Epidemiological and Cytokine Profile of Patients with Pulmonary and Extrapulmonary Tuberculosis in a Population of the Brazilian Amazon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Characteristics and Collection
2.2. Plasma Cytokine Measurement
2.3. Tuberculin Skin Test (TST)
2.4. Statistical Analysis
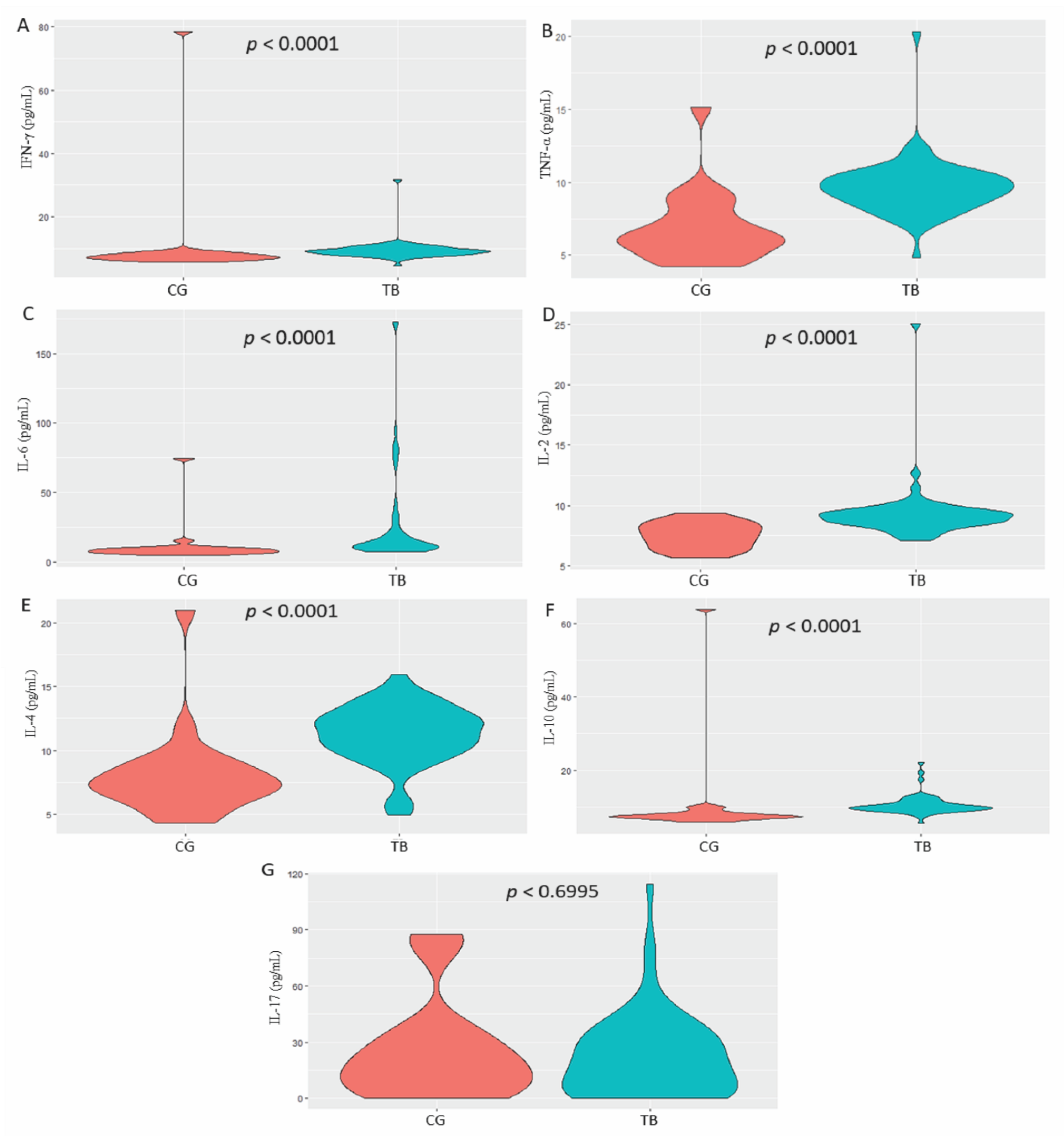
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2020. Available online: https://www.who.int/publications/i/item/9789240013131 (accessed on 13 January 2022).
- Lönnroth, K.; Jaramillo, E.; Williams, B.G.; Dye, C.; Raviglione, M. Drivers of tuberculosis epidemics: The role of risk factors and social determinants. Soc. Sci. Med. 2009, 68, 2240–2246. [Google Scholar] [CrossRef]
- Vallinoto, A.C.; Graça, E.S.; Araújo, M.S.; Azevedo, V.N.; Cayres-Vallinoto, I.; Machado, L.F.; Ishak, M.O.; Ishak, R. IFNG +874T/A polymorphism and cytokine plasma levels are associated with susceptibility to Mycobacterium tuberculosis infection and clinical manifestation of tuberculosis. Hum. Immunol. 2010, 71, 692–696. [Google Scholar] [CrossRef]
- Madhvi, A.; Mishra, H.; Chegou, N.N.; Tromp, G.; Van Heerden, C.J.; Pietersen, R.D.; Leisching, G.; Baker, B. Distinct host-immune response toward species related intracellular mycobacterial killing: A transcriptomic study. Virulence 2020, 11, 170–182. [Google Scholar] [CrossRef] [Green Version]
- Brasil, Ministério Da Saúde. Boletim Epidemiológico Tuberculose 2020; Secretaria De Vigilância Em Saúde—SVS: Brasília, Brazil. 39p. Available online: http://www.aids.gov.br/pt-br/pub/2020/boletim-epidemiologico-de-turbeculose-2020 (accessed on 10 January 2022).
- Getahun, H.; Matteelli, A.; Chaisson, R.E.; Raviglione, M. Latent Mycobacterium tuberculosis infection. N. Engl. J. Med. 2015, 372, 2127–2135. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Latent Tuberculosis Infection: Updated and Consolidated Guidelines for Programmatic Management. 2018. Available online: https://apps.who.int/iris/handle/10665/260233 (accessed on 13 January 2022).
- Dheda, K.; Barry, C.E., 3rd; Maartens, G. Tuberculosis. Lancet 2016, 87, 1211–1226. [Google Scholar] [CrossRef]
- Ray, S.; Talukdar, A.; Kundu, S.; Khanra, D.; Sonthalia, N. Diagnosis and management of miliary tuberculosis: Current state and future perspectives. Ther. Clin. Risk Manag. 2013, 9, 9–26. [Google Scholar] [CrossRef] [Green Version]
- Boonsarngsuk, V.; Mangkang, K.; Santanirand, P. Prevalence and risk factors of drug-resistant extrapulmonary tuberculosis. Clin. Respir. J. 2018, 12, 2101–2109. [Google Scholar] [CrossRef]
- Liu, C.H.; Liu, H.; Ge, B. Innate immunity in tuberculosis: Host defense vs. pathogen evasion. Cell Mol. Immunol. 2017, 14, 963–975. [Google Scholar] [CrossRef] [Green Version]
- Scriba, T.J.; Coussens, A.K.; Fletcher, H.A. Human Immunology of Tuberculosis. Microbiol. Spectr. 2017, 5, 1–24. [Google Scholar]
- da Silva, M.V.; Massaro Junior, V.J.; Machado, J.R.; Silva, D.A.; Castellano, L.R.; Alexandre, P.B.; Rodrigues, D.B.; Rodrigues, V. Expression pattern of transcription factors and intracellular cytokines reveals that clinically cured tuberculosis is accompanied by an increase in Mycobacterium-specific Th1, Th2, and Th17 cells. Biomed. Res. Int. 2015, 2015, 591237. [Google Scholar] [CrossRef] [PubMed]
- Green, A.M.; Difazio, R.; Flynn, J.L. IFN-γ from CD4 T cells is essential for host survival and enhances CD8 T cell function during Mycobacterium tuberculosis infection. J. Immunol. 2013, 190, 270–277. [Google Scholar] [CrossRef]
- Prezzemolo, T.; Guggino, G.; La Manna, M.P.; Di Liberto, D.; Dieli, F.; Caccamo, N. Functional Signatures of Human CD4 and CD8 T Cell Responses to Mycobacterium tuberculosis. Front. Immunol. 2014, 5, 180. [Google Scholar] [CrossRef] [Green Version]
- Walzl, G.; Ronacher, K.; Hanekom, W.; Scriba, T.J.; Zumla, A. Immunological biomarkers of tuberculosis. Nat. Rev. Immunol. 2011, 11, 343–354. [Google Scholar] [CrossRef]
- Ghanavi, J.; Farnia, P.; Farnia, P.; Velayati, A.A. The role of interferon-gamma and interferon-gamma receptor in tuberculosis and nontuberculous mycobacterial infections. Int. J. Mycobacteriol 2021, 10, 349–357. [Google Scholar]
- Jasenosky, L.D.; Scriba, T.J.; Hanekom, W.A.; Goldfeld, A.E. T cells and adaptive immunity to Mycobacterium tuberculosis in humans. Immunol. Rev. 2015, 264, 74–87. [Google Scholar] [CrossRef]
- Rossouw, M.; Nel, H.J.; Cooke, G.S.; van Helden, P.D.; Hoal, E.G. Association between tuberculosis and a polymorphic NFkappaB binding site in the interferon gamma gene. Lancet 2003, 361, 1871–1872. [Google Scholar] [CrossRef]
- Srivastava, S.; Grace, P.S.; Ernst, J.D. Antigen Export Reduces Antigen Presentation and Limits T Cell Control of M. tuberculosis. Cell Host Microbe 2016, 19, 44–54. [Google Scholar] [CrossRef] [Green Version]
- Chai, Q.; Wang, L.; Liu, C.H.; Ge, B. New insights into the evasion of host innate immunity by Mycobacterium tuberculosis. Cell Mol. Immunol. 2020, 17, 901–913. [Google Scholar] [CrossRef]
- Dlamini, Z.; Alaouna, M.; Cholo, M.C.; Hull, R. Is targeting dysregulation in apoptosis splice variants in Mycobacterium tuberculosis (MTB) host interactions and splicing factors resulting in immune evasion by MTB strategies a possibility? Tuberculosis (Edinb) 2020, 124, 101964. [Google Scholar] [CrossRef]
- Brasil. Ministério Da Saúde. Manual De Recomendações Para o Controle Da Tuberculose No Brasil. 2019. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/manual_recomendacoes_controle_tuberculose_brasil_2_ed.pdf (accessed on 12 October 2022).
- Brasil. Ministério Da Saúde. Técnicas De Aplicação e Leitura Da Prova Tuberculínica. 2014. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/tecnicas_aplicacao_leitura_prova_tuberculinica.pdf (accessed on 27 September 2022).
- Andrews, J.R.; Basu, S.; Dowdy, D.W.; Murray, M.B. The epidemiological advantage of preferential targeting of tuberculosis control at the poor. Int. J. Tuberc. Lung Dis. 2015, 19, 375–380. [Google Scholar] [CrossRef] [Green Version]
- Amelio, P.; Portevin, D.; Reither, K.; Mhimbira, F.; Mpina, M.; Tumbo, A.; Nickel, B.; Marti, H.; Knopp, S.; Ding, S.; et al. Mixed Th1 and Th2 Mycobacterium tuberculosis-specific CD4 T cell responses in patients with active pulmonary tuberculosis from Tanzania. PLoS Negl. Trop. Dis. 2017, 11, e0005817. [Google Scholar] [CrossRef] [Green Version]
- Gardner Toren, K.; Spitters, C.; Pecha, M.; Bhattarai, S.; Horne, D.J.; Narita, M. Tuberculosis in Older Adults: Seattle and King County, Washington. Clin. Infect. Dis. 2020, 70, 1202–1207. [Google Scholar] [CrossRef]
- Pangrazzi, L.; Weinberger, B. T cells, aging and senescence. Exp. Gerontol. 2020, 134, 110887. [Google Scholar] [CrossRef]
- Norbis, L.; Alagna, R.; Tortoli, E.; Codecasa, L.R.; Migliori, G.B.; Cirillo, D.M. Challenges and perspectives in the diagnosis of extrapulmonary tuberculosis. Expert Rev. Anti-Infect. Ther. 2014, 12, 633–647. [Google Scholar] [CrossRef]
- Jørstad, M.D.; Aβmus, J.; Marijani, M.; Sviland, L.; Mustafa, T. Diagnostic delay in extrapulmonary tuberculosis and impact on patient morbidity: A study from Zanzibar. PLoS ONE 2018, 13, e0203593. [Google Scholar] [CrossRef] [Green Version]
- Banta, J.E.; Ani, C.; Bvute, K.M.; Lloren, J.I.C.; Darnell, T.A. Pulmonary vs. extra-pulmonary tuberculosis hospitalizations in the US [1998–2014]. J. Infect. Public Health. 2020, 13, 131–139. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, M.; Liao, M.; Graner, M.W.; Wu, C.; Yang, Q.; Liu, H.; Zhou, B. Reduced Th17 response in patients with tuberculosis correlates with IL-6R expression on CD4+ T Cells. Am. J. Respir. Crit. Care Med. 2010, 181, 734–742. [Google Scholar] [CrossRef]
- Masood, K.I.; Rottenberg, M.E.; Salahuddin, N.; Irfan, M.; Rao, N.; Carow, B.; Islam, M.; Hussain, R.; Hasan, Z. Expression of M. tuberculosis-induced suppressor of cytokine signaling (SOCS) 1, SOCS3, FoxP3 and secretion of IL-6 associates with differing clinical severity of tuberculosis. BMC Infect. Dis. 2013, 13, 13. [Google Scholar] [CrossRef] [Green Version]
- Arrigucci, R.; Lakehal, K.; Vir, P.; Handler, D.; Davidow, A.L.; Herrera, R.; Estrada-Guzmán, J.D.; Bushkin, Y.; Tyagi, S.; Lardizabal, A.A.; et al. Active Tuberculosis Is Characterized by Highly Differentiated Effector Memory Th1 Cells. Front. Immunol. 2018, 9, 2127. [Google Scholar] [CrossRef] [Green Version]
- O’Garra, A.; Redford, P.S.; McNab, F.W.; Bloom, C.I.; Wilkinson, R.J.; Berry, M.P. The immune response in tuberculosis. Annu. Rev. Immunol. 2013, 31, 475–527. [Google Scholar] [CrossRef]
- Talat, N.; Shahid, F.; Perry, S.; Dawood, G.; Hussain, R. Th1/Th2 cytometric bead array can discriminate cytokine secretion from endogenously activated cells in pulmonary disease, recent and remote infection in tuberculosis. Cytokine 2011, 54, 136–143. [Google Scholar] [CrossRef]
- Joshi, L.; Ponnana, M.; Sivangala, R.; Chelluri, L.K.; Nallari, P.; Penmetsa, S.; Valluri, V.; Gaddam, S. Evaluation of TNF-α, IL-10 and IL-6 Cytokine Production and Their Correlation with Genotype Variants amongst Tuberculosis Patients and Their Household Contacts. PLoS ONE 2015, 10, e0137727. [Google Scholar] [CrossRef]
- Clifford, V.; Tebruegge, M.; Zufferey, C.; Germano, S.; Forbes, B.; Cosentino, L.; Matchett, E.; McBryde, E.; Eisen, D.; Robins-Browne, R.; et al. Cytokine biomarkers for the diagnosis of tuberculosis infection and disease in adults in a low prevalence setting. Tuberculosis (Edinb) 2019, 114, 91–102. [Google Scholar] [CrossRef]
- Dheda, K.; Chang, J.S.; Breen, R.A.; Haddock, J.A.; Huggett, J.F.; Johnson, M.A.; Rook, G.A.; Zumla, A. In vivo and in vitro studies of a novel cytokine, interleukin 4δ2, in pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 2005, 172, 501–508. [Google Scholar] [CrossRef] [Green Version]
- Pooran, A.; Davids, M.; Nel, A.; Shoko, A.; Blackburn, J.; Dheda, K. IL-4 subverts mycobacterial containment in Mycobacterium tuberculosis-infected human macrophages. Eur. Respir. J. 2019, 54, 1802242. [Google Scholar] [CrossRef]
- Turner, J.; Gonzalez-Juarrero, M.; Ellis, D.L.; Basaraba, R.J.; Kipnis, A.; Orme, I.M.; Cooper, A.M. In vivo IL-10 production reactivates chronic pulmonary tuberculosis in C57BL/6 mice. J. Immunol. 2022, 69, 6343–6351. [Google Scholar] [CrossRef] [Green Version]
- Lyadova, I.V.; Panteleev, A.V. Th1 and Th17 cells in tuberculosis: Protection, pathology, and biomarkers. Mediat. Inflamm. 2015, 2015, 854507. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Kong, Y. The bacterial and host factors associated with extrapulmonary dissemination of Mycobacterium tuberculosis. Front. Biol. 2015, 10, 252–261. [Google Scholar] [CrossRef] [Green Version]
- de Almeida, A.S.; Fiske, C.T.; Sterling, T.R.; Kalams, S.A. Increased frequency of regulatory T cells and T lymphocyte activation in persons with previously treated extrapulmonary tuberculosis. Clin. Vaccine Immunol. 2012, 19, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Fiske, C.T.; de Almeida, A.S.; Shintani, A.K.; Kalams, S.A.; Sterling, T.R. Abnormal immune responses in persons with previous extrapulmonary tuberculosis in an in vitro model that simulates in vivo infection with Mycobacterium tuberculosis. Clin. Vaccine Immunol. 2012, 19, 1142–1149. [Google Scholar] [CrossRef] [Green Version]
- Ranaivomanana, P.; Raberahona, M.; Rabarioelina, S.; Borella, Y.; Machado, A.; Randria, M.J.D.; Rakotoarivelo, R.A.; Rasolofo, V.; Rakotosamimanana, N. Cytokine Biomarkers Associated with Human Extra-Pulmonary Tuberculosis Clinical Strains and Symptoms. Front. Microbiol. 2018, 9, 275. [Google Scholar] [CrossRef]


| Characteristic | Tuberculosis Group n = 89 n (%) | Control Group n = 45 n (%) | p |
|---|---|---|---|
| Age | |||
| 18–38 | 39 (43.8) | 35 (77.8) | 0.0002 * |
| 39–59 | 33 (37.1) | 9 (20.0) | |
| >59 | 17 (19.1) | 1 (2.2) | |
| Sex | |||
| Female | 43 (48.3) | 25 (55.6) | 0.5426 ** |
| Male | 46 (51.7) | 20 (44.4) | |
| Marital status | |||
| Married | 32 (36.0) | 8 (17.8) | 0.0212 * |
| Single | 43 (48.3) | 33 (73.3) | |
| Separated/divorced/widowed | 14 (15.7) | 4 (8.9) | |
| Education level | |||
| Primary education/Illiterate | 40 (44.9) | 2 (4.4) | <0.0001 * |
| Secondary education | 39 (43.8) | 24 (53.4) | |
| Higher education | 10 (11.3) | 19 (42.2) | |
| Family income (minimum wage) a | |||
| <1 | 17 (19.1) | 1 (2.2) | <0.0001 * |
| 1–3 | 65 (73.0) | 24 (53.4) | |
| 4–6 | 7 (7.9) | 15 (33.3) | |
| >10 | 0 | 5 (11.1) | |
| Number of people living in the home | |||
| 1–3 | 31 (31.4) | 23 (51.1) | 0.2009 * |
| 4–6 | 49 (60.0) | 19 (42.2) | |
| ≥7 | 9 (8.6) | 3 (6.7) | |
| Have you been vaccinated against TB? | |||
| Yes | 75 (84.3) | 45 (100) | 0.1106 * |
| No | 6 (6.7) | 0 | |
| Does not know | 8 (9.0) | 0 | |
| Characteristic | Pulmonary TB n = 75 n (%) | Extrapulmonary TB n = 14 n (%) | p |
|---|---|---|---|
| Age | |||
| 18–38 | 31 (41.3) | 8 (57.2) | 0.3526 * |
| 39–59 | 28 (37.4) | 5 (35.7) | |
| >59 | 16 (21.3) | 1 (7.1) | |
| Sex | |||
| Female | 35 (46.7) | 8 (50.0) | 0.5656 ** |
| Male | 40 (53.3) | 6 (50.0) | |
| Marital status | |||
| Married | 28 (37.3) | 4 (28.6) | 0.1179 * |
| Single | 36 (48.0) | 7 (50.0) | |
| Separated/divorced/widowed | 11 (14.7) | 3 (21.4) | |
| Education level | |||
| Primary education/Illiterate | 30 (40.0) | 10 (71.4) | 0.0449 * |
| Secondary education | 35 (46.7) | 4 (28.6) | |
| Higher education | 10 (13.3) | 0 (0.0) | |
| Family income (minimum wage) a | |||
| < 1 | 13 (17.8) | 4 (28.6) | 0.2484 * |
| 1–3 | 55 (73.3) | 10 (71.4) | |
| 4–6 | 7 (8.9) | 0 (0.0) | |
| Are you working? | |||
| Yes | 33 (44.0) | 0 (0.0) | 0.0008 ** |
| No | 42 (55.0) | 14 (100) | |
| Reason for not working | |||
| Treatment/disease | 28 (66.7) | 13 (92.9) | 0.0822 ** |
| Other | 14 (33.3) | 1 (7.1) | |
| Number of people living in the home | |||
| 1–3 | 24 (31.4) | 7 (50.0) | 0.0917 * |
| 4–6 | 45 (60.0) | 4 (28.6) | |
| ≥7 | 6 (8.6) | 3 (21.4) | |
| Have you been vaccinated against TB? | |||
| Yes | 65 (86.6) | 10 (71.5) | 0.3307 * |
| No | 5 (6.7) | 1 (7.1) | |
| Does not know | 5 (6.7) | 3 (21.4) | |
| Cytokines/Ages | TB Median (IIQ) | p1 | CG Median (IIQ) | p2 |
|---|---|---|---|---|
| IFN-γ | ||||
| 18–38 | 9.36 (2.07) | 0.1229 | 8.01 (1.66) | 0.0706 |
| 39–59 | 9.23 (2.08) | 7.27 (0.90) | ||
| >59 | 7.98 (2.00) | 6.62 (0.00) * | ||
| TNF-α | ||||
| 18–38 | 9.96 (1.46) | 0.1317 | 6.71 (1.92) | 0.0294 |
| 39–59 | 9.34 (1.72) | 5.85 (1.76) | ||
| >59 | 8.82 (2.68) | 4.41 (0.00) * | ||
| IL-6 | ||||
| 18–38 | 12.21 (29.19) | 0.4948 | 7.01 (1.82) | 0.1023 |
| 39–59 | 13.10 (11.03) | 7.97 (1.41) | ||
| >59 | 11.03 (4.59) | 6.89 (0.00) * | ||
| IL-2 | ||||
| 18–38 | 9.05 (1.26) | 0.0344 | 7.33 (1.39) | 0.4127 |
| 39–59 | 9.25 (0.88) | 7.44 (1.74) | ||
| >59 | 8.23 (1.15) | 6.01 (0.00) * | ||
| IL-4 | ||||
| 18–38 | 12.29 (3.71) | 0.0355 | 6.85 (1.04) | 0.0333 |
| 39–59 | 11.17 (2.48) | 7.74 (1.81) | ||
| >59 | 10.66 (3.84) | 7.13 (0.00) * | ||
| IL-10 | ||||
| 18–38 | 10.02 (2.66) | 0.0106 | 7.53 (0.82) | 0.5973 |
| 39–59 | 10.27 (1.74) | 7.31 (1.14) | ||
| >59 | 9.48 (1.15) | 7.12 (0.00) * | ||
| IL-17 | ||||
| 18–38 | 21.77 (26.34) | 0.4272 | 17.14 (16.67) | 0.9605 |
| 39–59 | 24.63 (34.92) | 16.65 (10.28) | ||
| >59 | 15.32 (20.68) | 16.11 (0.00) * |
| Cytokine | Simple Logistic Regression | Multiple Logistic Regression * | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| IFN-γ | 2.98 | 1.69–5.28 | 0.0002 | 4.06 | 1.79–9.21 | 0.0008 |
| TNF-α | 2.28 | 1.55–3.34 | <0.0001 | - | - | - |
| IL-6 | 1.06 | 0.98–1.13 | 0.1383 | - | - | - |
| IL-2 | 5.02 | 2.34–10.72 | <0.0001 | - | - | - |
| IL-4 | 1.61 | 1.27–2.06 | 0.0001 | 2.62 | 1.58–4.33 | 0.0002 |
| IL-10 | 4.13 | 2.15–7.94 | <0.0001 | - | - | - |
| IL-17 | 0.99 | 0.97–1.01 | 0.3349 | - | - | - |
| Cytokine | Simple Logistic Regression | Multiple Logistic Regression * | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| IFN-γ | 0.1994 | 0.0752–0.5286 | 0.0012 | 0.1064 | 0.0223–0.5080 | 0.0050 |
| TNF-α | 0.9613 | 0.6937–1.3321 | 0.8125 | - | - | - |
| IL-6 | 1.0146 | 0.9975–1.0319 | 0.0942 | - | - | - |
| IL-2 | 0.4834 | 0.2239–1.0437 | 0.0642 | - | - | - |
| IL-4 | 1.2302 | 0.907–1.6685 | 0.1827 | - | - | - |
| IL-10 | 0.8233 | 0.5861–1.1566 | 0.2623 | - | - | - |
| IL-17 | 0.954 | 0.9123–0.9976 | 0.0388 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Queiroz, M.A.F.; Lima, S.S.; Amoras, E.d.S.G.; Sousa, F.D.M.d.; Souza, I.d.P.; Nunes, J.A.L.; Brasil-Costa, I.; Cayres-Vallinoto, I.M.V.; Ishak, R.; Vallinoto, A.C.R. Epidemiological and Cytokine Profile of Patients with Pulmonary and Extrapulmonary Tuberculosis in a Population of the Brazilian Amazon. Microorganisms 2022, 10, 2075. https://doi.org/10.3390/microorganisms10102075
Queiroz MAF, Lima SS, Amoras EdSG, Sousa FDMd, Souza IdP, Nunes JAL, Brasil-Costa I, Cayres-Vallinoto IMV, Ishak R, Vallinoto ACR. Epidemiological and Cytokine Profile of Patients with Pulmonary and Extrapulmonary Tuberculosis in a Population of the Brazilian Amazon. Microorganisms. 2022; 10(10):2075. https://doi.org/10.3390/microorganisms10102075
Chicago/Turabian StyleQueiroz, Maria Alice Freitas, Sandra Souza Lima, Ednelza da Silva Graça Amoras, Francisca Dayse Martins de Sousa, Iury de Paula Souza, Juliana Abreu Lima Nunes, Igor Brasil-Costa, Izaura Maria Vieira Cayres-Vallinoto, Ricardo Ishak, and Antonio Carlos Rosário Vallinoto. 2022. "Epidemiological and Cytokine Profile of Patients with Pulmonary and Extrapulmonary Tuberculosis in a Population of the Brazilian Amazon" Microorganisms 10, no. 10: 2075. https://doi.org/10.3390/microorganisms10102075
APA StyleQueiroz, M. A. F., Lima, S. S., Amoras, E. d. S. G., Sousa, F. D. M. d., Souza, I. d. P., Nunes, J. A. L., Brasil-Costa, I., Cayres-Vallinoto, I. M. V., Ishak, R., & Vallinoto, A. C. R. (2022). Epidemiological and Cytokine Profile of Patients with Pulmonary and Extrapulmonary Tuberculosis in a Population of the Brazilian Amazon. Microorganisms, 10(10), 2075. https://doi.org/10.3390/microorganisms10102075









