Antibiotic Susceptibility Profiles and Resistance Mechanisms to β-Lactams and Polymyxins of Escherichia coli from Broilers Raised under Intensive and Extensive Production Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Bacterial Isolates
2.2. Antimicrobial Susceptibility Testing
2.3. DNA Extraction
2.4. Identification of Integrons by Polymerase Chain Reaction
2.5. Molecular Characterization of Antimicrobial Resistance Genes
2.6. Whole Genome Sequencing
2.7. Statistical Analysis
3. Results
3.1. Phenotypic Characterization of Antimicrobial Resistance
3.2. Genotypic Characterization of Antimicrobial Resistance Determinants
3.3. Mobilizable Genetic Elements
3.4. Genomic Characterization of Isolates Selected for Whole Genome Sequencing (WGS)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ECDC. European Centre for Disease Prevention and Control. Antimicrobial Resistance in the EU/EEA (EARS-Net). Annual Epidemiological Report 2019. 2020. Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2019 (accessed on 30 June 2022).
- Hao, H.; Sander, P.; Iqbal, Z.; Wang, Y.; Cheng, G.; Yuan, Z. The Risk of Some Veterinary Antimicrobial Agents on Public Health Associated with Antimicrobial Resistance and their Molecular Basis. Front. Microbiol. 2016, 7, 1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Organization for Animal Health. The OIE Strategy on Antimicrobial Resistance and the Prudent Use of Antimicrobials. 2016. Available online: https://www.oie.int/app/uploads/2021/03/en-oie-amrstrategy.pdf (accessed on 30 June 2022).
- Magnusson, U.; Sternberg, S.; Eklund, G.; Rozstalnyy, A. Prudent and efficient use of antimicrobials in pigs and poultry. FAO Anim. Prod. Health Man. 2019, 23, 1–44. [Google Scholar]
- WHO (World Health Organization); FAO (Food and Agriculture Organization); OIE (World Organization for Animal Health). Antimicrobial Resistance: A Manual for Developing National Action Plans, Version 1. 2016. Available online: https://apps.who.int/iris/handle/10665/204470 (accessed on 30 June 2022).
- Becker, E.; Projahn, M.; Burow, E.; Käsbohrer, A. Are There Effective Intervention Measures in Broiler Production against the ESBL/AmpC Producer Escherichia coli? Pathogens 2021, 10, 608. [Google Scholar] [CrossRef]
- ECDC (European Centre for Disease Prevention and Control); EFSA (European Food Safety Authority); EMA (European Medicines Agency). Third joint inter-agency report on integrated analysis of consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food-producing animals in the EU/EEA. EFSA J. 2021, 19, 6712. [Google Scholar] [CrossRef]
- Ray, S.; Das, S.; Suar, M. Molecular Mechanism of Drug Resistance. In Drug Resistance in Bacteria, Fungi, Malaria, and Cancer; Springer: Cham, Switzerland, 2017; Volume 22, pp. 47–110. [Google Scholar]
- WHO. World Health Organization. Tackling Antibiotic Resistance from a Food Safety Perspective in Europe. 2011. Available online: https://www.euro.who.int/en/publications/abstracts/tackling-antibiotic-resistance-from-a-food-safety-perspective-in-europe (accessed on 30 June 2022).
- Ceccarelli, D.; Kant, A.; van Essen-Zandbergen, A.; Dierikx, C.; Hordijk, J.; Wit, B.; Mevius, D.J.; Veldman, K.T. Diversity of Plasmids and Genes Encoding Resistance to Extended Spectrum Cephalosporins in Commensal Escherichia coli From Dutch Livestock in 2007–2017. Front. Microbiol. 2019, 10, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Depoorter, P.; Persoons, D.; Uyttendaele, M.; Butaye, P.; de Zutter, L.; Dierick, K.; Herman, L.; Imberechts, H.; van Huffel, X.; Dewulf, J. Assessment of human exposure to 3rd generation cephalosporin resistant E. coli (CREC) through consumption of broiler meat in Belgium. Int. J. Food Microbiol. 2012, 159, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Clemente, L.; Leão, C.; Moura, L.; Albuquerque, T.; Amaro, A. Prevalence and Characterization of ESBL/AmpC Producing Escherichia coli from Fresh Meat in Portugal. Antibiotics 2021, 10, 1333. [Google Scholar] [CrossRef]
- Gazal, L.; Medeiros, L.; Dibo, M.; Nishio, E.; Koga, V.; Gonçalves, B.; Grassotti, T.; Leal de Camargo, T.; Pinheiro, J.; Vespero, E.; et al. Detection of ESBL/AmpC-Producing and Fosfomycin-Resistant Escherichia coli From Different Sources in Poultry Production in Southern Brazil. Front. Microbiol. 2021, 11, 604544. [Google Scholar] [CrossRef] [PubMed]
- Babakhani, S.; Oloomi, M. Transposons: The agents of antibiotic resistance in bacteria. J. Basic Microbiol. 2018, 58, 905–917. [Google Scholar] [CrossRef]
- Mehdi, Y.; Létourneau-Montminy, M.-P.; Gaucher, M.-L.; Chorfi, Y.; Suresh, G.; Rouissi, T.; Brar, S.K.; Côté, C.; Ramirez, A.A.; Godbout, S. Use of antibiotics in broiler production: Global impacts and alternatives. Anim. Nutr. 2018, 4, 170–178. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority); ECDC (European Centre for Disease Prevention and Control). The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2018/2019. EFSA J. 2021, 19, 6490. [Google Scholar]
- Rodrigues, C.; Machado, E.; Peixe, L.; Novais, A. IncI1/ST3 and IncN/ST1 plasmids drive the spread of blaTEM-52 and blaCTX-M-1/-32 in diverse Escherichia coli clones from different piggeries. J. Antimicrob. Chemother. 2013, 68, 2245–2248. [Google Scholar] [CrossRef] [PubMed]
- Clemente, L.; Manageiro, V.; Jones-Dias, D.; Correia, I.; Themudo, P.; Albuquerque, T.; Geraldes, M.; Matos, F.; Almendra, C.; Ferreira, E.; et al. Antimicrobial susceptibility and oxymino-β-lactam resistance mechanisms in Salmonella enterica and Escherichia coli isolates from different animal sources. Res. Microbiol. 2015, 166, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Jones-Dias, D.; Manageiro, V.; Martins, A.P.; Ferreira, E.; Caniça, M. New Class 2 Integron In2-4 Among IncI1-Positive Escherichia coli Isolates Carrying ESBL and PMAβ Genes from Food Animals in Portugal. Foodborne Pathog. Dis. 2016, 13, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.; Costa, L.; Gonçalves, A.; Sousa, M.; Radhouani, H.; Brito, F.; Igrejas, G.; Poeta, P. Genetic characterization of extended-spectrum β-lactamases in Escherichia coli isolated from retail chicken products including CTX-M-9 containing isolates: A food safety risk factor. Br. Poult. Sci. 2012, 53, 747–755. [Google Scholar] [CrossRef]
- Manageiro, V.; Clemente, L.; Graça, R.; Correia, I.; Albuquerque, T.; Ferreira, E.; Caniça, M. New insights into resistance to colistin and third-generation cephalosporins of Escherichia coli in poultry, Portugal: Novel blaCTX-M-166 and blaESAC genes. Int. J. Food Microbiol. 2017, 263, 67–73. [Google Scholar] [CrossRef]
- Saliu, E.; Vahjen, W.; Zentek, J. Types and prevalence of extended–spectrum beta–lactamase producing Enterobacteriaceae in poultry. Anim. Health Res. Rev. 2017, 18, 46–57. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Álvarez, S.; Sanz, S.; Olarte, C.; Hidalgo-Sanz, R.; Carvalho, I.; Fernández-Fernández, R.; Campaña-Burguet, A.; Latorre-Fernández, X.; Zarazaga, M.; Torres, C. Antimicrobial Resistance in Escherichia coli from the Broiler Farm Environment, with Detection of SHV-12-Producing Isolates. Antibiotics 2022, 11, 444. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Wang, Y.; Walsh, T.R.; Yi, L.-X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Caniaux, I.; van Belkum, A.; Zambardi, G.; Poirel, L.; Gros, M.F. MCR: Modern colistin resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 36, 415–420. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Liu, J.-H. Monitoring colistin resistance in food animals: An Urgent Threat. Expert Rev. Anti. Infect. Ther. 2018, 16, 443–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemente, L.; Manageiro, V.; Correia, I.; Amaro, A.; Albuquerque, T.; Themudo, P.; Ferreira, E.; Caniça, M. Revealing mcr-1-positive ESBL-producing Escherichia coli strains among Enterobacteriaceae from food-producing animals (bovine, swine and poultry) and meat (bovine and swine), Portugal, 2010–2015. Int. J. Food Microbiol. 2019, 296, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Hussein, N.H.; AL-Kadmy, I.M.S.; Taha, B.M.; Hussein, J.D. Mobilized colistin resistance (mcr) genes from 1 to 10: A comprehensive review. Mol. Biol. Rep. 2021, 48, 2897–2907. [Google Scholar] [CrossRef] [PubMed]
- Apostolakos, I.; Piccirillo, A. A review on the current situation and challenges of colistin resistance in poultry production. Avian Pathol. 2018, 47, 546–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Commission Implementing Decision 652 of 12 November 2013 on surveillance and reporting of antimicrobial resistance in zoonotic and commensal bacteria. In Official Journal of the European Union; Publications Office of the European Union: Luxembourg, 2013.
- European Reference Laboratory for Antimicrobial Resistance. Protocols. Available online: https://www.eurl-ar.eu/protocols.aspx (accessed on 30 June 2022).
- European Committee of Antimicrobial Susceptibility Testing (EUCAST). Available online: https://www.eucast.org/mic_distributions_and_ecoffs/ (accessed on 1 July 2022).
- Schwarz, S.; Silley, P.; Simjee, S.; Woodford, N.; van Duijkeren, E.; Johnson, A.; Gaastra, W. Assessing the antimicrobial susceptibility of bacteria obtained from animals. Vet. Microbiol. 2010, 65, 601–604. [Google Scholar] [CrossRef]
- Kargar, M.; Mohammadalipour, Z.; Doosti, A.; Lorzadeh, S.; Japoni-Nejad, A. High Prevalence of Class 1 to 3 Integrons Among Multidrug-Resistant Diarrheagenic Escherichia coli in Southwest of Iran. Osong Public Health Res. Perspect. 2014, 5, 193–198. [Google Scholar] [CrossRef] [Green Version]
- Machado, E.; Canton, R.; Baquero, F.; Galan, J.-C.; Rollan, A.; Peixe, L.; Coque, T.M. Integron Content of Extended-Spectrum- -Lactamase-Producing Escherichia coli Strains over 12 Years in a Single Hospital in Madrid, Spain. Antimicrob. Agents Chemother. 2005, 49, 1823–1829. [Google Scholar] [CrossRef] [Green Version]
- Dallenne, C.; Da Costa, A.; Decré, D.; Favier, C.; Arlet, G. Development of a set of multiplex PCR assays for the detection of genes encoding important β-lactamases in Enterobacteriaceae. J. Antimicrob. Chemother. 2010, 65, 490–495. [Google Scholar] [CrossRef] [Green Version]
- NCBI. National Center for Biotechnology Information. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi?PRGRAM=blastn&BLAST_SPEC=GeoBlast&PAGE_TYPE=BlastSearch (accessed on 30 June 2022).
- CARD. The Comprehensive Antibiotic Resistance Database. Available online: https://card.mcmaster.ca/analyze/blast (accessed on 30 June 2022).
- CGE. Center for Genomic Epidemiology. Available online: https://cge.cbs.dtu.dk/services/ (accessed on 30 June 2022).
- Bortolaia, V.; Kaas, R.F.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.R.; et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef]
- Rebelo, A.R.; Bortolaia, V.; Kjeldgaard, J.S.; Pedersen, S.K.; Leekitcharoenphon, P.; Hansen, I.M.; Guerra, B.; Malorny, B.; Borowiak, M.; Hammerl, J.A.; et al. Multiplex PCR for detection of plasmid-mediated colistin resistance determinants, mcr-1, mcr-2, mcr-3, mcr-4 and mcr-5 for surveillance purposes. Eurosurveillence 2018, 23, 17. [Google Scholar] [CrossRef] [Green Version]
- Borowiak, M.; Baumann, B.; Fischer, J.; Thomas, K.; Deneke, C.; Hammerl, J.; Szabo, I.; Malorny, B. Development of a Novel mcr-6 to mcr-9 Multiplex PCR and Assessment of mcr-1 to mcr-9 Occurrence in Colistin-Resistant Salmonella enterica Isolates From Environment, Feed, Animals and Food (2011–2018) in Germany. Front. Microbiol. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Critically Important Antimicrobials for Human Medicine, 6th ed.; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Hasman, H.; Saputra, D.; Sicheritz-Ponten, T.; Lund, O.; Svendsen, C.A.; Frimodt-Møller, N.; Aarestrup, F.M. Rapid whole-genome sequencing for detection and characterization of microorganisms directly from clinical samples. J. Clin. Microbiol. 2014, 52, 139–146. [Google Scholar] [CrossRef]
- Larsen, M.; Cosentino, S.; Rasmussen, S.; Rundsten, C.; Hasman, H.; Marvig, R.; Jelsbak, L.; Sicheritz-Pontén, T.; Ussery, D.; Aarestrup, F.; et al. Multilocus Sequence Typing of Total Genome Sequenced Bacteria. J. Clin. Microbiol. 2012, 50, 1355–1361. [Google Scholar] [CrossRef] [Green Version]
- Carattoli, A.; Zankari, E.; Garcia-Fernandez, A.; Voldby, L.M.; Lund, O.; Villa, L.; Aarestrup, F.M.; Hasman, H. PlasmidFinder and pMLST: In silico detection and typing of plasmids. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joensen, K.G.; Tetzschner, A.M.; Iguchi, A.; Aarestrup, F.M.; Scheutz, F. Rapid and easy in silico serotyping of Escherichia coli using whole genome sequencing (WGS) data. J. Clin. Microbiol. 2015, 53, 2410–2426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roer, L.; Johannesen, T.B.; Hansen, F.; Stegger, M.; Tchesnokova, V.; Sokurenko, E.; Garibay, N.; Allesøe, R.; Thomsen, M.C.F.; Lund, O.; et al. CHTyper, a Web Tool for Subtyping of Extraintestinal Pathogenic Escherichia coli Based on the fumC and fimH Alleles. J. Clin. Microbiol. 2018, 56, e00063-18. [Google Scholar] [CrossRef] [Green Version]
- Cosentino, S.; Voldby Larsen, M.; Møller Aarestrup, F.; Lund, O. PathogenFinder—Distinguishing Friend from Foe Using Bacterial Whole Genome Sequence Data. PLoS ONE 2013, 8, e77302. [Google Scholar] [CrossRef]
- Johansson, M.H.K.; Bortolaia, V.; Tansirichaiya, S.; Aarestrup, F.M.; Roberts, A.P.; Petersen, T.N. Detection of mobile genetic elements associated with antibiotic resistance in Salmonella enterica using a newly developed web tool: MobileElementFinder. J. Antimicrob. Chemother. 2021, 76, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Beghain, J.; Bridier-Nahmias, A.; Le Nagard, H.; Denamur, E.; Clermont, O. ClermonTyping: An easy-to-use and accurate in silico method for Escherichia genus strain phylotyping. Microb. genomics. 2018, 4, e000192. [Google Scholar] [CrossRef] [PubMed]
- Roth, N.; Kasbohrer, A.; Mayrhofer, S.; Zitz, U.; Hofacre, C.; Doming, K.J. The application in broiler production and resulting antibiotic resistance in Escherichia coli: A global Overview. Poultry Sci. 2019, 98, 1791–1804. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Jiménez, D.; García-Meniño, I.; Herrera, A.; García, V.; López-Beceiro, A.M.; Alonso, M.P.; Blanco, J.; Mora, A. Genomic Characterization of Escherichia coli Isolates Belonging to a New Hybrid aEPEC/ExPEC Pathotype O153:H10-A-ST10 eae-beta1 Occurred in Meat, Poultry, Wildlife and Human Diarrheagenic Samples. Antibiotics 2020, 9, 192. [Google Scholar] [CrossRef] [PubMed]
- Stedt, J.; Bonnedahl, J.; Hernandez, J.; Waldenström, J.; McMahon, B.J.; Tolf, C.; Olsen, B.; Drobni, M. Carriage of CTX-M type extended spectrum β-lactamases (ESBLs) in gulls across Europe. Acta Vet. Scand. 2015, 57, 74. [Google Scholar] [CrossRef]
- Lalak, A.; Wasyl, D.; Zając, M.; Skarżyńska, M.; Hoszowski, A.; Samcik, I.; Woźniakowski, G.; Szulowski, K. Mechanisms of cephalosporin resistance in indicator Escherichia coli isolated from food animals. Vet. Microbiol. 2016, 194, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Machado, E.; Coque, T.M.; Cantón, R.; Sousa, J.C.; Peixe, L. Antibiotic resistance integrons and extended-spectrum β-lactamases among Enterobacteriaceae isolates recovered from chickens and swine in Portugal. J. Antimicrob. Chemother. 2008, 62, 296–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewers, C.; de Jong, A.; Prenger-Berninghoff, E.; El Garch, F.; Leidner, U.; Tiwari, S.K.; Semmler, T. Genomic Diversity and Virulence Potential of ESBL-and AmpC-β-Lactamase-Producing Escherichia coli Strains From Healthy Food Animals Across Europe. Front. Microbiol. 2021, 12, 626774. [Google Scholar] [CrossRef]
- Huang, Y.; Zeng, L.; Doi, Y.; Lv, L.; Liu, J.H. Extended-spectrum β-lactamase-producing Escherichia coli. Lancet Infect. Dis. 2020, 20, 404–405. [Google Scholar] [CrossRef]
- Na, S.; Moon, D.; Choi, M.; Oh, S.; Jung, D.; Sung, E.; Kang, H.; Hyun, B.H.; Lim, S.K. Antimicrobial Resistance and Molecular Characterization of Extended-Spectrum β-Lactamase-Producing Escherichia coli Isolated from Ducks in South Korea. Foodborne Pathog. Dis. 2019, 16, 799–806. [Google Scholar] [CrossRef]
- Cunha, M.; Lincopan, N.; Cerdeira, L.; Dropa, M.; Franco, L.; Moreno, A.; Knöbl, T. Coexistence of CTX-M-2, CTX-M-55, CMY-2, FosA3, and QnrB19 in Extraintestinal Pathogenic Escherichia coli from Poultry in Brazil. Antimicrob. Agents Chemother. 2017, 61, e02474-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega-Paredes, D.; de Janon, S.; Villavicencio, F.; Ruales, K.; Torre, K.; Villacís, J.; Wagenaar, J.; Matheu, J.; Bravo- Vallejo, C.; Fernández-Moreira, E.; et al. Broiler Farms and Carcasses Are an Important Reservoir of Multi-Drug Resistant Escherichia coli in Ecuador. Front. Vet. Sci. 2020, 7, 547843. [Google Scholar] [CrossRef]
- Carvalho, I.; Chenouf, N.; Cunha, R.; Martins, C.; Pimenta, P.; Pereira, A.R.; Martínez-Álvarez, S.; Ramos, S.; Silva, V.; Igrejas, G.; et al. Antimicrobial Resistance Genes and Diversity of Clones among ESBL- and Ac- 578 quired AmpC-Producing Escherichia coli Isolated from Fecal Samples of Healthy and Sick Cats in Portugal. Antibiotics 2021, 10, 262. [Google Scholar] [CrossRef] [PubMed]
- Blaak, H.; van Hoek, A.H.A.M.; Hamidjaja, R.A.; van der Plaats, R.Q.J.; Kerkhof-de Heer, L.; de Roda Husman, A.M.; Schets, F.M. Distribution, Numbers, and Diversity of ESBL-Producing E. coli in the Poultry Farm Environment. PLOS ONE 2015, 10, e0135402. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.; Tolmasky, M. Aminoglycoside Modifying Enzymes. Drug Resist. Updat. 2010, 13, 151–171. [Google Scholar] [CrossRef]
- Deng, Y.; Bao, X.; Ji, L.; Chen, L.; Liu, J.; Miao, J.; Chen, D.; Bian, H.; Li, Y.; Yu, G. Resistance integrons: Class 1, 2 and 3 integrons. Ann. Clin. Microbiol. Antimicrob. 2015, 14, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunde, M.; Simonsen, G.S.; Slettemeas, J.S.; Bockerman, I.; Norstrom, M. Integron, Plasmid and Host Strain Characteristics of Escherichia coli from Humans and Food Included in the Norwegian Antimicrobial Resistance Monitoring Programs. PloS ONE 2015, 10, e0128797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, J.Y.; Oh, J.E. Distribution and characterization of integrons in Enterobacteriaceae isolates from chickens in Korea. J. Microbiol. Biotechnol. 2014, 24, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Racewicz, P.; Majewski, M.; Biesiada, H.; Nowaczewski, S.; Wilczyński, J.; Wystalska, D.; Kubiak, M.; Pszczoła, M.; Madeja, Z.E. Prevalence and characterisation of antimicrobial resistance genes and class 1 and 2 integrons in multiresistant Escherichia coli isolated from poultry production. Sci. Rep. 2022, 12, 6062. [Google Scholar] [CrossRef]
- Kaushik, M.; Kumar, S.; Kapoor, R.; Virdi, J.; Gulati, P. Integrons in Enterobacteriaceae: Diversity, distribution and epidemiology. Int. J. Antimicrob. Agents. 2018, 51, 167–176. [Google Scholar] [CrossRef]
- ESVAC. Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2019 and 2020; Trends from 2010 to 2020, Eleventh ESVAC Report; European Medicines Agency: Luxembourg, 2021. [Google Scholar]
- Pesciaroli, M.; Magistrali, C.F.; Filippini, G.; Epifanio, E.M.; Lovito, C.; Marchi, L.; Maresca, C.; Massacci, F.R.; Orsini, S.; Scoccia, E.; et al. Antibiotic-resistant commensal Escherichia coli are less frequently isolated from poultry raised using non-conventional management systems than from conventional broiler. Int. J. Food Microbiol. 2020, 314, 108391. [Google Scholar] [CrossRef]
- Salerno, B.; Furlan, M.; Sabatino, R.; Di Cesare, A.; Leati, M.; Volanti, M.; Barco, L.; Orsini, M.; Losasso, C.; Cibin, V. Antibiotic resistance genes load in an antibiotic free organic broiler farm. Poultry Sci. 2022, 101, 101675. [Google Scholar] [CrossRef] [PubMed]
- Dandachi, I.; Chabou, S.; Daoud, Z.; Rolain, J.-M. Prevalence and Emergence of Extended-Spectrum Cephalosporin-, Carbapenem- and Colistin-Resistant Gram-Negative Bacteria of Animal Origin in the Mediterranean Basin. Front. Microbiol. 2018, 9, 2299. [Google Scholar] [CrossRef] [Green Version]
- Jones-Dias, D.; Manageiro, V.; Ferreira, E.; Barreiro, P.; Vieira, L.; Moura, I.B.; Caniça, M. Architecture of Class 1, 2, and 3 Integrons from Gram Negative Bacteria Recovered among Fruits and Vegetables. Front. Microbiol. 2016, 7, 1400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Zhang, H.; Liu, Y.-H.; Feng, Y. Towards Understanding MCR-like Colistin Resistance. Trends Microbiol. 2018, 26, 794–808. [Google Scholar] [CrossRef] [PubMed]
- Javed, H.; Saleem, S.; Zafar, A.; Ghafoor, A.; Shahzad, A.B.; Ejaz, H.; Junaid, K.; Jahan, S. Emergence of plasmid-mediated mcr genes from Gram-negative bacteria at the human-animal interface. Gut Pathogens. 2020, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Haenni, M.; Beyrouthy, R.; Lupo, A.; Châtre, P.; Madec, J.-Y.; Bonnet, R. Epidemic spread of Escherichia coli ST744 isolates carrying mcr-3 and blaCTX-M-55 in cattle in France. J. Antimicrob. Chemother. 2017, 73, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Perrin-Guyomard, A.; Jouy, E.; Urban, D.; Chauvin, C.; Granier, S.A.; Mourand, G.; Chevance, A.; Adam, C.; Moulin, G.; Kempf, I. Decrease in fluoroquinolone use in French poultry and pig production and changes in resistance among E. coli and Campylobacter. Vet. Microbiol. 2020, 243, 108637. [Google Scholar] [CrossRef] [PubMed]
- Rozwandowicz, M.; Brouwer MS, M.; Fischer, J.; Wagenaar, J.A.; Gonzalez-Zorn, B.; Guerra, B.; Mevius, D.J.; Hordijk, J. Plasmids carrying antimicrobial resistance genes in Enterobacteriaceae. J. Antimicrob. Chemother. 2018, 73, 1121–1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poirel, L.; Madec, J.-Y.; Lupo, A.; Schink, A.-K.; Kieffer, N.; Nordmann, P.; Schwarz, S. Antimicrobial Resistance in Escherichia coli. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huijbers, P.M.C.; Graat, E.A.M.; Haenen, A.P.J.; van Santen, M.G.; van Essen-Zandbergen, A.; Mevius, D.J.; van Duijkeren, E.; van Hoek, A.H.A.M. Extended-spectrum and AmpC β-lactamase-producing Escherichia coli in broilers and people living and/or working on broiler farms: Prevalence, risk factors and molecular characteristics. J. Antimicrob. Chemother. 2014, 69, 2669–2675. [Google Scholar] [CrossRef]
- Alonso, C.A.; Michael, G.B.; Li, J.; Somalo, S.; Simón, C.; Wang, Y.; Kaspar, H.; Kadlec, K.; Torres, C.; Schwarz, S. Analysis of blaSHV-12-carrying Escherichia coli clones and plasmids from human, animal and food sources. J. Antimicrob. Chemother. 2017, 72, 1589–1596. [Google Scholar] [CrossRef] [Green Version]
- Ageevets, V.; Lazareva, I.; Mrugova, T.; Gostev, V.; Lobzin, Y.; Sidorenko, S. IncX4 plasmids harboring mcr-1 genes: Further dissemination. J Global Antimicrob. Res. 2019, 18, 166–167. [Google Scholar] [CrossRef]
- Manageiro, V.; Clemente, L.; Romão, R.; Silva, C.; Vieira, L.; Ferreira, E.; Caniça, M. IncX4 Plasmid Carrying the New mcr-1.9 Gene Variant in a CTX-M-8-Producing Escherichia coli Isolate Recovered From Swine. Front. Microbiol. 2019, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Mourand, G.; Le Devendec, L.; Delannoy, S.; Fach, P.; Keita, A.; Amelot, M.; Jaunet, H.; Dia, M.E.H.; Kempf, I. Variations of the Escherichia coli population in the digestive tract of broilers. Avian Pathol. 2020, 49, 678–688. [Google Scholar] [CrossRef]
- Oteo, J.; Diestra, K.; Juan, C.; Bautista, V.; Novais, Â.; Pérez-Vázquez, M.; Moyá, B.; Miró, E.; Coque, T.M.; Oliver, A.; et al. Extended-spectrum β-lactamase-producing Escherichia coli in Spain belong to a large variety of multilocus sequence typing types, including ST10 complex/A, ST23 complex/A and ST131/B2. Int. J. Antimicrob. Agents 2009, 34, 173–176. [Google Scholar] [CrossRef] [PubMed]
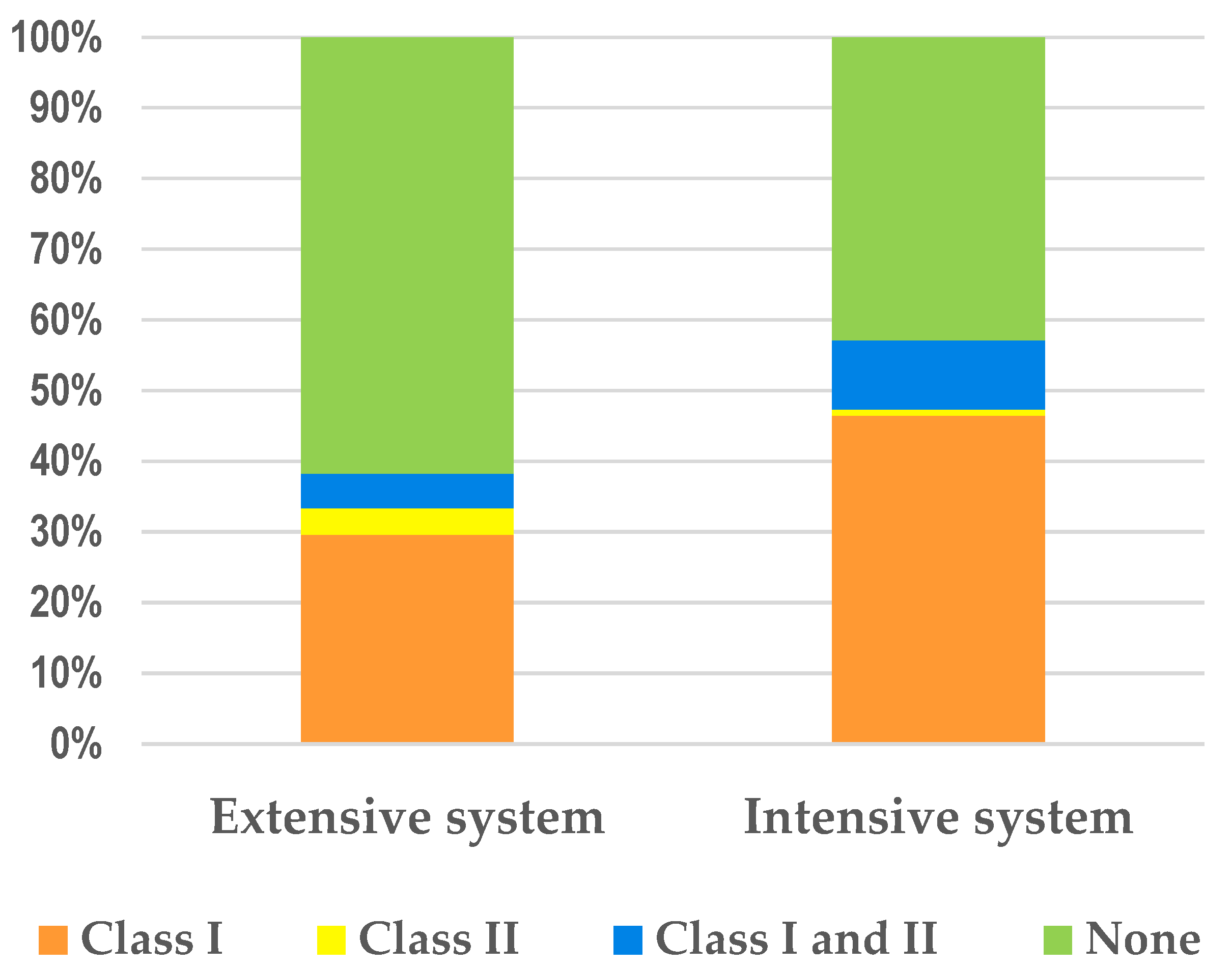
| Antimicrobial | Extensive System (n = 81) | Intensive System (n= 112) | ECOFF (mg/L) | ||
|---|---|---|---|---|---|
| Commensal (n =68) | CTXr (n = 13) | Commensal (n = 71) | CTXr (n = 41) | ||
| β-lactams | |||||
| Ampicillin | |||||
| MIC50 | >64 | >64 | >64 | >64 | |
| MIC90 | >64 | >64 | >64 | >64 | 8 |
| %RS | 64.7 | 100 | 69 | 100 | |
| Cefotaxime | |||||
| MIC50 | ≤0.25 | 16 | ≤0.25 | 16 | |
| MIC90 | ≤0.25 | 64 | ≤0.25 | >64 | 0.25 |
| %RS | 5.9 | 100 | 1.4 | 100 | |
| Ceftazidime | 0.5 | ||||
| MIC50 | ≤0.5 | 4 | ≤0.5 | 16 | |
| MIC90 | ≤0.5 | 32 | ≤0.5 | 32 | |
| %RS | 5.9 | 92.3 | 1.4 | 100 | |
| Cefoxitin | |||||
| MIC50 | * | 8 | * | 8 | 8 |
| MIC90 | * | 32 | * | 16 | |
| %RS | 0 | 23.1 | 0 | 22 | |
| Meropenem | 0.125 | ||||
| MIC50 | ≤0.03 | ≤0.03 | ≤0.03 | ≤0.03 | |
| MIC90 | ≤0.03 | ≤0.03 | ≤0.03 | ≤0.03 | |
| %RS | 0 | 0 | 0 | 0 | |
| Ertapenem | 0.06 | ||||
| MIC50 | * | ≤0.015 | * | ≤0.015 | |
| MIC90 | * | 0.03 | * | 0.06 | |
| %RS | 0 | 0 | 0 | 4.9 | |
| Imipenem | 0.5 | ||||
| MIC50 | * | 0.25 | * | 0.25 | |
| MIC90 | * | 0.25 | * | 0.5 | |
| %RS | 0 | 0 | 0 | 2.4 | |
| Quinolones | |||||
| Nalidixic Ácid | 16 | ||||
| MIC50 | >128 | >128 | >128 | >128 | |
| MIC90 | >128 | >128 | >128 | >128 | |
| %RS | 69.1 | 76.9 | 85.9 | 78 | |
| Ciprofloxacin | 0.064 | ||||
| MIC50 | 0.25 | 8 | 2 | 2 | |
| MIC90 | 8 | >8 | >8 | >8 | |
| %RS | 73.5 | 76.9 | 81.7 | 90.2 | |
| Macrolides | |||||
| Azithromycin | 16 | ||||
| MIC50 | 8 | 8 | 8 | 8 | |
| MIC90 | 8 | 64 | 32 | 32 | |
| %RS | 1.5 | 30.8 | 11.3 | 14.6 | |
| Phenicols | |||||
| Chloramphenicol | 16 | ||||
| MIC50 | ≤8 | ≤8 | ≤8 | 32 | |
| MIC90 | ≤8 | >128 | 128 | >128 | |
| %RS | 4.4 | 30.8 | 23.9 | 65.9 | |
| Polymixins | |||||
| Colistin | 2 | ||||
| MIC50 | ≤1 | ≤1 | ≤1 | ≤1 | |
| MIC90 | ≤1 | ≤1 | ≤1 | ≤1 | |
| %RS | 0 | 0 | 0 | 2.4 | |
| Tetracyclines | |||||
| Tetracycline | 8 | ||||
| MIC50 | 4 | 64 | 64 | 64 | |
| MIC90 | >64 | >64 | >64 | >64 | |
| %RS | 50 | 53.8 | 56.3 | 73.2 | |
| Sulfonamides | |||||
| Sulfamethoxazole | 64 | ||||
| MIC50 | ≤8 | >1024 | 1024 | >1024 | |
| MIC90 | >1024 | >1024 | >1024 | >1024 | |
| %RS | 36.8 | 61.5 | 50.7 | 87.8 | |
| Dihydrofolate reductase inhibitors | |||||
| Trimethoprim | 2 | ||||
| MIC50 | ≤0.25 | >32 | 0.5 | 0.5 | |
| MIC90 | >32 | >32 | >32 | >32 | |
| %RS | 27.9 | 53.8 | 46.5 | 34.1 | |
| Glycylcyclines | |||||
| Tigecycline | ND | ||||
| MIC50 | ≤0.25 | ≤0.25 | 0.5 | ≤0.25 | |
| MIC90 | 0.5 | ≤0.25 | 1 | 0.5 | |
| %RS | 0 | 0 | 0 | 0 | |
| MDR (%) | 50 | 61.5 | 60.6 | 87.8 | |
| Sample | Strain | Year | Phenotype | Inhibition by CLA | bla Resistance Genes | Integrons (Class) |
|---|---|---|---|---|---|---|
| 1fc | Commensal | 2014 | AMP-FOT-TAZ-CIP-NAL-SMX-TET-FEP | Yes | blaCTX-M-1 | Negative |
| 2fc | Commensal | 2014 | AMP-FOT-TAZ-CIP-NAL-SMX-TET-TMP-FEP | Yes | blaTEM-1; blaSHV-12 | I and II |
| 3fc | Commensal | 2014 | AMP-FOT-TAZ-CIP-NAL-SMX-TET-FEP | Yes | blaCTX-M-1 | Negative |
| 4fc | Commensal | 2014 | AMP-FOT-TAZ-FEP | Yes | blaSHV-12 | Negative |
| 5fc | CTXr | 2018 | AMP-FOT-CIP-NAL-FEP | Yes | blaCTX-M-1 | I |
| 6fc * | CTXr | 2018 | AMP-AZI-FOT-TAZ-CHL-CIP-NAL-SMX-TET-TMP-FEP | Yes | blaCTX-M-14; blaTEM-1 | I |
| 7fc | CTXr | 2018 | AMP-FOT-TAZ-FOX | No | blaCMY-2; blaTEM-1 | Negative |
| 8fc | CTXr | 2018 | AMP-FOT-TAZ-CIP-NAL-SMX-TMP-FEP | Yes | blaSHV-12 | I and II |
| 9fc | CTXr | 2018 | AMP-FOT-TAZ-FOX | No | blaCMY-2 | Negative |
| 10fc * | CTXr | 2018 | AMP-AZI-FOT-TAZ-CHL-CIP-NAL-SMX-TET-TMP-FEP | Yes | blaCTX-M-14; blaTEM-1B | I |
| 11fc | CTXr | 2018 | AMP-FOT-TAZ-FOX | No | blaCMY-2 | Negative |
| 12fc | CTXr | 2018 | AMP-FOT-TAZ-CIP-NAL-FEP | Yes | blaSHV-12; blaTEM-type | Negative |
| 13fc | CTXr | 2018 | AMP-FOT-TAZ-CIP-NAL-SMX-TET-TMP-FEP | Yes | blaCTX-M-1; blaTEM-1 | I |
| 14fc | CTXr | 2018 | AMP-FOT-TAZ-CHL-CIP-NAL-SMX-TET-FEP | Yes | blaSHV-12 | I |
| 15fc | CTXr | 2020 | AMP-AZI-FOT-TAZ-CIP-NAL-SMX-TET-TMP-FEP | Yes | blaSHV-12 | I and II |
| 16fc | CTXr | 2020 | AMP-AZI-FOT-TAZ-CHL-CIP-GEN-NAL-SMX-TET-TMP-FEP | Yes | blaCTX-M-55; blaTEM-type | I |
| 17fc | CTXr | 2020 | AMP-FOT-TAZ-CIP-NAL-SMX-TET-TMP-FEP | Yes | blaSHV-12 | I |
| Sample | Strain | Year | Phenotype | Inhibition by CLA | bla Resistance Genes | Integrons (Class) |
|---|---|---|---|---|---|---|
| 1fi | commensal | 2018 | AMP-AZI-FOT-TAZ-CHL-CIP-GEN-NAL-SMX-TET-TMP-FEP | Yes | blaCTX-M-55; blaTEM-type | I |
| 2fi * | CTXr | 2018 | AMP-FOT-TAZ-CHL-CIP-COL-NAL-AMX-TET-FEP | Yes | blaSHV-12 | I |
| 3fi | CTXr | 2018 | AMP-FOT-TAZ-CIP-NAL-SMX-TET-FEP-FOX | Yes | blaTEM-52C | I |
| 4fi | CTXr | 2018 | AMP-FOT-TAZ-CIP-NAL-FEP | Yes | blaSHV-12 | I and II |
| 5fi | CTXr | 2018 | AMP-FOT-TAZ-CIP-NAL-FEP-FOX-ETP | No | blaCMY-2; blaTEM-type | Negative |
| 6fi | CTXr | 2018 | AMP-FOT-TAZ-CHL-SMX-TET-FEP-FOX | Yes | blaSHV-12 | I and II |
| 7fi | CTXr | 2018 | AMP-FOT-TAZ-CHL-CIP-NAL-SMX-TET-FEP | Yes | blaSHV-12 | I and II |
| 8fi | CTXr | 2018 | AMP-FOT-TAZ-CHL-CIP-NAL-SMX-TET-FEP | Yes | blaSHV-12 | I |
| 9fi | CTXr | 2018 | AMP-AZI-FOT-TAZ-CHL-CIP-GEN-NAL-SMX-TET-TMP-FEP | Yes | blaCTX-M-55; blaTEM-type | I |
| 10fi | CTXr | 2018 | AMP-FOT-TAZ-CHL-CIP-NAL-SMX-TET-TMP-FEP | Yes | blaCTX-M-1; blaTEM-type | I |
| 11fi | CTXr | 2018 | AMP-FOT-TAZ-CIP-NAL-SMX-TMP-FEP-FOX | No | blaCMY-2 | I |
| 12fi | CTXr | 2018 | AMP-FOT-TAZ-CIP-NAL-FEP | Yes | blaSHV-12 | Negative |
| 13fi | CTXr | 2018 | AMP-FOT-TAZ-CIP-NAL-FEP-IMI | Yes | blaCTX-M-1 | Negative |
| 14fi | CTXr | 2018 | AMP-FOT-TAZ-CHL-CIP-NAL-SMX-TET-FEP | Yes | blaCTX-M-55 | I |
| 15fi | CTXr | 2018 | AMP-AZI-FOT-TAZ-CHL-CIP-GEN-NAL-SMX-TET-TMP-FEP | Yes | blaCTX-M-55; blaTEM-type | I |
| 16fi | CTXr | 2018 | AMP-FOT-TAZ-CIP-SMX-FEP | Yes | blaSHV-12 | I |
| 17fi | CTXr | 2018 | AMP-FOT-TAZ-TET-FEP | Yes | blaCTX-M-1 | I |
| 18fi | CTXr | 2018 | AMP-FOT-TAZ-CIP-SMX-TMP-FEP-FOX | No | blaCMY-2; blaTEM-1 | Negative |
| 19fi | CTXr | 2018 | AMP-FOT-TAZ-CIP-NAL-SMX-TMP-FEP | Yes | blaSHV-12 | II |
| 20fi | CTXr | 2018 | AMP-FOT-TAZ-CHL-CIP-NAL-SMX-TET-FEP | Yes | blaCTX-M-55 | Negative |
| 21fi | CTXr | 2020 | AMP-FOT-TAZ-CHL-SMX-TET-FEP | Yes | blaSHV-12 | I |
| 22fi | CTXr | 2020 | AMP-FOT-TAZ-CHL-CIP-NAL-SMX-TET-FEP | Yes | blaSHV-12 | Negative |
| 23fi | CTXr | 2020 | AMP-FOT-TAZ-CHL-CIP-NAL-SMX-TET-FEP | Yes | blaSHV-12 | Negative |
| 24fi | CTXr | 2020 | AMP-FOT-TAZ-CHL-CIP-NAL-SMX-TET-FEP-FOX | Yes | blaCTX-M-55 | I |
| 25fi | CTXr | 2020 | AMP-FOT-TAZ-CHL-CIP-NAL-SMX-TET-FEP | Yes | blaSHV-12 | I |
| 26fi | CTXr | 2020 | AMP-FOT-TAZ-CHL-CIP-NAL-SMX-TET-FEP | Yes | blaSHV-12; blaTEM-1 | I |
| 27fi | CTXr | 2020 | AMP-FOT-TAZ-CHL-CIP-NAL-SMX-FEP | Yes | blaSHV-12 | I |
| 28fi | CTXr | 2020 | AMP-FOT-TAZ-CIP-NAL-SMX-TET-FEP | Yes | blaSHV-12 | I |
| 29fi | CTXr | 2020 | AMP-AZI-FOT-TAZ-CHL-CIP-GEN-NAL-SMX-TET-TMP-FEP | Yes | blaCTX-M-55; blaTEM-type | I |
| 30fi | CTXr | 2020 | AMP-FOT-TAZ-CIP-NAL-SMX-TMP-FEP-FOX-ETP | Yes | blaCTX-M-32; mutation on the AmpC promotor | I |
| 31fi | CTXr | 2020 | AMP-FOT-TAZ-CHL-CIP-NAL-SMX-TET-FEP | Yes | blaSHV-12 | I |
| 32fi | CTXr | 2020 | AMP-FOT-TAZ-CIP-NAL-SMX-TET-TMP-FEP | Yes | blaCTX-M-9 | Negative |
| 33fi | CTXr | 2020 | AMP-FOT-TAZ-CIP-NAL-SMX-TET-FEP | Yes | blaCTX-M-1 | Negative |
| 34fi | CTXr | 2020 | AMP-FOT-TAZ-CHL-CIP-NAL-SMX-TET-FEP | Yes | blaSHV-12 | Negative |
| 35fi | CTXr | 2020 | AMP-AZI-FOT-TAZ-CHL-CIP-GEN-NAL-SMX-TET-TMP-FEP | Yes | blaCTX-M-55; TEM-1 | I |
| 36fi | CTXr | 2020 | AMP-FOT-TAZ-CHL-CIP-SMX-TET-FEP | Yes | blaSHV-12 | I |
| 37fi | CTXr | 2020 | AMP-FOT-TAZ-CHL-GEN-SMX-FEP | Yes | blaCTX-M-32; SHV-12 | I and II |
| 38fi | CTXr | 2020 | AMP-FOT-TAZ-CHL-CIP-GEN-NAL-SMX-TET-TMP-FEP + FOX | Yes | blaTEM-1; blaCMY-2 | I and II |
| 39fi | CTXr | 2020 | AMP-AZI-FOT-TAZ-CHL-CIP-SMX-TET-TMP-FEP | Yes | blaCTX-M-1; blaTEM-1 | I and II |
| 40fi | CTXr | 2020 | AMP-FOT-TAZ-CHL-CIP-SMX-TET-FEP | Yes | blaSHV-12 | I |
| 41fi | CTXr | 2020 | AMP-AZI-FOT-TAZ-CHL-CIP-GEN-NAL-SMX-TET-TMP-FEP-FOX | Yes | blaCTX-M-55; blaTEM-type | I |
| 42fi | CTXr | 2020 | AMP-FOT-TAZ-CHL-CIP-NAL-SMX-TET-TMP-FEP | Yes | blaSHV-12 | I |
| Isolate | 2fi | 6fc | 10fc | ||||
|---|---|---|---|---|---|---|---|
| Production system | Intensive | Extensive | Extensive | ||||
| MLST | ST10 | ST744 | ST744 | ||||
| Serotype | O166; H25 | O101: H10 | O101: H9 | ||||
| Phylogroup | A | A | A | ||||
| fumC/fimH type | fumC11:fimH54 | fumC11:fimH54 | fumC11:fimH54 | ||||
| Plasmids | Col; IncB/O/K/Z; IncI1; IncX4; p0111 | IncFIB; IncI1; IncW | IncFIB; IncI1; IncI2 | ||||
| Virulence Genes | astA, capU, cba, cea, cma, hra, iha, iss, iucC, iutA, sitA, terC, traT | cia, cvaC, etsC, etsC, hlyF, iroN, iss, iucC, iutA, mchF, ompT, sitA, terC, traT | cma, cvaC, etsC, hlyF, iroN, iss, iucC, iutA, ompT, sitA, terC, traT | ||||
| Pathogenicity | 84.1% | 86.0% | 82.4% | ||||
| Antibiotics | MIC (mg/L) | Resistance genes | MIC (mg/L) | Resistance genes | MIC (mg/L) | Resistance genes | |
| Sulfonamides | ˃1024 | sul3 | ˃1024 | sul1; sul2 | ˃1024 | sul1; sul2 | |
| Polymyxins: | Colistin | 8 | mcr-1.1 | ≤1 | - | ≤1 | - |
| β-lactams: | Cefotaxime | 4 | blaSHV-12 | ˃64 | blaCTX-M-14; blaTEM-1B | 64 | blaCTX-M-14; blaTEM-1B |
| Ceftazidime | 8 | blaSHV-12 | 1 | blaCTX-M-14; blaTEM-1B | 1 | blaCTX-M-14; blaTEM-1B | |
| Macrolides: | Azithromycin | 8 | - | ˃64 | mph(A) | 32 | mph(A) |
| Phenicols: | Chloramphenicol | 32 | cmlA1 | ˃128 | catA1 | ˃128 | catA1 |
| Tetracycline | 32 | tet(A) | ˃64 | tet(B) | ˃64 | tet(B) | |
| Trimethoprim | ≤0.25 | - | ˃32 | dfrA17; dfrA5 | ˃32 | dfrA17 | |
| Aminoglycosides | aadA1; aadA2b | aadA13; aadA5; aph(6)-Id; aph(3″)-Ib; aph(3′)-Ia; | aadA5; aph(6)-Id; aph(3″)-Ib; aph(3′)-Ia; | ||||
| Biocides | qacE | qacEdelta1 | qacEdelta1 | ||||
| Efflux pumps | mdf(A); marA | mdf(A) | mdf(A) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, M.; Leão, C.; Clemente, L.; Albuquerque, T.; Amaro, A. Antibiotic Susceptibility Profiles and Resistance Mechanisms to β-Lactams and Polymyxins of Escherichia coli from Broilers Raised under Intensive and Extensive Production Systems. Microorganisms 2022, 10, 2044. https://doi.org/10.3390/microorganisms10102044
Ferreira M, Leão C, Clemente L, Albuquerque T, Amaro A. Antibiotic Susceptibility Profiles and Resistance Mechanisms to β-Lactams and Polymyxins of Escherichia coli from Broilers Raised under Intensive and Extensive Production Systems. Microorganisms. 2022; 10(10):2044. https://doi.org/10.3390/microorganisms10102044
Chicago/Turabian StyleFerreira, Mariana, Célia Leão, Lurdes Clemente, Teresa Albuquerque, and Ana Amaro. 2022. "Antibiotic Susceptibility Profiles and Resistance Mechanisms to β-Lactams and Polymyxins of Escherichia coli from Broilers Raised under Intensive and Extensive Production Systems" Microorganisms 10, no. 10: 2044. https://doi.org/10.3390/microorganisms10102044
APA StyleFerreira, M., Leão, C., Clemente, L., Albuquerque, T., & Amaro, A. (2022). Antibiotic Susceptibility Profiles and Resistance Mechanisms to β-Lactams and Polymyxins of Escherichia coli from Broilers Raised under Intensive and Extensive Production Systems. Microorganisms, 10(10), 2044. https://doi.org/10.3390/microorganisms10102044





