Incidence of Common Herpesviruses in Colonic Mucosal Biopsies Following Hematopoietic Stem Cell Transplantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Patients
2.3. Endoscopic Sampling and General Examination
2.4. PCR Diagnostics
2.5. Statistics
3. Results
3.1. Relative Frequency of Common Herpesviruses in the Tissue Biopsies
3.2. Incidence of Epstein–Barr Virus and Post-Transplant Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thiagarajan, S.; Neurath, M.F.; Hildner, K. Resolution of acute intestinal graft-versus-host disease. Semin. Immunopathol. 2019, 41, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Whangbo, J.; Ritz, J.; Bhatt, A. Antibiotic-mediated modification of the intestinal microbiome in allogeneic hematopoietic stem cell transplantation. Bone Marrow Transpl. 2017, 52, 183–190. [Google Scholar] [CrossRef]
- Pankratova, O.S.; Chukhlovin, A.B. Time course of immune recovery and viral reactivation following hematopoietic stem cell transplantation. Cell. Ther. Transplant. 2016, 5, 32–43. [Google Scholar] [CrossRef][Green Version]
- Ross, W.A.; Ghosh, S.; Dekovich, A.A.; Liu, S.; Ayers, G.D.; Cleary, K.R.; Lee, J.H.; Couriel, D. Endoscopic biopsy diagnosis of acute gastrointestinal graft-versus-host disease: Rectosigmoid biopsies are more sensitive than upper gastrointestinal biopsies. Am. J. Gastroenterol. 2008, 103, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Terdiman, J.P.; Linker, C.A.; Ries, C.A.; Damon, L.E.; Rugo, H.S.; Ostroff, J.W. The role of endoscopic evaluation in patients with suspected intestinal graft-versus-host disease after allogeneic bone-marrow transplantation. Endoscopy 1996, 28, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Westerhoff, M.; Lamps, L.W. Mucosal biopsy after bone marrow transplantation. Surg. Pathol. Clin. 2017, 10, 909–930. [Google Scholar] [CrossRef]
- Yerushalmy-Feler, A.; Padlipsky, J.; Cohen, S. Diagnosis and management of CMV colitis. Curr. Infect. Dis. Rep. 2019, 21, 5. [Google Scholar] [CrossRef]
- Wang, W.; Chen, X.; Pan, J.; Zhang, X.; Zhang, L. Epstein-Barr virus and human cytomegalovirus infection in intestinal mucosa of Chinese patients with inflammatory bowel disease. Front. Microbiol. 2022, 13, 915453. [Google Scholar] [CrossRef]
- Glucksberg, H.; Storb, R.; Fefer, A.; Buckner, C.D.; Neiman, P.E.; Clift, R.A.; Lerner, K.G.; Thomas, E.D. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 1974, 18, 295–304. [Google Scholar] [CrossRef]
- Lewis, S.J.; Heaton, K.W. Stool form scale as a useful guide to intestinal transit time. Scand. J. Gastroenterol. 1997, 32, 920–924. [Google Scholar] [CrossRef]
- Ganzenmueller, T.; Henke-Gendo, C.; Schlué, J.; Wedemeyer, J.; Huebner, S.; Heim, A. Quantification of cytomegalovirus DNA levels in intestinal biopsies as a diagnostic tool for CMV intestinal disease. J. Clin. Virol. 2009, 46, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Q.; Zeng, L.; Zhang, Q.; Wu, X.Y.; Zhang, M.L.; Jing, X.T.; Wang, Y.F.; Gan, H.T. Clinical features of Epstein-Barr virus in the intestinal mucosa and blood of patients with inflammatory bowel disease. Saudi. J. Gastroenterol. 2020, 26, 312–320. [Google Scholar] [CrossRef]
- Liu, J.; Yan, C.; Zhang, C.; Xu, L.; Liu, Y.; Huang, X. Late-onset Epstein-Barr virus-related disease in acute leukemia patients after haploidentical hematopoietic stem cell transplantation is associated with impaired early recovery of T and B lymphocytes. Clin. Transplant. 2015, 29, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, E.; Satou, A.; Nakamura, M.; Nakamura, S.; Fujishiro, M. Epstein-Barr virus-positive B-cell lymphoproliferative disorder of the gastrointestinal tract. Cancers 2021, 13, 3815. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.L.; Shen, Y.J.; Morgan, D.R.; Thorne, L.; Kenney, S.; Dominguez, R.L.; Gulley, M.L. Epstein-Barr virus infection is common in inflamed gastrointestinal mucosa. Dig. Dis. Sci. 2012, 57, 1887–1898. [Google Scholar] [CrossRef][Green Version]
- Morales, F.; Sánchez-Ponce, Y.; Castorena-Villa, I.; López-Martínez, B.; Parra-Ortega, I.; Escamilla-Núñez, M.C.; Méndez-Tenorio, A.; Pompa-Mera, E.N.; Martinez-Ruiz, G.U.; Fuentes-Pananá, E.M.; et al. Herpesvirus Screening in Childhood Hematopoietic Transplant Reveals High Systemic Inflammation in Episodes of Multiple Viral Detection and an EBV Association with Elevated IL-1_, IL-8 and Graft-Versus-Host Disease. Microorganisms 2022, 10, 1685. [Google Scholar] [CrossRef]
- Gianella, S.; Chaillon, A.; Mutlu, E.A.; Engen, P.A.; Voigt, R.M.; Keshavarzian, A.; Losurdo, J.; Chakradeo, P.; Lada, S.M.; Nakazawa, M.; et al. Effect of cytomegalovirus and Epstein-Barr virus replication on intestinal mucosal gene expression and microbiome composition of HIV-infected and uninfected individuals. AIDS 2017, 31, 2059–2067. [Google Scholar] [CrossRef]
- Styczynski, J.; Tridello, G.; Gil, L.; Ljungman, P.; Hoek, J.; Iacobelli, S.; Ward, K.N.; Cordonnier, C.; Einsele, H.; Socie, G.; et al. Impact of donor Epstein-Barr virus serostatus on the incidence of graft-versus-host disease in patients with acute leukemia after hematopoietic stem-cell transplantation: A study from the Acute Leukemia and Infectious Diseases Working Parties of the European Society for Blood and Marrow Transplantation. J. Clin. Oncol. 2016, 34, 2212–2220. [Google Scholar] [CrossRef]
- Kołodziejczak, M.; Gil, L.; de la Camara, R.; Styczyński, J. Impact of donor and recipient Epstein-Barr Virus serostatus on outcomes of allogeneic hematopoietic cell transplantation: A systematic review and meta-analysis. Ann. Hematol. 2021, 100, 763–777. [Google Scholar] [CrossRef]
- Hosoi, H.; Matsuyama, Y.; Murata, S.; Mushino, T.; Sonoki, T. Prolonged Epstein-Barr virus reactivation coincident with chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Leuk. Lymphoma 2022, 63, 1009–1012. [Google Scholar] [CrossRef]
- Legoff, J.; Resche-Rigon, M.; Bouquet, J.; Robin, M.; Naccache, S.N.; Mercier-Delarue, S.; Federman, S.; Samayoa, E.; Rousseau, C.; Piron, P.; et al. The eukaryotic gut virome in hematopoietic stem cell transplantation: New clues in enteric graft-versus-host disease. Nat. Med. 2017, 23, 1080–1085. [Google Scholar] [CrossRef] [PubMed]
- Jansen, S.A.; Nijhuis, W.; Leavis, H.L.; Riezebos-Brilman, A.; Lindemans, C.A.; Schuurman, R. Broad virus detection and variant discovery in fecal samples of hematopoietic transplant recipients using targeted sequence capture metagenomics. Front. Microbiol. 2020, 11, 560179. [Google Scholar] [CrossRef] [PubMed]

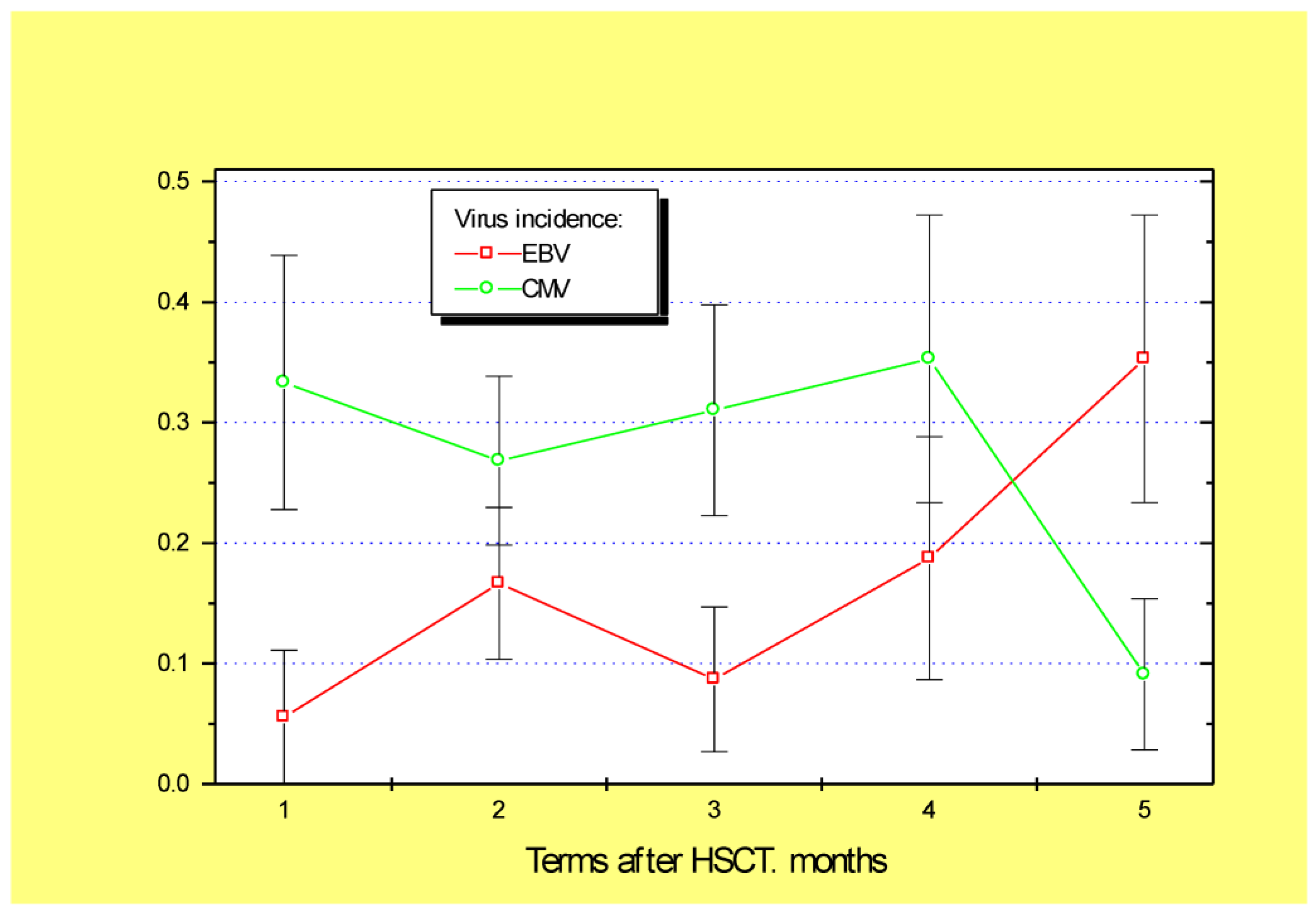
| Demographic Data, Primary Diagnosis | Parameters of Hematopoietic Transplantation | ||
|---|---|---|---|
| Number of patients | 119 | HSCT mode: | |
| Mean age ± SD | 28.3 ± 19.0 | Haploidentical related | 48.2% |
| Median age | 24 (1–72) | Allogeneic unrelated | 40.8% |
| Sex, males females | 56% 44% | Allogeneic related | 11.0% |
| Body mass index ± SD | 23.4 ± 12.5 | CD34+ cells/kg (M ± SD) | 5.9 + 3.0 (0.7–17; Med. 6) |
| Primary clinical diagnosis: | Number of cases: | Stem cell source: | |
| Acute myeloid leukemia | 34 | Bone marrow | 41.6% |
| Acute lymphoblastic leukemia | 36 | Peripheral blood stem cells | 58.4% |
| Chronic myeloid leukemia | 8 | Oral mucositis, grade | 0: 67.1% 1–2: 19.7% 3–4: 13.2% |
| Severe aplastic anemia | 15 | ||
| Hodgkin’s disease | 10 | ||
| Non-Hodgkin’s lymphoma | 7 | ||
| Other disorders | 9 | ||
| Herpesvirus-positive biopsies | 27.7% | Skin GVHD grade | 0: 35.6% 1: 12.4% 2: 21.7% 3: 30.3% |
| CMV | 25.8% | ||
| EBV | 18.7% | ||
| HSV | 3.9% | ||
| HHV type 6 | 62.4% | ||
| Conditioning chemotherapy: Myeloablative | 56.5% | Intestinal GVHD grade | 0: 41.7% 1: 5.3% 2: 15.4% 3–4: 37.6% |
| Non-myeloablative | 43.5% | ||
| HSCT Parameters | HHV6-Positive Samples, % | p Levels | CMV-Positive Samples, % | p Levels | EBV Positive Samples, % | p Levels |
|---|---|---|---|---|---|---|
| PCR positivity with different HSC sources: | ||||||
| Bone marrow | 65.6% (n = 40/61) | 0.564 | 16.1% (n = 17/62) | 0.737 | 28.1% (n = 16/57) | 0.015 |
| Peripheral stem cells | 60.9% (n = 57/94) | 23.9% (n = 23/92) | 11.6% (n = 9/78) | |||
| CD34+ stem cells/kg: | ||||||
| Virus-positive | 5.91 ± 0.31 (93) | 0.818 | 6.12 ± 0.48 (40) | 0.562 | 5.97 ± 0.54 | 0.675 |
| Virus-negative | 5.94 ± 0.42 (55) | 5.7 ± 0.28 (113) | 5.89 ± 0.29 | |||
| Leukocyte recovery, d: | ||||||
| Virus-positive | 21.01 + 0.80 (87) | 0.362 | 19.29 ± 0.91 (38) | 0.431 | 22.04 + 1.17 | 0.06 |
| Virus-negative | 19.56 + 0.91 (53) | 20.94 ± 0.73 (109) | 20.06 + 0.73 | |||
| Platelet recovery (>20/µL), days | ||||||
| Virus-positive | 24.71 + 2.28 (74) | 0.195 | 19.27 + 2.22 (33) | 0.381 | 24.77 + 3.18 (22) | 0.023 |
| Virus-negative | 18.98 + 1.87 (46) | 23.83 + 1.90 (94) | 21.24 + 1.76 (86) | |||
| Clinical complications: | ||||||
| Virus-positive, oral mucositis, | ||||||
| Grade 0 | 65.0% (26/40) | 0.369 | 31.0% (13/42) | 0.07 | 25.7% (9/35) | 0.76 |
| Grade I–II | 75.0% (9/12) | 36.3% (4/11) | 18.2% (2/11) | |||
| Grade III–IV | 87.5.0% (7/8) | 25.0% (2/8) | 42.9% (3/7) | |||
| Skin GVHD, virus-positive tests, % | ||||||
| Grade 0 | 71.7% (33/46) | 0.140 | 34.8% (16/46) | 0.275 | 7.3% (3/41) | 0.04 |
| Grade I–II | 50% (21/42) | 17.0 % (8/47) | 30.0% (15/40) | |||
| Grade III–IV | 68.4% (26/38) | 28.2% (11/39) | 19.4% (7/36) | |||
| Intestinal GVHD, virus-positive tests, % | ||||||
| Grade 0 | 65.4% (34/52) | 0.127 | 30.9% (17/55) | 0.286 | 18.4% (9/49) | 0.12 |
| Grade I–II | 48.0% (12/25) | 14.5% (4/28) | 4.3% (1/23) | |||
| Grade III–IV | 69.4% (34/49) | 28.6 (14/49) | 26.7% (12/45) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goloshchapov, O.V.; Shvetsov, A.N.; Chukhlovin, A.B.; Spiridonova, A.A.; Vladovskaya, M.D.; Zubarovskaya, L.S.; Kulagin, A.D. Incidence of Common Herpesviruses in Colonic Mucosal Biopsies Following Hematopoietic Stem Cell Transplantation. Microorganisms 2022, 10, 2128. https://doi.org/10.3390/microorganisms10112128
Goloshchapov OV, Shvetsov AN, Chukhlovin AB, Spiridonova AA, Vladovskaya MD, Zubarovskaya LS, Kulagin AD. Incidence of Common Herpesviruses in Colonic Mucosal Biopsies Following Hematopoietic Stem Cell Transplantation. Microorganisms. 2022; 10(11):2128. https://doi.org/10.3390/microorganisms10112128
Chicago/Turabian StyleGoloshchapov, Oleg V., Alexander N. Shvetsov, Alexey B. Chukhlovin, Anna A. Spiridonova, Maria D. Vladovskaya, Ludmila S. Zubarovskaya, and Alexander D. Kulagin. 2022. "Incidence of Common Herpesviruses in Colonic Mucosal Biopsies Following Hematopoietic Stem Cell Transplantation" Microorganisms 10, no. 11: 2128. https://doi.org/10.3390/microorganisms10112128
APA StyleGoloshchapov, O. V., Shvetsov, A. N., Chukhlovin, A. B., Spiridonova, A. A., Vladovskaya, M. D., Zubarovskaya, L. S., & Kulagin, A. D. (2022). Incidence of Common Herpesviruses in Colonic Mucosal Biopsies Following Hematopoietic Stem Cell Transplantation. Microorganisms, 10(11), 2128. https://doi.org/10.3390/microorganisms10112128






