal colorectal cancers. Proc. Natl. Acad. Sci. USA 2014, 111, 18321–18326. [Google Scholar] [CrossRef] [PubMed]
- Ellermann, M.; Arthur, J.C. Siderophore-mediated iron acquisition and modulation of host-bacterial interactions. Free. Radic. Biol. Med. 2017, 105, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.; Hudspeth, M.; Meganathan, R. Anaerobic biosynthesis of enterobactin Escherichia coli: Regulation of entC gene expression and evidence against its involvement in menaquinone (vitamin K2) biosynthesis. J. Bacteriol. 1996, 178, 3252–3259. [Google Scholar] [CrossRef][Green Version]
- Golonka, R.; Yeoh, B.S.; Vijay-Kumar, M. The iron tug-of-war between bacterial siderophores and innate immunity. J. Innate Immun. 2019, 11, 249–262. [Google Scholar] [CrossRef]
- Aksan, A.; Farrag, K.; Aksan, S.; Schroeder, O.; Stein, J. Flipside of the coin: Iron deficiency and colorectal cancer. Front. Immunol. 2021, 12, 644. [Google Scholar] [CrossRef]
- Fu, D.; Richardson, D.R. Iron chelation and regulation of the cell cycle: 2 mechanisms of posttranscriptional regulation of the universal cyclin-dependent kinase inhibitor p21CIP1/WAF1 by iron depletion. Am. J. Hematol. 2007, 110, 752–761. [Google Scholar] [CrossRef]

| Coefficients a | |||||
|---|---|---|---|---|---|
| Model | Unstandardized Coefficients | Standardized Coefficients | T | Sig. | |
| B | Std. Error | Beta | |||
| (Constant) | −0.005 | 0.008 | −0.550 | 0.584 | |
| Type 1 Fimbriae Regulatory protein FimB | 0.014 | 0.057 | 0.014 | 0.239 | 0.812 |
| Type 1 Fimbriae Regulatory protein FimE | 0.017 | 0.040 | 0.017 | 0.425 | 0.672 |
| Type-1 Fimbrial protein, A chain precursor | −0.081 | 0.032 | −0.080 | −2.510 | 0.014 |
| iron-enterobactin ABC transporter permease | 0.501 | 0.053 | 0.505 | 9.498 | 0.000 |
| phosphopantetheinyl transferase component of enterobactin synthase multienzyme complex | −0.072 | 0.034 | −0.075 | −2.139 | 0.036 |
| FimG protein precursor | 0.067 | 0.030 | 0.069 | 2.211 | 0.030 |
| ferrienterobactin ABC transporter periplasmic binding protein | 0.041 | 0.038 | 0.042 | 1.092 | 0.278 |
| E. coli common pilus usher EcpC | 0.354 | 0.060 | 0.351 | 5.881 | 0.000 |
| enterobactin/ferric enterobactin esterase | −0.038 | 0.081 | −0.039 | −0.468 | 0.641 |
| enterobactin exporter, iron-regulated | 0.213 | 0.054 | 0.216 | 3.910 | 0.000 |
| general secretion pathway protein D | 0.078 | 0.035 | 0.074 | 2.207 | 0.030 |
| general secretion pathway protein K | −0.111 | 0.035 | −0.109 | −3.146 | 0.002 |
| aerobactin synthesis protein IucB | 0.020 | 0.022 | 0.015 | 0.879 | 0.382 |
| Early Stage CRC | Advance Stage CRC | |
|---|---|---|
| Functions of Virulence factors |
|
|
| Pathway of virulence factor |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathlouthi, N.E.H.; Kriaa, A.; Keskes, L.A.; Rhimi, M.; Gdoura, R. Virulence Factors in Colorectal Cancer Metagenomes and Association of Microbial Siderophores with Advanced Stages. Microorganisms 2022, 10, 2365. https://doi.org/10.3390/microorganisms10122365
Mathlouthi NEH, Kriaa A, Keskes LA, Rhimi M, Gdoura R. Virulence Factors in Colorectal Cancer Metagenomes and Association of Microbial Siderophores with Advanced Stages. Microorganisms. 2022; 10(12):2365. https://doi.org/10.3390/microorganisms10122365
Chicago/Turabian StyleMathlouthi, Nour El Houda, Aicha Kriaa, Leila Ammar Keskes, Moez Rhimi, and Radhouane Gdoura. 2022. "Virulence Factors in Colorectal Cancer Metagenomes and Association of Microbial Siderophores with Advanced Stages" Microorganisms 10, no. 12: 2365. https://doi.org/10.3390/microorganisms10122365
APA StyleMathlouthi, N. E. H., Kriaa, A., Keskes, L. A., Rhimi, M., & Gdoura, R. (2022). Virulence Factors in Colorectal Cancer Metagenomes and Association of Microbial Siderophores with Advanced Stages. Microorganisms, 10(12), 2365. https://doi.org/10.3390/microorganisms10122365








