Abstract
(1) Background: Hyaluronic acid (HA) is a polyanionic mucopolysaccharide extensively used in biomedical and cosmetic industries due to its unique rheological properties. Recombinant HA production using other microbial platforms has received increasing interest to avoid potential toxin contamination associated with its production by streptococcal fermentation. In this study, the Gram-negative strains Escherichia coli (pLysY/Iq), E. coli Rosetta2, E. coli Rosetta (DE3) pLysS, E. coli Rosetta2 (DE3), E. coli Rosetta gammiB(DE3)pLysS, and the Gram-positive Bacillus megaterium (MS941) were investigated as new platforms for the heterologous production of HA. (2) Results: The HA biosynthesis gene hasA, cloned from Streptococcus equi subsp. zoopedemicus, was ligated into plasmid pMM1522 (MoBiTec), resulting in pMM1522 hasA, which was introduced into E. coli Rosetta-2(DE3) and B. megaterium (MS941). The initial HA titer by the two hosts in the LB medium was 5 mg/L and 50 mg/L, respectively. Streptococcal hasABC and hasABCDE genes were ligated into plasmid pPT7 (MoBiTec) and different E. coli host strains were then transformed with the resulting plasmids pPT7hasABC and pPT7hasABCDE. For E. coli Rosetta-gamiB(DE3)pLysS transformed with pPT7hasABC, HA production was 500 ± 11.4 mg/L in terrific broth (TB) medium. Productivity was slightly higher (585 ± 2.9 mg/L) when the same host was transformed with pPT7 carrying the entire HA operon. We also transformed B. megaterium (MS941) protoplasts carrying T7-RNAP with pPT7hasABC and pPT7hasABCDE. In comparison, the former plasmid resulted in HA titers of 2116.7 ± 44 and 1988.3 ± 19.6 mg/L in LB media supplemented with 5% sucrose and A5 medium + MOPSO, respectively; the latter plasmid boosted the titer final concentration further to reach 2476.7 ± 14.5 mg/L and 2350 ± 28.8 mg/L in the two media, respectively. The molecular mass of representative HA samples ranged from 105 − 106 Daltons (Da), and the polydispersity index (PDI) was <2. Fourier transform infrared spectroscopy (FTIR) spectra of the HA product were identical to those obtained for commercially available standard polymers. Finally, scanning electron microscopic examination revealed the presence of extensive HA capsules in E. coli Rosetta-gamiB(DE3)pLysS, while no HA capsules were produced by B. megaterium. (3) Conclusions: Our results suggested that Gram-positive bacteria are probably superior host strains for recombinant HA production over their Gram-negative counters. The titers and the molecular weight (MW) of HA produced by B. megaterium were significantly higher than those obtained by different E. coli host strains used in this study.
1. Introduction
HA is a linear mucopolysaccharide with a polyanionic nature and consists of disaccharide repeats of D-glucuronic acid (GlcUA), and N-acetylglucosamine (GlcNAc) joined alternatively by β-1,3 and β-1,4 glycosidic bonds [1,2,3]. Due to its excellent water-binding capacity and viscoelasticity, HA possesses unique physicochemical and rheological properties. Accordingly, HA has been used in many biomedical applications, including chondro-protection, orthopedic surgery, visco-supplementation, anti-adhesion therapy, dermatology, wound-healing in ophthalmology, tissue regeneration in the cardiovascular system, and anti-aging cosmetics [3,4,5,6,7,8]. In addition, HA has been used as a chemotherapeutic drug carrier in conjugated complexes. The HA-Paclitaxel (PTX) antitumor conjugate is an example in which HA provides the dual advantage of accumulation at the tumor site and a receptor-mediated uptake [3,9,10]. HA can also be coupled with fibrin in hydrogel to function as a delivery system for mesenchymal stem-cell injection [11].
Currently, there are two competing methods for the industrial production of HA including the extraction from animal sources, such as bovine eyes and rooster combs, and the large-scale expression in microbial host strains, e.g., in Streptococcus [7,12,13]. HA extracted from animal sources suffers from two significant limitations. First, in animal tissues, HA is complexed with proteoglycans and often contaminated with HA-degrading enzymes, making isolating high-purity and large-molecular-size polysaccharides difficult and costly [14]. Second, using animal-derived biomolecules for biopharmaceutical applications is facing growing concerns because of the risk of cross-species viral and other adventitious agent contaminations. The FDA has not approved HA production from animal-derived sources for these reasons. Since the 1980s, recombinant HA microbial production has gradually replaced extraction from animal tissues [2,14]. The commonly used strain for HA production is Streptococcus equi subsp. zooepidemicus [7,11,15]. In these bacteria, the HA capsule is a virulence factor, which contributes to the pathogenicity, probably providing an unknown immunological response that fails the immune system to recognize the HA capsule as a foreign entity [16,17]. However, HA production by Streptococcus equi subsp. zooepidemicus is facing some drawbacks. First, lactic acid is the main byproduct of HA fermentation, and its accumulation causes potent inhibition of cell growth and HA synthesis. Second, fastidious microorganisms require high-cost media from animal origins for growth and fermentation [18,19,20]. Furthermore, HA fermentation by the streptococcal source is frequently accompanied by endotoxin contamination, a restriction that limits the application of the HA product in the biomedical field. Accordingly, recombinant HA production from other microbial platforms has been investigated as an alternative to avoid the safety concerns mentioned above [5,7].
Several reports focused on recombinant HA production with E. coli [7,21,22,23,24,25] and Agrobacterium sp. [7,26], with HA titers amounting to 3.8 and 0.3 g/L, respectively. E. coli-Rosetta host strains (Novagen) allowed researchers to improve low-protein-expression titers caused by codon-usage bias [27].
In addition, the Gram-positive HA (to 6.8 g/L) produced is generally recognized as safe for the GRAS bacterium, Bacillus subtilis [28,29], and also for Corynebacterium glutamicum [30,31]. Recently, Bacillus megaterium was also established as an industrially recognized host. It is non-pathogenic, does not possess any alkaline proteases degrading recombinant gene products, can replicate stably, and can maintain recombinant plasmids even without antibiotic selection [32,33,34]. B. megaterium efficiently secretes proteins directly into the culture medium; in some cases, the recombinant protein makes up 30% of the total soluble protein [32,34]. Consequently, B. megaterium was used to produce several alpha and beta amylases, which are used for starch modification in the baking industry [33,34], along with penicillin acylase [34,35,36,37] and in aerobic and anaerobic production of vitamin B12 [38,39].
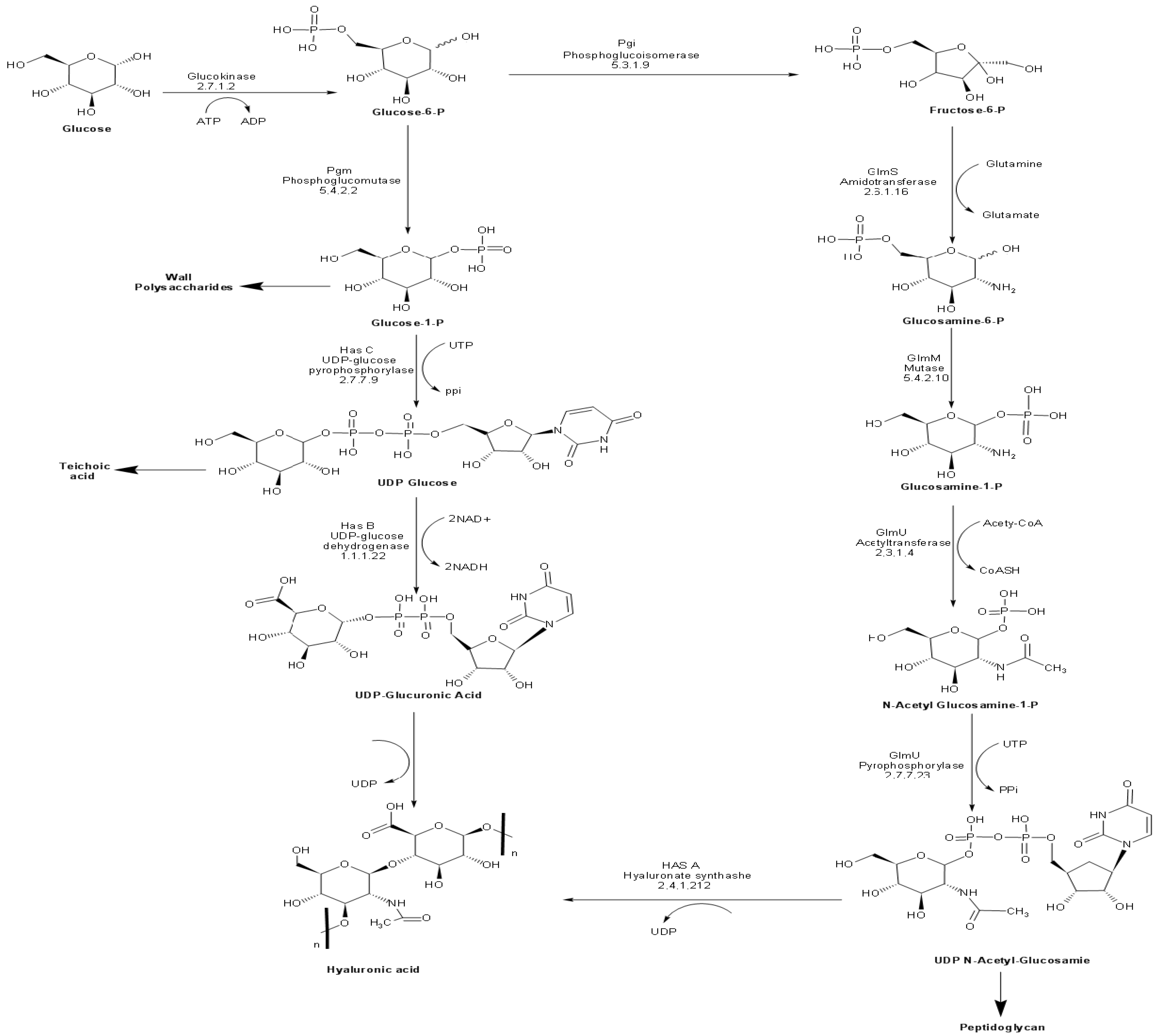
Ten enzymes are involved in the HA biosynthetic pathway from glucose (Figure 1). Most of these enzymes possess overlapping functions at the interface between catabolic pathways (glycolysis and pentose phosphate pathway), anabolic production of structural polysaccharides (peptidoglycan and teichoic acid), and the extracellular matrix (HA). Accordingly, it is no surprise to find homologs of most of these enzymes in bacterial species, which do not naturally produce HA. The unique enzyme to HA biosynthetic pathway is the plasma membrane-bound enzyme hyaluronan synthase (HasA), encoded by the hasA gene. HasA is an integral membrane protein responsible for catalyzing the polymerization of UDP-glucuronic acid and N-acetyl glucosamine and the simultaneous translocation of the growing HA chain across the membrane [40,41].
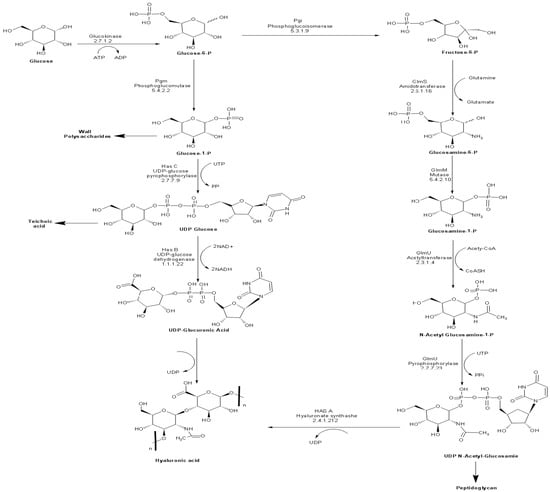
Figure 1.
Biosynthetic pathway of HA in S. equi subsp. zooepidemicus.
In this study, we investigated the applicability of four E. coli strains (pLysY/Iq, Rosetta2, Rosetta2 (DE3) pLysS, Rosetta-gami B (DE3)pLysS) and B. megaterium (MS941) as potential platforms for the heterologous production of HA. Episomal expression systems were constructed for hasA along with other genes in the operon involved in the HA biosynthetic pathway. For all host strains, HA production was confirmed by Fourier transform infrared spectroscopy (FTIR), its titer was monitored in different culture media by turbidimetric assay, and its molecular weight was characterized by gel permeation chromatography. Selected recombinant strains were also examined by scanning electron microscopy (SEM).
2. Materials and Methods
Following the materials and methods shown in (Figure S1), all chemicals were purchased from Sigma-Aldrich (Labor Chemie GmbH, Steinheim, Germany) or VWR International GmbH (Darmstadt, Germany). Genomic DNA digestion, ligation, and transformation procedures were isolated using standard techniques [42]. Plasmid and PCR purification kits were purchased from Qiagen (Hilden, Germany). Restriction enzymes and T4 DNA-ligase were purchased from New England Biolabs (Frankfurt am Main, Germany). Oligonucleotides were synthesized by Eurofins MWG (Ebersberg, Germany). Phusion Hot Start DNA polymerase was purchased from Thermo Scientific (Dreieich, Germany).
2.1. Bacterial Strains, Plasmids Media, and Culture Conditions
E. coli strains (Table S1) were cultivated in LB medium [43], while plasmid-harboring E.coli strains were grown in triplicates in 50 mL LB medium containing 1% glucose, super optimal broth with catabolite repression (SOC) [44] or TB media [45], supplemented with 100 mg ampicillin/L and 34 mg chloramphenicol/L. Bacterial cultures were grown at 37 °C until an optical density at 600 nm (OD600) = 1 was reached, and gene expression was induced by adding β-D-1-thiogalactopyranoside (IPTG) at a final concentration of 0.5 mM in addition to 10 mM MgCl2, 2.5 g K2HpO4/L and 1 g sorbitol/L. Cultures were incubated at 37 °C or 30 °C for 48 h after induction under shaking at 170 rpm in an orbital shaker.
B. megaterium MS941 and B. megaterium MS941 pre-transformed with T7-RNAP (Mobitec, Munich, Germany) were grown aerobically in either complex or semi-defined media. Complex growth media were either LB-medium supplemented with 1%, 3%, or 5% glucose, sucrose, or TB medium [46]. Semi-defined media were either A5 medium, A5 medium + 4 or A5 medium + MOPSO [37]. For antibiotic selection, media were supplemented with 4.5 mg chloramphenicol/L and 10 mg tetracycline/L. Bacterial cultures were induced at an OD578 = 1 by adding 0.25–0.5% xylose in addition to 10 mM MgCl2, 2.5 g K2HpO4/L, and 1 g sorbitol/L, and then the cultures were further incubated at 25 °C for 48 h under shaking at 170 rpm. Genomic DNA was extracted from S. equi subsp. zooepidemicus ATCC 39920 pre-grown in BHI medium (Oxoid).
2.2. Plasmid Construction
Genomic DNA was extracted from Streptococcus equi subsp. zoopedemicus ATCC 39920 (pre-grown in BHI medium from Oxoid) using the Genomic DNA extraction kit (Qiagen). hasA, hasABC, and the entire operon hasABCDE were PCR-amplified using Phusion polymerase (Thermo Scientific). The primers are listed in Table S2. DNA was digested using restriction endonucleases purchased from New England BioLabs (NEB; Ipswich; USA) or Thermo Fisher Scientific Inc., Rockford, IL, USA.
PCR reactions of a total volume of 20 μL were prepared to amplify the DNA of interest. For the amplification of DNA fragments more prominent than 1000 bp, the PhusionTM polymerase (Finnzymes; Espoo; Finland) was used for colony PCR, Taq DNA polymerase (BioTherm®; genecraft; Lüdinghausen; Germany) was used. The Phusion polymerase has a proofreading function that avoids mistakes during amplification. The Taq DNA polymerase is known to create one mismatch in 1000 base pairs.
The ‘5’ phosphate groups of the linearized vector were removed before the ligation reaction to avoid the re-circularization of a previously digested DNA vector. The dephosphorylation was achieved by adding 1 unit of calf intestinal alkaline phosphatase (CIP; New England BioLabs; Ipswich; USA) per μg of DNA to the sample immediately after restriction. Incubation at 37 °C for 3 h followed. DNA was purified using the PCR purification kit (Qiagen; Hilden; Germany) following the manufacturer’s instructions. In one ligation reaction, 25–200 ng of plasmid DNA was used. Insert DNA was added in excess (insert-to-vector ratio concerning molar concentrations of 2: 1 to 10: 1), and a reaction buffer 1:10 of the total volume and 200 U of T4 DNA ligase were added to a final volume of 20 μL (New England BioLabs; Ipswich; MA; USA).
hasA and hasABC were cloned into plasmid pJet1.2 (Qiagen) to form pJethasA and pJethasABC, respectively. hasA was subcloned into plasmid pMM1522 to generate plasmid pMM1522hasA, and hasABC was ligated into pPT7 to construct plasmid pPT7hasABC. Finally, the entire operon was cloned into pPT7 to generate plasmid pPT7hasABCDE. Sequences of all plasmid constructs, Figure S2, were verified using Sanger’s chain-termination-sequencing method.
2.3. Bacterial Transformation
Competent E. coli cells were prepared and transformed using standard techniques [28]. Competent B. megaterium MS941 protoplasts were purchased from MoBiTec GmbH; Göttingen, Germany and transformed according to the manufacturer’s guidelines.
For both E. coli and B. megaterium, only colonies with stable mucoid consistency over several generations were used in HA production (Figure S1).
2.4. Growth Experiments and Induction of HA Production
All growth experiments were performed using 50 mL culture medium in 250 mL baffled (1 cm throw) Erlenmeyer flasks in triplicates. HA production by E. coli cultures was induced by adding 1 mM–0.05 mM IPTG, while for B. megaterium, induction was carried out by adding 0.5–0.1% xylose. In addition to the inducer, 10 mM MgCl2, 1 g sorbitol/L, and 2.5 g K2HPO4/L were added to all culture media [23]. HA was quantified in 1 mL samples withdrawn during the exponential growth phase at different time intervals after induction.
2.5. HA Extraction and Quantification in Cell-Free Culture Media
We followed the previously described HA extraction protocol [37]. In brief, 1 mL culture samples were taken at different time intervals in 15 mL Falcon tubes, fivefold diluted with sterile distilled water, and incubated for 10 min with 0.1% sodium dodecyl sulfate (SDS) before being centrifuged at 3000× g for 20 min. Supernatants were filtered through 0.22 μm membrane filters to ensure that no bacterial cells were left with 1 M NaCl before adding 2.5 volumes of 100% ethanol. The mixtures were then centrifuged again at 10,000× g for 10 min. The clear white HA precipitate was separated and air-dried overnight at room temperature. HA was quantified using the turbidimetric assay [47,48].
2.6. Quantification and Characterization of Recombinant HA
For the quantification of HA, a turbidimetric assay was used in which HA formed a complex with CTAB (cetyl-trimethyl-ammonium bromide). The results were not significantly different from those obtained using an ELISA assay (see below). The CTAB assay was recommended due to its robustness, high specificity, and simplicity, and it was suggested to be a superior substitute to the colorimetric carbazole assay, which requires further purification before HA quantification [49].
To test the sensitivity of the assay, we compared the obtained HA levels with those determined in random samples assayed for HA using ELISA (Corgenix, Broomfield, CO, USA), which utilizes an HA-binding protein (HABP) [47,48]. In the ELISA assay, properly diluted HA samples or HA reference solutions were incubated in HABP-coated micro-wells, permitting HA present in the samples to react with immobilized binding protein (HABP). After removing unbound serum molecules by washing, HABP conjugated with horseradish peroxide (HRP) solution was added to the micro-wells to form complexes with bound HA. After another washing step, a chromogenic substrate of tetramethylbenzidine and hydrogen peroxide was added to develop a colored reaction, the intensity of which was measured at 450 nm.
2.7. HA Purification for Molecular Weight Determination and FTIR Analysis
Forty-eight hours after induction, cells were separated from the media by centrifugation at 10,000× g for 15 min. The clarified spent media were extracted in CHCl3 to deproteinize the mixture before quaternary amine detergent precipitation (1% cetylpyridinium chloride [CPC]), and the resulting pellets were washed in water, re-dissolved in 1 M NaCl, the solution was clarified by centrifugation, and the polymer was precipitated by the addition of 2.5 volumes of absolute ethanol. The washing and ethanol-precipitation steps were repeated twice, and the final pellets were then re-dissolved in water, treated with DNase and RNase (1 µg/mL final) for 1 h, then extracted again with CHCl3 [49]. The aqueous phase was harvested and eluted with 500 µL Milli-Q water system (Millipore) in an Amicon column (MW cut off >10,000 Da) and was centrifuged at 7000 rpm for 20 min to remove traces of CPC and protein. The solution left above was used for molecular weight determination.
2.8. Molecular Weight Determination of HA
The molecular weight of HA was determined using a Waters 515/2410 Gel Permeation Chromatograph (GPC, Waters, Milford, MA, USA) with an ultra-hydrogel column calibrated with polyethylene glycol standards and a series 2410 refractive index detector. Mobile phase: water, sodium nitrate (0.10 M). Solvent: water, sodium azide 0.05%; flow rate: 1 mL/ min; temperature: 25 °C.
2.9. Scanning Electron Microscopy of HA Capsules
Dried bacterial cultures were processed by suspending them in 3% glutaraldehyde of 0.1 M phosphate buffer for 1 h, pH 7.2. After washing three times, post-fixing was conducted in 1% osmium tetroxide (in H2O) for 1 h. Afterward, the cells were dehydrated in 30%, 50%, 75%, 90%, and 2 × 100% for 5 min each. A 0.2-micron polycarbonate filter was used for purifying the dried bacteria, sucked by a vacuum pump in 100% ethanol [50]. Dried bacterial cultures were coated with metal stubs of the thin layer of heavy metal to increase the secondary electron signal (usually gold or gold palladium). The dried bacterial cultures were imaged in a JCM-5700 Scanning Electron Microscope (JEOL USA, Peabody, MA, USA), equipped with a mobile biological containment (Dycor Technologies Ltd., Edmonton, AB, Canada). Images of the dried bacterial cultures were taken after they had been processed under high vacuum at 6 kV, with an 8 mm working distance and a 30 μm objective lens aperture. A secondary electron detector was the tool used to collect the images, with which the acquisition time per image was 160 s, and each image was 2560 × 1920 pixels. Images of the ionic-liquid-stained samples were obtained using the above-noted settings, except that the acceleration voltage was adjusted to 4 kV. SEM images were recorded at magnifications ranging from 3000× to 20,000×.
2.10. Characterization of HA Using FTIR
The characterization of representative HA samples [51] was performed using FTIR spectrophotometry (Jasco, Hachioji, Japan), and the spectra were compared to a standard sample of HA (Sigma Aldrich Chemicals, Burlington, MA, USA) between 4000–400 cm−1 (2.5–25 μm). All organic molecules can absorb FTIR radiation between 4000 cm−1 and 400 cm−1, corresponding to energy absorption between 11 kcal/M and 1 kcal/M. This amount of energy initiates transitions between vibrational states of bonds contained within the molecule.
2.11. Data Processing and Statistical Analysis
Statistical analysis was performed using the statistical program SPSS V.17 [52]. Data are represented as means ± SEM. To compare differences between groups’ odds ratios, a nonparametric Student’s t-test (Mann–Whitney), Wilcoxon signed-rank test, and nonparametric one-way ANOVA (Kruskal–Wallis) were used. A two-tailed p-value ≤ 0.05 was considered statistically significant in all statistical tests.
3. Results
3.1. HA Production with E. coli
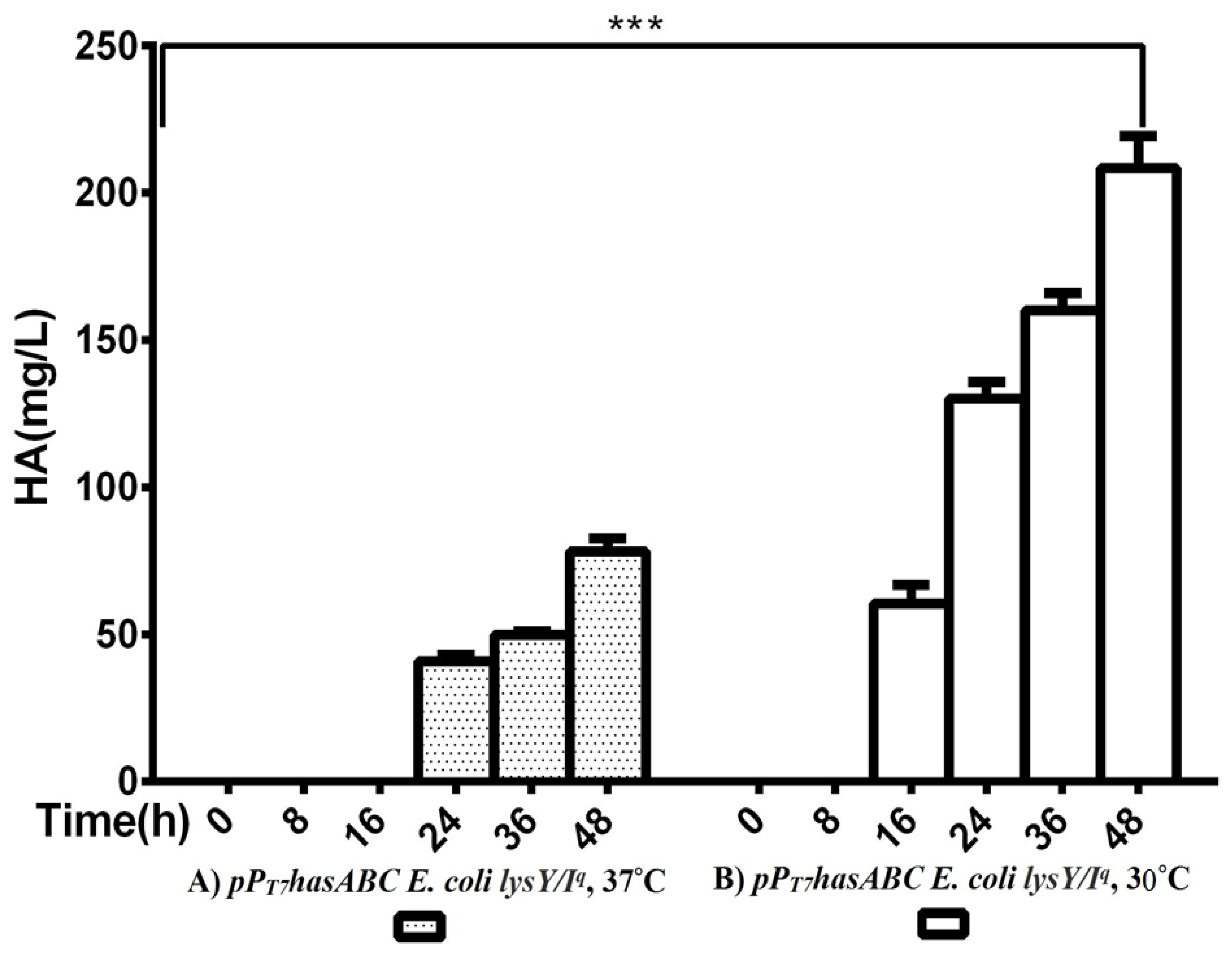
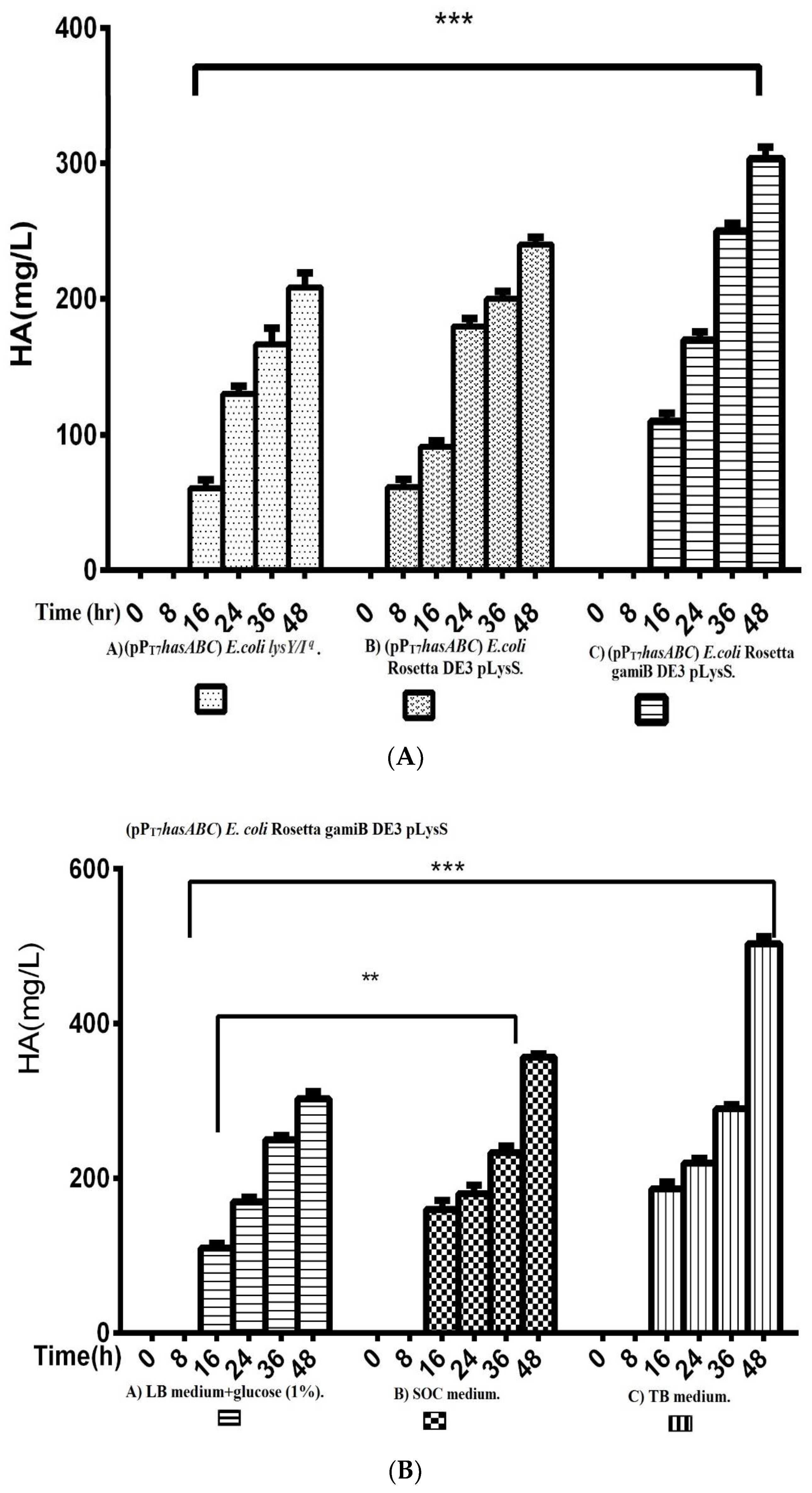
The hasA gene from Streptococcus equi subsp. zooepidemicus ATCC 39920 was amplified and ligated into plasmid pMM1522 under the control of a xylose-inducible promoter. E. coli Rosetta2 was transformed with the resulting plasmid pMMhasA, and the most HA-producing colony of a stable mucoid consistency was chosen. Unexpectedly, HA production by this strain did not exceed 8.8 ± 0.8 mg/L after 48 h of incubation at 37 °C in an LB medium. Accordingly, we explored the strategy of cloning hasABC, and the entire has operon hasABCDE under the control of the firm and tightly regulated T7 promoter. We used E. coli INV alpha F’, which is known to harbor a soft copy of the foreign plasmid [53] for hasABC and hasABCDE cloning; this, together with the addition of glucose (1%) to the growth medium, seems to completely shut off the leaky expression of the hasABC and overcome potential gene toxicity. hasABC from S. equi subsp. zooepidemicus was successfully ligated into plasmid pPT7, resulting in plasmid pPT7hasABC. E. coli LysY/Iq cells being transformed with pPT7hasABC. This E. coli strain carries the T7 RNA polymerase gene under the control of LysY, a variant of T7 lysozyme lacking amidase activity; thus, cells are less susceptible to lysis during induction, according to the manufacturer [54]. The plate-assay method was used to choose the mucoid colony that grew the most in the presence of IPTG and ampicillin. At 1 mM IPTG, the growth was prolonged, and the HA titer was very low. By reducing the IPTG concentration to 0.5 mM and supplementing the LB medium with 10 mM MgCl2, 2.5 g K2HPO4/L, and 1 g sorbitol/L, the HA titer was 78.3 ± 4.4 mg/L after 48 h at 37 °C, which was still lower than the HA concentrations that were reported earlier [19,33]. After induction, the titer of the polymer increased to 208.3 ± 10.9 mg/L after 48 h with a temperature of 30 °C (Figure 2). Therefore, since the hasABC gene was introduced into E. coli lysY/Iq cells was not codon-optimized, we attributed the low HA productivity by this host strain to a bias in codon usage. Accordingly, we tested the use of the E. coli strains Rosetta2 (DE3) plysS and Rosetta-gami B (DE3)pLysS, which both enhanced the expression of heterologous (non-codon-optimized) genes. Both strains were grown in LB media supplemented with 1% glucose, and the HA titer was determined at different time points (Figure 3A). Under these conditions, Rosetta-gami B(DE3)pLysS exhibited a slightly higher HA titer, probably due to enhanced disulfide bond formation and improved protein folding [54,55]. Further improvements were attained when SOC and TB media were used after 48 h of incubation, resulting in HA concentrations of 346.7 ± 3.3 mg/L and 500 ± 11.4 mg/L, respectively (p < 0.001; Figure 3B). No detectable effect was observed in our study when lysozyme was applied at a concentration of 500 U/mL, as recommended for Streptococcus [37]. The lysozyme encoding plasmid pLysS in Rosetta-gamiB cells as a control system to prevent leaky expression reported for T7 promoter systems has led to further suppression of the HA expression. In this respect, adding lysozyme to the TB medium has led to declining HA production compared to the TB medium.
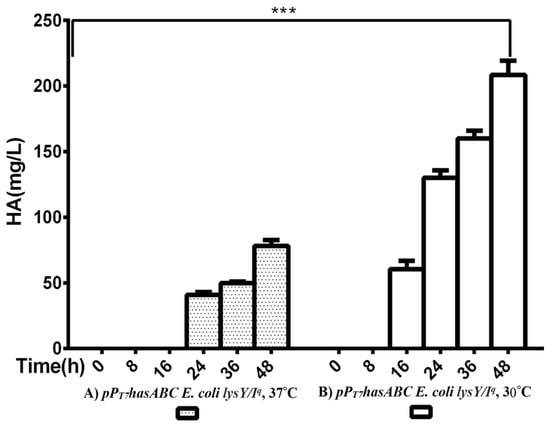
Figure 2.
Comparison of HA productivity between pPT7hasABC E. coli lysY/Iq at (A) 37 °C and at (B) 30 °C, (***): p ˂ 0.0001.
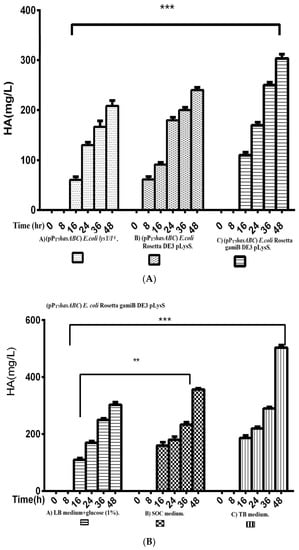
Figure 3.
Comparison of HA productivity presented by (Figure 3A); A) (pPT7hasABC) E.colilysY/Iq, B) Rosetta DE3 pLysS, and C) Rosetta gamiB DE3 pLysS, (***): p ˂ 0.0001. (Figure 3B) Comparison of HA productivity by E. coli Rosetta gamiB DE3 pLysS transformed with (pPT7hasABC) in A) LB medium + glucose (1%), B) SOC medium, and C) TB medium, (**): p < 0.001, (***): p ˂ 0.0001.
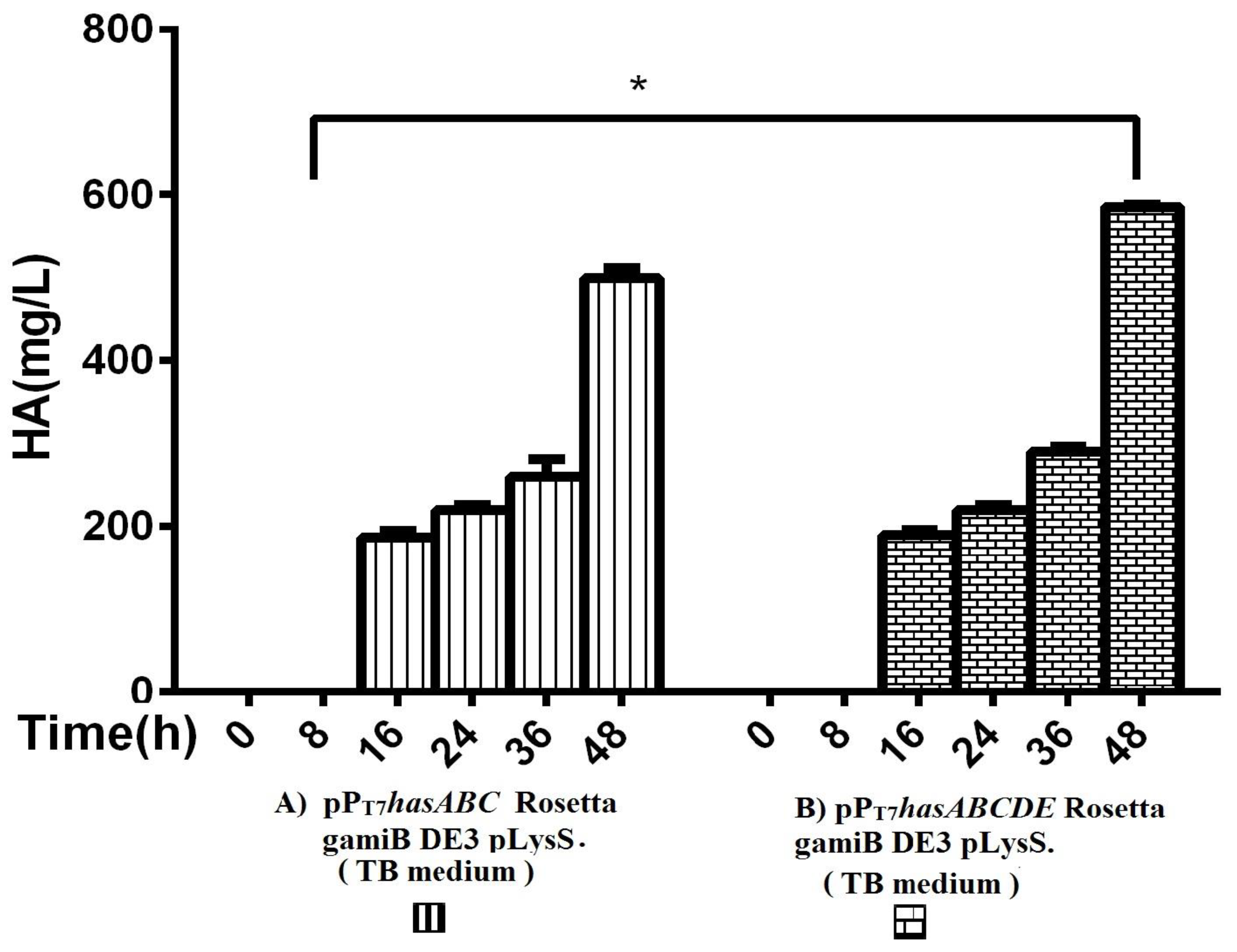
The entire operon hasABCDE from S. equi subsp. zooepidemicus was successfully ligated into plasmid pPT7 to construct plasmid pPT7hasABCDE. After verification of the sequence by Sanger sequencing, E. coli Rosetta-gamiB (DE3) pLysS cells were transformed, and transformants were grown in TB medium. The HA titers reached 585 ± 2.9 mg/L, significantly higher than the HA titer obtained for pPT7hasABC (Figure 4).
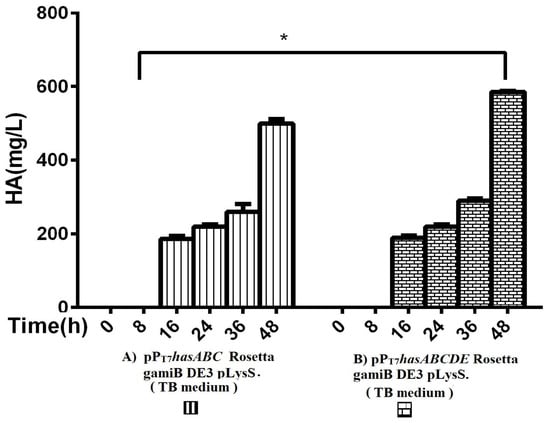
Figure 4.
Comparison of HA productivity in TB medium between (A) pPT7hasABC Rosetta gamiB (DE3) pLysS and (B) (pPT7hasABCDE) Rosetta gamiB (DE3) pLysS, (*): p ˂ 0.05.
E. coli JM109 was used for the co-expression of hasA from Pasteurella multocida and the UDP-glucose dehydrogenase gene (kfiD), a hasB analog from E. coli K5. In shake flasks, the HA titer of this strain was at 500 mg/L [19,22]. In a latter study, glucosamine was added to the feeding solution to de-couple growth and capsular polysaccharide (CPS.) synthesis, and it was determined that insufficient precursor supply was detrimental to HA synthesis [20,21].
3.2. HA Production with B. megaterium
A potential B. megaterium expression platform was suggested for recombinant HA production due to its ability to secrete recombinant proteins directly into the culture medium. Plasmid pMM1522-hasA was transformed into B. megaterium MS941 protoplasts pre-transformed with pT7-RNAP. Cells were grown to an OD578 = 1 before being induced using a mixture containing 0.25% xylose, 10 mM MgCl2, 10 mM K2HPO4, and 1 g sorbitol/L (see Methods). The presence of sorbitol has been shown to promote the mucoid phenotype of the transformed colonies. The productivity of the HA was only 50 ± 5.7 mg/L after 48 h. The low HA titer was expected when only hasA was expressed, implying a limitation of essential HA precursors, as reported for B. subtilis cells expressing hasA only [22]. Subsequently, plasmid pPT7hasABC was used, and the LB culture medium was supplemented with 1%, 3%, or 5% glucose (Table S3).
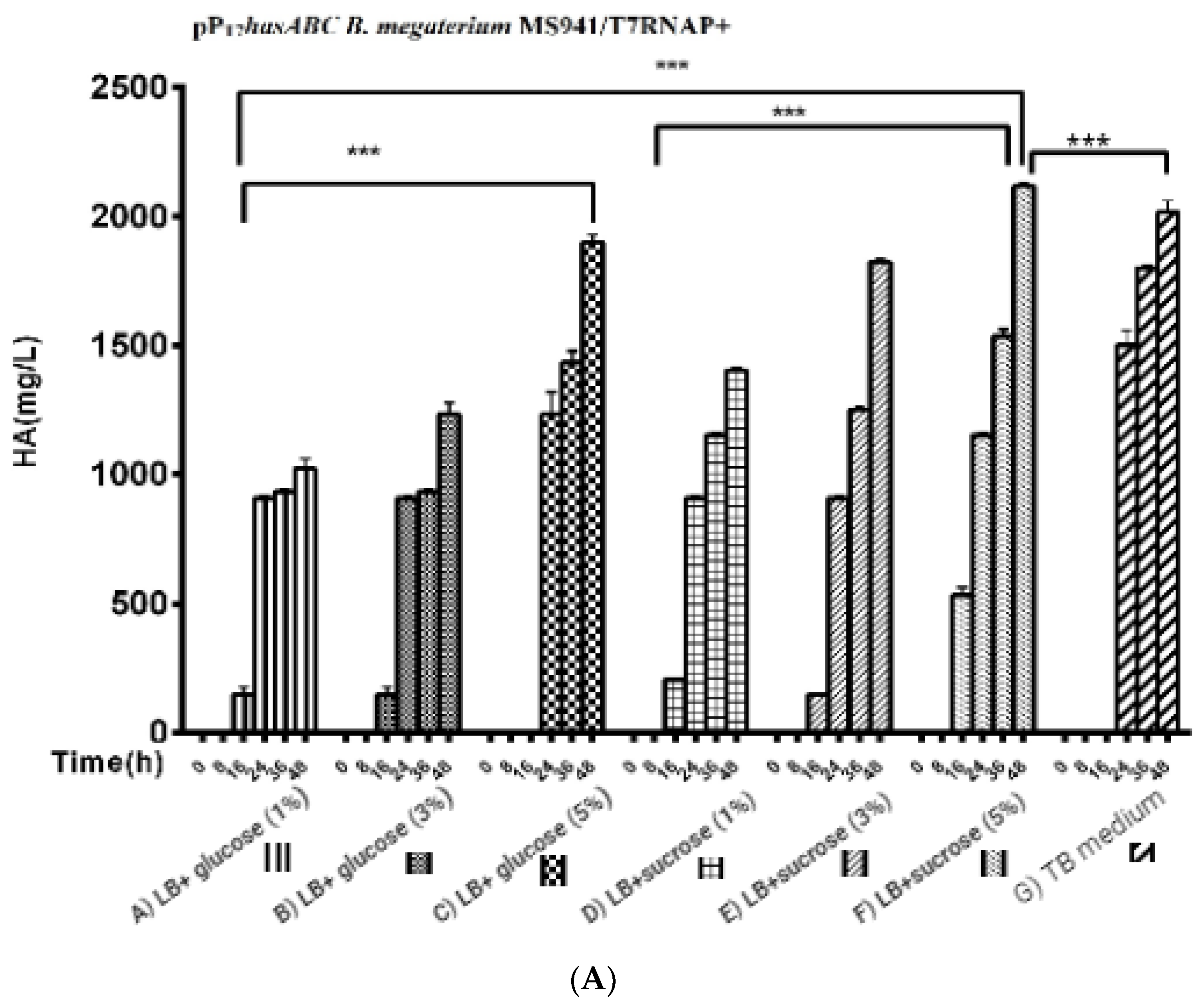
For the three used glucose concentrations (Figure 5A): After induction with 0.5% xylose, the HA titers reached 1023.3 ± 39.2 mg/L, 1200 ± 57.7 mg/L, and 1833 ± 60.1 mg/L, respectively. A further increase in HA productivity was achieved by the LB medium supplemented with different sucrose concentrations (1%, 3%, and 5%) to reach 1403 ± 8.8 mg/L, 1823.3 ± 14.5 mg/L, and 2116.7 ± 44 mg/L, respectively, while in the TB medium, the HA titer was 2016 ± 44 mg/L (Figure 5B).
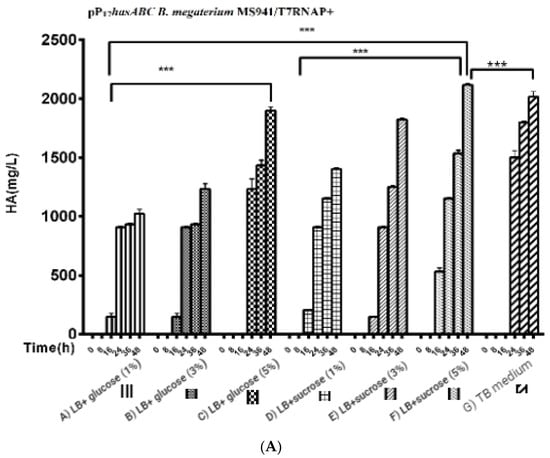
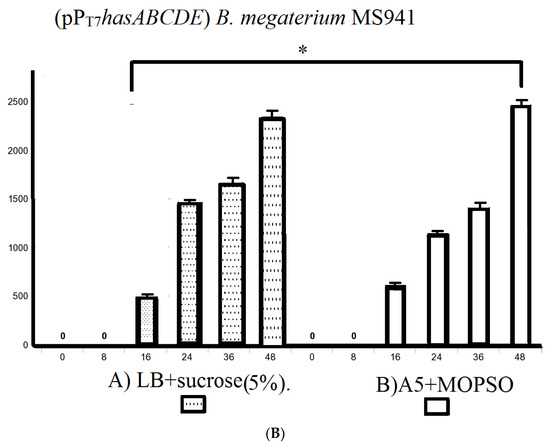
Figure 5.
Comparison of HA production presented by (Figure 5A); pPT7hasABC B. megaterium MS941 pre-transformed with T7RNAP in complex media of A) LB + glucose (1%), B) glucose (3%), C) glucose (5%), D) LB + sucrose (1%), E) LB + sucrose (3%), F) LB + sucrose (5%), and G) TB medium, (***): p < 0.0001. (Figure 5B): Comparison of HA production by PpT7hasABC B. megaterium MS941 pre-transformed with T7RNAP in semi minimal media A) A5, B) A5 + K, and C) A5 + MOPSO, (*): p ˂ 0.05.
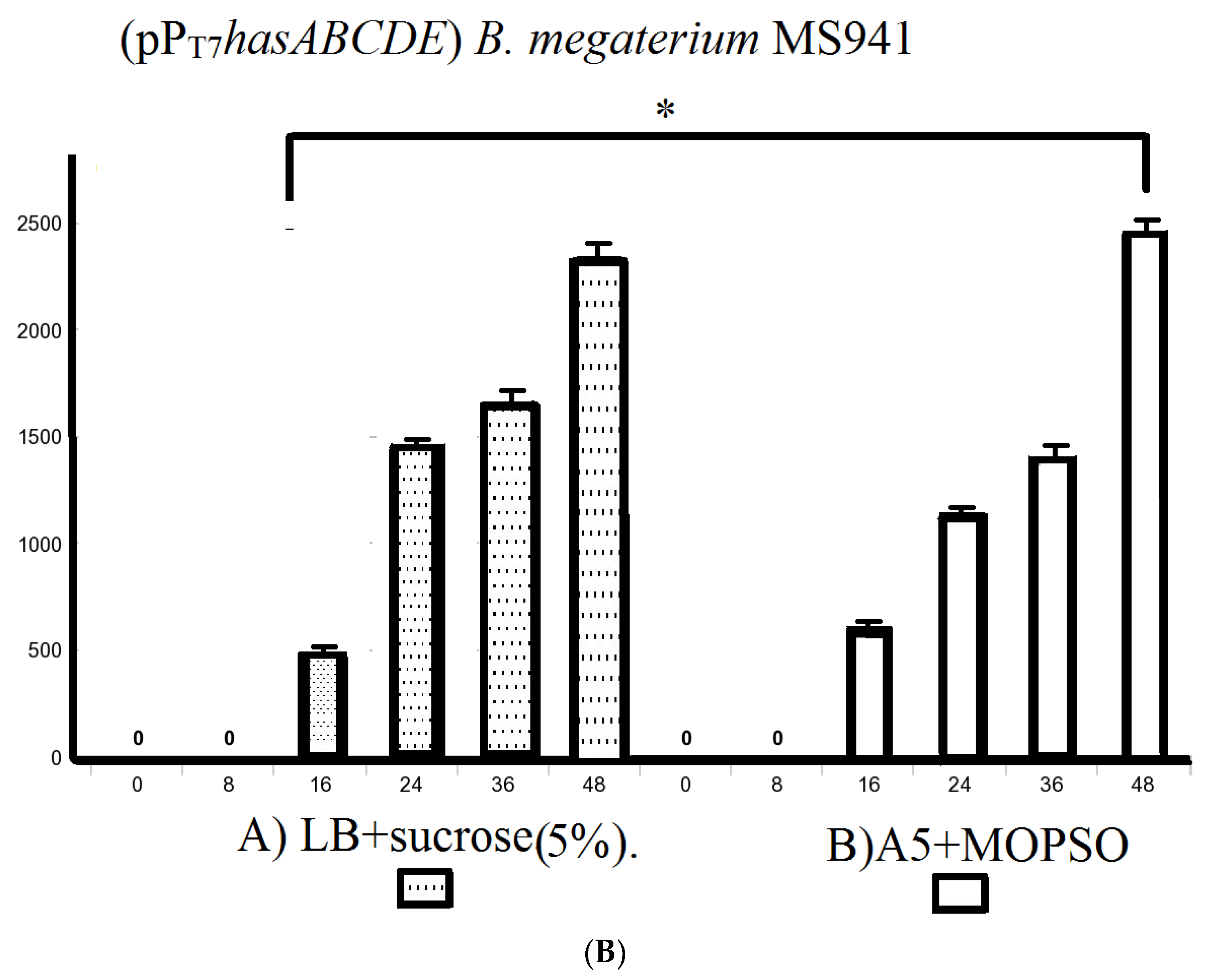
We also explored using the semi-minimal growth media A5, A5 + 4, and A5 supplemented with the buffering agent MOPSO (2-hydroxy-3-morpholinopropanesulfonic acid; 2-hydroxy-3-morpholinopropane-1-sulfonic acid) as a nitrogenous base for optimal pH constant for bacterial growth. The incubation of B. megaterium MS941 protoplasts pre-transformed with pT7-RNAP in these media resulted in HA titers of 1900 ± 25.1 mg/L, 1873.3 ± 14.5 mg/L, and 1988.3 ± 19.6 mg/L, respectively (Figure S3). A5 + MOPSO medium supported the highest HA productivity compared to the other semi-minimal media (Figure 5B).
As B. megaterium MS941 (pPT7hasABC) cultured in LB medium supplemented with 5% sucrose or in A5 medium + MOPSO exhibited by far the highest HA titers in complex media and semi-minimal media. These media were also used for growing B. megaterium MS941 harboring the entire HA operon (pPT7hasABCDE). The HA titer was 2350 ± 28.9 mg/L in LB medium containing 5% sucrose and 2476.7 ± 14.5 mg/L in A5 medium + MOPSO (Figure 5B). The higher titers indicated that the co-expression of hasDE relieved the limitation in HA precursors when only a part of the operon was used.
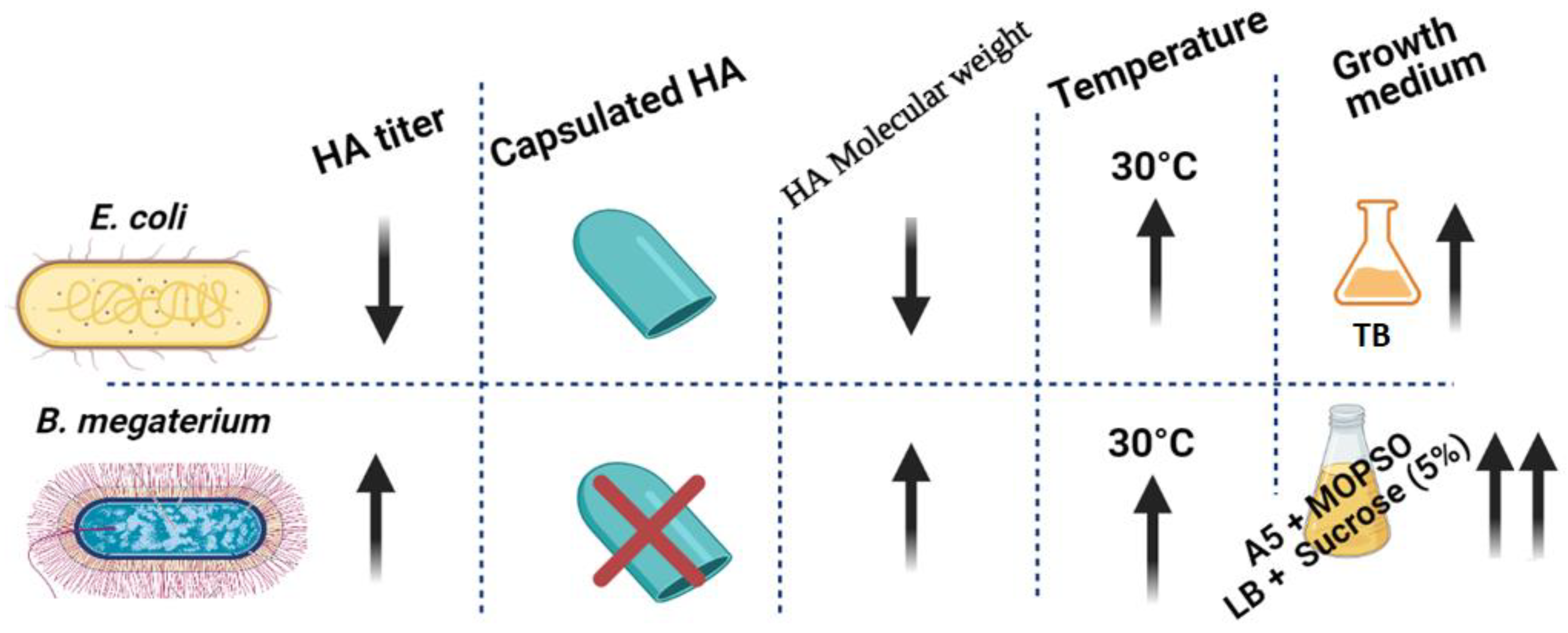
Finally, the HA production by B. megaterium was almost fourfold higher than the highest HA levels produced by any of the E. coli strains investigated (Figure 6).
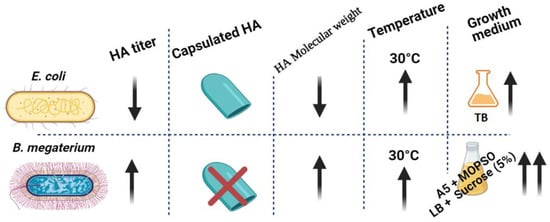
Figure 6.
Graphical conclusion of the superiority of HA productivity in B. megaterium over E. coli. HA productivity using B. megaterium platform showed higher HA titer with lower polydispersity and no capsulated HA in the culturing media of A5 + MOPSO and LB + sucrose (5%) (up-arrow represents “increase”, down-arrow represents “decrease”).
3.3. Molecular Weight and Polydispersity Index (PDI) of Recombinant HA
Table 1 summarizes the average MW and PDI values for the HA products, which were obtained in all experiments. For E. coli strains, the highest HA MW values were obtained for cells expressing the entire operon (pPT7hasABCDE) and grown in TB medium (8.3 × 105 ± 78.4 Da), and the lowest MWs for cells expressing hasA only (pMM1522hasA) and grown in LB medium supplemented with 1% glucose (1.2 × 105 ± 83.5 Da). In contrast to previous reports, the presence of hasE (pgi) seems to have little or no significant effect on HA MW [19]. However, a good comparison may not be possible due to the different methodologies used in MW determination. The PDI levels followed a reverse pattern, lowest for the entire operon (1.3) and highest for hasA (1.5). The addition of lysozyme resulted in lower molecular weights and higher PDI.

Table 1.
Summary of HA quantification and molecular weight determination in all strains used in this study.
For B. megaterium, HA MW obtained by overexpression of hasA (pMM1522hasA) was 6.8 × 105 ± 28.4 Da, almost sixfold higher than the corresponding E. coli levels expressing the same gene, while the overexpression of hasABC (pPT7hasABC) resulted in HA MW ranging from 9.9 × 105 ± 6.5 to 1.2 × 106 ± 30.5 Da. In semi-minimal media, (A5, A5 + 4, A5 + MOPSO) HA MW was 1.2 × 106, 1.2 × 106, and 1.4 × 106 Da, respectively, and PDI was 1.2. Thus, A5 + MOPSO showed the highest MW observed with a minor PDI. It was also encouraging to overexpress hasABCDE in the same medium where the observed HA MW was 1.9 ± 52 × 106 Da and PDI was 1.2. The presence of hasDE genes resulted in significant differences in HA MWs. It increased the flux of the gene product, UDP-GlcNAc, which is a crucial player in determining HA MW [39].
However, the HA MWs obtained in the present study were lower than those previously reported for B. subtilis expressing hasA from P. multocidia in combination with the B. subtilis analogs of hasB (tua D), hasC (gtaB) and hasE (pgi:glucose-6-phosphate isomerase) [23]. In summary, B. megaterium expressing recombinant has genes produced a higher quality of the polymer (higher MW and lower PDI) than E. coli strains to harbor identical plasmids. Whereas the availability of UDP-GlcNAc, the product of hasDE genes, resulted in higher HA MW in B. megaterium, hasDE expression in E. coli did not lead to the same effect. Furthermore, a scanning electron microscope was used for HA capsule detection to investigate the potentiality of B. megaterium over E. coli in HA production.
3.4. Scanning Electron Microscopic (SEM) Examination
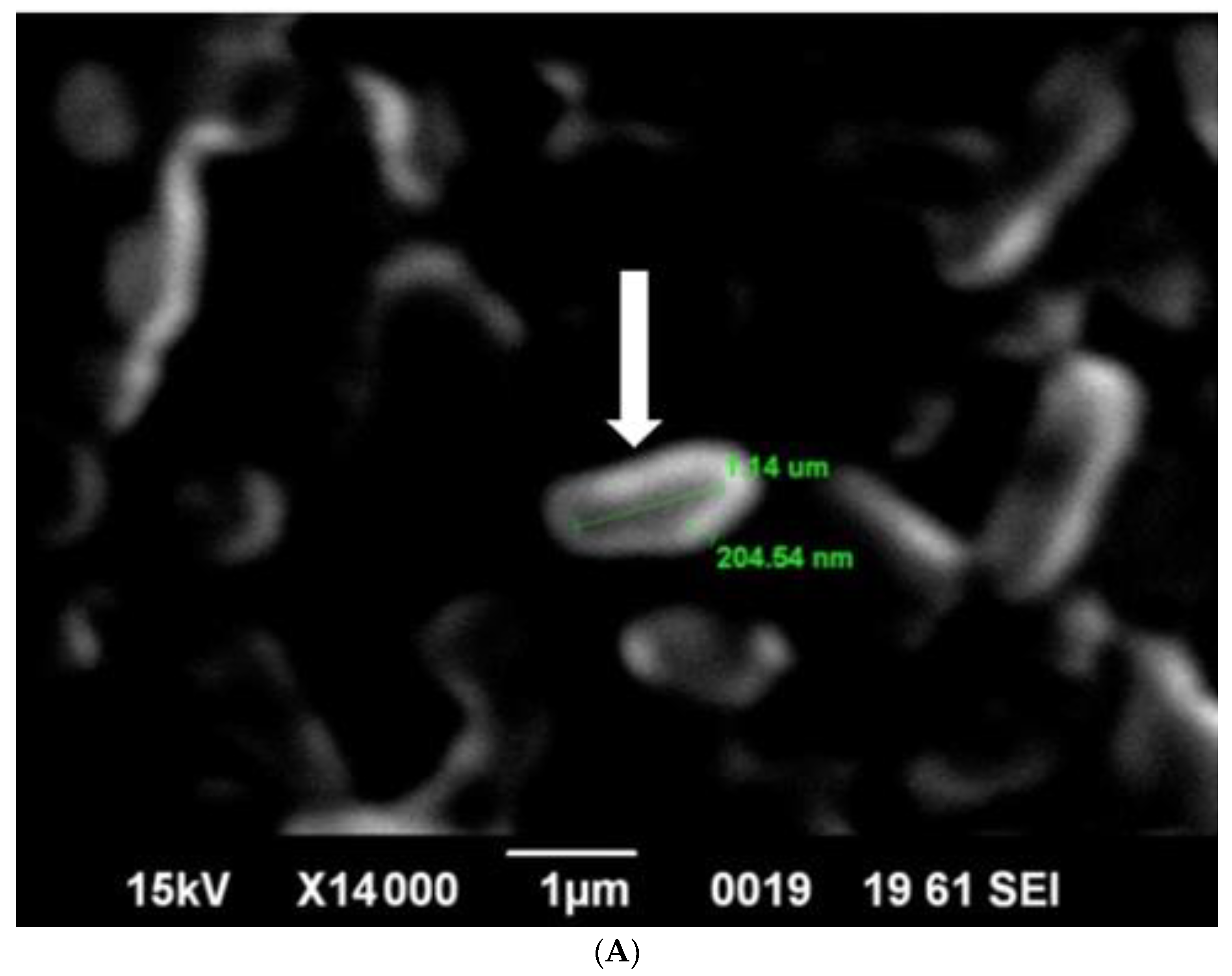
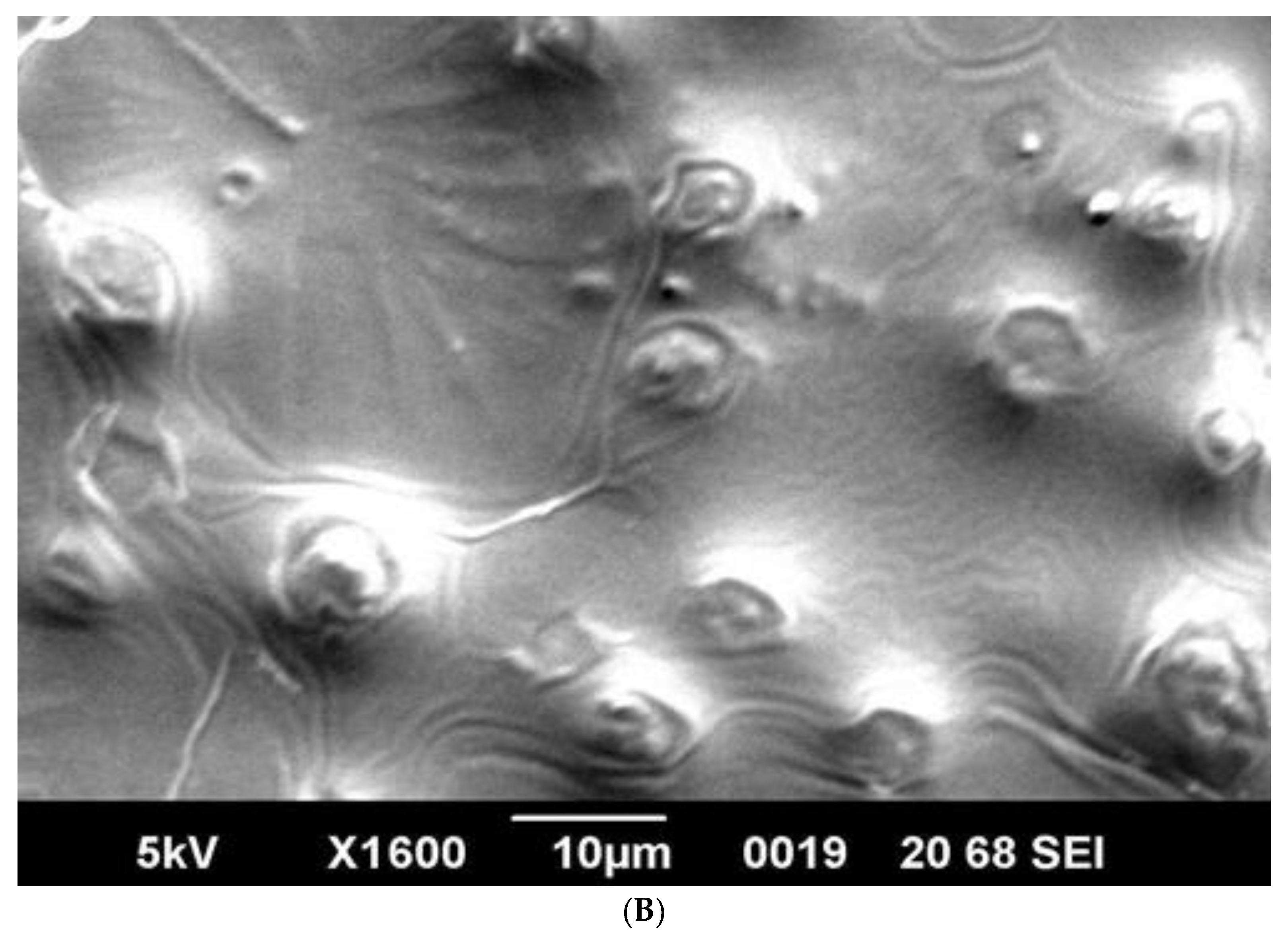
SEM analysis showed that induced E. coli Rosetta-gami B(DE3)pLysS cells carrying pPT7hasABCDE formed a capsule (Figure 7A). It disappeared after incubation with hyaluronidase (0.1% in PBS for 30 min at room temperature). It indicated its apparent nature as HA (Figure 7B). B. megaterium harboring pPT7hasABCDE and T7RNAP, on the other hand, did not accumulate capsules and seemed to shed HA into the medium, after being incubated with hyaluronidase under the same conditions (Figure 8A,B). Corynebacterium glutamicum has been also reported to shed the HA polymer into the culture medium without forming a distinct capsule [23].
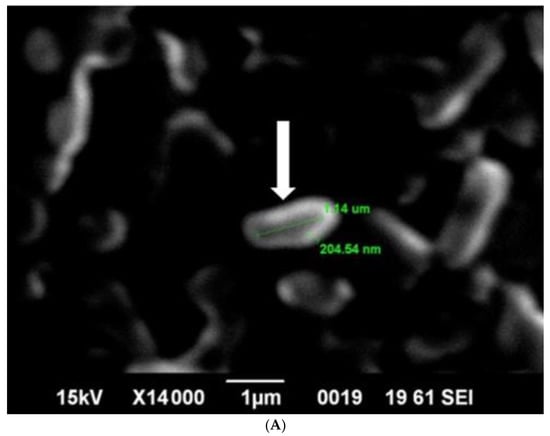

Figure 7.
(A): SEM photo of induced pPT7hasABCDE E. coli Rosetta-gamiBpLysS. HA capsule was found intact in the cell, with white arrow. (B): SEM: Photo of induced pPT7hasABCDE E. coli Rosetta-gamiBpLysS after incubation for 20 min with hyaluronidase enzyme dissolved in phosphate buffered saline.

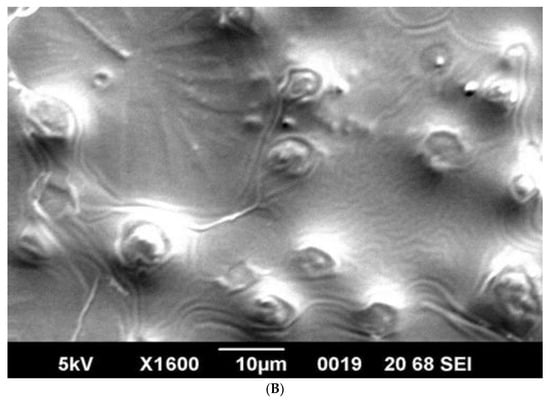
Figure 8.
(A): SEM photo of induced pPT7hasABCDE B. megaterium MS941. It was clear that HA particles were outside the protoplasts and do not form a distinct capsule. They appeared as white particles (black arrow). (B): SEM photo of induced pPT7hasABCDE B. megaterium MS941 after incubation for 20 min with hyaluronidase enzyme dissolved in phosphate-buffered saline.
Capsule disruption is an essential step for HA quantification that should precede the assay procedure. The low HA titers obtained for E. coli compared to B. megaterium likely result from inefficient capsule dissolution.
3.5. FTIR Spectrum of HA Produced by Recombinant E. coli and B. megaterium Strains
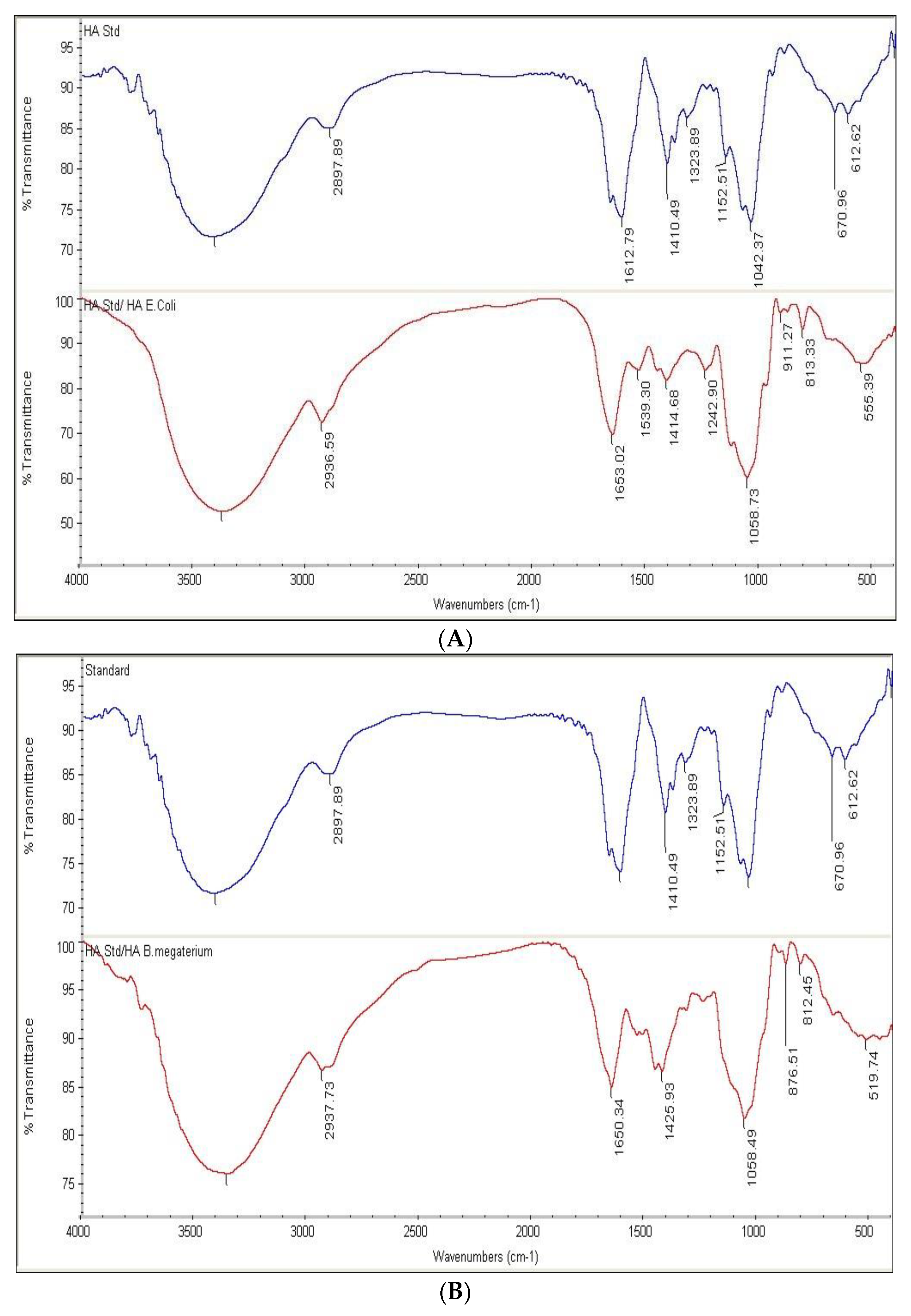
HA samples prepared as described in the “Methods” section showed an FTIR spectrum similar to the HA standard (Table S4). The positions of the six peaks, in terms of wave number (cm−1), of both the reference standard and HA produced by pPT7hasABCDE E. coli Rosetta-gami B pLysS and pPT7hasABCDE B. megaterium, under optimal conditions, were identical (Figure 9A,B). It indicated the efficiency of the purification method employed in this study.
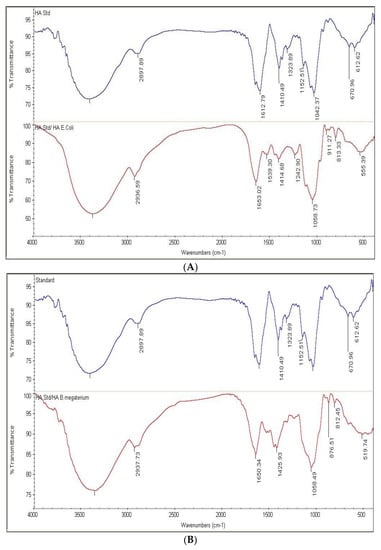
Figure 9.
(A): FTIR of HA standard versus HA extracted from pPT7hasABCDE E. coli Rosetta-gamiBpLysS. (B): FTIR of HA standard versus HA extracted from pPT7hasABCDE B. megaterium.
4. Discussion
Our attempts to obtain stable clones carrying hasAB or hasABC in pMM1522 were unsuccessful, probably because of the leaky nature of the xylose promoter in E. coli. It is based on xylAB genes, as reported earlier [32]. All subsequent culturing conditions for E. coli were performed at a temperature of 37 °C after induction. It was previously indicated that temperature switches improve the HA titer in Streptococcus equi subsp. zooepidemicus, probably because the slower growth rate provides enough time for correct protein folding via the weakening of the glycolytic process, enhancing the HA synthesis process, which reduces the biomass-formation rate [55]. HA productivity titers in E. coli strains Rosetta2 (DE3) plysS and Rosetta-gami B (DE3)pLysS were higher than those previously reported for the same media [18,34]. The improved productivity is probably attributed to the solid T7 promoter and the buffering of the TB medium, which could maintain the neutral pH crucial to HA production [56]. However, lysozyme has been previously suggested to increase HA productivity of Lactococcus lactis when applied 24 h after induction [19]. This suppressive effect of lysozyme on HA production has similarly been reported for Streptococcus equi [40], indicating the expression of the entire operon hasABCDE indeed increased the availability of HA precursors UDP-GlcA and UDP-N-GlcNA, in agreement with previous recommendations that emphasize the importance of the expression of hasE (pgi) together with hasABC for optimized HA productivity [19,39]. HA production in E. coli was significantly affected by factors such as culture temperature, the codon usage of the heterologous genes, promoter strength, and the specific type of E. coli host strain. These factors can influence the solubility of the expressed proteins and control the HA biosynthesis enzymes (Figure 1). However, the obtained HA titers in E. coli were still significantly lower than those reported for the Gram-positive bacterial hosts [7,23,40,41].
Although TB medium resulted in a relatively high HA titer in B. megaterium, HA production was not observed until 16 h from the onset of growth. The enhancing effects of glucose and sucrose on HA productivity have been previously reported in several studies [16,54,55,56]. The solid buffering capacity of MOPSO seems to play a significant role in optimizing the culture conditions by maintaining a neutral pH throughout the entire growth period, eventually leading to improved HA production [44,57,58]. It is noteworthy that the advantages of B. megaterium over Lactococcus lactis in producing HA are direct to the choice of the growth media. Lactococcus lactis typically produces HA in a capsulated form, which requires more time and cost for HA purification. B. megaterium can be a better industrial host strain for HA production. The vector systems used in our study were commercially available. We also concluded from our study that HA production needs well-controlled expression systems, as hasA is toxic, and it is challenging to clone genes when the expression system is not well-controlled. We have also tried to use the shuttle vectors E. coli/Corynebacterium glutamicum, but the cloning failed due to the leaky expression of the pTac system used. In conclusion, it would be better to use the pPt7 system when we want to produce HA by Corynebacterium glutamicum; this is our outlook for the future.
From our study, we suggest that the following adjustments have contributed to the high HA productivity obtained with B. megaterium, whether from hasABC or hasABCDE expression; the different optimization factors between B. megaterium and E. coli showed a potential influence on HA-production characteristics:
- The initial lack of cell walls in the B. megaterium protoplasts MS941 favors HA production by limiting the competition between HA production and cell-wall biosynthesis.
- Decreasing the temperature after induction from 30 °C to 25 °C helps decrease the growth rate, thus directing the carbon flow from the available substrates (glucose or sucrose) to HA production.
- Using the potent buffering agent MOPSO in semi-minimal media (A5 + MOPSO) supplemented with 5% sucrose was essential.
The high productivity of HA by B. megaterium compared with E. coli may be attributed to the ability of B. megaterium to maintain several stable extra-chromosomal DNA elements replicating in parallel [59,60]. It has been crucial to express more genes of the HA biosynthetic pathway without encountering plasmid-instability events. In addition, UDP-GlcNAc is limited in E. coli, and this substrate plays an essential role in the HA titer.
The molecular weight (MW) of HA is an essential criterion in the characterization of the polymer that defines its physiochemical and biological properties and applications. Although the HA biosynthetic mechanism is well established, little is known about the molecular mechanisms that control chain termination. Hence, the MW of produced HA is true not only for hyaluronan synthases but also for other β-polysaccharide synthases, e.g., cellulose, chitin and 1,3-betaglucan synthases [44]. High MW HA polymers, capable of reaching an HMW of 108 Da, are suitable as dermal fillers, anti-angiogenic agents, and potentiality as immunosuppressives via fibrinogen binding, and enhance inflammatory cytokines as well as stem-cell mitigation. Medium-size hyaluronan chains (between 2 × 104 and 105 Da) are involved in ovulation, embryogenesis, and wound repair. Oligosaccharides with 15 to 50 repeating disaccharide units (between 6 × 103 and 2 × 104 Da) are anti-inflammatory, immuno-stimulatory, and angiogenic, while small hyaluronan oligomers (from 400 to 4000 Da) are anti-apoptotic and inducers of heat-shock proteins [2,61,62].
Another critical parameter is the polydispersity index (PDI) of the polymer. The reported molecular weight of isolated HA polymers may be under-evaluated depending on the extraction, isolation, and analysis methods used. Consequently, the molecular weight of HA is experimentally determined as displaying the polydispersity(Mw/Mn) of the polymer. It depends on both the method of extraction and the method of analysis used [63].
In the present study, HA MW was determined using gel permeation chromatography (GPC), which has been proven to be the most accurate method for MW determination. In this context, it is worth saying that PDI is a pivotal factor in determining the appropriate applications of HA, with a low PDI suggesting a priority in medical/industrial applications due to its few discrepancies or narrow size distribution, that is, a low heterogeneity index for the HA [48].
In conclusion, there are a lot of research opportunities and challenges in recombinant HA. HA production can be achieved by Gram-negative (E. coli) or Gram-positive (B. megaterium) bacterial platforms. However, the superiority of Gram-positive bacteria (B. megaterium) can be attributed to their capacity for HA-production delivery to the medium without displaying a capsulated form. However, the media composition, the buffering system (pH neutralization), and temperature could play a critical role in the chosen plasmid system, whether Ptac or pPT7. The study’s limitations were the choice of stable mucoid colonies and the possibility of using B. megaterium protoplasts at an industrial scale, as well as media optimization and the use of low-cost media and those suitable for increasing the HA yield. More efforts are recommended to reach optimal and stable HA production, starting from genomics (gene-code optimization) to proteomics (controlling HA synthase genes, together with other genes involved in HA biosynthesis), and from there to the proper choice of bacterial strains.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms10122347/s1, Figure S1: The graphical abstract of Materials and Methods; Figure S2: Plasmid construction of hasaABC/DE cloned into Pjet and pPT7; Figure S3: HA production by pPT7hasABCDE B. megaterium MS941 pre-transformed with T7RNAP in (A) A5, (B) A5 + K and (C) A5 + MOPSO, (***): p ˂ 0.0001.
Author Contributions
Conceptualization, H.N., K.A.-A., M.E.-A., B.J.E.; Investigation, H.N., M.M.T., Resources, B.J.E., K.A.-A.; Writing—original draft preparation, H.N.; Writing—review and editing, B.J.E., M.M.T., M.E.-A., K.A.-A.; All authors have read and agreed to the published version of the manuscript.
Funding
Not applicable.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All Data are contained within the article and supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Falcone, S.J.; Palmeri, D.M.; Berg, R.A. Rheological and cohesive properties of hyaluronic acid. J. Biomed. Mater. Res. A 2006, 76, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Boeriu, C.G.; Springer, J.; Kooy, F.K.; van den Broek, L.A.; Eggink, G. Production Methods for Hyaluronan. Int. J. Carbohydr. Chem. 2013, 2013, 624967. [Google Scholar] [CrossRef]
- Arpicco, S.; Milla, P.; Stella, B.; Dosio, F. Hyaluronic Acid Conjugates as Vectors for the Active Targeting of Drugs, Genes and Nanocomposites in Cancer Treatment. Molecules 2014, 19, 3193–3230. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Liu, L.; Liu, S. Microbial production of hyaluronic acid: Current state, challenges, and perspectives. In Functional Carbohydrates; CRC Press: Boca Raton, FL, USA, 2017; pp. 21–42. [Google Scholar]
- Liu, L.; Liu, Y.; Li, J.; Du, G.; Chen, J. Microbial production of hyaluronic acid: Current state, challenges, and perspectives. Microb. Cell Fact. 2011, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef]
- Sze, J.H.; Brownlie, J.C.; Love, C.A. Biotechnological production of hyaluronic acid: A mini review. 3 Biotech 2016, 6, 67. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Derm. Endocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef]
- Rosato, A.; Banzato, A.; De Luca, G.; Renier, D.; Bettella, F.; Pagano, C.; Esposito, G.; Zanovello, P.; Bassi, P. HYTAD1-p20: A new paclitaxel-hyaluronic acid hydrosoluble bioconjugate for treatment of superficial bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2006, 24, 207–215. [Google Scholar] [CrossRef]
- Gallo, N.; Nasser, H.; Salvatore, L.; Natali, M.L.; Campa, L.; Mahmoud, M.; Capobianco, L.; Sannino, A.; Madaghiele, M. Hyaluronic acid for advanced therapies: Promises and challenges. Eur. Polym. J. 2019, 117, 134–147. [Google Scholar] [CrossRef]
- Snyder, T.N.; Madhavan, K.; Intrator, M.; Dregalla, R.C.; Park, D. A fibrin/hyaluronic acid hydrogel for the delivery of mesenchymal stem cells and potential for articular cartilage repair. J. Biol. Eng. 2014, 8, 10. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Pastrana, L.; Piñeiro, C.; Teixeira, J.A.; Pérez-Martín, R.I.; Amado, I.R. Production of Hyaluronic Acid by Streptococcus zooepidemicus on Protein Substrates Obtained from Scyliorhinus canicula Discards. Mar. Drugs 2015, 13, 6537–6549. [Google Scholar] [CrossRef]
- Pan, N.C.; Pereira, H.C.B.; Silva, M.D.L.C.D.; Vasconcelos, A.F.D.; Celligoi, M.A.P.C. Improvement Production of Hyaluronic Acid by Streptococcus zooepidemicus in Sugarcane Molasses. Appl. Biochem. Biotechnol. 2016, 182, 276–293. [Google Scholar] [CrossRef] [PubMed]
- O’Regan, M.; Martini, I.; Crescenzi, F.; De Luca, C.; Lansing, M. Molecular mechanisms and genetics of hyaluronan biosynthesis. Int. J. Biol. Macromol. 1994, 16, 283–286. [Google Scholar] [CrossRef]
- Marcellin, E.; Chen, W.Y.; Nielsen, L.K. Metabolic pathway engineering for hyaluronic acid production. In Handbook of Carbohydrate-Modifying Biocatalysts; Jenny Stanford Publishing: New York, NY, USA, 2016; Volume 2, pp. 683–696. [Google Scholar]
- Schmidt, K.-H.; Günther, E.; Courtney, H.S. Expression of both M protein and hyaluronic acid capsule by group A streptococcal strains results in a high virulence for chicken embryos. Med. Microbiol. Immunol. 1996, 184, 169–173. [Google Scholar] [CrossRef]
- Xie, Z.; Meng, K.; Yang, X.; Liu, J.; Yu, J.; Zheng, C.; Cao, W.; Liu, H. Identification of a Quorum Sensing System Regulating Capsule Polysaccharide Production and Biofilm Formation in Streptococcus zooepidemicus. Front. Cell. Infect. Microbiol. 2019, 9, 121. [Google Scholar] [CrossRef]
- Chien, L.; Lee, C. Enhanced Hyaluronic Acid Production in Bacillus subtilis by Coexpressing Bacterial Hemoglobin. Biotechnol. Prog. 2007, 23, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Chien, L.-J.; Lee, C.-K. Hyaluronic acid production by recombinant Lactococcus lactis. Appl. Microbiol. Biotechnol. 2007, 77, 339–346. [Google Scholar] [CrossRef]
- Zakeri, A.; Rasaee, M.J.; Pourzardosht, N. Enhanced hyluronic acid production in Streptococcus zooepidemicus by over expressing HasA and molecular weight control with Niscin and glucose. Biotechnol. Rep. 2017, 16, 65–70. [Google Scholar] [CrossRef]
- Yu, H.; Stephanopoulos, G. Metabolic engineering of Escherichia coli for biosynthesis of hyaluronic acid. Metab. Eng. 2008, 10, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Yoo, S.-J.; Oh, D.-K.; Kweon, Y.-G.; Park, D.-W.; Lee, C.-H.; Gil, G.-H. Selection of a Streptococcus equi mutant and optimization of culture conditions for the production of high molecular weight hyaluronic acid. Enzym. Microb. Technol. 1996, 19, 440–445. [Google Scholar] [CrossRef]
- Woo, J.E.; Seong, H.J.; Lee, S.Y.; Jang, Y.-S. Metabolic Engineering of Escherichia coli for the Production of Hyaluronic Acid From Glucose and Galactose. Front. Bioeng. Biotechnol. 2019, 7, 351. [Google Scholar] [CrossRef]
- Mao, Z.; Shin, H.-D.; Chen, R. A recombinant E. coli bioprocess for hyaluronan synthesis. Appl. Microbiol. Biotechnol. 2009, 84, 63–69. [Google Scholar] [CrossRef]
- Lai, Z.-W.; Rahim, R.A.; Ariff, A.B.; Mohamad, R. Comparison of hyaluronic acid biosynthesis by the recombinant escherichia coli strains in different mode of bioreactor operation. J. Microbiol. Biotechnol. Food Sci. 2016, 6, 905–910. [Google Scholar] [CrossRef]
- Mao, Z.; Chen, R.R. Recombinant Synthesis of Hyaluronan by Agrobacterium sp. Biotechnol. Prog. 2007, 23, 1038–1042. [Google Scholar] [CrossRef]
- Terpe, K. Overview of bacterial expression systems for heterologous protein production: From molecular and biochemical fundamentals to commercial systems. Appl. Microbiol. Biotechnol. 2006, 72, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Widner, B.; Behr, R.; Von Dollen, S.; Tang, M.; Heu, T.; Sloma, A.; Sternberg, D.; DeAngelis, P.L.; Weigel, P.H.; Brown, S. Hyaluronic acid production in Bacillus subtilis. Appl. Environ. Microbiol. 2005, 71, 3747–3752. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhu, J.; Chen, X.; Tang, D.; Su, D.; Yao, W.; Gao, X. Metabolic engineering of Bacillus subtilis for the efficient biosynthesis of uniform hyaluronic acid with controlled molecular weights. Bioresour. Technol. 2013, 132, 427–431. [Google Scholar] [CrossRef]
- Hoffmann, J.; Altenbuchner, J. Hyaluronic acid production with Corynebacterium glutamicum: Effect of media composition on yield and molecular weight. J. Appl. Microbiol. 2014, 117, 663–678. [Google Scholar] [CrossRef]
- Cheng, F.; Luozhong, S.; Guo, Z.; Yu, H.; Stephanopoulos, G. Enhanced biosynthesis of hyaluronic acid using engineered Corynebacterium glutamicum via metabolic pathway regulation. Biotechnol. J. 2017, 12, 1700191. [Google Scholar] [CrossRef]
- Knobloch, D.; Clemens, A.; Ostermann, K.; Rödel, G. The xylA promoter of Bacillus megaterium mediates constitutive gene expression in Escherichia coli. Eng. Life Sci. 2011, 11, 458–462. [Google Scholar] [CrossRef]
- Biedendieck, R.; Borgmeier, C.; Bunk, B.; Stammen, S.; Scherling, C.; Meinhardt, F.; Wittmann, C.; Jahn, D. systems biology of recombinant protein production using Bacillus megaterium. Methods Enzymol. 2011, 500, 165–195. [Google Scholar] [CrossRef]
- Bunk, B.; Schulz, A.; Stammen, S.; Münch, R.; Warren, M.J.; Jahn, D.; Biedendieck, R. A short story about a big magic bug. Bioeng. Bugs 2010, 1, 85–91. [Google Scholar] [CrossRef]
- Panbangred, W.; Weeradechapon, K.; Udomvaraphant, S.; Fujiyama, K.; Meevootisom, V. High expression of the penicillin G acylase gene (pac) from Bacillus megaterium UN1 in its own pac minus mutant. J. Appl. Microbiol. 2000, 89, 152–157. [Google Scholar] [CrossRef]
- Sarian, F.D.; Janeček, Š.; Pijning, T.; Ihsanawati; Nurachman, Z.; Radjasa, O.K.; Dijkhuizen, L.; Natalia, D.; Van Der Maarel, M.J.E.C. A new group of glycoside hydrolase family 13 α-amylases with an aberrant catalytic triad. Sci. Rep. 2017, 7, srep44230. [Google Scholar] [CrossRef]
- Yang, Y.; Biedendieck, R.; Wang, W.; Gamer, M.; Malten, M.; Jahn, D.; Deckwer, W.-D. High yield recombinant penicillin G amidase production and export into the growth medium using Bacillus megaterium. Microb. Cell Fact. 2006, 5, 36. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Raux, E.; Lanois, A.; Warren, M.; Rambach, A.; Thermes, C. Cobalamin (vitamin B12) biosynthesis: Identification and characterization of a Bacillus megaterium cobI operon. Biochem. J. 1998, 335, 159–166. [Google Scholar] [CrossRef]
- Moore, S.J.; Mayer, M.J.; Biedendieck, R.; Deery, E.; Warren, M.J. Towards a cell factory for vitamin B12 production in Bacillus megaterium: Bypassing of the cobalamin riboswitch control elements. New Biotechnol. 2014, 31, 553–561. [Google Scholar] [CrossRef]
- Viola, M.; Vigetti, D.; Genasetti, A.; Rizzi, M.; Karousou, E.; Moretto, P.; Clerici, M.; Bartolini, B.; Pallotti, F.; De Luca, G.; et al. Molecular Control of the Hyaluronan Biosynthesis. Connect. Tissue Res. 2008, 49, 111–114. [Google Scholar] [CrossRef]
- Rosano, G.L.; Ceccarelli, E.A. Recombinant protein expression in Escherichia coli: Advances and challenges. Front. Microbiol. 2014, 5, 172. [Google Scholar] [CrossRef] [PubMed]
- Wood, E.J. Molecular Cloning. A Laboratory Manual. Biochem. Educ. 1983, 11, 82. [Google Scholar] [CrossRef]
- Sezonov, G.; Joseleau-Petit, D.; D’Ari, R. Escherichia coli Physiology in Luria-Bertani Broth. J. Bacteriol. 2007, 189, 8746–8749. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J. Molecular cloning: A Laboratory Manual. In Transformation of E. coli Byelectroporation, 3rd ed.; Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Tartof, K.D. Improved media for growing plasmid and cosmid clones. Bethesda Res. Lab. Focus 1987, 9, 12. [Google Scholar]
- Malten, M.; Hollmann, R.; Deckwer, W.-D.; Jahn, D. Production and secretion of recombinantLeuconostoc mesenteroides dextransucrase DsrS in Bacillus megaterium. Biotechnol. Bioeng. 2004, 89, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Di Ferrante, N. Turbidimetric measurement of acid mucopoly-saccharides and hyaluronidase activity. J. Biol. Chem. 1956, 220, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Oueslati, N.; Leblanc, P.; Harscoat-Schiavo, C.; Rondags, E.; Meunier, S.; Kapel, R.; Marc, I. CTAB turbidimetric method for assaying hyaluronic acid in complex environments and under cross-linked form. Carbohydr. Polym. 2014, 112, 102–108. [Google Scholar] [CrossRef]
- DeAngelis, P.L.; Gunay, N.S.; Toida, T.; Mao, W.-J.; Linhardt, R.J. Identification of the capsular polysaccharides of Type D and F Pasteurella multocida as unmodified heparin and chondroitin, respectively. Carbohydr. Res. 2002, 337, 1547–1552. [Google Scholar] [CrossRef]
- Murtey, M.D.; Ramasamy, P. Sample Preparations for Scanning Electron Microscopy–Life Sciences. In Modern Electron Microscopy in Physical and Life Sciences; Janecek, M., Kral, R., Eds.; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Lee, D.G.; Nurunnabi, M.; Hwang, J.; Chung, E.S.; Rahmatullah, M.; Huh, K.M.; Kwak, K.S.; Lee, Y.K. Synthesis and Characterization of Hyaluronic Acid for Biomedical Application. Adv. Mater. Res. 2012, 581–582, 185–188. [Google Scholar] [CrossRef]
- Statistics IBMS. IBM SPSS Statistics for Windows; IBM Corp.: Armonk, NY, USA, 2011. [Google Scholar]
- Hubbard, C.; McNamara, J.T.; Azumaya, C.; Patel, M.S.; Zimmer, J. The Hyaluronan Synthase Catalyzes the Synthesis and Membrane Translocation of Hyaluronan. J. Mol. Biol. 2012, 418, 21–31. [Google Scholar] [CrossRef]
- Bryksin, A.V.; Matsumura, I. Rational Design of a Plasmid Origin That Replicates Efficiently in Both Gram-Positive and Gram-Negative Bacteria. PLoS ONE 2010, 5, e13244. [Google Scholar] [CrossRef]
- Jagannath, S.; Ramachandran, K. Influence of competing metabolic processes on the molecular weight of hyaluronic acid synthesized by Streptococcus zooepidemicus. Biochem. Eng. J. 2010, 48, 148–158. [Google Scholar] [CrossRef]
- Schiraldi, C.; Andreozzi, L.; Marzaioli, I.; Vinciguerra, S.; D’Avino, A.; Volpe, F.; Panariello, A.; De Rosa, M. Hyaluronic acid degradation during initial steps of downstream processing. Biocatal. Biotransform. 2009, 28, 83–89. [Google Scholar] [CrossRef]
- Ferreira, C.M.H.; Pinto, I.; Soares, E.V.; Soares, H.M.V.M. (Un)suitability of the use of pH buffers in biological, biochemical and environmental studies and their interaction with metal ions—A review. RSC Adv. 2015, 5, 30989–31003. [Google Scholar] [CrossRef]
- Liu, Y.; Liao, X.; Zhang, R. An Enhanced MOPSO Algorithm for Energy-Efficient Single-Machine Production Scheduling. Sustainability 2019, 11, 5381. [Google Scholar] [CrossRef]
- Chen, J.; Kang, Z.; Du, G.; Jin, P. Method of Constructing a Recombinant Bacillus subtilis That Can Produce Specific-Molecular-Weight Hyaluronic Acids. U.S. Patent 9,771,607, 26 September 2017. [Google Scholar]
- Kim, J.-Y. Overproduction and secretion of Bacillus circulans endo-β-1,3-1,4-glucanase gene (bglBC1) in B. subtilis and B. megaterium. Biotechnol. Lett. 2003, 25, 1445–1449. [Google Scholar] [CrossRef]
- Zhang, K.; Su, L.; Duan, X.; Liu, L.; Wu, J. High-level extracellular protein production in Bacillus subtilis using an optimized dual-promoter expression system. Microb. Cell Fact. 2017, 16, 32. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.; Asari, A.A.; Sugahara, K.N. Hyaluronan fragments: An information-rich system. Eur. J. Cell Biol. 2006, 85, 699–715. [Google Scholar] [CrossRef]
- Jahanian-Najafabadi, A.; Shafiee, F.; Rabbani, M. Optimization of the Expression of DT386-BR2 Fusion Protein in Escherichia coli using Response Surface Methodology. Adv. Biomed. Res. 2017, 6, 22. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).