Legacy Effects of Biochar and Compost Addition on Arbuscular Mycorrhizal Fungal Community and Co-Occurrence Network in Black Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Description and Environmental Design
2.2. Soil Sampling, Aggregate Fractionation and Determination of Soil Variables
2.3. Miseq Sequencing and Bioinformatics
2.4. Data Analysis
3. Results
3.1. Soil Physiochemical Variables
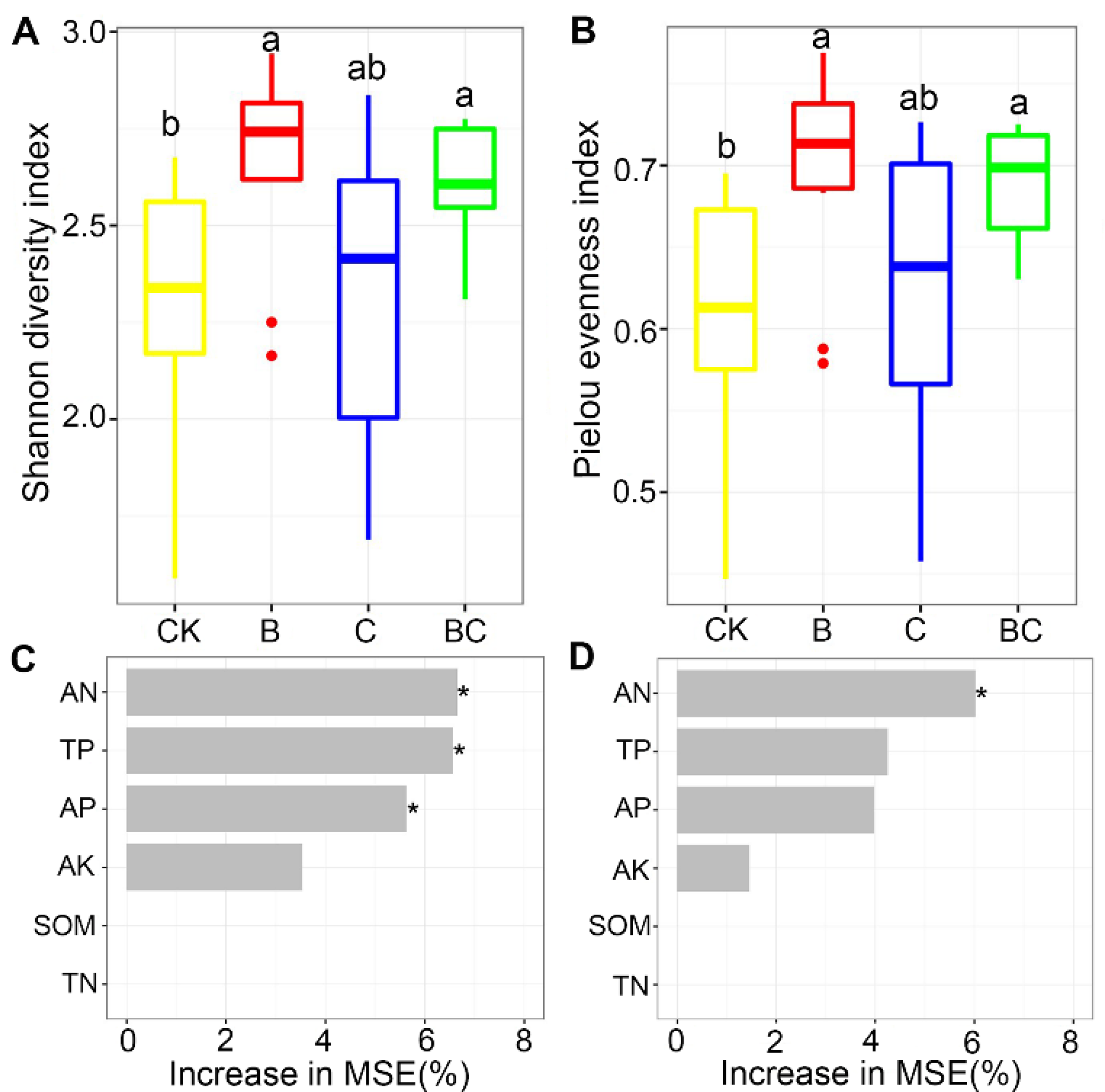
3.2. AM Fungal Diversity
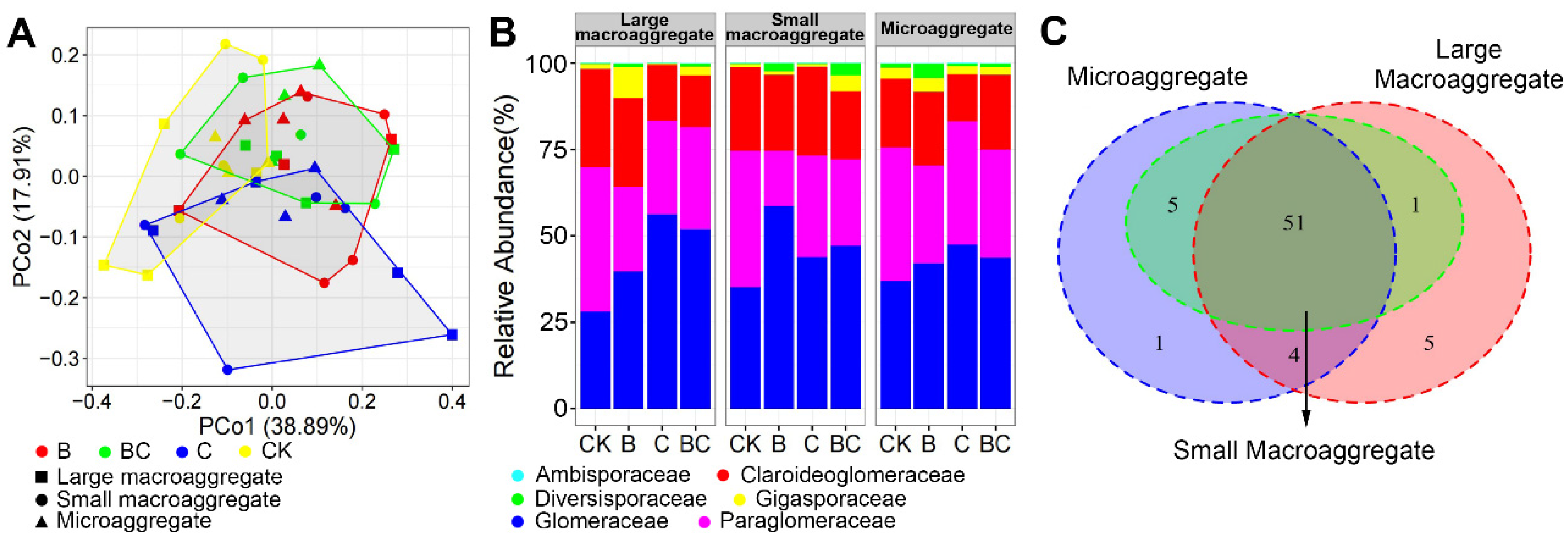
3.3. AM Fungal Community Composition
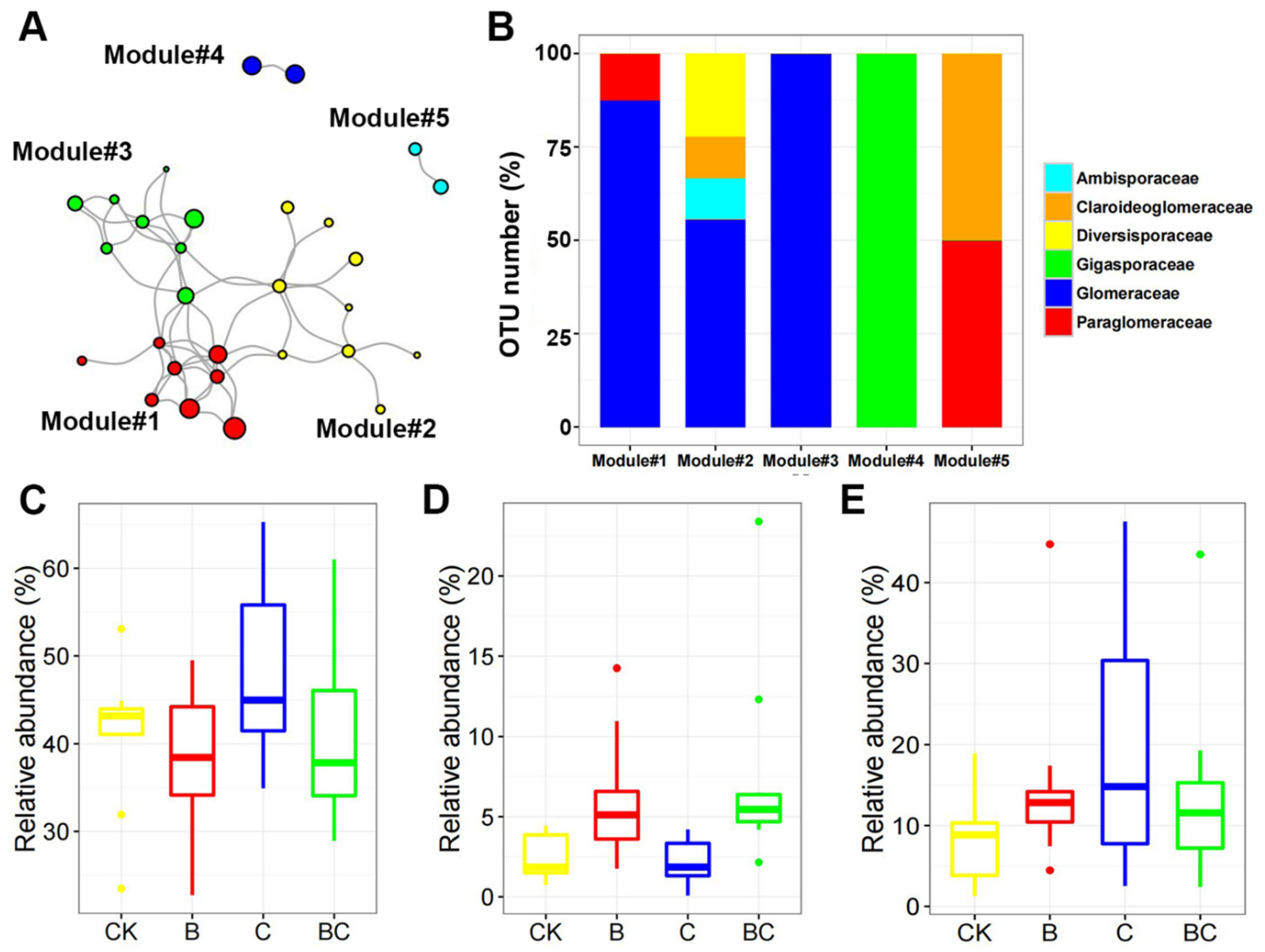
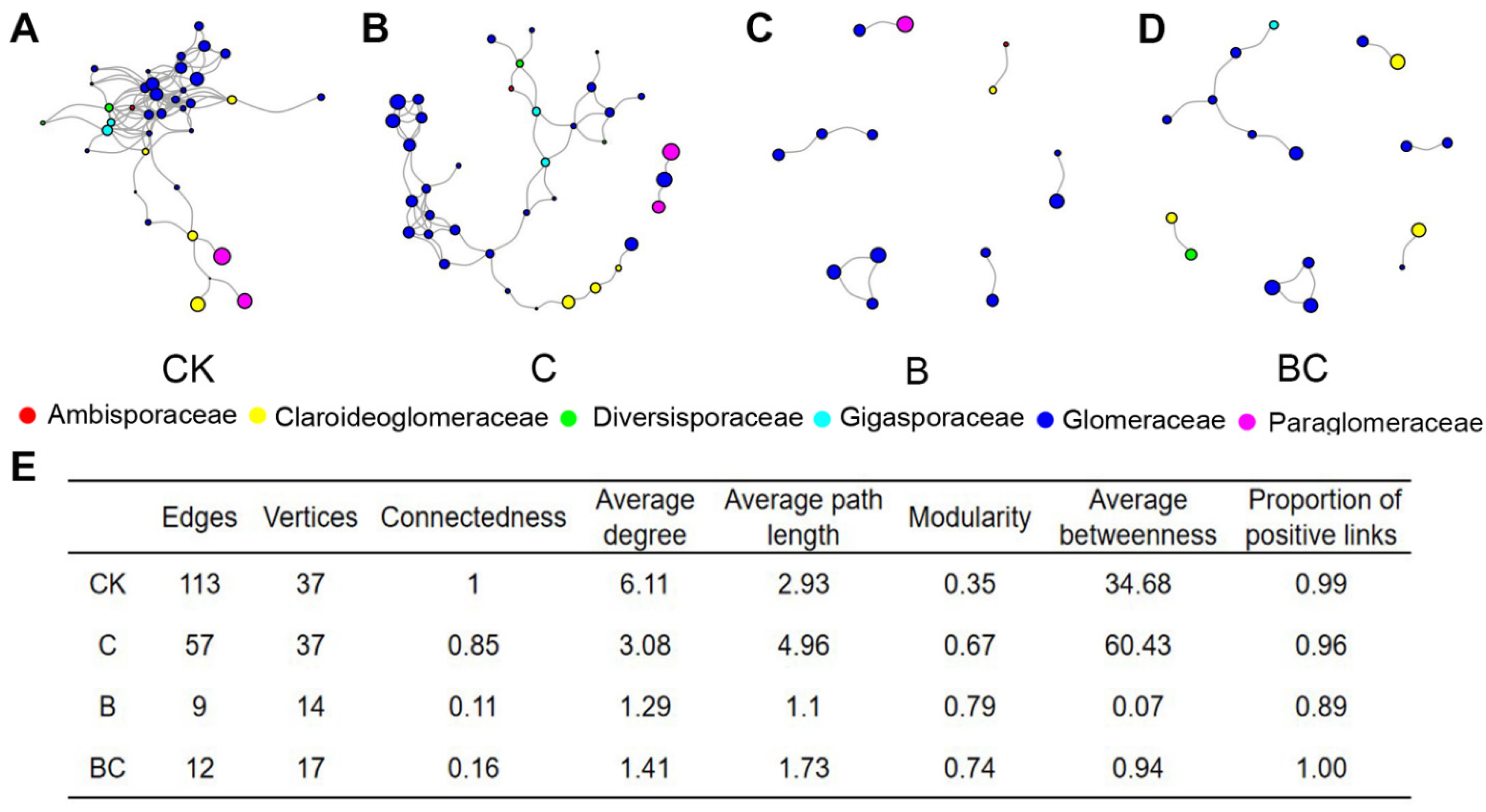
3.4. AM Fungal Co-Occurrence Network
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ding, X.; Han, X.; Liang, Y.; Qiao, Y.; Li, L.; Li, N. Changes in soil organic carbon pools after 10 years of continuous manuring combined with chemical fertilizer in a Mollisol in China. Soil Till. Res. 2012, 122, 36–41. [Google Scholar] [CrossRef]
- Liu, X.; Burras, C.L.; Kravchenko, Y.S.; Duran, A.; Huffman, T.; Morras, H.; Studdert, G.; Zhang, X.; Cruse, R.M.; Yuan, X. Overview of Mollisols in the world: Distribution, land use and management. Can. J. Soil Sci. 2017, 92, 383–402. [Google Scholar] [CrossRef]
- Yao, Q.; Liu, J.; Yu, Z.; Li, Y.; Jin, J.; Liu, X.; Wang, G. Changes of bacterial community compositions after three years of biochar application in a black soil of northeast China. Appl. Soil Ecol. 2017, 113, 11–21. [Google Scholar] [CrossRef]
- Hussain, S.S.; Ara, T.; Ahmad, F.R.; Gani, G.; Hussain, N.; Hussain, M.; Dar, S.R. Quality evaluation of different forms of compost and their effect in comparison with inorganic fertilizers on growth and yield attributes of wheat (Triticum aestivum L.). J. Agric. Sci. 2015, 7, 154–160. [Google Scholar] [CrossRef][Green Version]
- Wei, D.; Yang, Q.; Zhang, J.; Wang, S.; Chen, X.; Zhang, X.; Li, W. Bacterial community structure and diversity in a black soil as affected by long-term fertilization. Pedosphere 2008, 18, 582–592. [Google Scholar] [CrossRef]
- Xie, H.T.; Li, J.W.; Zhu, P.; Peng, C.; Wang, J.K.; He, H.B.; Zhang, X.D. Long-term manure amendments enhance neutral sugar accumulation in bulk soil and particulate organic matter in a Mollisol. Soil Biol. Biochem. 2014, 78, 45–53. [Google Scholar] [CrossRef]
- Hammesfahr, U.; Bierl, R.; Thiele-Bruhn, S. Combined effects of the antibiotic sulfadiazine and liquid manure on the soil microbial-community structure and functions. J. Plant Nutr. Soil Sci. 2011, 174, 614–623. [Google Scholar] [CrossRef]
- Insam, H.; Gómez-Brandón, M.; Ascher, J. Manure-based biogas fermentation residues—Friend or foe of soil fertility? Soil Biol. Biochem. 2015, 84, 1–14. [Google Scholar] [CrossRef]
- Xie, H.; Li, J.; Zhang, B.; Wang, L.; Wang, J.; He, H.; Zhang, X. Long-term manure amendments reduced soil aggregate stability via redistribution of the glomalin-related soil protein in macroaggregates. Sci. Rep. 2015, 5, 14687. [Google Scholar] [CrossRef]
- Bello, A.; Deng, L.; Sheng, S.; Jiang, X.; Yang, W.; Meng, Q.; Wu, X.; Han, Y.; Zhu, H.; Xu, X. Biochar reduces nutrient loss and improves microbial biomass of composted cattle manure and maize straw. Biotechnol. Appl. Biochem. 2020, 67, 799–811. [Google Scholar] [CrossRef]
- Sánchez-Monedero, M.A.; Cayuela, M.L.; Roig, A.; Jindo, K.; Mondini, C.; Bolan, N. Role of biochar as an additive in organic waste composting. Bioresour. Technol. 2018, 247, 1155–1164. [Google Scholar] [CrossRef]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef]
- Novak, J.M.; Cantrell, K.B.; Watts, D.W. Compositional and thermal evaluation of lignocellulosic and poultry litter chars via high and low temperature pyrolysis. Bioenerg. Res. 2013, 6, 114–130. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H.Y. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Van Laer, T.; de Smedt, P.; Ronsse, F.; Ruysschaert, G.; Boeckx, P.; Verstraete, W.; Buysse, J.; Lavrysen, L.J. Legal constraints and opportunities for biochar: A case analysis of EU law. GCB Bioenergy 2015, 7, 14–24. [Google Scholar] [CrossRef]
- Godlewska, P.; Ok, Y.S.; Oleszczuk, P. The dark side of black gold: Ecotoxicological aspects of biochar and biochar-amended soils. J. Hazard. Mater. 2021, 403, 123833. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.P.; Cowie, A.L.; Smernik, R.J. Biochar Carbon Stability in a Clayey Soil as a Function of Feedstock and Pyrolysis Temperature. Environ. Sci. Technol. 2012, 46, 11770–11778. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Song, W.; Tian, J. Biochar-facilitated soil remediation: Mechanisms and efficacy variations. Front. Environ. Sci. 2020, 8, 521512. [Google Scholar] [CrossRef]
- Dong, X.; Singh, B.P.; Li, G.; Lin, Q.; Zhao, X. Biochar increased field soil inorganic carbon content five years after application. Soil Till. Res. 2019, 186, 36–41. [Google Scholar] [CrossRef]
- Steiner, C.; Teixeira, W.G.; Lehmann, J.; Nehls, T.; Macêdo, J.L.V.d.; Blum, W.E.H.; Zech, W. Long term eVects of manure, charcoal and mineral fertilization on crop production and fertility on a highly weathered Central Amazonian upland soil. Plant Soil 2007, 291, 275–290. [Google Scholar] [CrossRef]
- Berruti, A.; Lumini, E.; Balestrini, R.; Bianciotto, V. Arbuscular mycorrhizal fungi as natural biofertilizers: Let’s benefit from past successes. Front. Microbiol. 2016, 6, 1559. [Google Scholar] [CrossRef] [PubMed]
- Cavagnaro, T.R. Impacts of compost application on the formation and functioning of arbuscular mycorrhizas. Soil Biol. Biochem. 2014, 78, 38–44. [Google Scholar] [CrossRef]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; De-Pascale, S.; Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic-Amst. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Püschel, D.; Janoušková, M.; Hujslová, M.; Slavíková, R.; Gryndlerová, H.; Jansa, J. Plant-fungus competition for nitrogen erases mycorrhizal growth benefits of Andropogon gerardii under limited nitrogen supply. Ecol. Evol. 2016, 6, 4332–4346. [Google Scholar] [CrossRef] [PubMed]
- Sikes, B.A.; Cottenie, K.; Klironomos, J.N. Plant and fungal identity determines pathogen protection of plant roots by arbuscular mycorrhizas. J. Ecol. 2009, 97, 1274–1280. [Google Scholar] [CrossRef]
- Igiehon, N.O.; Babalola, O.O.; Cheseto, X.; Torto, B. Effects of rhizobia and arbuscular mycorrhizal fungi on yield, size distribution and fatty acid of soybean seeds grown under drought stress. Microbiol. Res. 2021, 242, 126640. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Miller, R.M. Carbon cycling by arbuscular mycorrhizal fungi in soil–plant systems. Trends Plant Sci. 2003, 8, 407–409. [Google Scholar] [CrossRef]
- Gu, S.; Wu, S.; Guan, Y.; Zhai, C.; Zhang, Z.; Bello, A.; Guo, X.; Yang, W. Arbuscular mycorrhizal fungal community was affected by tillage practices rather than residue management in black soil of northeast China. Soil Till. Res. 2020, 198, 104552. [Google Scholar] [CrossRef]
- Agbodjato, N.A.; Assogba, S.A.; Babalola, O.O.; Koda, A.D.; Aguegue, R.M.; Sina, H.; Dagbenonbakin, G.D.; Adjanohoun, A.; Baba-Moussa, L. Formulation of biostimulants based on arbuscular mycorrhizal fungi for maize growth and yield. Front. Agron. 2022, 4, 894489. [Google Scholar] [CrossRef]
- Fasusi, O.A.; Amoo, A.E.; Babalola, O.O. Propagation and characterization of viable arbuscular mycorrhizal fungal spores within maize plant. J. Sci. Food Agric. 2021, 101, 5834–5841. [Google Scholar] [CrossRef]
- Emmanuel, O.C.; Babalola, O.O. Productivity and quality of horticultural crops through co-inoculation of arbuscular mycorrhizal fungi and plant growth-promoting bacteria. Microbiol. Res. 2020, 239, 126569. [Google Scholar] [CrossRef]
- Igiehon, N.O.; Babalola, O.O. Biofertilizers and sustainable agriculture: Exploring arbuscular mycorrhizal fungi. Appl. Microbiol. Biot. 2017, 101, 4871–4881. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Gu, S.; Xin, Y.; Bello, A.; Sun, W.; Xu, X. Compost addition enhanced hyphal growth and sporulation of arbuscular mycorrhizal fungi without affecting their community composition in the soil. Front. Microbiol. 2018, 9, 169. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, A.; Aggarwal, A.; Yadav, A.; Parkash, V. Screening and selection of efficient host and sugarcane bagasse as substrate for mass multiplication of Funneliformis mosseae. Biol. Agric. Hortic. 2013, 29, 107–117. [Google Scholar] [CrossRef]
- Cavagnaro, T.R. Biologically regulated nutrient supply systems: Compost and arbuscular mycorrhizas-a review. Adv. Agron. 2015, 129, 293–321. [Google Scholar]
- Gryndler, M.; Hrselova, H.; Cajthaml, T.; Havrankova, M.; Rezacova, V.; Gryndlerova, H.; Larsen, J. Influence of soil organic matter decomposition on arbuscular mycorrhizal fungi in terms of asymbiotic hyphal growth and root colonization. Mycorrhiza 2009, 19, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.X.; Douds, D.D.; Boateng, A.A. Effect of biochar soil-amendments on Allium porrum growth and arbuscular mycorrhizal fungus colonization. J. Plant Nutr. 2016, 39, 1654–1662. [Google Scholar] [CrossRef]
- Amendola, C.; Montagnoli, A.; Terzaghi, M.; Trupiano, D.; Oliva, F.; Baronti, S.; Miglietta, F.; Chiatante, D.; Scippa, G.S. Short-term effects of biochar on grapevine fine root dynamics and arbuscular mycorrhizae production. Agric. Ecosyst. Environ. 2017, 239, 236–245. [Google Scholar] [CrossRef]
- Efthymiou, A.; Jensen, B.; Jakobsen, I. The roles of mycorrhiza and Penicillium inoculants in phosphorus uptake by biochar-amended wheat. Soil Biol. Biochem. 2018, 127, 168–177. [Google Scholar] [CrossRef]
- Figueiredo, C.C.d.; Farias, W.M.; Coser, T.R.; Monteiro de Paula, A.; Sartori da Silva, M.R.; Paz-Ferreiro, J. Sewage sludge biochar alters root colonization of mycorrhizal fungi in a soil cultivated with corn. Eur. J. Soil Biol. 2019, 93, 103092. [Google Scholar] [CrossRef]
- Yan, T.; Xue, J.; Zhou, Z.; Wu, Y. Impacts of biochar-based fertilization on soil arbuscular mycorrhizal fungal community structure in a karst mountainous area. Environ. Sci. Pollut. R. 2021, 28, 66420–66434. [Google Scholar] [CrossRef]
- Davison, J.; Moora, M.; Semchenko, M.; Adenan, S.B.; Ahmed, T.; Akhmetzhanova, A.A.; Alatalo, J.M.; Al-Quraishy, S.; Andriyanova, E.; Anslan, S.; et al. Temperature and pH define the realised niche space of arbuscular mycorrhizal fungi. New Phytol. 2021, 231, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hart, M.; Kong, Z. The distribution of arbuscular mycorrhizal fungal communities at soil aggregate level in subtropical grasslands. Arch. Agron. Soil Sci. 2021, 68, 1755–1767. [Google Scholar] [CrossRef]
- Yang, W.; Guo, Y.; Wang, X.; Chen, C.; Hu, Y.; Cheng, L.; Gu, S.; Xu, X. Temporal variations of soil microbial community under compost addition in black soil of Northeast China. Appl. Soil Ecol. 2017, 121, 214–222. [Google Scholar] [CrossRef]
- Bach, E.M.; Hofmockel, K.S. Soil aggregate isolation method affects measures of intra-aggregate extracellular enzyme activity. Soil Biol. Biochem. 2014, 69, 54–62. [Google Scholar] [CrossRef]
- Schwarzott, D.; Schüßler, A. A simple and reliable method for SSU rRNA gene DNA extraction, amplification, and cloning from single AM fungal spores. Mycorrhiza 2001, 10, 203–207. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Young, J.P.W. Improved PCR primers for the detection and identification of arbuscular mycorrhizal fungi. FEMS Microbiol. Ecol. 2008, 65, 339–349. [Google Scholar] [CrossRef]
- Simon, L.; Lalonde, M.; Bruns, T.D. Specific amplification of 18S fungal ribosomal genes from vesicular-arbuscular endomycorrhizal fungi colonizing roots. Appl. Environ. Microbiol. 1992, 58, 291–295. [Google Scholar] [CrossRef]
- Lumini, E.; Orgiazzi, A.; Borriello, R.; Bonfante, P.; Bianciotto, V. Disclosing arbuscular mycorrhizal fungal biodiversity in soil through a land-use gradient using a pyrosequencing approach. Environ. Microbiol. 2010, 12, 2165–2179. [Google Scholar] [CrossRef]
- Yang, W.; Yang, Z.; Guan, Y.; Zhai, C.; Shi, D.; Chen, J.; Wang, T.; Gu, S. Dose-dependent effect of compost amendment on soil bacterial community composition and co-occurrence network patterns in soybean agroecosystem. Arch. Agron. Soil Sci. 2019, 66, 1027–1041. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Opensource, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microb. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive tree of life (iTOL) v3: An online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016, 44, W242–W245. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random forest. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and regression by randomForest. R News. 2002, 2, 18–22. [Google Scholar]
- Goslee, S.C.; Urban, D.L. The ecodist package for dissimilarity-based analysis of ecological data. J. Stat. Softw. 2007, 22, 1–19. [Google Scholar] [CrossRef]
- Widder, S.; Besemer, K.; Singer, G.A.; Ceola, S.; Bertuzzo, E.; Quince, C.; Sloan, W.T.; Rinaldo, A.; Battin, T.J. Fluvial network organization imprints on microbial co-occurrence networks. Proc. Natl. Acad. Sci. USA 2014, 111, 12799–12804. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Feng, Y. The effects of biochar addition on soil physicochemical properties: A review. Catena 2021, 202, 105284. [Google Scholar] [CrossRef]
- Major, J.; Rondon, M.; Molina, D.; Riha, S.J.; Lehmann, J. Maize yield and nutrition during 4 years after biochar application to a Colombian savanna oxisol. Plant Soil 2010, 333, 117–128. [Google Scholar] [CrossRef]
- Laird, D.A.; Novak, J.M.; Collins, H.P.; Ippolito, J.A.; Karlen, D.L.; Lentz, R.D.; Sistani, K.R.; Spokas, K.; Van Pelt, R.S. Multi-year and multi-location soil quality and crop biomass yield responses to hardwood fast pyrolysis biochar. Geoderma 2017, 289, 46–53. [Google Scholar] [CrossRef]
- Zhang, M.; Riaz, M.; Xia, H.; Li, Y.; Wang, X.; Jiang, C. Four-year biochar study: Positive response of acidic soil microenvironment and citrus growth to biochar under potassium deficiency conditions. Sci. Total Environ. 2022, 813, 152515. [Google Scholar] [CrossRef] [PubMed]
- Curaqueo, G.; Roldán, A.; Mutis, A.; Panichini, M.; Pérez-San Martín, A.; Meier, S.; Mella, R. Effects of biochar amendment on wheat production, mycorrhizal status, soil microbial community, and properties of an Andisol in Southern Chile. Field Crop. Res. 2021, 273, 108306. [Google Scholar] [CrossRef]
- Zackrisson, O.; Nilsson, M.C.; Wardle, D.A. Key ecological function of charcoal from wildfire in the Boreal forest. Oikos 1996, 77, 10–19. [Google Scholar] [CrossRef]
- Pietikainen, J.; Kiikkila, O.; Fritze, H. Charcoal as a habitat for microbes and its effect on the microbial community of the underlying humus. Oikos 2000, 89, 231–242. [Google Scholar] [CrossRef]
- Warnock, D.D.; Lehmann, J.; Kuyper, T.W.; Rillig, M.C. Mycorrhizal responses to biochar in soil—Concepts and mechanisms. Plant Soil 2007, 300, 9–20. [Google Scholar] [CrossRef]
- Thies, J.E.; Rillig, M.C. Characteristics of biochar: Biological properties. In Biochar for Environmental Management: Science and Technology; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2009; pp. 85–105. [Google Scholar]
- Quilliam, R.S.; Glanville, H.C.; Wade, S.C.; Jones, D.L. Life in the ‘charosphere’—Does biochar in agricultural soil provide a significant habitat for microorganisms? Soil Biol. Biochem. 2013, 65, 287–293. [Google Scholar] [CrossRef]
- Jaafar, N.M.; Clode, P.L.; Abbott, L.K. Microscopy observations of habitable space in biochar for colonization by fungal hyphae from soil. J. Integr. Agric. 2014, 13, 483–490. [Google Scholar] [CrossRef]
- Lourenço, K.S.; Suleiman, A.K.A.; Pijl, A.; van Veen, J.A.; Cantarella, H.; Kuramae, E.E. Resilience of the resident soil microbiome to organic and inorganic amendment disturbances and to temporary bacterial invasion. Microbiome 2018, 6, 142. [Google Scholar] [CrossRef]
- Lord, R.; Sakrabani, R. Ten-year legacy of organic carbon in non-agricultural (brownfield) soils restored using green waste compost exceeds 4 per mille per annum: Benefits and trade-offs of a circular economy approach. Sci. Total Environ. 2019, 686, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Annabi, M.; Houot, S.; Francou, C.; Poitrenaud, M.; Bissonnais, Y.L. Soil Aggregate Stability Improvement with Urban Composts of Different Maturities. Soil Sci. Soc. Am. J. 2007, 71, 413–423. [Google Scholar] [CrossRef]
- Tripathy, R.; Singh, A.K. Effect of water and nitrogen management on aggregate size and carbon enrichment of soil in rice-wheat cropping system. J. Plant Nutr. Soil Sci. 2004, 167, 216–228. [Google Scholar] [CrossRef]
- Singh, G.; Jalota, S.K.; Singh, Y. Manuring and residue management effects on physical properties of a soil under the rice-wheat system in Punjab. India Soil Till. Res. 2007, 94, 229–238. [Google Scholar] [CrossRef]
- Whalen, J.K.; Hu, Q.; Liu, A. Compost applications increase water-stable aggregates in conventional and no-tillage systems. Soil Sci. Soc. Am. J. 2003, 67, 1842–1847. [Google Scholar] [CrossRef]
- Muneer, M.A.; Wang, P.; Zhang, J.; Li, Y.; Munir, M.Z.; Ji, B. Formation of common mycorrhizal networks significantly affects plant biomass and soil properties of the neighboring plants under various nitrogen levels. Microorganisms 2020, 8, 230. [Google Scholar] [CrossRef]
- Deveau, A.; Bonito, G.; Uehling, J.; Paoletti, M.; Becker, M.; Bindschedler, S.; Hacquard, S.; Hervé, V.; Labbé, J.; Lastovetsky, O.A.; et al. Bacterial-fungal interactions: Ecology, mechanisms and challenges. FEMS Microbiol. Rev. 2018, 42, 335–352. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Subbotina, I.; Chen, H.; Bogomolova, I.; Xu, X. Black carbon decomposition and incorporation into soil microbial biomass estimated by 14C labeling. Soil Biol. Biochem. 2009, 41, 210–219. [Google Scholar] [CrossRef]
- Warnock, D.D.; Mummey, D.L.; McBride, B.; Major, J.; Lehmann, J.; Rillig, M.C. Influences of non-herbaceous biochar on arbuscular mycorrhizal fungal abundances in roots and soils: Results from growth-chamber and field experiments. Appl. Soil Ecol. 2010, 46, 450–456. [Google Scholar] [CrossRef]
- Qian, L.B.; Chen, B.L. Interactions of Aluminum with Biochars and Oxidized Biochars: Implications for the Biochar Aging Process. J. Agric. Food Chem. 2014, 62, 373–380. [Google Scholar] [CrossRef]
- Sheng, Y.Q.; Zhu, L.Z. Biochar alters microbial community and carbon sequestration potential across different soil pH. Sci. Total Environ. 2018, 622, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Mardatin, N.F.; Leifheit, E.F.; Antunes, P.M. Mycelium of arbuscular mycorrhizal fungi increases soil water repellency and is sufficient to maintain water-stable soil aggregates. Soil Biol. Biochem. 2010, 42, 1189–1191. [Google Scholar] [CrossRef]
- Jeewani, P.H.; Luo, Y.; Yu, G.H.; Fu, Y.Y.; He, X.H.; Zwieten, L.; Liang, C.; Kumar, A.; He, Y.; Kuzyakov, Y.; et al. Arbuscular mycorrhizal fungi and goethite promote carbon sequestration via hyphal-aggregate mineral interactions. Soil Biol. Biochem. 2021, 162, 108417. [Google Scholar] [CrossRef]
- Leifheit, E.F.; Verbruggen, E.; Rillig, M.C. Arbuscular mycorrhizal fungi reduce decomposition of woody plant litter while increasing soil aggregation. Soil Biol. Biochem. 2015, 81, 323–328. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, Y.; Fan, Y.; Babalola, O.O.; Zhang, X.; Yang, W. Legacy Effects of Biochar and Compost Addition on Arbuscular Mycorrhizal Fungal Community and Co-Occurrence Network in Black Soil. Microorganisms 2022, 10, 2137. https://doi.org/10.3390/microorganisms10112137
Xin Y, Fan Y, Babalola OO, Zhang X, Yang W. Legacy Effects of Biochar and Compost Addition on Arbuscular Mycorrhizal Fungal Community and Co-Occurrence Network in Black Soil. Microorganisms. 2022; 10(11):2137. https://doi.org/10.3390/microorganisms10112137
Chicago/Turabian StyleXin, Ying, Yi Fan, Olubukola Oluranti Babalola, Ximei Zhang, and Wei Yang. 2022. "Legacy Effects of Biochar and Compost Addition on Arbuscular Mycorrhizal Fungal Community and Co-Occurrence Network in Black Soil" Microorganisms 10, no. 11: 2137. https://doi.org/10.3390/microorganisms10112137
APA StyleXin, Y., Fan, Y., Babalola, O. O., Zhang, X., & Yang, W. (2022). Legacy Effects of Biochar and Compost Addition on Arbuscular Mycorrhizal Fungal Community and Co-Occurrence Network in Black Soil. Microorganisms, 10(11), 2137. https://doi.org/10.3390/microorganisms10112137






