Significant Shifts in Microbial Communities Associated with Scleractinian Corals in Response to Algae Overgrowth
Abstract
1. Introduction
2. Material and Methods
2.1. Study Site and Coral Sample Collection
2.2. DNA Extraction, Polymerase Chain Reaction (PCR) Amplification, and Next-Generation Sequencing
2.3. Next-Generation Sequencing Data Processing and Analysis
2.4. Statistical Analysis
3. Results
3.1. Alpha and Beta Diversity of Coral-Associated Bacteria
3.2. Shift in Taxonomic Assignment of Coral-Associated Bacteria
3.3. Microbial Gene Function Variation among Coral Tissues and Species
4. Discussion
4.1. Algae Overgrowth Significantly Alters the Structure of Coral-Associated Bacterial Communities
4.2. Algae Overgrowth Impacts the Microbial Function of Corals
4.3. Ecological Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Premarathna, A.D.; Sarvananda, L.; Jayasooriya, A.P.; Amarakoon, S. A Review on Pathogenic Diseases on Corals Associated Risk Factors and Possible Devastations in Future in the Globe. J. Mar. Sci. Technol. 2019, 9, 269. [Google Scholar]
- Moberg, F.; Folke, C. Ecological goods and services of coral reef ecosystems. Ecol. Econ. 1999, 29, 215–233. [Google Scholar] [CrossRef]
- Boilard, A.; Dubé, C.E.; Gruet, C.; Mercière, A.; Hernandez-Agreda, A.; Derome, N. Defining Coral Bleaching as a Microbial Dysbiosis within the Coral Holobiont. Microorganisms 2020, 8, 1682. [Google Scholar] [CrossRef]
- Ainsworth, T.D.; Fordyce, A.J.; Camp, E.F. The Other Microeukaryotes of the Coral Reef Microbiome. Trends Microbiol. 2017, 25, 980–991. [Google Scholar] [CrossRef] [PubMed]
- Putnam, H.M.; Barott, K.L.; Ainsworth, T.D.; Gates, R.D. The Vulnerability and Resilience of Reef-Building Corals. Curr. Biol. 2017, 27, R528. [Google Scholar] [CrossRef] [PubMed]
- Howells, E.J.; Vaughan, G.O.; Work, T.M.; Burt, J.A.; Abrego, D. Annual outbreaks of coral disease coincide with extreme seasonal warming. Coral Reefs 2020, 39, 771–781. [Google Scholar] [CrossRef]
- Moriarty, T.; Leggat, W.; Huggett, M.J.; Ainsworth, T.D. Coral Disease Causes, Consequences, and Risk within Coral Restoration. Trends Microbiol. 2020, 28, 793–807. [Google Scholar] [CrossRef]
- Pootakham, W.; Mhuantong, W.; Putchim, L.; Yoocha, T.; Sonthirod, C.; Kongkachana, W.; Sangsrakru, D.; Naktang, C.; Jomchai, N.; Thongtham, N.; et al. Dynamics of coral-associated microbiomes during a thermal bleaching event. MicrobiologyOpen 2018, 7, e00604. [Google Scholar] [CrossRef]
- Hoytema, N.V.; Bednarz, V.N.; Cardini, U.; Naumann, M.S.; Al-Horani, F.A.; Wild, C. The influence of seasonality on benthic primary production in a Red Sea coral reef. Mar. Biol. 2016, 163, 52. [Google Scholar] [CrossRef]
- McCook, L.; Jompa, J.; Diaz-Pulido, G. Competition between corals and algae on coral reefs: A review of evidence and mechanisms. Coral Reefs 2001, 19, 400–417. [Google Scholar] [CrossRef]
- Barott, K.L.; Rodriguez-Mueller, B.; Youle, M.; Marhaver, K.L.; Vermeij, M.J.A.; Smith, J.E.; Rohwer, F.L. Microbial to reef scale interactions between the reef-building coral Montastraea annularis and benthic algae. Proc. R. Soc. B Biol. Sci. 2011, 279, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Roth, F.; El-Khaled, Y.C.; Karcher, D.B.; Rädecker, N.; Carvalho, S.; Duarte, C.M.; Silva, L.; Calleja, M.L.; Morán, X.A.G.; Jones, B.H.; et al. Nutrient pollution enhances productivity and framework dissolution in algae- but not in coral-dominated reef communities. Mar. Pollut. Bull. 2021, 168, 112444. [Google Scholar] [CrossRef]
- Sotka, E.E.; Hay, M.E. Effects of herbivores, nutrient enrichment, and their interactions on macroalgal proliferation and coral growth. Coral Reefs 2009, 28, 555–568. [Google Scholar] [CrossRef]
- Evensen, N.R.; Vanwonterghem, I.; Doropoulos, C.; Gouezo, M.; Botté, E.S.; Webster, N.S.; Mumby, P.J. Benthic micro- and macro-community succession and coral recruitment under overfishing and nutrient enrichment. Ecology 2021, 102, e03536. [Google Scholar] [CrossRef] [PubMed]
- Vermeij, M.; Imke, V.M.; Sarah, E.; Christine, H.; Vonk, S.M.; Visser, P.M.; Frank, U. The Effects of Nutrient Enrichment and Herbivore Abundance on the Ability of Turf Algae to Overgrow Coral in the Caribbean. PLoS ONE 2010, 5, e14312. [Google Scholar] [CrossRef]
- Adam, T.C.; Burkepile, D.E.; Holbrook, S.J.; Carpenter, R.C.; Claudet, J.; Loiseau, C.; Thiault, L.; Brooks, A.J.; Washburn, L.; Schmitt, R.J. Landscape-scale patterns of nutrient enrichment in a coral reef ecosystem: Implications for coral to algae phase shifts. Ecol. Appl. 2020, 31, e2227. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.L. Associational refuge facilitates phase shifts to macroalgae in a coral reef ecosystem. Ecosphere 2018, 9, e02272. [Google Scholar] [CrossRef]
- Graham, N.; Jennings, S.; Macneil, M.A.; Mouillot, D.; Wilson, S.K. Predicting climate-driven regime shifts versus rebound potential in coral reefs. Nature 2015, 518, 94–97. [Google Scholar] [CrossRef]
- Nelson, C.E.; Goldberg, S.J.; Wegley Kelly, L.; Haas, A.F.; Smith, J.E.; Rohwer, F.; Carlson, C.A. Coral and macroalgal exudates vary in neutral sugar composition and differentially enrich reef bacterioplankton lineages. ISME J. 2013, 7, 962–979. [Google Scholar] [CrossRef]
- Wells, C.D.; Quintana, A.M.; Tonra, K.J.; Lasker, H.R. Algal turf negatively affects recruitment of a Caribbean octocoral. Coral Reefs 2021, 40, 1045–1053. [Google Scholar] [CrossRef]
- Harris, J.L. The Ecology of Turf Algae on Coral Reefs; University of California: San Diego, CA, USA, 2015. [Google Scholar]
- Ritson-Williams, R.; Paul, V.J.; Arnold, S.N.; Steneck, R.S. Larval settlement preferences and post-settlement survival of the threatened Caribbean corals Acropora palmata and A. cervicornis. Coral Reefs 2010, 29, 71–81. [Google Scholar] [CrossRef]
- Harrington, L.; Fabricius, K.; De’Ath, G.; Negri, A. Recognition And Selection Of Settlement Substrata Determine Post-Settlement Survival In Corals. Ecology 2004, 85, 3428–3437. [Google Scholar] [CrossRef]
- Vermeij, M.J.A.; Dailer, M.L.; Smith, C.M. Crustose coralline algae can suppress macroalgal growth and recruitment on Hawaiian coral reefs. Mar. Ecol. Prog. Ser. 2011, 422, 1–7. [Google Scholar] [CrossRef]
- Smith, J.E.; Shaw, M.; Edwards, R.A.; Obura, D.; Pantos, O.; Sala, E.; Sandin, S.A.; Smriga, S.; Hatay, M.; Rohwer, F.L. Indirect effects of algae on coral: Algae-mediated, microbe-induced coral mortality. Ecol. Lett. 2006, 9, 835–845. [Google Scholar] [CrossRef]
- Jompa, J.; Mccook, L.J. Contrasting effects of turf algae on corals: Massive Porites spp. are unaffected by mixed-species turfs, but killed by the red alga Anotrichium tenue. Mar. Ecol. Prog. Ser. 2003, 258, 79–86. [Google Scholar]
- Pratte, Z.A.; Longo, G.O.; Burns, A.S.; Hay, M.E.; Stewart, F.J. Contact with turf algae alters the coral microbiome: Contact versus systemic impacts. Coral Reefs 2018, 37, 1–13. [Google Scholar] [CrossRef]
- Peixoto, R.S.; Rosado, P.M.; Assis, L.D.C.D.; Rosado, A.S.; Bourne, D.G. Beneficial Microorganisms for Corals (BMC): Proposed Mechanisms for Coral Health and Resilience. Front. Microbiol. 2017, 7, 341. [Google Scholar] [CrossRef]
- Rajasabapathy, R.; Priyan, K.; Manikandan, B.; Mohandass, C.; James, R.A. Bacterial Communities Associated With Healthy and Diseased (Skeletal Growth Anomaly) Reef Coral Acropora cytherea from Palk Bay, India. Front. Mar. Sci. 2020, 7, 92. [Google Scholar] [CrossRef]
- Ziegler, M.; Grupstra, C.; Barreto, M.M.; Eaton, M.; Voolstra, C.R. Coral bacterial community structure responds to environmental change in a host-specific manner. Nat. Commun. 2019, 10, 3092. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Glckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.; Lesniewski, R.A.; Oakley, B.; Parks, D.H.; Robinson, C.J. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.; Yurgel, S.N.; Langille, M. PICRUSt2: An improved and extensible approach for metagenome inference. bioRxiv 2019. [Google Scholar] [CrossRef]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef]
- Rosenberg, E. Coral Microbiology. Oceanography 2007, 20, 146–154. [Google Scholar] [CrossRef]
- Lesser, M.P.; Mazel, C.H.; Gorbunov, M.Y.; Falkowski, P.G. Discovery of Symbiotic Nitrogen-Fixing Cyanobacteria in Corals. Science 2004, 305, 997–1000. [Google Scholar] [CrossRef]
- Casey, J.M.; Connolly, S.R.; Ainsworth, T.D. Coral transplantation triggers shift in microbiome and promotion of coral disease associated potential pathogens. Sci. Rep. 2015, 6, 11903. [Google Scholar] [CrossRef]
- Patel, N.P.; Shimpi, G.G.; Haldar, S. A comparative account of resistance and antagonistic activity of healthy and bleached coral-associated bacteria as an indicator of coral health status. Ecol. Indic. 2021, 120, 106886. [Google Scholar] [CrossRef]
- Koren, O.; Rosenberg, E. Bacteria Associated with the Bleached and Cave Coral Oculina patagonica. Microb. Ecol. 2008, 55, 523–529. [Google Scholar] [CrossRef]
- Harvell, D.; Aronson, R.; Baron, N.; Connell, J.; Dobson, A.; Ellner, S.; Gerber, L.; Kim, K.; Kuris, A.; McCallum, H.; et al. The rising tide of ocean diseases: Unsolved problems and research priorities. Front. Ecol. Environ. 2004, 2, 375–382. [Google Scholar] [CrossRef]
- Nugues, M.M.; Smith, G.W.; Hooidonk, R.J.; Seabra, M.I.; Bak, R.P.M. Algal contact as a trigger for coral disease. Ecol. Lett. 2004, 7, 919–923. [Google Scholar] [CrossRef]
- Pollock, F.J.; McMinds, R.; Smith, S.; Bourne, D.G.; Willis, B.L.; Medina, M.; Thurber, R.V.; Zaneveld, J.R. Coral-associated bacteria demonstrate phylosymbiosis and cophylogeny. Nat. Commun. 2018, 9, 4921. [Google Scholar] [CrossRef]
- Pootakham, W.; Mhuantong, W.; Yoocha, T.; Putchim, L.; Tangphatsornruang, S. Heat-induced shift in coral microbiome reveals several members of the Rhodobacteraceae family as indicator species for thermal stress in Porites lutea. MicrobiologyOpen 2019, 8, e935. [Google Scholar] [CrossRef] [PubMed]
- Ben-Haim, Y.; Thompson, F.L.; Thompson, C.; Cnockaert, M.C.; Rosenberg, E. Vibrio coralliilyticus sp. nov., a temperature-dependent pathogen of the coral Pocillopora damicornis. Int. J. Syst. Evol. Microbiol. 2003, 53, 309–315. [Google Scholar] [CrossRef]
- Meron, D.; Atias, E.; Kruh, L.I.; Elifantz, H.; Banin, E. The impact of reduced pH on the microbial community of the coral Acropora eurystoma. ISME J. 2011, 5, 51–60. [Google Scholar] [CrossRef]
- Selje, N.; Simon, M.; Brinkhoff, T. A newly discovered Roseobacter cluster in temperate and polar oceans. Nature 2004, 427, 445–448. [Google Scholar] [CrossRef]
- Giebel, H.A.; Kalhoefer, D.; Lemke, A.; Thole, S.; Brinkhoff, T. Distribution of Roseobacter RCA and SAR11 lineages in the North Sea and characteristics of an abundant RCA isolate. ISME J. 2011, 5, 8–19. [Google Scholar] [CrossRef]
- Rosenberg, E.; Falkovitz, L. The Vibrio shiloi/Oculina patagonica Model System of Coral Bleaching. Annu. Rev. Microbiol. 2004, 58, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Klindworth, A.; Mann, A.J.; Huang, S.; Wichels, A.; Quast, C.; Waldmann, J.; Teeling, H.; Glöckner, F.O. Diversity and activity of marine bacterioplankton during a diatom bloom in the North Sea assessed by total RNA and pyrotag sequencing. Mar. Genom. 2014, 18, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Buchan, A.; Lecleir, G.R.; Gulvik, C.A.; González, J. Master recyclers: Features and functions of bacteria associated with phytoplankton blooms. Nat. Rev. Microbiol. 2014, 12, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Kamjunke, N.; Koehler, B.; Wannicke, N.; Tittel, J. Algae as competitors of heterotrophic bacteria for glucose. J. Phycol. 2008, 44, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Mouchka, M.E.; Hewson, I.; Harvell, C.D. Coral-Associated Bacterial Assemblages: Current Knowledge and the Potential for Climate-Driven Impacts. Integr. Comp. Biol. 2010, 50, 662–674. [Google Scholar] [CrossRef]
- Kviatkovski, I.; Minz, D. A member of the Rhodobacteraceae promotes initial biofilm formation via the secretion of extracellular factor(s). Aquat. Microb. Ecol. 2015, 75, 155–167. [Google Scholar] [CrossRef]
- Roder, C.; Arif, C.; Bayer, T.; Aranda, M.; Daniels, C.; Shibl, A.; Chavanich, S.; Voolstra, C.R. Bacterial profiling of White Plague Disease in a comparative coral species framework. ISME J. 2014, 8, 31–39. [Google Scholar] [CrossRef]
- Sunagawa, S.; Desantis, T.Z.; Piceno, Y.M.; Brodie, E.L.; Desalvo, M.K.; Voolstra, C.R.; Weil, E.; Andersen, G.L.; Medina, M. Bacterial diversity and White Plague Disease-associated community changes in the Caribbean coral Montastraea faveolata. ISME J. 2009, 3, 512–521. [Google Scholar] [CrossRef]
- Rud, I.; Kolarevic, J.; Holan, A.B.; Berget, I.; Calabrese, S.; Terjesen, B.F. Deep-sequencing of the bacterial microbiota in commercial-scale recirculating and semi-closed aquaculture systems for Atlantic salmon post-smolt production. Aquac. Eng. 2016, 78, 50–62. [Google Scholar] [CrossRef]
- Rebecca, V.T.; Burkepile, D.E.; Correa, A.; Thurber, A.R.; Shantz, A.A.; Rory, W.; Catharine, P.; Stephanie, R.; Voolstra, C.R. Macroalgae Decrease Growth and Alter Microbial Community Structure of the Reef-Building Coral, Porites astreoides. PLoS ONE 2012, 7, e44246. [Google Scholar]
- Wichard, T. Exploring bacteria-induced growth and morphogenesis in the green macroalga order Ulvales (Chlorophyta). Front. Plant Sci. 2015, 6, 86. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Feng, Q.; Zhang, B.; Gao, J.; Zhang, X. Marinobacter alexandrii sp. nov., a novel yellow-pigmented and algae growth-promoting bacterium isolated from marine phycosphere microbiota. Antonie Van Leeuwenhoek 2021, 114, 709–718. [Google Scholar] [CrossRef]
- Dungan, A.M.; Bulach, D.; Lin, H.; van Oppen, M.J.H.; Blackall, L.L. Development of a free radical scavenging bacterial consortium to mitigate oxidative stress in cnidarians. Microb. Biotechnol. 2021, 14, 2025–2040. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.M.; Maher, R.L.; Thurber, R.V.; Burkepile, D.E. Different nitrogen sources speed recovery from corallivory and uniquely alter the microbiome of a reef-building coral. PeerJ 2019, 7, e8056. [Google Scholar] [CrossRef] [PubMed]
- Zozaya-Valdés, E.; Roth-Schulze, J.A.; Egan, S.; Thomas, T. Microbial community function in the bleaching disease of the marine macroalgae Delisea pulchra. Environ. Microbiol. 2017, 19, 3012–3024. [Google Scholar] [CrossRef]
- Chimetto, T.; Thompson, J.R.; Moreira, A.; Garcia, G.D.; Kevin, P.; Rachelle, L.; Berlinck, R.; Thompson, C.C.; Thompson, F.L. Quantitative Detection of Active Vibrios Associated with White Plague Disease in Mussismilia braziliensis Corals. Front. Microbiol. 2017, 8, 2272. [Google Scholar] [CrossRef]
- Rosenberg, E.; Ben-Haim, Y. Microbial diseases of corals and global warming. Environ. Microbiol. 2002, 4, 318–328. [Google Scholar] [CrossRef]
- Sweet, M.; Bythell, J. Ciliate and bacterial communities associated with White Syndrome and Brown Band Disease in reef-building corals. Environ. Microbiol. 2012, 14, 3288. [Google Scholar] [CrossRef]
- Sweet, M.J.; Bythell, J.C.; Nugues, M.M. Algae as Reservoirs for Coral Pathogens. PLoS ONE 2013, 8, e69717. [Google Scholar] [CrossRef]
- Rahmi; Jompa, J.; Tahir, A. Transmission of Desulfovibrio salexigens DSM2638 bacteria in Pachyseris involuta-infection rate and changes in coral morphology at different temperatures. IOP Conf. Ser. Earth Environ. Sci. 2021, 763, 012055. [Google Scholar] [CrossRef]
- Scott, G.; Elizabeth, B.; James, B.; Lily, P.; Mónica, M. The Role of Coral-Associated Bacterial Communities in Australian Subtropical White Syndrome of Turbinaria mesenterina. PLoS ONE 2012, 7, e44243. [Google Scholar]
- Cooney, R.P.; Pantos, O.; le Tissier, M.D.A.; Barer, M.R.; O’Donnell, A.G.; Bythell, J.C. Characterization of the bacterial consortium associated with black band disease in coral using molecular microbiological techniques. Environ. Microbiol. 2002, 4, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Bourne, D.G.; Morrow, K.M.; Webster, N.S. Insights into the Coral Microbiome: Underpinning the Health and Resilience of Reef Ecosystems. Annu. Rev. Microbiol. 2016, 70, 317–340. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Portillo, E.; Santos, F.; Martínez-García, M.; Ríos, A.d.L.; Anton, J. Structure and temporal dynamics of the bacterial communities associated to microhabitats of the coral Oculina patagonica. Environ. Microbiol. 2016, 18, 4564–4578. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Yu, K.; Liang, J.; Yao, Q.; Chen, B. Significant Changes in Microbial Communities Associated With Reef Corals in the Southern South China Sea During the 2015/2016 Global-Scale Coral Bleaching Event. J. Geophys. Res. Oceans 2020, 125, e2019jc015579. [Google Scholar] [CrossRef]
- Ziegler, M.; Seneca, F.O.; Yum, L.K.; Palumbi, S.R.; Voolstra, C.R. Bacterial community dynamics are linked to patterns of coral heat tolerance. Nat. Commun. 2017, 8, 14213. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, N.M.; Kline, D.I.; Sandin, S.A.; Rohwer, F. Pathologies and mortality rates caused by organic carbon and nutrient stressors in three Caribbean coral species. Mar. Ecol. Prog. Ser. 2005, 294, 173–180. [Google Scholar] [CrossRef]
- Mitchell, R.; Chet, I. Bacterial attack of corals in polluted seawater. Microb. Ecol. 1975, 2, 227–233. [Google Scholar] [CrossRef]
- Fong, J.; Lim, Z.W.; Bauman, A.G.; Valiyaveettil, S.; Liao, L.M.; Yip, Z.T.; Todd, P.A. Allelopathic effects of macroalgae on Pocillopora acuta coral larvae. Mar. Environ. Res. 2019, 151, 104745.1–104745.8. [Google Scholar] [CrossRef]
- Nagelkerken, I.; Goldenberg, S.U.; Coni, E.; Connell, S.D. Microhabitat change alters abundances of competing species and decreases species richness under ocean acidification. Sci. Total Environ. 2018, 645, 615–622. [Google Scholar] [CrossRef]
- Diaz-Pulido, G.; Gouezo, M.; Tilbrook, B.; Dove, S.; Anthony, K.R.N. High CO2 enhances the competitive strength of seaweeds over corals. Ecol. Lett. 2011, 14, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.M.; Putnam, H.M.; Burkepile, D.E.; Adam, T.C.; Thurber, R.V.; Silbiger, N.J. Chronic low-level nutrient enrichment benefits coral thermal performance in a fore reef habitat. Coral Reefs 2021, 40, 1637–1655. [Google Scholar] [CrossRef]
- Shaver, E.C.; Shantz, A.A.; McMinds, R.; Burkepile, D.E.; Vega Thurber, R.L.; Silliman, B.R. Effects of predation and nutrient enrichment on the success and microbiome of a foundational coral. Ecology 2017, 98, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, J.P.; Smith, L.D.; Heyward, A.J.; Baird, A.H.; Pratchett, M.S. Recovery of an Isolated Coral Reef System Following Severe Disturbance. Science 2013, 340, 69–71. [Google Scholar] [CrossRef]
- Muthukrishnan, R.; Fong, P. Rapid recovery of a coral dominated Eastern Tropical Pacific reef after experimentally produced anthropogenic disturbance. Mar. Environ. Res. 2018, 139, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Duran, A.; Collado-Vides, L.; Palma, L.; Burkepile, D.E. Correction to: Interactive effects of herbivory and substrate orientation on algal community dynamics on a coral reef. Mar. Biol. 2018, 165, 156. [Google Scholar] [CrossRef]
- Gamazo, A.A.; Randle, J.L.; Garcia, F.C.; Rossbach, S.; Ellis, J.I.; Weinzierl, M.; Duarte, C.M. Differential thermal tolerance between algae and corals may trigger the proliferation of algae in coral reefs. Glob. Chang. Biol. 2020, 26, 4316–4327. [Google Scholar]
- Williams, S.M. The reduction of harmful algae on Caribbean coral reefs through the reintroduction of a keystone herbivore, the long-spined sea urchin Diadema antillarum. Restor. Ecol. 2021, 30, e13475. [Google Scholar] [CrossRef]
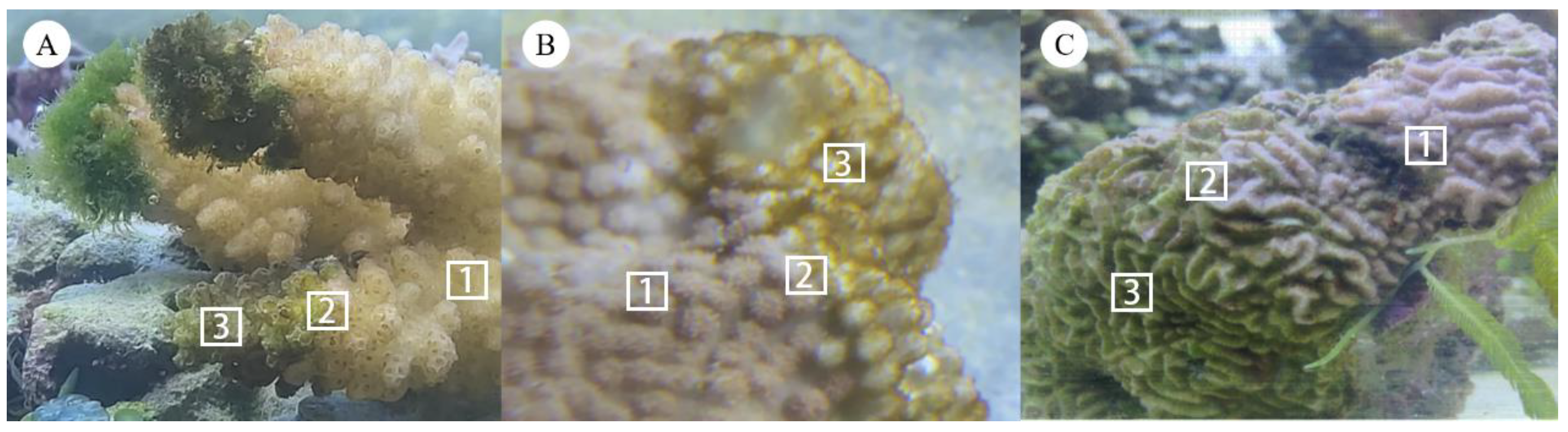
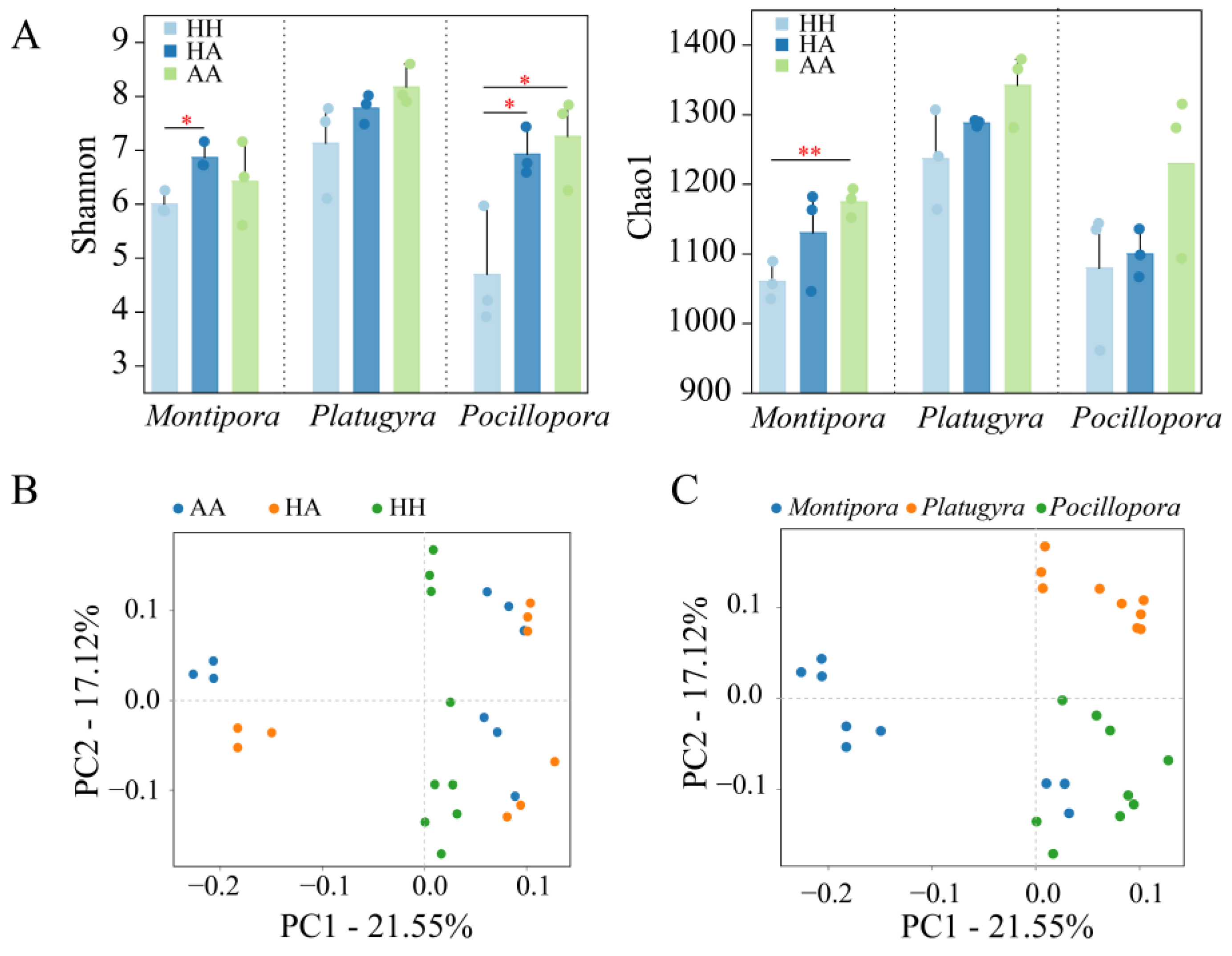

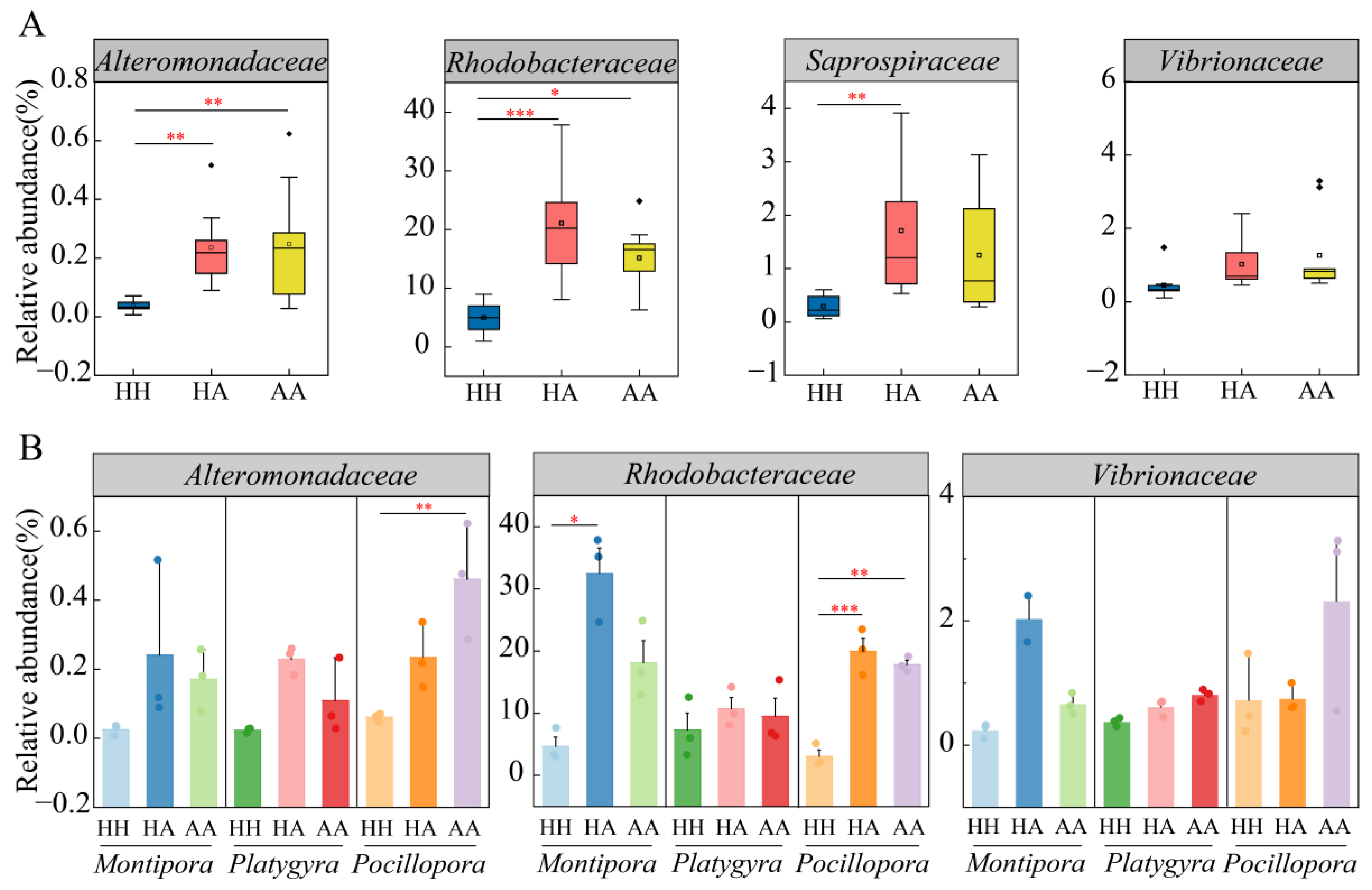

| Coral Genus | Condition | No. of Replicates | Richness Estimates | Diversity Estimates |
|---|---|---|---|---|
| OTUs | Simpson | |||
| Mean | ||||
| Montipora sp. | HH | 3 | 1048 ± 37 | 0.94 ±0.00 |
| HA | 3 | 1196 ± 16 | 0.97 ± 0.01 | |
| AA | 3 | 1209 ± 21 | 0.95 ± 0.02 | |
| Platygyra sp. | HH | 3 | 929 ± 71 | 0.96 ± 0.02 |
| HA | 3 | 970 ± 36 | 0.97 ± 0.01 | |
| AA | 3 | 995 ± 25 | 0.99 ± 0.00 | |
| Pocillopora sp. | HH | 3 | 714 ± 91 | 0.86 ± 0.04 |
| HA | 3 | 974 ± 19 | 0.97 ± 0.01 | |
| AA | 3 | 1106 ± 90 | 0.97 ± 0.01 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, C.; Zhang, Q.; Huang, Q.; Wang, S.; Qin, X.; Ren, T.; Xie, R.; Su, H. Significant Shifts in Microbial Communities Associated with Scleractinian Corals in Response to Algae Overgrowth. Microorganisms 2022, 10, 2196. https://doi.org/10.3390/microorganisms10112196
Lu C, Zhang Q, Huang Q, Wang S, Qin X, Ren T, Xie R, Su H. Significant Shifts in Microbial Communities Associated with Scleractinian Corals in Response to Algae Overgrowth. Microorganisms. 2022; 10(11):2196. https://doi.org/10.3390/microorganisms10112196
Chicago/Turabian StyleLu, Chunrong, Qi Zhang, Qinyu Huang, Shuying Wang, Xiao Qin, Tianfei Ren, Rufeng Xie, and Hongfei Su. 2022. "Significant Shifts in Microbial Communities Associated with Scleractinian Corals in Response to Algae Overgrowth" Microorganisms 10, no. 11: 2196. https://doi.org/10.3390/microorganisms10112196
APA StyleLu, C., Zhang, Q., Huang, Q., Wang, S., Qin, X., Ren, T., Xie, R., & Su, H. (2022). Significant Shifts in Microbial Communities Associated with Scleractinian Corals in Response to Algae Overgrowth. Microorganisms, 10(11), 2196. https://doi.org/10.3390/microorganisms10112196






