Molecular Detection of Tick-Borne Bacteria from Amblyomma (Acari: Ixodidae) Ticks Collected from Reptiles in South Africa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. PCR and Phylogenetic Analysis
| Species | Assay | Primer Sequence | Annealing (°C) | Product Size | Target Gene | References |
|---|---|---|---|---|---|---|
| Universal bacterial primers | PCR | TCAAAKGAATTGACGGGGGC GCCCGGGAACGTATTCAC | 50 | 478 bp | 16S rRNA | [26] |
| Anaplasma spp. | PCR | CAGAGTTTGATCCTGGCTCAGAACG GAGTTTGCCGGGACTTCTTCTGAA | 50 | 467 bp | 16S rRNA | [27] |
| Borrelia spp. | PCR | AGAGCAACTTACAGACGAAATTAAT CAAGTCTATTTTGGAAAGCACCTAA | 54 | 482 bp | Flagellin gene | [28] |
| Borrelia spp. | PCR | TGCGTCTTAAGCATGCAAGT GTACAAAGGCCCGAGAACGTA | 56 | 1344 bp | 16S rRNA | [29] |
| nPCR | CATGCAAGTCAAACGGAATG ACTGTTTCGCTTCGCTTTGT | 56 | 1205 bp | 16S rRNA | [29] | |
| Rickettsia spp. | PCR | GGGGGCCTGCTCACGGCGG ATTGCAAAAAGTACAGTGAAC | 48 | 382 bp | gltA gene | [30] |
| Rickettsia spp. | PCR | TGGCGAATATT TCTCCAAAAA GTTCCGTTA ATGGCAGCATCT | 46 | 631 bp | ompA gene | [31] |
| Coxiella spp. | PCR | CGTAGGAATCTACCTTRTAGWGG GCCTACCCGCTTCTGGTACAATT | 52 | 832–939 bp | 16S rRNA | [32] |
| nPCR | CGTAGGAATCTACCTTRTAGWGG TGAGAACTAGCTGTTGGTTAGT | 52 | 624–627 bp | 16S rRNA | [32] | |
| Coxiella spp. | PCR | GCCTGCGAWAAGCTTCGGGGAG CTCCTAKCCACASCTCATCCCC | 56 | 694–1188 bp | 23S rRNA | [32] |
| nPCR | GATCCGGAGATWTCYGAATGGGG TCGYTCGGTTTCGGGTCKACTC | 56 | 583–867 bp | 23S rRNA | [32] | |
| Coxiella burnetii | PCR | ACT CAA CGC ACT GGA ACC GC TAG CTG AAG CCA ATT CGC C | 50 | 257 bp | htpAB | [33] |
2.3. Statistical Analysis
3. Results
3.1. Overall Prevalence
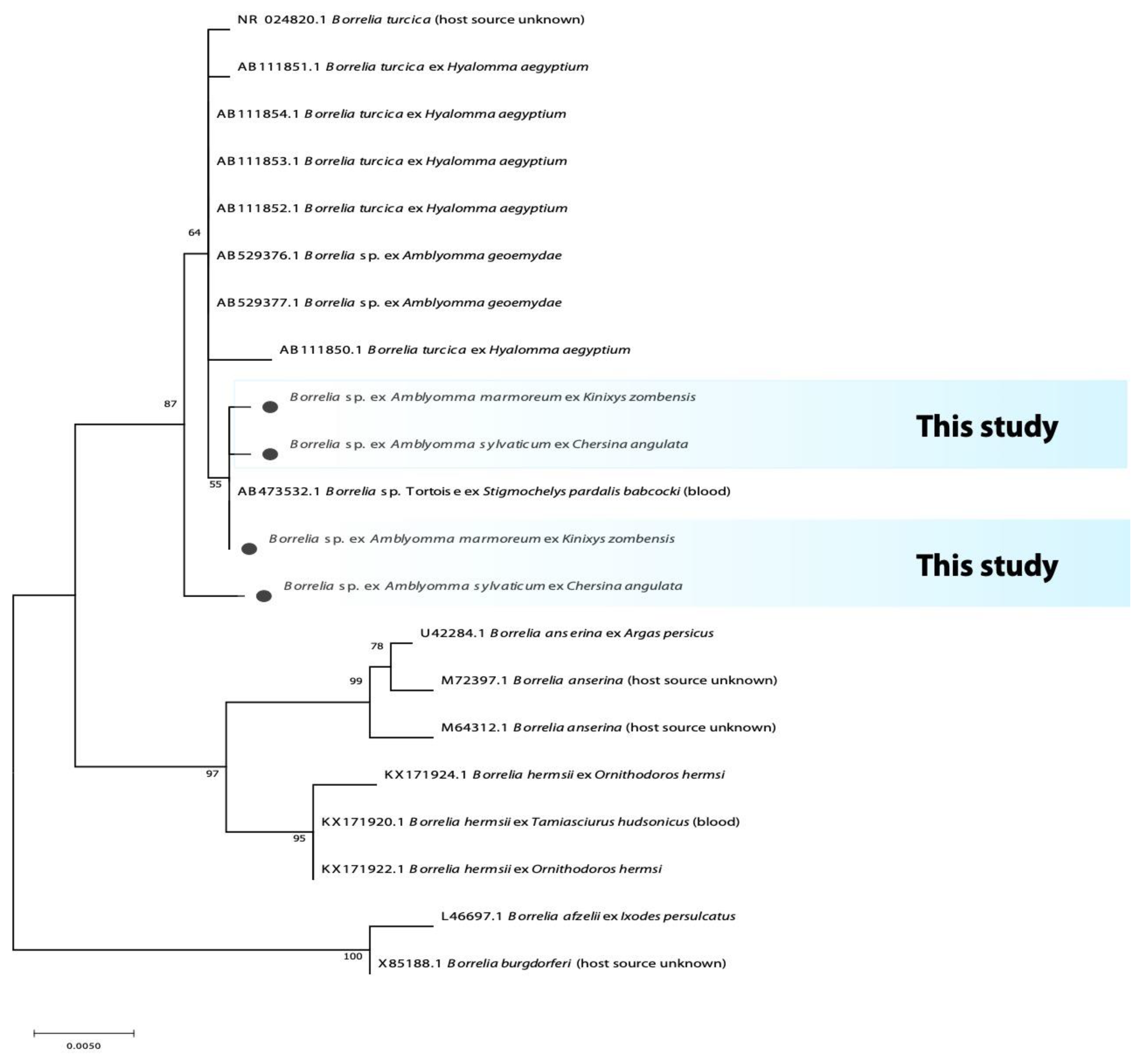
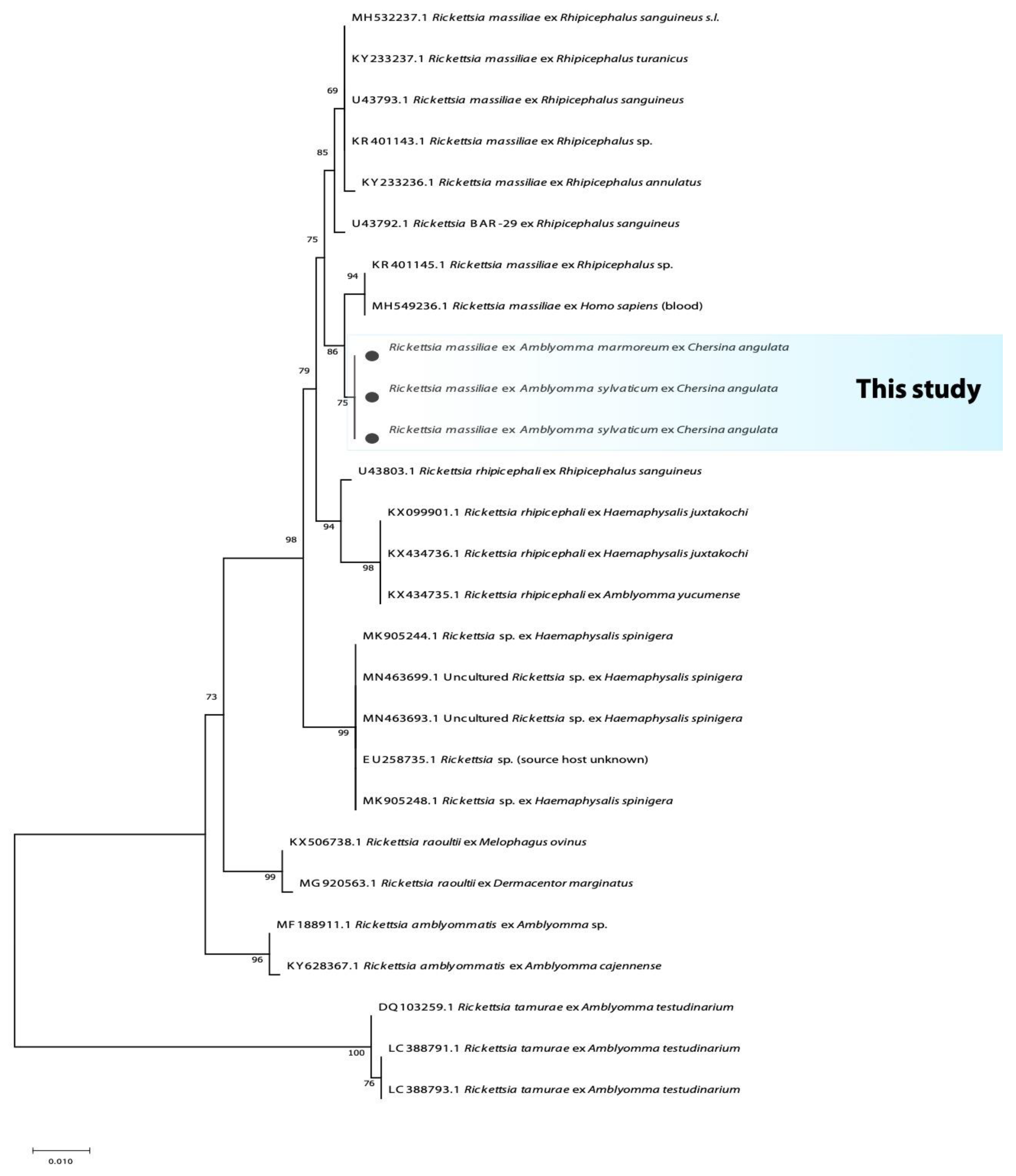
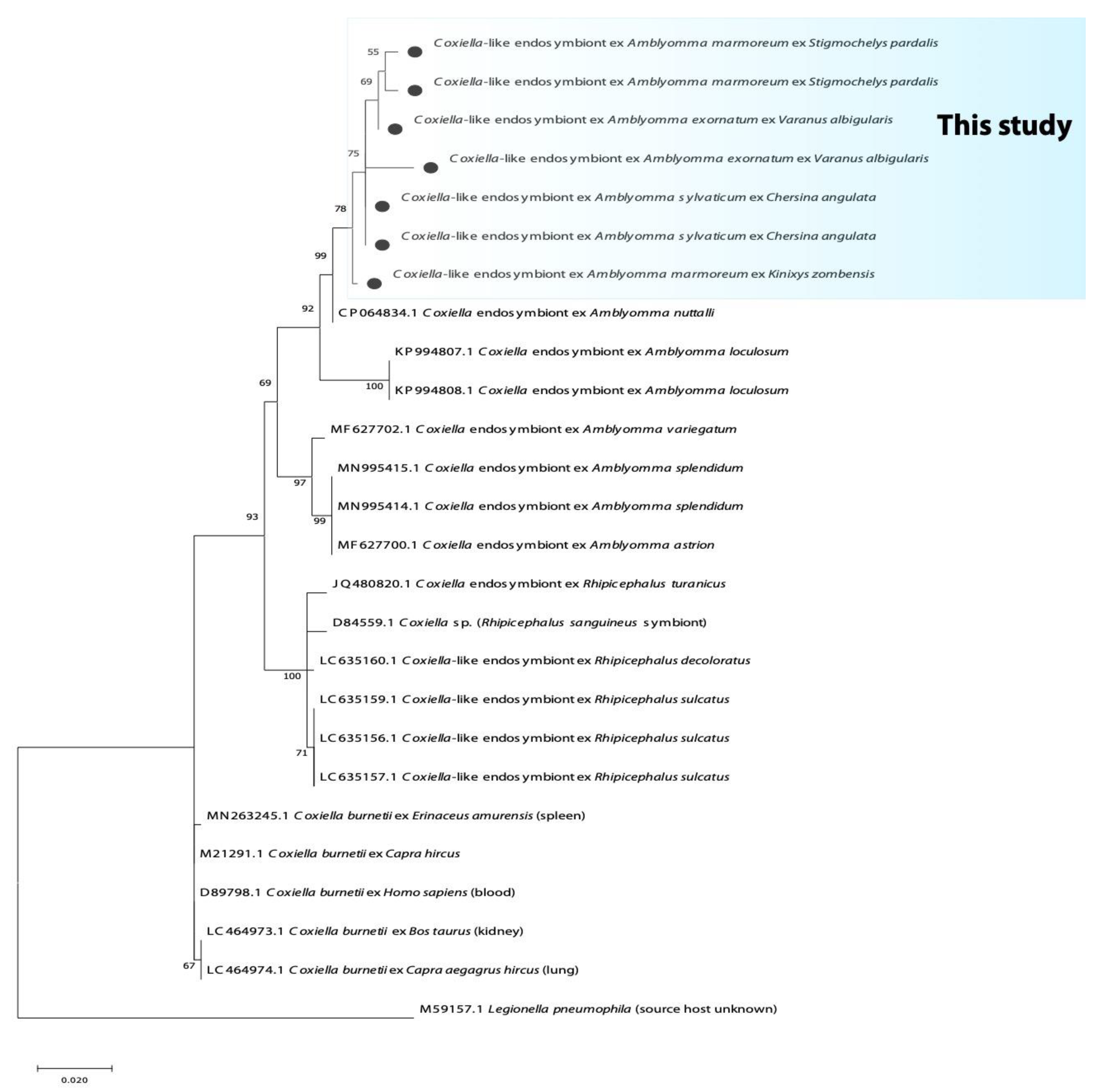
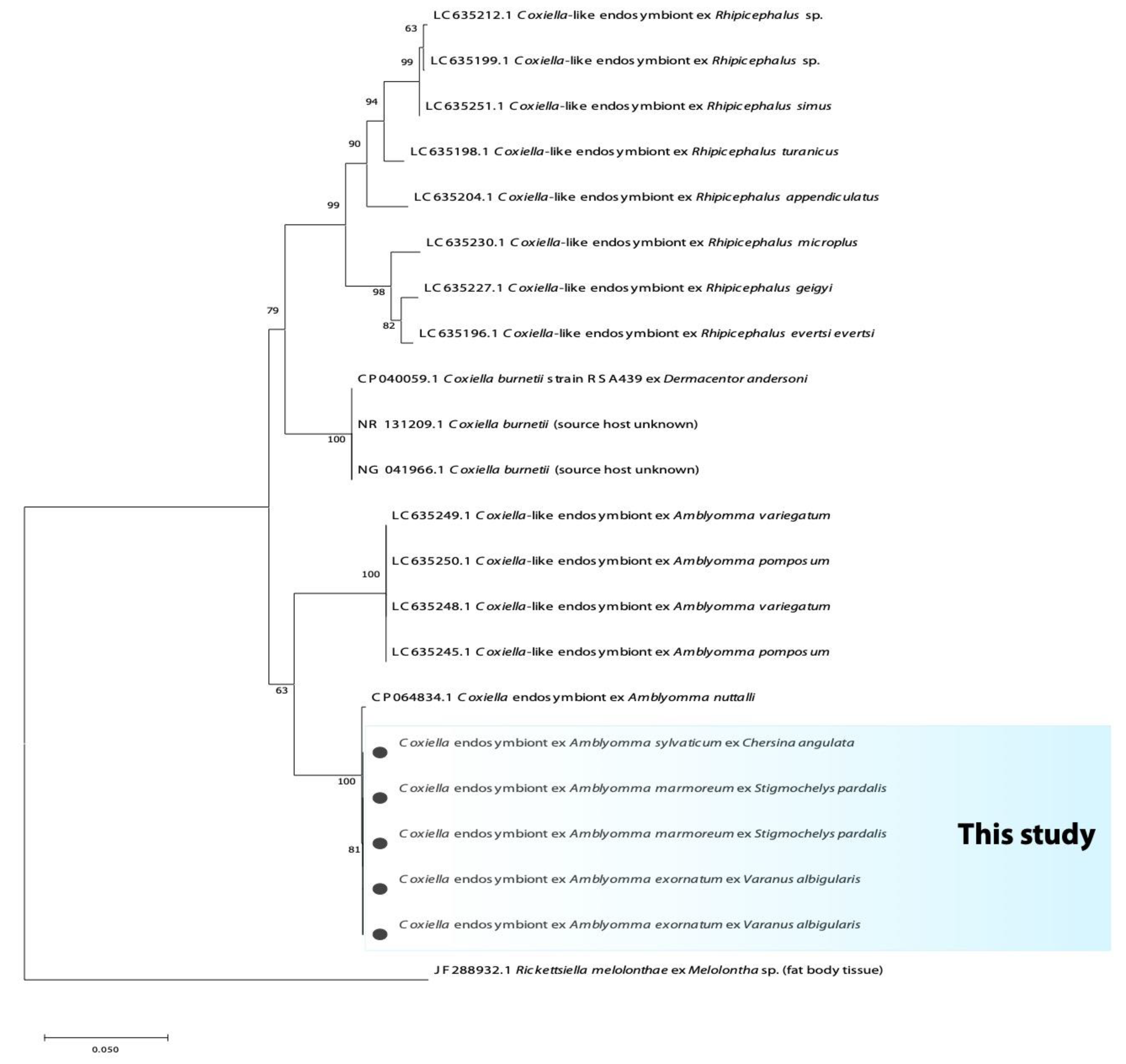
3.2. Phylogenetic Analysis of Bacterial Microorganisms in Amblyomma Ticks
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parola, P.; Paddock, C.D.; Raoult, D. Tick-borne rickettsioses around the world: Emerging diseases challenging old concepts. Clin. Microbiol. 2005, 18, 719–756. [Google Scholar] [CrossRef]
- Omondi, D.; Masiga, D.K.; Fielding, B.C.; Kariuki, E.; Ajamma, Y.U.; Mwamuye, M.M.; Ouso, D.O.; Villinger, J. Molecular detection of tick-borne pathogen diversities in ticks from livestock and reptiles along the shores and adjacent islands of Lake Victoria and Lake Baringo, Kenya. Front. Vet. Sci. 2017, 4, 73. [Google Scholar] [CrossRef] [PubMed]
- Uilenberg, G. International collaborative research: Significance of tick-borne hemoparasitic diseases to world animal health. Vet. Parasitol. 1995, 57, 19–41. [Google Scholar] [CrossRef]
- Warwick, C.; Corning, S. Managing patients for zoonotic disease in hospitals. J. R. Soc. Med. Sh. Rep. 2013, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, A.M.; Birtles, R.J. Rickettsia aeschlimannii: A new pathogenic spotted fever group rickettsia, South Africa. Emerg. Infect. Dis. 2002, 8, 874. [Google Scholar] [CrossRef]
- Pretorius, A.M.; Birtles, R.J. Rickettsia mongolotimonae infection in South Africa. Emerg. Infect. Dis. 2004, 10, 125. [Google Scholar] [CrossRef]
- Parola, P.; Paddock, C.D.; Socolovschi, C.; Labruna, M.B.; Mediannikov, O.; Kernif, T.; Abdad, M.Y.; Stenos, J.; Bitam, I.; Fournier, P.E.; et al. Update on tick-borne rickettsioses around the world: A geographic approach. Clin. Microbiol. 2013, 26, 657–702. [Google Scholar] [CrossRef]
- Halajian, A.; Palomar, A.M.; Portillo, A.; Heyne, H.; Luus-Powell, W.J.; Oteo, J.A. Investigation of Rickettsia, Coxiella burnetii and Bartonella in ticks from animals in South Africa. Ticks Tick Borne Dis. 2016, 7, 361–366. [Google Scholar] [CrossRef]
- Santodomingo, A.; Cotes-Perdomo, A.; Foley, J.; Castro, L.R. Rickettsial infection in ticks (Acari: Ixodidae) from reptiles in the Colombian Caribbean. Ticks Tick Borne Dis. 2018, 9, 623–628. [Google Scholar] [CrossRef]
- Peter, T.F.; Burridge, M.J.; Mahan, S.M. Competence of the African tortoise tick, Amblyomma marmoreum (acari: Ixodidae), as a vector of the agent of heartwater (Cowdria ruminantium). J. Parasitol. 2000, 86, 438–441. [Google Scholar]
- Daveu, R.; Laurence, C.; Bouju-Albert, A.; Sassera, D.; Plantard, O. Symbiont dynamics during the blood meal of Ixodes ricinus nymphs differ according to their sex. Ticks Tick Borne Dis. 2021, 12, 101707. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Chatanga, E.; Qiu, Y.; Simuunza, M.; Kajihara, M.; Hang’ombe, B.M.; Eto, Y.; Saasa, N.; Mori-Kajihara, A.; Simulundu, E.; et al. Molecular Detection and Genotyping of Coxiella-Like Endosymbionts in Ticks Collected from Animals and Vegetation in Zambia. Pathogens 2021, 10, 779. [Google Scholar] [CrossRef] [PubMed]
- Rio, R.V.; Attardo, G.M.; Weiss, B.L. Grandeur alliances: Symbiont metabolic integration and obligate arthropod hematophagy. Trends Parasitol. 2016, 32, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Nardi, T.; Olivieri, E.; Kariuki, E.; Sassera, D.; Castelli, M. Sequence of a Coxiella endosymbiont of the tick Amblyomma nuttalli suggests a pattern of convergent genome reduction in the Coxiella genus. Genome Biol. Evol. 2021, 13, evaa253. [Google Scholar] [CrossRef]
- Moreira, L.A.; Iturbe-Ormaetxe, I.; Jeffery, J.A.; Lu, G.; Pyke, A.T.; Hedges, L.M.; Rocha, B.C.; Hall-Mendelin, S.; Day, A.; Riegler, M.; et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 2009, 139, 1268–1278. [Google Scholar] [CrossRef]
- Sánchez-Montes, S.; Isaak-Delgado, A.B.; Guzmán-Cornejo, C.; Rendón-Franco, E.; Muñoz-García, C.I.; Bermúdez, S.; Morales-Diaz, J.; Cruz-Romero, A.; Romero-Salas, D.; Dzul-Rosado, K.; et al. Rickettsia species in ticks that parasitize amphibians and reptiles: Novel report from Mexico and review of the worldwide record. Ticks Tick Borne Dis. 2019, 10, 987–994. [Google Scholar] [CrossRef]
- Andoh, M.; Sakata, A.; Takano, A.; Kawabata, H.; Fujita, H.; Une, Y.; Goka, K.; Kishimoto, T.; Ando, S. Detection of Rickettsia and Ehrlichia spp. in ticks associated with exotic reptiles and amphibians imported into Japan. PLoS ONE 2015, 10, e0133700. [Google Scholar] [CrossRef]
- Muñoz-Leal, S.; Tarragona, E.L.; Martins, T.F.; Martín, C.M.; Burgos-Gallardo, F.; Nava, S.; Labruna, M.B.; González-Acuña, D. Liolaemus lizards (Squamata: Liolaemidae) as hosts for the nymph of Amblyomma parvitarsum (Acari: Ixodidae), with notes on Rickettsia infection. Exp. Appl. Acarol. 2016, 70, 253–259. [Google Scholar] [CrossRef]
- Desser, S.S.; Hong, H.; Martin, D.S. The life history, ultrastructure, and experimental transmission of Hepatozoon catesbianae n. comb., an apicomplexan parasite of the bullfrog, Rana catesbeiana and the mosquito, Culex territans in Algonquin Park, Ontario. J. Parasitol. 1995, 81, 212–222. [Google Scholar] [CrossRef]
- Mofokeng, L.S.; Smit, N.J.; Cook, C.A. Molecular screening of ticks of the genus Amblyomma (Acari: Ixodidae) infesting South African reptiles with comments on their potential to act as vectors for Hepatozoon fitzsimonsi (Dias, 1953) (Adeleorina: Hepatozoidae). Int. J. Parasitol. Parasites Wildl. 2021, 16, 163–167. [Google Scholar] [CrossRef]
- Theiler, G. Ticks in the South African zoological survey collection. Part III. The ornate Aponommas. Onderstepoort J. Vet. Sci. Anim. Ind. 1945, 20, 165–178. Available online: http://hdl.handle.net/2263/59279 (accessed on 21 July 2020).
- Theiler, G.; Salisbury, L.E. Ticks in the South African Zoological Survey Collection-Part IX-The Amblyomma marmoreum Group. Onderstepoort J. Vet. Sci. Anim. Ind. 1959, 28, 54–98. Available online: http://hdl.handle.net/2263/57905 (accessed on 21 July 2021).
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Guindon, S.; Gascuel, O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Relman, D.A.; Schmidt, T.M.; MacDermott, R.P.; Falkow, S. Identification of the uncultured Bacillus of Whipple’s disease. N. Engl. J. Med. 1992, 327, 293–301. [Google Scholar] [CrossRef]
- Stuen, S.; Nevland, S.; Moum, T. Fatal cases of tick-borne fever (TBF) in sheep caused by several 16S rRNA gene variants of Anaplasma phagocytophilum. Ann. N. Y. Acad. Sci. 2003, 990, 433–434. [Google Scholar] [CrossRef]
- Skotarczak, B.; Wodecka, B.; Cichocka, A. Coexistence DNA of Borrelia burgdorferi sensu lato and Babesia microti in Ixodes ricinus ticks from north-western Poland. Ann. Agric. Environ. Med. 2002, 9, 25–28. [Google Scholar]
- Loh, S.M.; Gillett, A.; Ryan, U.; Irwin, P.; Oskam, C. Molecular characterization of ‘Candidatus Borrelia tachyglossi’ (family Spirochaetaceae) in echidna ticks, Bothriocroton concolor. Int. J. Syst. Evol. 2017, 67, 1075. [Google Scholar] [CrossRef]
- Dupont, H.T.; Cornet, J.P.; Raoult, D. Identification of rickettsiae from ticks collected in the Central African Republic using the polymerase chain reaction. Am. J. Trop. Med. 1994, 50, 373–380. [Google Scholar] [CrossRef]
- Roux, V.; Fournier, P.E.; Raoult, D. Differentiation of spotted fever group rickettsiae by sequencing and analysis of restriction fragment length polymorphism of PCR-amplified DNA of the gene encoding the protein rOmpA. J. Clin. Microbiol. 1996, 34, 2058–2065. [Google Scholar] [CrossRef]
- Duron, O.; Noël, V.; McCoy, K.D.; Bonazzi, M.; Sidi-Boumedine, K.; Morel, O.; Vavre, F.; Zenner, L.; Jourdain, E.; Durand, P.; et al. The recent evolution of a maternally-inherited endosymbiont of ticks led to the emergence of the Q fever pathogen, Coxiella burnetii. PLoS Pathog. 2015, 11, e1004892. [Google Scholar] [CrossRef]
- Stein, A.; Raoult, D. Detection of Coxiella burnetti by DNA amplification using polymerase chain reaction. J. Clin. Microbiol. 1992, 30, 2462–2466. [Google Scholar] [CrossRef]
- Lane, R.S.; Mun, J.; Eisen, L.; Eisen, R.J. Refractoriness of the western fence lizard (Sceloporus occidentalis) to the Lyme disease group spirochete Borrelia bissettii. J. Parasitol. 2006, 92, 691–696. [Google Scholar] [CrossRef]
- Beati, L.; Finidori, J.P.; Gilot, B.; Raoult, D. Comparison of serologic typing, sodium dodecyl sulfate-polyacrylamide gel electrophoresis protein analysis, and genetic restriction fragment length polymorphism analysis for identification of rickettsiae: Characterization of two new rickettsial strains. J. Clin. Microbiol. 1992, 30, 1922–1930. [Google Scholar] [CrossRef]
- Blanda, V.; Torina, A.; La Russa, F.; D’Agostino, R.; Randazzo, K.; Scimeca, S.; Giudice, E.; Caracappa, S.; Cascio, A.; de la Fuente, J. A retrospective study of the characterization of Rickettsia species in ticks collected from humans. Ticks Tick Borne. Dis. 2017, 8, 610–614. [Google Scholar] [CrossRef]
- Demir, S.; Erkunt Alak, S.; Köseoğlu, A.E.; Ün, C.; Nalçacı, M.; Can, H. Molecular investigation of Rickettsia spp. and Francisella tularensis in ticks from three provinces of Turkey. Exp. Appl. Acarol. 2020, 81, 239–253. [Google Scholar] [CrossRef]
- Vitale, G.; Mansueto, S.; Rolain, J.M.; Raoult, D. Rickettsia massiliae human isolation. Emerg. Infect. Dis. 2006, 12, 174. [Google Scholar] [CrossRef]
- García-García, J.C.; Portillo, A.; Núñez, M.J.; Santibáñez, S.; Castro, B.; Oteo, J.A. Case report: A patient from Argentina infected with Rickettsia massiliae. Am. J. Trop. Med. Hyg. 2010, 82, 691. [Google Scholar] [CrossRef]
- Cascio, A.; Torina, A.; Valenzise, M.; Blanda, V.; Camarda, N.; Bombaci, S.; Iaria, C.; De Luca, F.; Wasniewska, M. Scalp eschar and neck lymphadenopathy caused by Rickettsia massiliae. Emerg. Infect. Dis. 2013, 19, 836. [Google Scholar] [CrossRef] [PubMed]
- Elelu, N.; Ola-Fadunsin, S.D.; Bankole, A.A.; Raji, M.A.; Ogo, N.I.; Cutler, S.J. Prevalence of tick infestation and molecular characterization of spotted fever Rickettsia massiliae in Rhipicephalus species parasitizing domestic small ruminants in north-central Nigeria. PLoS ONE 2022, 17, e0263843. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Yang, J.F.; Mukhtar, M.U.; Chen, Z.; Niu, Q.L.; Lin, Y.Q.; Liu, G.Y.; Luo, J.X.; Yin, H.; Liu, Z.J. Molecular detection of Anaplasma infections in ixodid ticks from the Qinghai-Tibet Plateau. Infect. Dis. Poverty 2019, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Nieto, N.C.; Foley, J.E.; Bettaso, J.; Lane, R.S. Reptile infection with Anaplasma phagocytophilum, the causative agent of granulocytic anaplasmosis. J. Parasitol. 2009, 95, 1165–1170. [Google Scholar] [CrossRef]
- Kho, K.L.; Koh, F.X.; Tay, S.T. Molecular evidence of potential novel spotted fever group rickettsiae, Anaplasma and Ehrlichia species in Amblyomma ticks parasitizing wild snakes. Parasites Vectors 2015, 8, 112. [Google Scholar] [CrossRef] [Green Version]
- Dupont, H.T.; Brouqui, P.; Faugere, B.; Raoult, D. Prevalence of antibodies to Coxiella burnetii, Rickettsia conorii, and Rickettsia typhi in seven African countries. Clin. Infect. Dis. 1995, 21, 1126–1133. [Google Scholar] [CrossRef]
- Vanderburg, S.; Rubach, M.P.; Halliday, J.E.; Cleaveland, S.; Reddy, E.A.; Crump, J.A. Epidemiology of Coxiella burnetii infection in Africa: A One-Health systematic review. PLoS Negl. Trop. Dis. 2014, 8, e2787. [Google Scholar] [CrossRef]
- Mtshali, K.; Nakao, R.; Sugimoto, C.; Thekisoe, O. Occurrence of Coxiella burnetii, Ehrlichia canis, Rickettsia species and Anaplasma phagocytophilum-like bacterium in ticks collected from dogs and cats in South Africa. J. S. Afr. Vet. Assoc. 2017, 88, 1390. [Google Scholar] [CrossRef]
- Halajian, A.; Palomar, A.M.; Portillo, A.; Heyne, H.; Romero, L.; Oteo, J.A. Detection of zoonotic agents and a new Rickettsia strain in ticks from donkeys from South Africa: Implications for travel medicine. Travel Med. Infect. Dis. 2018, 26, 43–50. [Google Scholar] [CrossRef]
- Maurin, M.; Raoult, D.F. Q fever. Clin. Microbiol. Rev. 1999, 12, 518–553. [Google Scholar] [CrossRef]
- Gottlieb, Y.; Lalzar, I.; Klasson, L. Distinctive genome reduction rates revealed by genomic analyses of two Coxiella-like endosymbionts in ticks. Genome Biol. Evol. 2015, 7, 1779–1796. [Google Scholar] [CrossRef]
- Guizzo, M.G.; Parizi, L.F.; Nunes, R.D.; Schama, R.; Albano, R.M.; Tirloni, L.; Oldiges, D.P.; Vieira, R.P.; Oliveira, W.H.C.; de Souza Leite, M.; et al. A Coxiella mutualist symbiont is essential to the development of Rhipicephalus microplus. Sci. Rep. 2017, 7, 17554. [Google Scholar] [CrossRef] [PubMed]
- Arthan, W.; Sumrandee, C.; Hirunkanokpun, S.; Kitthawee, S.; Baimai, V.; Trinachartvanit, W.; Ahantarig, A. Detection of Coxiella-like endosymbiont in Haemaphysalis tick in Thailand. Ticks Tick Borne Dis. 2015, 6, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Rahal, M.; Medkour, H.; Diarra, A.Z.; Bitam, I.; Parola, P.; Mediannikov, O. Molecular identification and evaluation of Coxiella-like endosymbionts genetic diversity carried by cattle ticks in Algeria. Ticks Tick Borne Dis. 2020, 11, 101493. [Google Scholar] [CrossRef] [PubMed]
- Guimard, T.; Amrane, S.; Prudent, E.; El Karkouri, K.; Raoult, D.; Angelakis, E. Case report: Scalp eschar and neck lymphadenopathy associated with bacteremia due to Coxiella-like bacteria. Am. J. Trop. Med. 2017, 97, 1319. [Google Scholar] [CrossRef]
- Shivaprasad, H.L.; Cadenas, M.B.; Diab, S.S.; Nordhausen, R.; Bradway, D.; Crespo, R.; Breitschwerdt, E.B. Coxiella-like infection in psittacines and a toucan. Avian Dis. 2008, 52, 426–432. [Google Scholar] [CrossRef]
- Vapniarsky, N.; Barr, B.C.; Murphy, B. Systemic Coxiella-like infection with myocarditis and hepatitis in an eclectus parrot (Eclectus roratus). Vet. Pathol. 2012, 49, 717–722. [Google Scholar] [CrossRef]
- Takano, A.; Goka, K.; Une, Y.; Shimada, Y.; Fujita, H.; Shiino, T.; Watanabe, H.; Kawabata, H. Isolation and characterization of a novel Borrelia group of tick-borne borreliae from imported reptiles and their associated ticks. Environ. Microbiol. 2010, 12, 134–146. [Google Scholar] [CrossRef]
- Panetta, J.L.; Šíma, R.; Calvani, N.E.; Hajdušek, O.; Chandra, S.; Panuccio, J.; Šlapeta, J. Reptile-associated Borrelia species in the goanna tick (Bothriocroton undatum) from Sydney, Australia. Parasites Vectors 2017, 10, 616. [Google Scholar] [CrossRef]
- Gofton, A.W.; Margos, G.; Fingerle, V.; Hepner, S.; Loh, S.M.; Ryan, U.; Irwin, P.; Oskam, C.L. Genome-wide analysis of Borrelia turcica and ‘Candidatus Borrelia tachyglossi’ shows relapsing fever-like genomes with unique genomic links to Lyme disease Borrelia. Infect. Genet. Evol. 2018, 66, 72–81. [Google Scholar] [CrossRef]
- Takano, A.; Fujita, H.; Kadosaka, T.; Konnai, S.; Tajima, T.; Watanabe, H.; Ohnishi, M.; Kawabata, H. Characterization of reptile-associated Borrelia sp. in the vector tick, Amblyomma geoemydae, and its association with Lyme disease and relapsing fever Borrelia spp. Environ. Microbiol. Rep. 2011, 3, 632–637. [Google Scholar] [CrossRef]
- Adeolu, M.; Gupta, R.S. A phylogenomic and molecular marker based proposal for the division of the genus Borrelia into two genera: The emended genus Borrelia containing only the members of the relapsing fever Borrelia, and the genus Borreliella gen. nov. containing the members of the Lyme disease Borrelia (Borrelia burgdorferi sensu lato complex). Antonie Leeuwenhoek 2014, 105, 1049–1072. [Google Scholar] [CrossRef]
- Horak, I.G.; McKay, I.J.; Henen, B.T.; Heyne, H.; Hofmeyr, M.D.; De Villiers, A.L. Parasites of Domestic and Wild Animals in South Africa. XLVII. Ticks of Tortoises and Other Reptiles. Onderstepoort J. Vet. Res. 2006, 73, 215–227. Available online: https://hdl.handle.net/10520/EJC86256 (accessed on 28 May 2020). [CrossRef]
- Margos, G.; Fingerle, V.; Cutler, S.; Gofton, A.; Stevenson, B.; Estrada-Peña, A. Controversies in bacterial taxonomy: The example of the genus Borrelia. Ticks Tick Borne Dis. 2020, 11, 101335. [Google Scholar] [CrossRef]
- Morales-Diaz, J.; Colunga-Salas, P.; Romero-Salas, D.; Sánchez-Montes, S.; Estrada-Souza, I.M.; Ochoa-Ochoa, L.M.; Becker, I.; Flores-Primo, A.; Cruz-Romero, A. Molecular detection of reptile-associated Borrelia in Boa constrictor (Squamata: Boidae) from Veracruz, Mexico. Acta Trop. 2020, 205, 105422. [Google Scholar] [CrossRef]
- Randolph, S.E.; Gern, L.; Nuttall, P.A. Co-feeding ticks: Epidemiological significance for tick-borne pathogen transmission. Parasitol. Today 1996, 12, 472–479. [Google Scholar] [CrossRef]
- Del Cerro, A.; Oleaga, A.; Somoano, A.; Barandika, J.F.; García-Pérez, A.L.; Espí, A. Molecular identification of tick-borne pathogens (Rickettsia spp., Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato, Coxiella burnetii and piroplasms) in questing and feeding hard ticks from North-Western Spain. Ticks Tick Borne Dis. 2022, 13, 101961. [Google Scholar] [CrossRef]
- Zhang, C.M.; Li, N.X.; Zhang, T.T.; Qiu, Z.X.; Li, Y.; Li, L.W.; Liu, J.Z. Endosymbiont CLS-HI plays a role in reproduction and development of Haemaphysalis longicornis. Exp. Appl. Acarol. 2017, 73, 429–438. [Google Scholar] [CrossRef]
- Duron, O.; Morel, O.; Noël, V.; Buysse, M.; Binetruy, F.; Lancelot, R.; Loire, E.; Ménard, C.; Bouchez, O.; Vavre, F.; et al. Tick-bacteria mutualism depends on B vitamin synthesis pathways. Curr. Biol. 2018, 28, 1896–1902. [Google Scholar] [CrossRef] [Green Version]

| Province | Tick Species | n | Host (n) | 16S rRNA Bacteria | Rickettsia spp. | Borrelia spp. | Coxiella spp. | |||
|---|---|---|---|---|---|---|---|---|---|---|
| gltA Gene | OmpA Gene | Flagellin Gene | 16S rRNA | 16S rRNA | 23S rRNA | |||||
| KZN | A. marmoreum | 4 | Varanus albigularis (1) | - | - | - | - | - | 50% (2/4) | - |
| A. marmoreum | 13 | Kinixys sp. (1) | - | - | - | 46.2% (6/13) | 46.2 (6/13) | 46.2% (6/13) | 30.8% (4/13) | |
| A. marmoreum | 11 | Kinixys zombensis (1) | - | - | - | 18.2% (2/11) | 18.2% (2/11) | 45.5% (5/11) | 45.45% (5/11) | |
| A. marmoreum | 12 | Stigmochelys pardalis (7) | - | - | - | - | - | 41.7% (5/12) | 16.7% (2/12) | |
| A. latum | 43 | Naja mossambica (3), Dendroapis spp. (4) | 4.7% (2/43) Francisella spp. | - | - | - | - | - | - | |
| A. exornatum | 37 | Varanus albigularis (5) | - | - | - | - | - | 32.4% (12/37) | 24.3% (9/37) | |
| WC | A. marmoreum | 21 | Kinixys zombesis (3) | - | - | - | 4.8% (1/21) | 4.8% (1/21) | 53.4% (11/21) | 38.1% (8/21) |
| A. marmoreum | 37 | Stigmochelys. pardalis (2) | - | - | - | - | - | 56.8% (21/37) | 40.5% (15/37) | |
| A. marmoreum | 15 | Chersina angulata (9) | - | 46.7% (7/15) | 33.3% (5/15) | 20% (3/15) | 20% (3/15) | 53.3% (8/15) | 53.3% (8/15) | |
| A. sylvaticum | 60 | Chersina angulata (9) | 1.7% (1/60) Rickettsia spp. | 20.0% (12/60) | 23.3% (14/60) | - | 6.7% (4/60) | 56.7% (34/60) | 23.3% (14/60) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mofokeng, L.S.; Smit, N.J.; Cook, C.A. Molecular Detection of Tick-Borne Bacteria from Amblyomma (Acari: Ixodidae) Ticks Collected from Reptiles in South Africa. Microorganisms 2022, 10, 1923. https://doi.org/10.3390/microorganisms10101923
Mofokeng LS, Smit NJ, Cook CA. Molecular Detection of Tick-Borne Bacteria from Amblyomma (Acari: Ixodidae) Ticks Collected from Reptiles in South Africa. Microorganisms. 2022; 10(10):1923. https://doi.org/10.3390/microorganisms10101923
Chicago/Turabian StyleMofokeng, Lehlohonolo S., Nico J. Smit, and Courtney A. Cook. 2022. "Molecular Detection of Tick-Borne Bacteria from Amblyomma (Acari: Ixodidae) Ticks Collected from Reptiles in South Africa" Microorganisms 10, no. 10: 1923. https://doi.org/10.3390/microorganisms10101923
APA StyleMofokeng, L. S., Smit, N. J., & Cook, C. A. (2022). Molecular Detection of Tick-Borne Bacteria from Amblyomma (Acari: Ixodidae) Ticks Collected from Reptiles in South Africa. Microorganisms, 10(10), 1923. https://doi.org/10.3390/microorganisms10101923





