Brucella Phagocytosis Mediated by Pathogen-Host Interactions and Their Intracellular Survival
Abstract
1. Introduction
2. Brucella Phagocytosis and Intracellular Survival
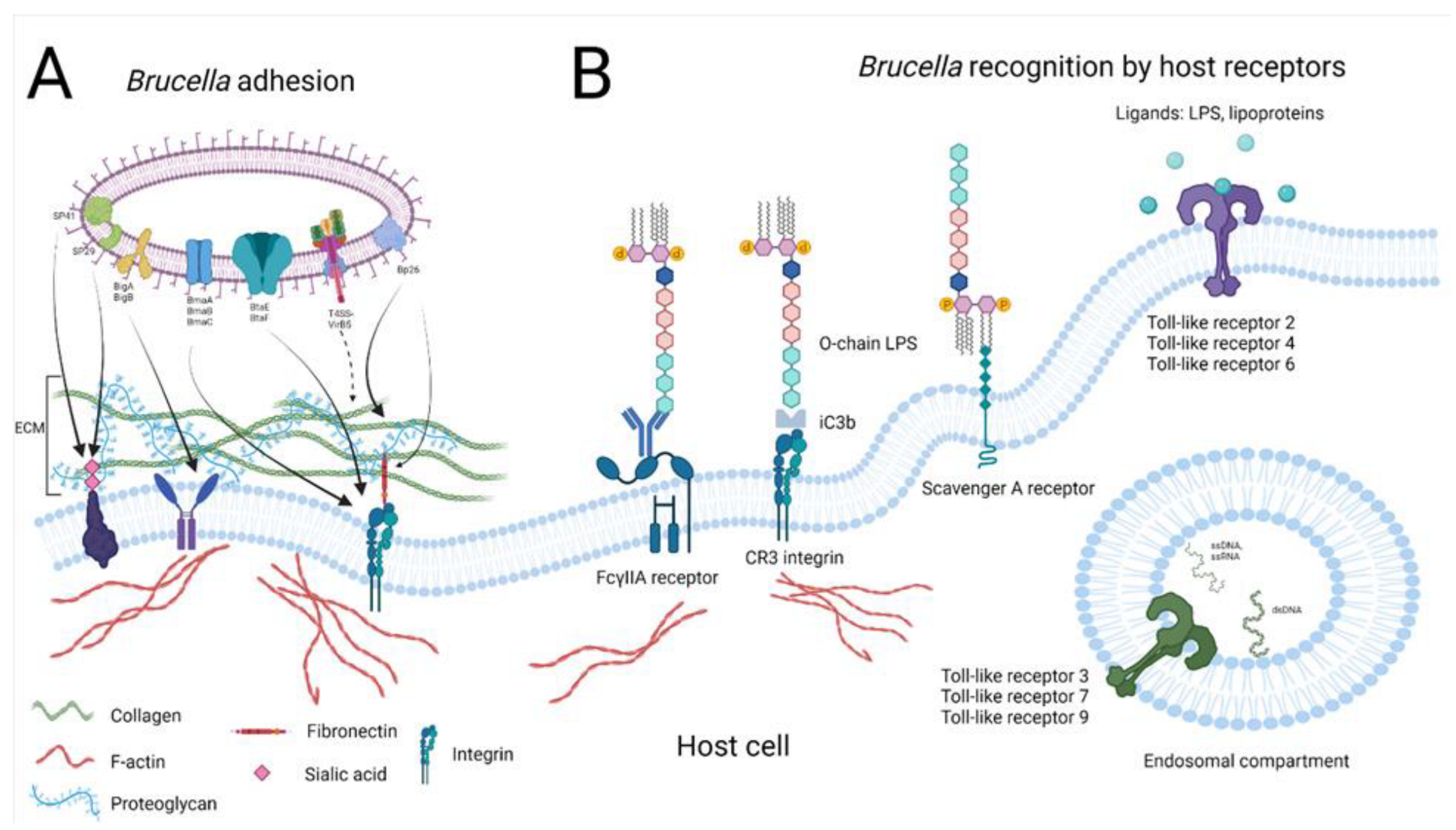
2.1. Pathogen Recognition
2.1.1. Bacterial Adhesion
2.1.2. Host Receptors
2.2. Engulfment and Internalization Process Activation
2.2.1. Bacterial Engulfment
2.2.2. Formation and Maturation of Brucella-Containing Vacuoles
2.3. Host Intracellular Killing or Bacterial Intracellular Growth
2.3.1. Host Bactericidal Effect against Brucella
2.3.2. Brucella Intracellular Survival
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CβG | Cyclic β-1,2-glucan |
| LPS | Lipopolysaccharide |
| DCs | Dendritic cells |
| MDCK | Madin-Darby canine kidney |
| CRs | Complement receptors |
| FcRs | Fc receptors |
| CR3 | Complement receptor 3 |
| SR-A | Scavenger receptor A |
| TLRs | Toll-like receptors |
| TcpB | A TIR domain-containing protein in Brucella |
| PrPc | The cellular prion protein |
| TfR | Transferrin receptor |
| ER | Endoplasmic reticulum |
| rBCVs | Replicative BCVs |
| eBCVs | Endosomal BCVs |
| ROS | Reactive oxygen species |
| NO | Nitric oxide |
| aBCVs | Autophagic BCVs |
References
- Gonzalez-Espinoza, G.; Arce-Gorvel, V.; Memet, S.; Gorvel, J.P. Brucella: Reservoirs and Niches in Animals and Humans. Pathogens 2021, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.; Zhou, Z.; Li, B.; Xiao, Y.; Li, M.; Zeng, H.; Guo, X.; Gu, G. The Mechanism of Facultative Intracellular Parasitism of Brucella. Int. J. Mol. Sci. 2021, 22, 3673. [Google Scholar] [CrossRef] [PubMed]
- Aliyev, J.; Alakbarova, M.; Garayusifova, A.; Omarov, A.; Aliyeva, S.; Fretin, D.; Godfroid, J. Identification and Molecular Characterization of Brucella abortus and Brucella melitensis Isolated from Milk in Cattle in Azerbaijan. BMC Vet. Res. 2022, 18, 71. [Google Scholar] [CrossRef]
- Laine, C.G.; Scott, H.M.; Arenas-Gamboa, A.M. Human Brucellosis: Widespread Information Deficiency Hinders an Understanding of Global Disease Frequency. PLoS Negl. Trop. Dis. 2022, 16, E0010404. [Google Scholar] [CrossRef]
- Solis Garcia Del Pozo, J.; Solera, J. Systematic Review and Meta-Analysis of Randomized Clinical Trials in The Treatment of Human Brucellosis. PLoS ONE 2012, 7, E32090. [Google Scholar] [CrossRef] [PubMed]
- Lalsiamthara, J.; Lee, J.H. Development and Trial of Vaccines Against Brucella. J. Vet. Sci. 2017, 18, 281–290. [Google Scholar] [CrossRef]
- Dadar, M.; Tiwari, R.; Sharun, K.; Dhama, K. Importance of Brucellosis Control Programs of Livestock on The Improvement of One Health. Vet. Q. 2021, 41, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Glowacka, P.; Zakowska, D.; Naylor, K.; Niemcewicz, M.; Bielawska-Drozd, A. Brucella—Virulence Factors, Pathogenesis and Treatment. Pol. J. Microbiol. 2018, 67, 151–161. [Google Scholar] [CrossRef]
- Gheitasi, R.; Keramat, F.; Khosravi, S.; Hajilooi, M.; Pletz, M.W.; Makarewicz, O. Evaluation of Th2 and Th17 Immunity-Related Factors As Indicators of Brucellosis. Front. Cell. Infect. Microbiol. 2021, 11, 786994. [Google Scholar] [CrossRef]
- He, Y. Analyses of Brucella Pathogenesis, Host Immunity, and Vaccine Targets Using Systems Biology and Bioinformatics. Front. Cell. Infect. Microbiol. 2012, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Roop, R.M., 2nd; Gaines, J.M.; Anderson, E.S.; Caswell, C.C.; Martin, D.W. Survival of The Fittest: How Brucella Strains Adapt to Their Intracellular Niche in The Host. Med. Microbiol. Immunol. 2009, 198, 221–238. [Google Scholar] [CrossRef]
- Bialer, M.G.; Sycz, G.; Munoz Gonzalez, F.; Ferrero, M.C.; Baldi, P.C.; Zorreguieta, A. Adhesins of Brucella: Their Roles in The Interaction with The Host. Pathogens 2020, 9, 942. [Google Scholar] [CrossRef]
- Munoz Gonzalez, F.; Sycz, G.; Alonso Paiva, I.M.; Linke, D.; Zorreguieta, A.; Baldi, P.C.; Ferrero, M.C. The Btaf Adhesin Is Necessary for Full Virulence during Respiratory Infection by Brucella suis and Is a Novel Immunogen for Nasal Vaccination Against Brucella Infection. Front. Immunol. 2019, 10, 1775. [Google Scholar] [CrossRef]
- Eltahir, Y.; Al-Araimi, A.; Nair, R.R.; Autio, K.J.; Tu, H.; Leo, J.C.; Al-Marzooqi, W.; Johnson, E.H. Binding of Brucella Protein, Bp26, to Select Extracellular Matrix Molecules. BMC Mol. Cell Biol. 2019, 20, 55. [Google Scholar] [CrossRef]
- Lopez, P.; Guaimas, F.; Czibener, C.; Ugalde, J.E. A Genomic Island in Brucella Involved in the Adhesion to Host Cells: Identification of a New Adhesin and a Translocation Factor. Cell. Microbiol. 2020, 22, E13245. [Google Scholar] [CrossRef] [PubMed]
- Castaneda-Roldan, E.I.; Ouahrani-Bettache, S.; Saldana, Z.; Avelino, F.; Rendon, M.A.; Dornand, J.; Giron, J.A. Characterization of Sp41, a Surface Protein of Brucella Associated with Adherence and Invasion of Host Epithelial Cells. Cell. Microbiol. 2006, 8, 1877–1887. [Google Scholar] [CrossRef]
- Ruiz-Ranwez, V.; Posadas, D.M.; Van Der Henst, C.; Estein, S.M.; Arocena, G.M.; Abdian, P.L.; Martin, F.A.; Sieira, R.; De Bolle, X.; Zorreguieta, A. Btae, An Adhesin That Belongs to the Trimeric Autotransporter Family, Is Required for Full Virulence and Defines a Specific Adhesive Pole of Brucella suis. Infect. Immun. 2013, 81, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Gracia, R.D.C.; Castañeda-Roldán, E.I.; Giono-Cerezo, S.; Girón, J.A. Brucella sp. Bind to Sialic Acid Residues on Human and Animal Red Blood Cells. Fems Microbiol. Lett. 2002, 213, 219–224. [Google Scholar] [CrossRef]
- Czibener, C.; Merwaiss, F.; Guaimas, F.; Del Giudice, M.G.; Serantes, D.A.; Spera, J.M.; Ugalde, J.E. Biga Is a Novel Adhesin of Brucella That Mediates Adhesion to Epithelial Cells. Cell. Microbiol. 2016, 18, 500–513. [Google Scholar] [CrossRef]
- Deng, H.; Zhou, J.; Gong, B.; Xiao, M.; Zhang, M.; Pang, Q.; Zhang, X.; Zhao, B.; Zhou, X. Screening and Identification of a Human Domain Antibody Against Brucella abortus Virb5. Acta Trop. 2019, 197, 105026. [Google Scholar] [CrossRef] [PubMed]
- Aly, K.A.; Baron, C. The Virb5 Protein Localizes to the T-Pilus Tips in Agrobacterium Tumefaciens. Microbiology 2007, 153, 3766–3775. [Google Scholar] [CrossRef] [PubMed]
- Eze, M.O.; Yuan, L.; Crawford, R.M.; Paranavitana, C.M.; Hadfield, T.L.; Bhattacharjee, A.K.; Warren, R.L.; Hoover, D.L. Effects of Opsonization and Gamma Interferon on Growth of Brucella melitensis 16 m in Mouse Peritoneal Macrophages In Vitro. Infect. Immun. 2000, 68, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Zheng, K.; Liu, Z.F. Establishment of Chronic Infection: Brucella’s Stealth Strategy. Front. Cell. Infect. Microbiol. 2016, 6, 30. [Google Scholar] [CrossRef]
- Lin, M.; Nielsen, K. Binding of the Brucella abortus Lipopolysaccharide O-Chain Fragment to a Monoclonal Antibody. Quantitative Analysis by Fluorescence Quenching and Polarization. J. Biol. Chem. 1997, 272, 2821–2827. [Google Scholar] [CrossRef] [PubMed]
- Bellaire, B.H.; Roop, R.M., 2nd; Cardelli, J.A. Opsonized Virulent Brucella abortus Replicates within Nonacidic, Endoplasmic Reticulum-Negative, Lamp-1-Positive Phagosomes in Human Monocytes. Infect. Immun. 2005, 73, 3702–3713. [Google Scholar] [CrossRef]
- Rittig, M.G.; Alvarez-Martinez, M.T.; Porte, F.; Liautard, J.P.; Rouot, B. Intracellular Survival of Brucella spp. in Human Monocytes Involves Conventional Uptake But Special Phagosomes. Infect. Immun. 2001, 69, 3995–4006. [Google Scholar] [CrossRef][Green Version]
- Fernandez-Prada, C.M.; Nikolich, M.; Vemulapalli, R.; Sriranganathan, N.; Boyle, S.M.; Schurig, G.G.; Hadfield, T.L.; Hoover, D.L. Deletion of Wboa Enhances Activation of the Lectin Pathway of Complement in Brucella abortus and Brucella melitensis. Infect. Immun. 2001, 69, 4407–4416. [Google Scholar] [CrossRef]
- Le Cabec, V.; Carreno, S.; Moisand, A.; Bordier, C.; Maridonneau-Parini, I. Complement Receptor 3 (Cd11b/Cd18) Mediates Type I and Type Ii Phagocytosis during Nonopsonic and Opsonic Phagocytosis, Respectively. J. Immunol. 2002, 169, 2003–2009. [Google Scholar] [CrossRef]
- Hosseini Khah, Z.; Bahadori, Z.; Rafiei, A.; Hajilooi, M. Association of Fcγriia (Cd32) Polymorphism with Susceptibility to Brucellosis. Res. Mol. Med. 2014, 2, 17–22. [Google Scholar] [CrossRef][Green Version]
- Bajic, G.; Yatime, L.; Sim, R.B.; Vorup-Jensen, T.; Andersen, G.R. Structural Insight on the Recognition of Surface-Bound Opsonins by the Integrin I Domain of Complement Receptor 3. Proc. Natl. Acad. Sci. USA 2013, 110, 16426–16431. [Google Scholar] [CrossRef]
- Kim, S.; Watarai, M.; Suzuki, H.; Makino, S.; Kodama, T.; Shirahata, T. Lipid Raft Microdomains Mediate Class a Scavenger Receptor-Dependent Infection of Brucella abortus. Microb. Pathog. 2004, 37, 11–19. [Google Scholar] [CrossRef]
- Martin-Martin, A.I.; Vizcaino, N.; Fernandez-Lago, L. Cholesterol, Ganglioside Gm1 and Class a Scavenger Receptor Contribute to Infection by Brucella ovis and Brucella canis in Murine Macrophages. Microbes Infect. 2010, 12, 246–251. [Google Scholar] [CrossRef]
- Pei, J.; Ding, X.; Fan, Y.; Rice-Ficht, A.; Ficht, T.A. Toll-Like Receptors Are Critical for Clearance of Brucella and Play Different Roles in Development of Adaptive Immunity Following Aerosol Challenge in Mice. Front. Cell. Infect. Microbiol. 2012, 2, 115. [Google Scholar] [CrossRef] [PubMed]
- Campos, P.C.; Gomes, M.T.; Guimaraes, E.S.; Guimaraes, G.; Oliveira, S.C. Tlr7 and Tlr3 Sense Brucella abortus Rna to Induce Proinflammatory Cytokine Production But They Are Dispensable for Host Control of Infection. Front. Immunol. 2017, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, L.A.; Macedo, G.C.; Marinho, F.A.; Gomes, M.T.; Corsetti, P.P.; Silva, A.M.; Cassataro, J.; Giambartolomei, G.H.; Oliveira, S.C. Toll-Like Receptor 6 Plays An Important Role in Host Innate Resistance to Brucella abortus Infection in Mice. Infect. Immun. 2013, 81, 1654–1662. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.T.; Campos, P.C.; Pereira Gde, S.; Bartholomeu, D.C.; Splitter, G.; Oliveira, S.C. Tlr9 Is Required for Mapk/Nf-Kappab Activation But Does Not Cooperate with Tlr2 Or Tlr6 to Induce Host Resistance to Brucella abortus. J. Leukoc. Biol. 2016, 99, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Kim, D.H.; Kim, D.G.; Lee, H.J.; Min, W.; Rhee, M.H.; Cho, J.Y.; Watarai, M.; Kim, S. Toll-Like Receptor 4-Linked Janus Kinase 2 Signaling Contributes to Internalization of Brucella abortus by Macrophages. Infect. Immun. 2013, 81, 2448–2458. [Google Scholar] [CrossRef]
- Delpino, M.V.; Barrionuevo, P.; Macedo, G.C.; Oliveira, S.C.; Genaro, S.D.; Scian, R.; Miraglia, M.C.; Fossati, C.A.; Baldi, P.C.; Giambartolomei, G.H. Macrophage-Elicited Osteoclastogenesis in Response To Brucella abortus Infection Requires Tlr2/Myd88-Dependent Tnf-Alpha Production. J. Leukoc. Biol. 2012, 91, 285–298. [Google Scholar] [CrossRef]
- Alaidarous, M.; Ve, T.; Casey, L.W.; Valkov, E.; Ericsson, D.J.; Ullah, M.O.; Schembri, M.A.; Mansell, A.; Sweet, M.J.; Kobe, B. Mechanism of Bacterial Interference with Tlr4 Signaling by Brucella Toll/Interleukin-1 Receptor Domain-Containing Protein Tcpb. J. Biol. Chem. 2014, 289, 654–668. [Google Scholar] [CrossRef]
- Ke, Y.; Li, W.; Wang, Y.; Yang, M.; Guo, J.; Zhan, S.; Du, X.; Wang, Z.; Yang, M.; Li, J.; et al. Inhibition of Tlr4 Signaling by Brucella Tir-Containing Protein Tcpb-Derived Decoy Peptides. Int. J. Med. Microbiol. 2016, 306, 391–400. [Google Scholar] [CrossRef]
- Amjadi, O.; Rafiei, A.; Mardani, M.; Zafari, P.; Zarifian, A. A Review of the Immunopathogenesis of Brucellosis. Infect. Dis. 2019, 51, 321–333. [Google Scholar] [CrossRef]
- Campos, M.A.; Rosinha, G.M.; Almeida, I.C.; Salgueiro, X.S.; Jarvis, B.W.; Splitter, G.A.; Qureshi, N.; Bruna-Romero, O.; Gazzinelli, R.T.; Oliveira, S.C. Role of Toll-Like Receptor 4 in Induction of Cell-Mediated Immunity and Resistance to Brucella abortus Infection in Mice. Infect. Immun. 2004, 72, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Weiss, D.S.; Takeda, K.; Akira, S.; Zychlinsky, A.; Moreno, E. Myd88, But Not Toll-Like Receptors 4 and 2, Is Required for Efficient Clearance of Brucella abortus. Infect. Immun. 2005, 73, 5137–5143. [Google Scholar] [CrossRef] [PubMed]
- Barquero-Calvo, E.; Chaves-Olarte, E.; Weiss, D.S.; Guzman-Verri, C.; Chacon-Diaz, C.; Rucavado, A.; Moriyon, I.; Moreno, E. Brucella abortus Uses a Stealthy Strategy to Avoid Activation of the Innate Immune System during the Onset of Infection. PLoS ONE 2007, 2, E631. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Bai, N.; Zhang, Z.H.; Liang, N.; Dong, L.; Xiang, R.; Liu, C.H. Tlr2 Signaling Subpathways Regulate Tlr9 Signaling for the Effective Induction of Il-12 Upon Stimulation by Heat-Killed Brucella abortus. Cell. Mol. Immunol. 2012, 9, 324–333. [Google Scholar] [CrossRef][Green Version]
- Watanabe, K.; Shin, E.-K.; Hashino, M.; Tachibana, M.; Watarai, M. Toll-Like Receptor 2 and Class B Scavenger Receptor Type I Are Required for Bacterial Uptake by Trophoblast Giant Cells. Mol. Immunol. 2010, 47, 1989–1996. [Google Scholar] [CrossRef] [PubMed]
- De Figueiredo, P.; Ficht, T.A.; Rice-Ficht, A.; Rossetti, C.A.; Adams, L.G. Pathogenesis and Immunobiology of Brucellosis: Review of Brucella-Host Interactions. Am. J. Pathol. 2015, 185, 1505–1517. [Google Scholar] [CrossRef] [PubMed]
- Watarai, M.; Makino, S.I.; Fujii, Y.; Okamoto, K.; Shirahata, T. Modulation of Brucella-Induced Macropinocytosis by Lipid Rafts Mediates Intracellular Replication. Cell. Microbiol. 2002, 4, 341–355. [Google Scholar] [CrossRef]
- Kusumawati, A.; Cazevieille, C.; Porte, F.; Bettache, S.; Liautard, J.P.; Sri Widada, J. Early Events and Implication of F-Actin and Annexin I Associated Structures in the Phagocytic Uptake of Brucella suis by the J-774a.1 Murine Cell Line and Human Monocytes. Microb. Pathog. 2000, 28, 343–352. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, C.J.; Chiarini, L.B.; Da Silva, J.P.; Pm, E.S.; Martins, M.A.; Linden, R. the Cellular Prion Protein Modulates Phagocytosis and Inflammatory Response. J. Leukoc. Biol. 2005, 77, 238–246. [Google Scholar] [CrossRef]
- Nakato, G.; Hase, K.; Suzuki, M.; Kimura, M.; Ato, M.; Hanazato, M.; Tobiume, M.; Horiuchi, M.; Atarashi, R.; Nishida, N.; et al. Cutting Edge: Brucella abortus Exploits a Cellular Prion Protein on Intestinal M Cells As An Invasive Receptor. J. Immunol. 2012, 189, 1540–1544. [Google Scholar] [CrossRef]
- Watarai, M. Interaction Between Brucella abortus and Cellular Prion Protein in Lipid Raft Microdomains. Microbes Infect. 2004, 6, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, S.; Duzguner, V.; Kucukgul, A.; Aslantas, O. Silencing of Prp C (Prion Protein) Expression Does Not Affect Brucella melitensis Infection in Human Derived Microglia Cells. Res. Vet. Sci. 2013, 95, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Verri, C.; Chaves-Olarte, E.; Von Eichel-Streiber, C.; Lopez-Goni, I.; Thelestam, M.; Arvidson, S.; Gorvel, J.P.; Moreno, E. Gtpases of the Rho Subfamily Are Required for Brucella abortus Internalization in Nonprofessional Phagocytes: Direct Activation of Cdc42. J. Biol. Chem. 2001, 276, 44435–44443. [Google Scholar] [CrossRef]
- Casanova, A.; Low, S.H.; Québatte, M.; Sedzicki, J.; Tschon, T.; Ketterer, M.; Smith, K.; Emmenlauer, M.; Ben-Tekaya, H.; Dehio, C. A Role for the Vps Retromer in Brucella Intracellular Replication Revealed by Genomewide Sirna Screening. Msphere 2019, 4, E00380-19. [Google Scholar] [CrossRef]
- Pei, J.; Turse, J.E.; Ficht, T.A. Evidence of Brucella abortus Ops Dictating Uptake and Restricting Nf-Kappab Activation in Murine Macrophages. Microbes Infect. 2008, 10, 582–590. [Google Scholar] [CrossRef]
- Pizarro-Cerdá, J.; Moreno, E.; Gorvel, J.-P. Invasion and Intracellular Trafficking of Brucella abortus in Nonphagocytic Cells. Microbes Infect. 2000, 2, 829–835. [Google Scholar] [CrossRef]
- Celli, J.; De Chastellier, C.; Franchini, D.M.; Pizarro-Cerda, J.; Moreno, E.; Gorvel, J.P. Brucella Evades Macrophage Killing Via Virb-Dependent Sustained Interactions with the Endoplasmic Reticulum. J. Exp. Med. 2003, 198, 545–556. [Google Scholar] [CrossRef]
- Carvalho Neta, A.V.; Mol, J.P.; Xavier, M.N.; Paixao, T.A.; Lage, A.P.; Santos, R.L. Pathogenesis of Bovine Brucellosis. Vet. J. 2010, 184, 146–155. [Google Scholar] [CrossRef] [PubMed]
- De Bolle, X.; Letesson, J.J.; Gorvel, J.P. Small Gtpases and Brucella Entry Into the Endoplasmic Reticulum. Biochem. Soc. Trans. 2012, 40, 1348–1352. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, M.G.; Ugalde, J.E.; Czibener, C. A Lysozyme-Like Protein in Brucella abortus Is Involved in the Early Stages of Intracellular Replication. Infect. Immun. 2013, 81, 956–964. [Google Scholar] [CrossRef][Green Version]
- Carrica, M.D.C.; Craig, P.O.; Alonso, S.D.V.; Goldbaum, F.A.; Cravero, S.L. Brucella abortus Mfp: A Trimeric Coiled-Coil Protein with Membrane Fusogenic Activity. Biochemistry 2008, 47, 8165–8175. [Google Scholar] [CrossRef]
- De Souza Filho, J.A.; De Paulo Martins, V.; Campos, P.C.; Alves-Silva, J.; Santos, N.V.; De Oliveira, F.S.; Menezes, G.B.; Azevedo, V.; Cravero, S.L.; Oliveira, S.C. Mutant Brucella abortus Membrane Fusogenic Protein Induces Protection Against Challenge Infection in Mice. Infect. Immun. 2015, 83, 1458–1464. [Google Scholar] [CrossRef]
- Naroeni, A.; Jouy, N.; Ouahrani-Bettache, S.; Liautard, J.P.; Porte, F. Brucella suis-Impaired Specific Recognition of Phagosomes by Lysosomes Due to Phagosomal Membrane Modifications. Infect. Immun. 2001, 69, 486–493. [Google Scholar] [CrossRef]
- Porte, F.; Naroeni, A.; Ouahrani-Bettache, S.; Liautard, J.P. Role of the Brucella suis Lipopolysaccharide O Antigen in Phagosomal Genesis and in Inhibition of Phagosome-Lysosome Fusion in Murine Macrophages. Infect. Immun. 2003, 71, 1481–1490. [Google Scholar] [CrossRef] [PubMed]
- Sedzicki, J.; Tschon, T.; Low, S.H.; Willemart, K.; Goldie, K.N.; Letesson, J.J.; Stahlberg, H.; Dehio, C. 3d Correlative Electron Microscopy Reveals Continuity of Brucella-Containing Vacuoles with the Endoplasmic Reticulum. J. Cell. Sci. 2018, 131, jcs210799. [Google Scholar] [CrossRef]
- Arellano-Reynoso, B.; Lapaque, N.; Salcedo, S.; Briones, G.; Ciocchini, A.E.; Ugalde, R.; Moreno, E.; Moriyon, I.; Gorvel, J.P. Cyclic Beta-1,2-Glucan Is a Brucella Virulence Factor Required for Intracellular Survival. Nat. Immunol. 2005, 6, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Guidolin, L.S.; Morrone Seijo, S.M.; Guaimas, F.F.; Comerci, D.J.; Ciocchini, A.E. Interaction Network and Localization of Brucella abortus Membrane Proteins Involved in the Synthesis, Transport, and Succinylation of Cyclic Beta-1,2-Glucans. J. Bacteriol. 2015, 197, 1640–1648. [Google Scholar] [CrossRef][Green Version]
- Starr, T.; Ng, T.W.; Wehrly, T.D.; Knodler, L.A.; Celli, J. Brucella Intracellular Replication Requires Trafficking through the Late Endosomal/Lysosomal Compartment. Traffic 2008, 9, 678–694. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Gendron-Fitzpatrick, A.; Splitter, G.A. Susceptibility of Ifn Regulatory Factor-1 and Ifn Consensus Sequence Binding Protein-Deficient Mice to Brucellosis. J. Immunol. 2002, 168, 2433–2440. [Google Scholar] [CrossRef]
- Uribe-Querol, E.; Rosales, C. Control of Phagocytosis by Microbial Pathogens. Front. Immunol. 2017, 8, 1368. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.M.S.; Reda, R.S.; Rania, I. Nitric Oxide and Lysozyme Activities As Early Monitors of the Immune Response of Brucella abortus Rb51 Vaccinated Cows.
- Hu, H.; Tian, M.; Li, P.; Guan, X.; Lian, Z.; Yin, Y.; Shi, W.; Ding, C.; Yu, S. Brucella Infection Regulates Thioredoxin-Interacting Protein Expression to Facilitate Intracellular Survival by Reducing the Production of Nitric Oxide and Reactive Oxygen Species. J. Immunol. 2020, 204, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Hornback, M.L.; Roop, R.M., 2nd. The Brucella abortus Xtha-1 Gene Product Participates in Base Excision Repair and Resistance to Oxidative Killing But Is Not Required for Wild-Type Virulence in the Mouse Model. J. Bacteriol. 2006, 188, 1295–1300. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rittig, M.G.; Kaufmann, A.; Robins, A.; Shaw, B.; Sprenger, H.; Gemsa, D.; Foulongne, V.; Rouot, B.; Dornand, J. Smooth and Rough Lipopolysaccharide Phenotypes of Brucella Induce Different Intracellular Trafficking and Cytokine/Chemokine Release in Human Monocytes. J. Leukoc. Biol. 2003, 74, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Köhler, S.; Michaux-Charachon, S.; Porte, F.; Ramuz, M.; Liautard, J.-P. What Is the Nature of the Replicative Niche of a Stealthy Bug Named Brucella? Trends Microbiol. 2003, 11, 215–219. [Google Scholar] [CrossRef]
- Celli, J. The Intracellular Life Cycle of Brucella spp. Microbiol. Spectr. 2019, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Li, B.; Zhou, Z.; Gu, G.; Li, M.; Liu, J.; Jiao, H. The Virb System Plays a Crucial Role in Brucella Intracellular Infection. Int. J. Mol. Sci. 2021, 22, 13637. [Google Scholar] [CrossRef]
- Ke, Y.; Wang, Y.; Li, W.; Chen, Z. Type Iv Secretion System of Brucella spp. and Its Effectors. Front. Cell. Infect. Microbiol. 2015, 5, 72. [Google Scholar] [CrossRef]
- Coronas-Serna, J.M.; Louche, A.; Rodriguez-Escudero, M.; Roussin, M.; Imbert, P.R.C.; Rodriguez-Escudero, I.; Terradot, L.; Molina, M.; Gorvel, J.P.; Cid, V.J.; et al. The Tir-Domain Containing Effectors Btpa and Btpb from Brucella abortus Impact Nad Metabolism. PLoS Pathog. 2020, 16, E1007979. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Nunez, C.; Altamirano-Silva, P.; Alvarado-Guillen, F.; Moreno, E.; Guzman-Verri, C.; Chaves-Olarte, E. The Two-Component System Bvrr/Bvrs Regulates the Expression of the Type Iv Secretion System Virb in Brucella abortus. J. Bacteriol. 2010, 192, 5603–5608. [Google Scholar] [CrossRef] [PubMed]
- Gorvel, J.P.; Moreno, E. Brucella Intracellular Life: From Invasion to Intracellular Replication. Vet. Microbiol. 2002, 90, 281–297. [Google Scholar] [CrossRef]
- Köhler, S.; Foulongne, V.; Ouahrani-Bettache, S.; Bourg, G.; Teyssier, J.; Ramuz, M.; Liautard, J.-P. The Analysis of the Intramacrophagic Virulome of Brucella suis Deciphers the Environment Encountered by the Pathogen Inside the Macrophage Host Cell. Proc. Natl. Acad. Sci. USA 2002, 99, 15711–15716. [Google Scholar] [CrossRef] [PubMed]
- Brumell, J.H. Brucella “Hitches a Ride” with Autophagy. Cell Host Microbe 2012, 11, 2–4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Starr, T.; Child, R.; Wehrly, T.D.; Hansen, B.; Hwang, S.; López-Otin, C.; Virgin, H.W.; Celli, J. Selective Subversion of Autophagy Complexes Facilitates Completion of the Brucella Intracellular Cycle. Cell Host Microbe 2012, 11, 33–45. [Google Scholar] [CrossRef]
- Hanwei, J.; Nie, X.; Zhu, H.; Li, B.; Pang, F.; Yang, X.; Cao, R.; Yang, X.; Zhu, S.; Peng, D. Mir-146b-5p Plays a Critical Role in the Regulation of Autophagy In∆ Per Brucella melitensis-Infected Raw264. 7 Cells. Biomed. Res. Int. 2020, 2020, 1953242. [Google Scholar] [CrossRef]
- Luizet, J.B.; Raymond, J.; Lacerda, T.L.S.; Barbieux, E.; Kambarev, S.; Bonici, M.; Lembo, F.; Willemart, K.; Borg, J.P.; Celli, J.; et al. The Brucella Effector Bspl Targets the Er-Associated Degradation (Erad) Pathway and Delays Bacterial Egress from Infected Cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2105324118. [Google Scholar] [CrossRef]
- Ma, Z.; Li, R.; Hu, R.; Deng, X.; Xu, Y.; Zheng, W.; Yi, J.; Wang, Y.; Chen, C. Brucella abortus Bspj Is a Nucleomodulin That Inhibits Macrophage Apoptosis and Promotes Intracellular Survival of Brucella. Front. Microbiol. 2020, 11, 599205. [Google Scholar] [CrossRef]
- Köhler, S.; Porte, F.; Jubier-Maurin, V.; Ouahrani-Bettache, S.; Teyssier, J.; Liautard, J.-P. The Intramacrophagic Environment of Brucella suis and Bacterial Response. Vet. Microbiol. 2002, 90, 299–309. [Google Scholar] [CrossRef]

| Brucella Proteins | Functions |
|---|---|
| Cyclic β-1,2-glucan | Important for circumventing host cell defenses, and modulate lipid raft organization |
| VirB T4SS | Mediating intracellular survival and circumventing host immune responses |
| SP29, SP41 | Sialic acid-binding proteins, in bacterial adherence |
| BigA, BigB | Proteins containing the immunoglobulin-like domain, in bacterial adherence |
| BmaA, BmaB, BmaC | The monomeric autotransporters, in bacterial adherence |
| BtaE, BtaF | The trimeric autotransporters, in bacterial adherence |
| Bp26 | Collagen, vitronectin-binding protein, in bacterial adherence |
| VirB5 | Effector of a well-known Brucella virulence factor T4SS, in bacterial adherence |
| SagA | A lysozyme-like protein SagA identified as a muramidase |
| VceC, VecA | T4SS, related to host autophagy and apoptosis |
| BtpA, BtpB | Modulate host immunity and energy metabolism |
| BvrS/R | Transcriptionally regulates T4SS VirB |
| VPS35, VPS26A | Related to the evasion of the lysosomal degradative pathway |
| BspL | Delay the formation of aBCVs that benefit the optimal intracellular replication before disseminating to other cells |
| BspJ | A nucleomodulin, directly or indirectly regulates host cell apoptosis to complete its intracellular cycle |
| DnaK, ClpB | Heat shock proteins, role in bacterial resistance against stresses |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huy, T.X.N.; Nguyen, T.T.; Kim, H.; Reyes, A.W.B.; Kim, S. Brucella Phagocytosis Mediated by Pathogen-Host Interactions and Their Intracellular Survival. Microorganisms 2022, 10, 2003. https://doi.org/10.3390/microorganisms10102003
Huy TXN, Nguyen TT, Kim H, Reyes AWB, Kim S. Brucella Phagocytosis Mediated by Pathogen-Host Interactions and Their Intracellular Survival. Microorganisms. 2022; 10(10):2003. https://doi.org/10.3390/microorganisms10102003
Chicago/Turabian StyleHuy, Tran X. N., Trang T. Nguyen, Heejin Kim, Alisha W. B. Reyes, and Suk Kim. 2022. "Brucella Phagocytosis Mediated by Pathogen-Host Interactions and Their Intracellular Survival" Microorganisms 10, no. 10: 2003. https://doi.org/10.3390/microorganisms10102003
APA StyleHuy, T. X. N., Nguyen, T. T., Kim, H., Reyes, A. W. B., & Kim, S. (2022). Brucella Phagocytosis Mediated by Pathogen-Host Interactions and Their Intracellular Survival. Microorganisms, 10(10), 2003. https://doi.org/10.3390/microorganisms10102003





