Antibacterial and Anti-Inflammatory Properties of Peptide KN-17
Abstract
1. Introduction
2. Materials and Methods
2.1. Basic and Antimicrobial Properties of Peptides
2.1.1. Peptide Synthesis
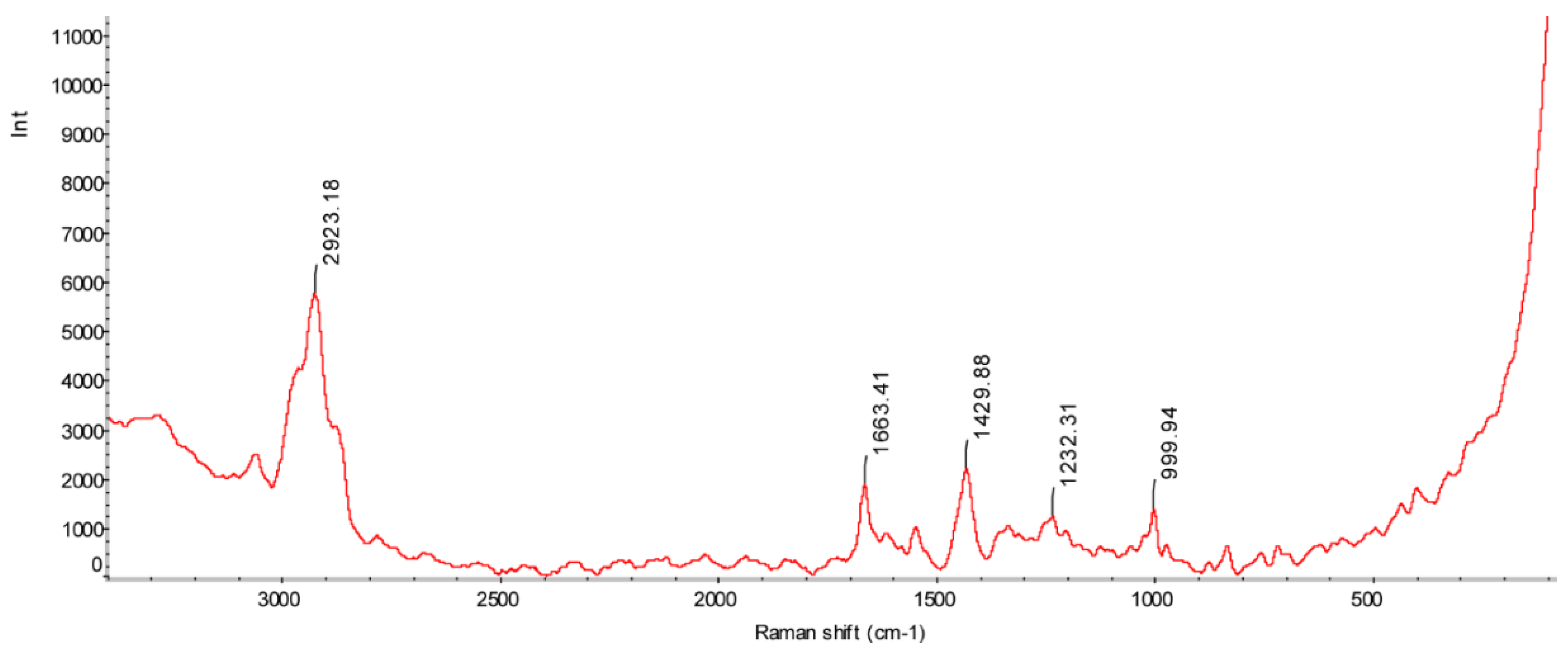
2.1.2. Raman Spectroscopy
2.2. Antibacterial Activity Test
2.2.1. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
2.2.2. Biofilm Susceptibility
2.2.3. Confocal Laser Scanning Microscopy (CLSM)
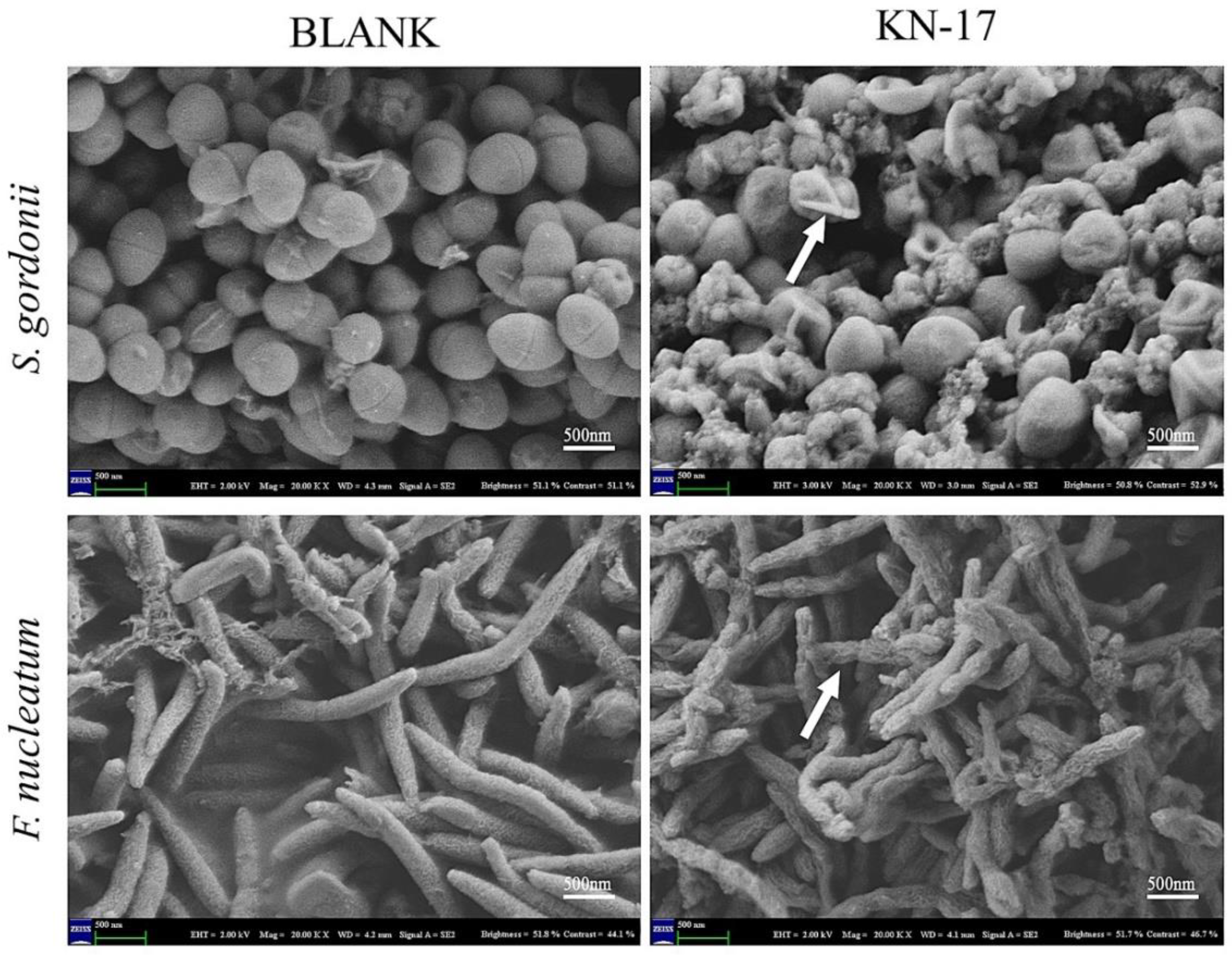
2.2.4. Scanning Electron Microscopy (SEM)
2.3. Human Bone Marrow Stromal Cells (hBMSCs) Cultured with KN-17 Peptide
2.3.1. Cell Culture
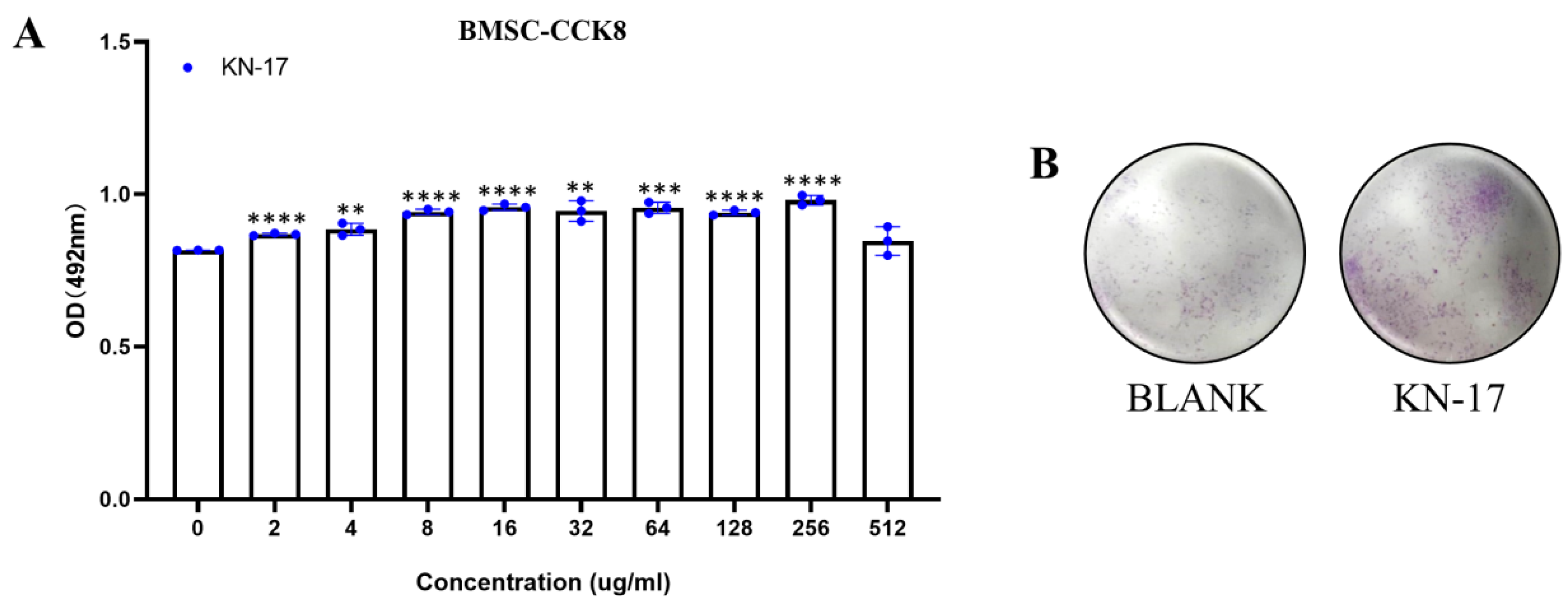
2.3.2. Cell Proliferation Assay
2.3.3. Cell Migration Assay
2.4. RAW264.7 Macrophages Cultured with KN-17
2.4.1. Cell Culture
2.4.2. Cell Proliferation Assay
2.4.3. Real-Time Polymerase Chain Reaction (RT-PCR)
2.4.4. Microscopic Cell Polarization Morphology
2.4.5. Cell Morphology and P65 Immunofluorescence Staining
2.5. Statistical Analysis
3. Results
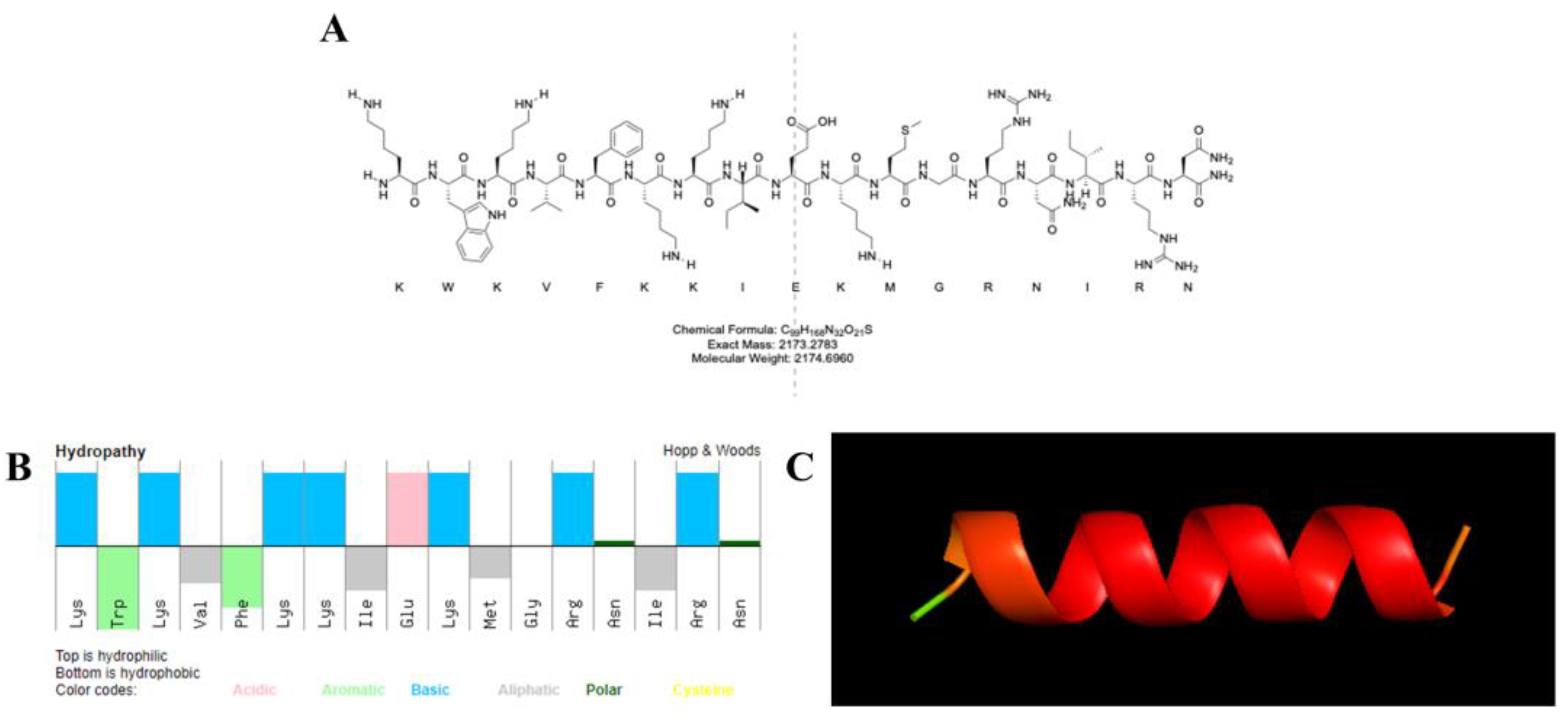
3.1. Peptide Properties
3.2. Raman Spectroscopy
3.3. Antibacterial Test
3.3.1. MIC and MBC Results of Different Strains
3.3.2. Biofilm Inhibition
3.3.3. Scanning Electron Microscopy (SEM)
3.4. Peptide Biocompatibility
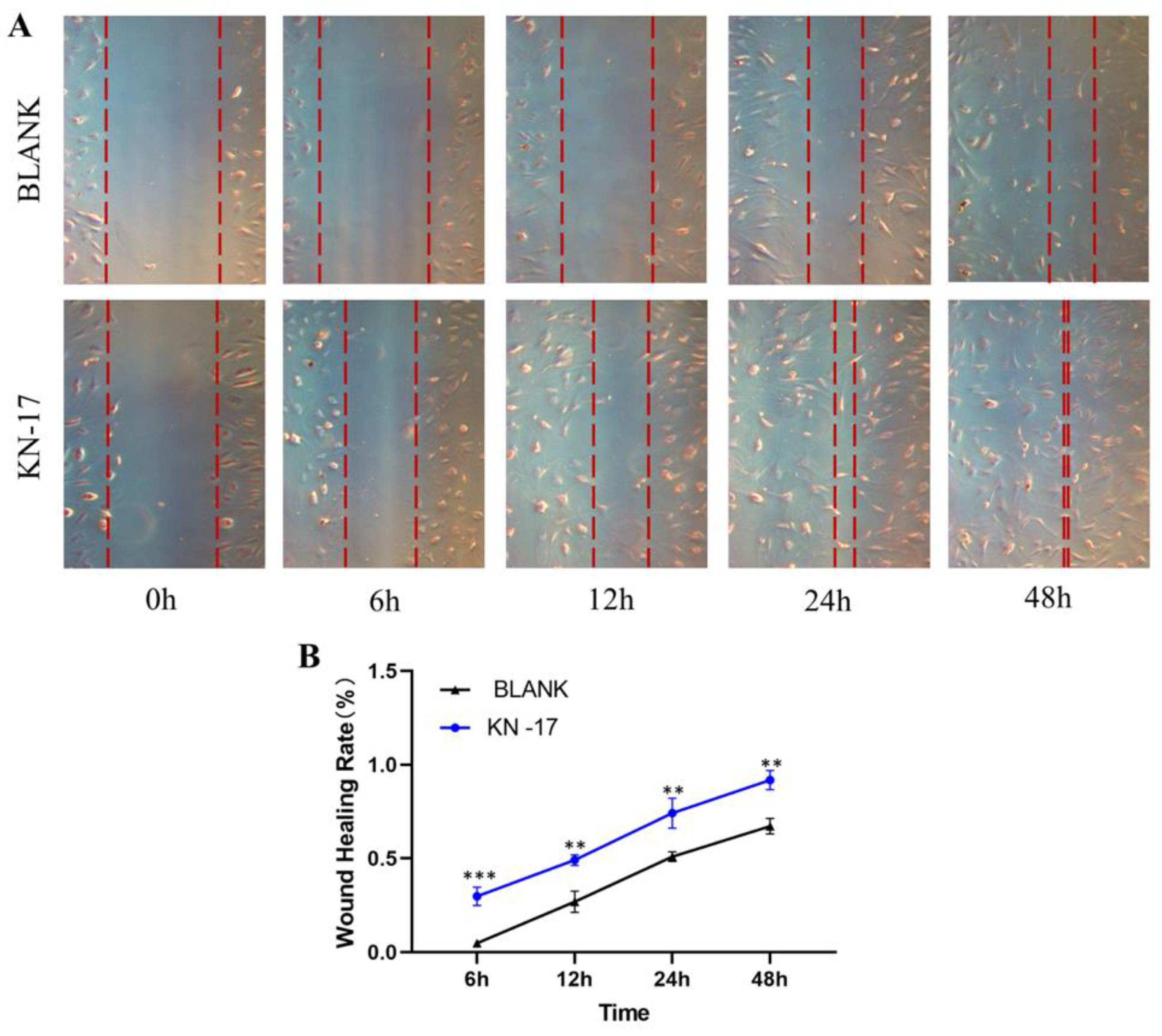
3.5. Wound Healing Assay
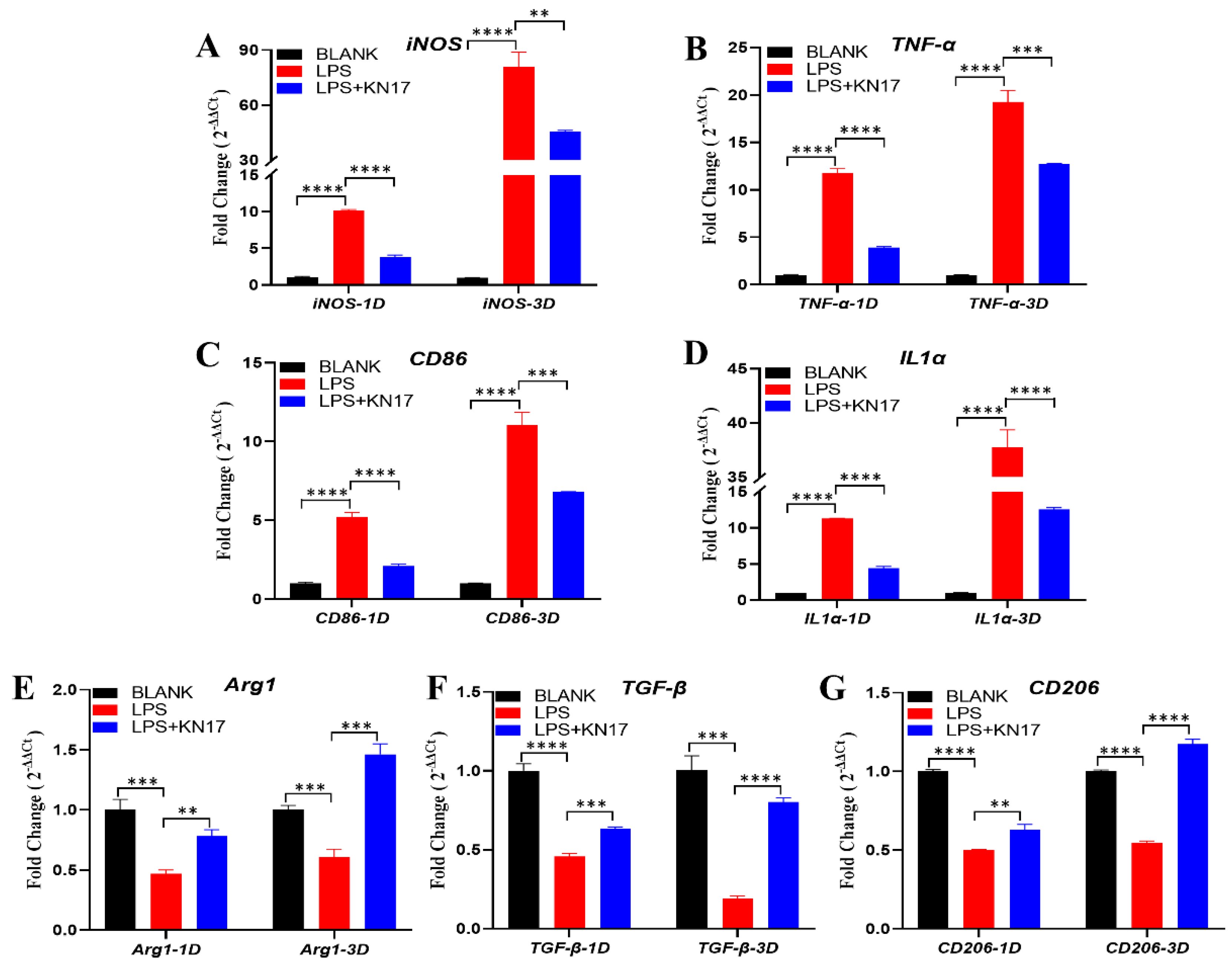
3.6. Effect of Peptides on Pro-inflammatory and Anti-Inflammatory Gene Expression in RAW 264.7
3.6.1. Non-Inflammatory Conditions
3.6.2. Inflammatory Conditions
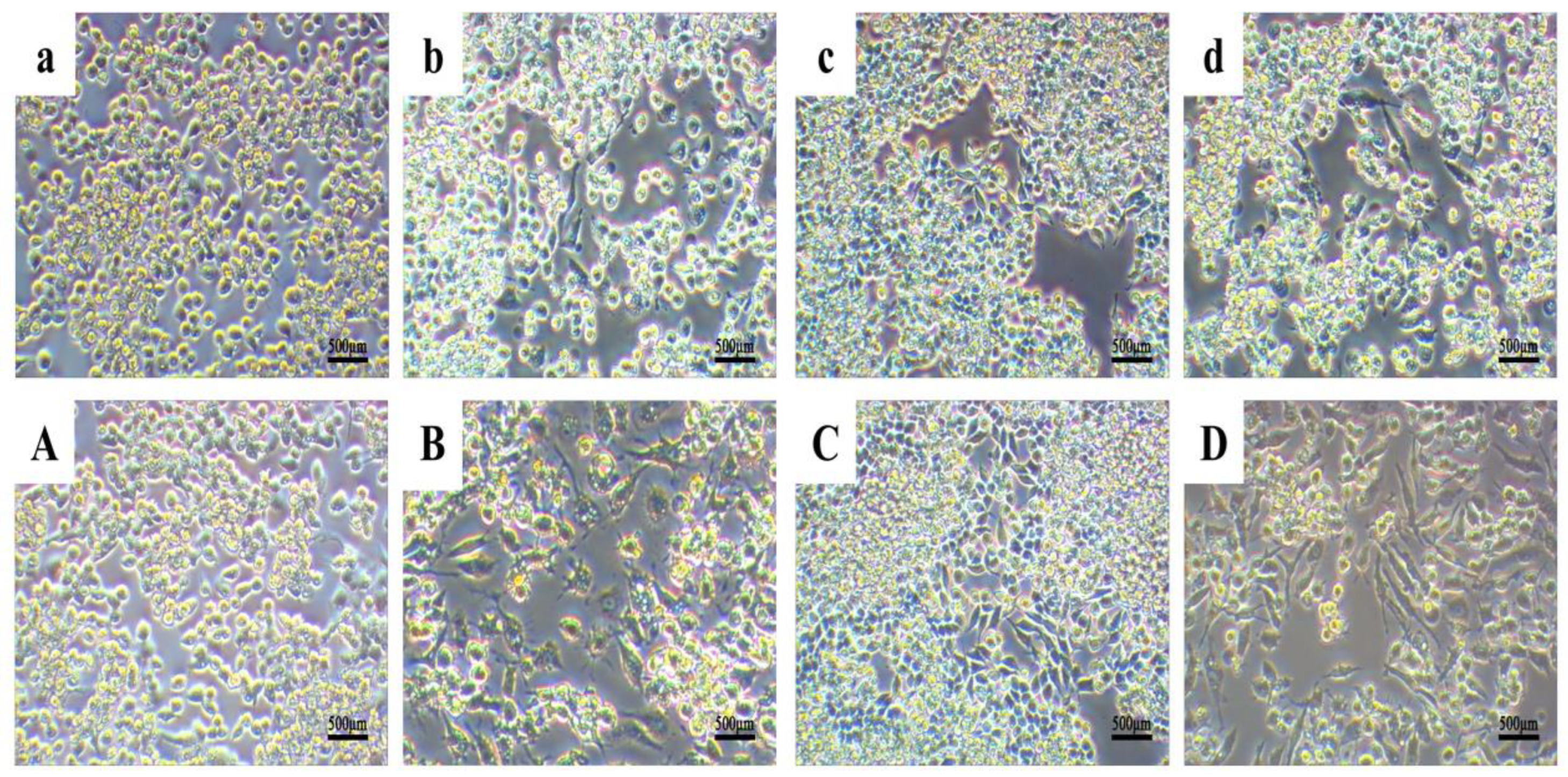
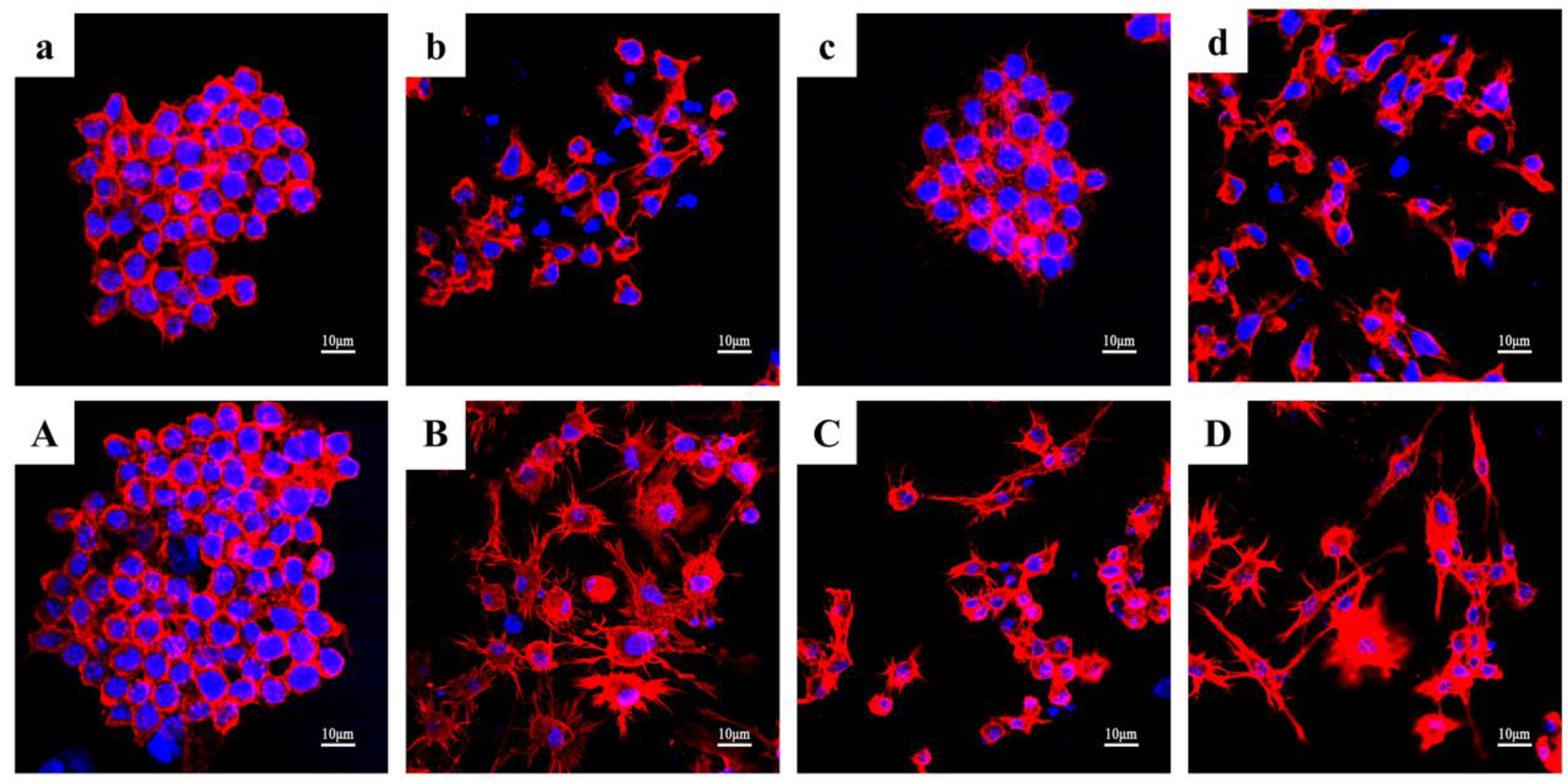
3.7. Impact of KN-17 on In Vitro Regulation of RAW 264.7 Phenotype, Cell Morphology, and Cytoskeleton Actin Staining
3.7.1. Cell Morphology
3.7.2. Cytoskeleton Actin Staining
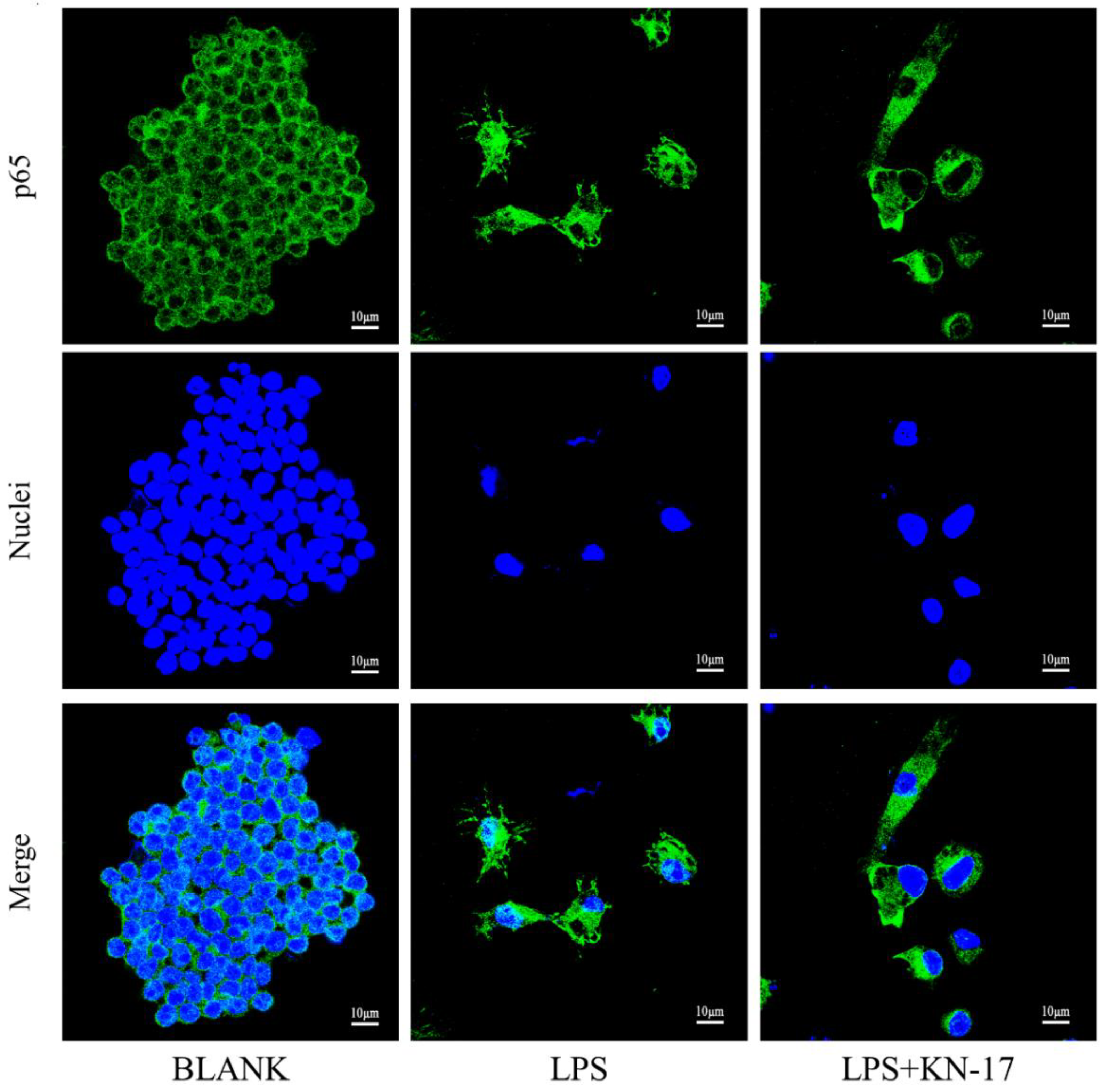
3.7.3. KN-17 Regulated NF-κB-P65 Signaling during RAW264.7 Polarization
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barkarmo, S.; Longhorn, D.; Leer, K.; Johansson, C.B.; Stenport, V.; Franco-Tabares, S.; Kuehne, S.A.; Sammons, R. Biofilm formation on polyetheretherketone and titanium surfaces. Clin. Exp. Dent. Res. 2019, 5, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Kormas, I.; Pedercini, C.; Pedercini, A.; Raptopoulos, M.; Alassy, H.; Wolff, L.F. Peri-Implant Diseases: Diagnosis, Clinical, Histological, Microbiological Characteristics and Treatment Strategies. A Narrative Review. Antibiotics 2020, 9, 835. [Google Scholar] [CrossRef] [PubMed]
- Rosing, C.K.; Fiorini, T.; Haas, A.N.; Muniz, F.; Oppermann, R.V.; Susin, C. The impact of maintenance on peri-implant health. Braz. Oral Res. 2019, 33 (Suppl. 1), e074. [Google Scholar] [CrossRef]
- Monje, A.; Blasi, G. Significance of keratinized mucosa/gingiva on peri-implant and adjacent periodontal conditions in erratic maintenance compliers. J. Periodontol. 2019, 90, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Pesce, P.; Menini, M.; Tommasato, G.; Patini, R.; Canullo, L. Influence of modified titanium abutment surface on peri-implant soft tissue behaviour: A systematic review of histological findings. Int. J. Oral Implantol. 2019, 12, 419–429. [Google Scholar]
- Belibasakis, G.N.; Manoil, D. Microbial Community-Driven Etiopathogenesis of Peri-Implantitis. J. Dent. Res. 2021, 100, 21–28. [Google Scholar] [CrossRef]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef]
- Zhong, C.; Zhu, N.; Zhu, Y.; Liu, T.; Gou, S.; Xie, J.; Yao, J.; Ni, J.M. Antimicrobial peptides conjugated with fatty acids on the side chain of D-amino acid promises antimicrobial potency against multidrug-resistant bacteria. Eur. J. Pharm. Sci. 2020, 141, 105123. [Google Scholar] [CrossRef]
- Romoli, O.R.; Mukherjee, S.; Mohid, S.A.; Dutta, A.; Montali, A.; Franzolin, E.; Brady, D.; Zito, F.; Bergantino, E.; Rampazzo, C.; et al. Enhanced Silkworm Cecropin B Antimicrobial Activity against Pseudomonas aeruginosa from Single Amino Acid Variation. ACS Infect. Dis. 2019, 5, 1200–1213. [Google Scholar] [CrossRef]
- Xu, D.; Yang, W.; Hu, Y.; Luo, Z.; Li, J.; Hou, Y.; Liu, Y.; Cai, K.Y. Surface functionalization of titanium substrates with cecropin B to improve their cytocompatibility and reduce inflammation responses. Colloids Surf. B Biointerfaces 2013, 110, 225–235. [Google Scholar] [CrossRef]
- Ziaja, M.; Dziedzic, A.; Szafraniec, K.; Piastowska-Ciesielska, A. Cecropins in cancer therapies-where we have been? Eur. J. Pharmacol. 2020, 882, 173317. [Google Scholar] [CrossRef]
- Denardi, L.B.; Weiblen, C.; Ianiski, L.B.; Stibbe, P.C.; Santurio, J.M. Activity of MSI-78, h-Lf1-11 and cecropin B antimicrobial peptides alone and in combination with voriconazole and amphotericin B against clinical isolates of Fusarium solani. J. Mycol. Med. 2021, 31, 101119. [Google Scholar] [CrossRef] [PubMed]
- Anasir, M.I.; Ramanathan, B.; Poh, C.L. Structure-Based Design of Antivirals against Envelope Glycoprotein of Dengue Virus. Viruses 2020, 12, 367. [Google Scholar] [CrossRef] [PubMed]
- Anasir, M.I.; Zarif, F.; Poh, C.L. Antivirals blocking entry of enteroviruses and therapeutic potential. J. Biomed. Sci. 2021, 28, 10. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Wu, Z.Y.; Liu, W.W.; Long, H.L.; Zhu, G.M.; Guo, G.; Wu, J.W. Antimicrobial functional divergence of the cecropin antibacterial peptide gene family in Musca domestica. Parasites Vectors. 2019, 12, 537. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.Y.; Li, N.Z.; Wang, W.M.; Liu, C.T.; Yu, C.H.; Chen, Y.C.; Lu, D.; Lin, P.S.; Huang, C.H.; Kono, O.; et al. Longer charged amino acids favor β-strand formation in hairpin peptides. J. Pept. Sci. 2021, 27, e3333. [Google Scholar] [CrossRef] [PubMed]
- Zarif, F.; Anasir, M.I.; Koh, J.X.; Chew, M.F.; Poh, C.L. Stability and antiviral activity of SP40 peptide in human serum. Virus Res. 2021, 303, 198456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Geng, H.J.; Gong, L.; Zhang, Q.; Li, H.J.; Zhang, X.; Wang, Y.L.; Gao, P. Modification of the surface of titanium with multifunctional chimeric peptides to prevent biofilm formation via inhibition of initial colonizers. Int. J. Nanomed. 2018, 13, 5361–5375. [Google Scholar] [CrossRef]
- Singh, H.; Gahane, A.; Singh, V.; Ghosh, S.; Thakur, A. Antibiofilm activity of Fmoc-phenylalanine against Gram-positive and Gram-negative bacterial biofilms. J. Antibiot. 2021, 74, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Jara, M.C.; Silva, A.C.A.; Ritter, M.; da Silva, A.F.; Goncalves, C.L.; Dos Santos, P.R.; Borja, L.S.; de Pereira, C.M.P.; da Silva Nascente, P. Dihydropyrimidinones against Multiresistant Bacteria. Front. Microbiol. 2022, 13, 743213. [Google Scholar] [CrossRef]
- Li, M.; Yang, Y.Y.; Lin, C.; Zhang, Q.; Gong, L.; Wang, Y.L.; Zhang, X. Antibacterial Properties of Small-Size Peptide Derived from Penetratin against Oral Streptococci. Materials 2021, 14, 2730. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Tan, L.S.; Wang, H.Y.; Kou, Y.R.; Shi, X.T.; Zhang, S.W.; Pan, Y.P. Fusobacterium nucleatum Interaction with Pseudomonas aeruginosa Induces Biofilm-Associated Antibiotic Tolerance via Fusobacterium Adhesin A. ACS Infect. Dis. 2020, 6, 1686–1696. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; You, Y.P.; Ma, A.B.; Song, Y.J.; Jiao, J.; Song, L.T.; Shi, E.Y.; Zhong, X.; Li, Y.; Li, C.Y. Zn-Incorporated TiO2 Nanotube Surface Improves Osteogenesis Ability Through Influencing Immunomodulatory Function of Macrophages. Int. J. Nanomed. 2020, 15, 2095–2118. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.R.U.; Augustine, R.; Zahid, A.A.; Ahmed, R.; Tariq, M.; Hasan, A. Reduced Graphene Oxide Incorporated GelMA Hydrogel Promotes Angiogenesis for Wound Healing Applications. Int. J. Nanomed. 2019, 14, 9603–9617. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.C.; Jiang, L.J.; Qiu, J.F.; Wang, Y. A comparative evaluation of the immunotoxicity and immunomodulatory effects on macrophages exposed to aromatic trihalogenated DBPs. Immunopharmacol. Immunotoxicol. 2019, 41, 319–326. [Google Scholar] [CrossRef]
- Devitt, G.; Rice, W.; Crisford, A.; Nandhakumar, I.; Mudher, A.; Mahajan, S. Conformational Evolution of Molecular Signatures during Amyloidogenic Protein Aggregation. ACS Chem. Neurosci. 2019, 10, 4593–4611. [Google Scholar] [CrossRef]
- Benevides, J.M.; Overman, S.A.; Thomas, G.J., Jr. Raman spectroscopy of proteins. Curr. Protoc. Protein Sci. 2004, 33. [Google Scholar] [CrossRef]
- Edwards, H.G.; Hunt, D.E.; Sibley, M.G. FT-Raman spectroscopic study of keratotic materials: Horn, hoof and tortoiseshell. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1998, 54A, 745–757. [Google Scholar] [CrossRef]
- Stawoska, I.; Wesełucha-Birczyńska, A.; Skoczowski, A.; Dziurka, M.; Waga, J. FT-Raman Spectroscopy as a Tool to Study the Secondary Structures of Wheat Gliadin Proteins. Molecules 2021, 26, 5388. [Google Scholar] [CrossRef]
- Guleken, Z.; Bulut, H.; Bulut, B.; Paja, W.; Orzechowska, B.; Parlinska-Wojtan, M.; Depciuch, J. Identification of polycystic ovary syndrome from blood serum using hormone levels via Raman spectroscopy and multivariate analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 273, 121029. [Google Scholar] [CrossRef] [PubMed]
- Lichtenecker, R.J.; Ellinger, B.; Han, H.M.; Jadhav, K.B.; Baumann, S.; Makarewicz, O.; Grabenbauer, M.; Arndt, H.D. Iterative antimicrobial candidate selection from informed d-/l-Peptide dimer libraries. ChemBioChem 2013, 14, 2492–2499. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.F.; Guan, M.; Ren, R.Y.; Gao, C.H.; Cheng, H.; Li, Y.; Gao, B.; Wei, Y.; Fu, J.J.; Sun, J.; et al. Improved Immunoregulation of Ultra-Low-Dose Silver Nanoparticle-Loaded TiO2 Nanotubes via M2 Macrophage Polarization by Regulating GLUT1 and Autophagy. Int. J. Nanomed. 2020, 15, 2011–2026. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Song, X.Y.; Xing, F.; Wang, Y.N.; Wang, Y.Z.; He, Z.Y.; Sun, L. A Review on Antimicrobial Coatings for Biomaterial Implants and Medical Devices. J. Biomed. Nanotechnol. 2020, 16, 789–809. [Google Scholar] [CrossRef]
- Astolfi, V.; Gómez-Menchero, A.; Ríos-Santos, J.V.; Bullón, P.; Galeote, F.; Ríos-Carrasco, B.; de la Fuente, B.B.; Herrero-Climent, M. Influence of Removing or Leaving the Prosthesis after Regenerative Surgery in Peri-Implant Defects: Retrospective Study: 32 Clinical Cases with 2 to 8 Years of Follow-Up. Int. J. Environ. Res. Public Health 2021, 8, 645. [Google Scholar] [CrossRef]
- Radaelli, M.T.B.; Federizzi, L.; Nascimento, G.G.; Leite, F.R.M.; Boscato, N. Early-predictors of marginal bone loss around morse taper connection implants loaded with single crowns: A prospective longitudinal study. J. Periodontal Res. 2020, 55, 174–181. [Google Scholar] [CrossRef]
- Körtvélyessy, G.; Tarjányi, T.; Baráth, Z.L.; Minarovits, J.; Tóth, Z. Bioactive coatings for dental implants: A review of alternative strategies to prevent peri-implantitis induced by anaerobic bacteria. Anaerobe 2021, 70, 102404. [Google Scholar] [CrossRef]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.L. Peri-implantitis. J. Clin. Periodontol. 2018, 45, S246–S266. [Google Scholar] [CrossRef]
- Vickery, K. Special Issue: Microbial Biofilms in Healthcare: Formation, Prevention and Treatment. Materials 2019, 12, 2001. [Google Scholar] [CrossRef]
- Yeo, I.L. Modifications of Dental Implant Surfaces at the Micro- and Nano-Level for Enhanced Osseointegration. Materials 2019, 13, 89. [Google Scholar] [CrossRef]
- Kardani, K.; Bolhassani, A. Antimicrobial/anticancer peptides: Bioactive molecules and therapeutic agents. Immunotherapy 2021, 13, 669–684. [Google Scholar] [CrossRef] [PubMed]
- Mahlapuu, M.; Björn, C.; Ekblom, J. Antimicrobial peptides as therapeutic agents: Opportunities and challenges. Crit. Rev. Biotechnol. 2020, 40, 978–992. [Google Scholar] [CrossRef] [PubMed]
- Mookherjee, N.; Anderson, M.A.; Haagsman, H.P.; Davidson, D.J. Antimicrobial host defence peptides: Functions and clinical potential. Nat. Rev. Drug Discov. 2020, 19, 311–332. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, K.; Ruan, M.S.; Wang, Y.J.; Li, Y.; Fu, Y.V.; Song, Y.H.; Sun, B.; Wang, J.F. A novel cecropin B-derived peptide with antibacterial and potential anti-inflammatory properties. PeerJ 2018, 6, e5369. [Google Scholar] [CrossRef]
- Chung, C.R.; Jhong, J.H.; Wang, Z.; Chen, S.; Wan, Y.; Horng, J.T.; Lee, T.Y. Characterization and Identification of Natural Antimicrobial Peptides on Different Organisms. Int. J. Mol. Sci. 2020, 21, 986. [Google Scholar] [CrossRef] [PubMed]
- Payne, T.D.; Moody, A.S.; Wood, A.L.; Pimiento, P.A.; Elliott, J.C.; Sharma, B. Raman spectroscopy and neuroscience: From fundamental understanding to disease diagnostics and imaging. Analyst 2020, 145, 3461–3480. [Google Scholar] [CrossRef]
- Jadhav, K.B.; Stein, C.; Makarewicz, O.; Pradel, G.; Lichtenecker, R.J.; Sack, H.; Heinemann, S.H.; Arndt, H.D. Bioactivity of topologically confined gramicidin A dimers. Bioorg. Med. Chem. 2017, 25, 261–268. [Google Scholar] [CrossRef]
- Pandit, G.; Chowdhury, N.; Abdul Mohid, S.; Bidkar, A.P.; Bhunia, A.; Chatterjee, S. Effect of Secondary Structure and Side Chain Length of Hydrophobic Amino Acid Residues on the Antimicrobial Activity and Toxicity of 14-Residue-Long de novo AMPs. ChemMedChem 2021, 16, 355–367. [Google Scholar] [CrossRef]
- Martinotti, S.; Ranzato, E. Scratch Wound Healing Assay. Methods Mol. Biol. 2020, 2109, 225–229. [Google Scholar]
- Muñoz, J.; Akhavan, N.S.; Mullins, A.P.; Arjmandi, B.H. Macrophage Polarization and Osteoporosis: A Review. Nutrients 2020, 12, 2999. [Google Scholar] [CrossRef]
- Latour, Y.L.; Gobert, A.P.; Wilson, K.T. The role of polyamines in the regulation of macrophage polarization and function. Amino Acids 2020, 52, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Troiano, G.; Lo Russo, L.; Canullo, L.; Ciavarella, D.; Lo Muzio, L.; Laino, L. Early and late implant failure of submerged versus non-submerged implant healing: A systematic review, meta-analysis and trial sequential analysis. J. Clin. Periodontol. 2018, 45, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Romanowska-Próchnicka, K.; Felis-Giemza, A.; Olesińska, M.; Wojdasiewicz, P.; Paradowska-Gorycka, A.; Szukiewicz, D. The Role of TNF-alpha and Anti-TNF-alpha Agents during Preconception, Pregnancy, and Breastfeeding. Int. J. Mol. Sci. 2021, 22, 2922. [Google Scholar] [CrossRef] [PubMed]
- Hira, K.; Sajeli Begum, A. Methods for Evaluation of TNF-alpha Inhibition Effect. Methods Mol. Biol. 2021, 2248, 271–279. [Google Scholar]
- Rajasekaran, G.; Kamalakannan, R.; Shin, S.Y. Enhancement of the anti-inflammatory activity of temporin-1Tl-derived antimicrobial peptides by tryptophan, arginine and lysine substitutions. J. Pept. Sci. 2015, 21, 779–875. [Google Scholar] [CrossRef]
- Barnabei, L.; Laplantine, E.; Mbongo, W.; Rieux-Laucat, F.; Weil, R. NF-κB: At the Borders of Autoimmunity and Inflammation. Front. Immunol. 2021, 12, 716469. [Google Scholar] [CrossRef]
- Ouyang, G.; Liao, Q.; Zhang, D.; Rong, F.; Cai, X.; Fan, S.; Zhu, J.; Wang, J.; Liu, X.; Xiao, W. Zebrafish NF-κB/p65 Is Required for Antiviral Responses. J. Immunol. 2020, 204, 3019–3029. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, H.B.; Pan, J.Y.; Hu, Z.Q.; Liu, L.L.; Liu, Y.L.; Yu, X.; Bai, X.C.; Cai, D.Z.; Zhang, H.Y. Fargesin ameliorates osteoarthritis via macrophage reprogramming by downregulating MAPK and NF-κB pathways. Arthritis Res. Ther. 2021, 23, 142. [Google Scholar] [CrossRef]
- Zhou, X.Q.; Li, X.W.; Wang, X.L.; Jin, X.; Shi, D.S.; Wang, J.; Bi, D.R. Cecropin B Represses CYP3A29 Expression through Activation of the TLR2/4-NF-κB/PXR Signaling Pathway. Sci. Rep. 2016, 6, 27876. [Google Scholar] [CrossRef]


| Gene | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) |
|---|---|---|
| iNOS | GAGACAGGGAAGTCTGAAGCAC | CCAGCAGTAGTTGCTCCTCTTC |
| TNF-α | GGTGCCTATGTCTCAGCCTCTT | GCCATAGAACTGATGAGAGGGAG |
| CD86 | ACGTATTGGAAGGAGATTACAGCT | TCTGTCAGCGTTACTATCCCGC |
| IL-1α | ACGGCTGAGTTTCAGTGAGAC | CACTCTGGTAGGTGTAAGGTGC |
| Arg-1 | CATTGGCTTGCGAGACGTAGAC | GCTGAAGGTCTCTTCCATCACC |
| TGF-β | TTGCTTCAGCTCCACAGAGA | TGGTTGTAGAGGGCAAGGAC |
| CD206 | GTTCACCTGGAGTGATGGTTCTC | GTTCACCTGGAGTGATGGTTCTC |
| GAPDH | CATCACTGCCACCCAGAAGACTG | ATGCCAGTGAGCTTCCCGTTCAG |
| Peptide | MW | E coef | Charge | PI |
|---|---|---|---|---|
| KN-17 | 2174.70 | 5690 | +6 | 11.63 |
| Bacteria | MIC (μg/mL) | MBC (μg/mL) |
|---|---|---|
| S. gordonii | 80 | 200 |
| F. nucleatum | 90 | 220 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Yu, S.; Hu, M.; Liu, Z.; Yu, P.; Li, C.; Zhang, X. Antibacterial and Anti-Inflammatory Properties of Peptide KN-17. Microorganisms 2022, 10, 2114. https://doi.org/10.3390/microorganisms10112114
Zhang Q, Yu S, Hu M, Liu Z, Yu P, Li C, Zhang X. Antibacterial and Anti-Inflammatory Properties of Peptide KN-17. Microorganisms. 2022; 10(11):2114. https://doi.org/10.3390/microorganisms10112114
Chicago/Turabian StyleZhang, Qian, Shuipeng Yu, Meilin Hu, Zhiyang Liu, Pei Yu, Changyi Li, and Xi Zhang. 2022. "Antibacterial and Anti-Inflammatory Properties of Peptide KN-17" Microorganisms 10, no. 11: 2114. https://doi.org/10.3390/microorganisms10112114
APA StyleZhang, Q., Yu, S., Hu, M., Liu, Z., Yu, P., Li, C., & Zhang, X. (2022). Antibacterial and Anti-Inflammatory Properties of Peptide KN-17. Microorganisms, 10(11), 2114. https://doi.org/10.3390/microorganisms10112114






