Abstract
The success of mine site restoration programs in arid and semi-arid areas poses a significant challenge and requires the use of high-quality seedlings capable of tolerating heavy metal stresses. The effect of ectomycorrhizal fungi on different physiological traits was investigated in Pinus halepensis seedlings grown in soil contaminated with heavy metals (Pb-Zn-Cd). Ectomycorrhizal (M) and non-ectomycorrhizal (NM) seedlings were subjected to heavy metals stress (C: contaminated, NC: control or non-contaminated) soils conditions for 12 months. Gas exchange, chlorophyll fluorescence, water relations parameters derived from pressure–volume curves and electrolyte leakage were evaluated at 4, 8 and 12 months. Ectomycorrhizal symbiosis promoted stronger resistance to heavy metals and improved gas exchange parameters and water-use efficiency compared to the non-ectomycorrhizal seedlings. The decrease in leaf osmotic potentials (Ψπ100: osmotic potential at saturation and Ψπ0: osmotic potential with loss of turgor) was higher for M-C seedling than NM-C ones, indicating that the ectomycorrhizal symbiosis promotes cellular osmotic adjustment and protects leaf membrane cell against leakage induced by Pb, Zn and Cd. Our results suggest that the use of ectomycorrhizal symbiosis is among the promising practices to improve the morphophysiological quality of seedlings produced in forest nurseries, their performance and their tolerance to multi-heavy metal stresses.
1. Introduction
Mining activities that are conducted in a Mediterranean climate can exert their greatest effects on the environment through water pollution, contamination and alteration of agricultural soil due to the spread of heavy metals [1,2]. In North Africa, land losses due to increasingly heavy metal-polluted soils are the highest in the world [1]. Therefore, rehabilitation strategies targeting mine-degraded areas have been launched to combat land degradation and have been recognized as one of several sustainable development goals [1]. However, phytoremediation of abandoned mine lands by installing forest plantation presents a great challenge [3]. Indeed, severe environmental stresses and their interactions can negatively affect the survival of forest trees [4,5,6], the sustainability of ecosystems and the success of reforestation programs on abandoned mine sites in the context of climate change. North Africa (Morocco, Algeria and Tunisia) is recognized as one of the Mediterranean region’s most vulnerable to climate change, given that it is characterized by a significant decrease in precipitation and a significant increase in temperature, which reinforce both the pressures and the phenomena that are associated with ecosystem degradation [5,7].
In North Africa, various efforts have been made to modernize the seedling production chain and forest nurseries that produce seedlings of high morphophysiological quality, which are capable of surviving, growing and tolerating various environmental stresses in reforestation sites [8,9,10]. The results of this modernization project have shown that growth of ectomycorrhizal seedlings, which are produced in containers in modern forest nurseries, was much higher than that of seedlings that were produced in polybags in traditional nurseries [9]. Yet, these restoration projects did not focus upon the rehabilitation of mining sites using adapted species and ectomycorrhizal seedlings that were produced in modern forest nurseries.
The development of mycorrhizoremediation technology involving the use ectomycorrhizal seedlings is seen as a way for enhancing their abilities to tolerate various multi-metal stresses [11,12,13]. Other studies have reported the efficiency of ectomycorrhizal seedlings in overcoming the detrimental effects of abiotic stresses, such as drought [14,15] and salinity [16,17]. In contrast, numerous studies have demonstrated the negative effects of heavy metal contamination on the survival and growth of a wide range of non-mycorrhizal plant species [18,19,20,21,22,23]. On one hand, an excess of heavy metals in soils limits the efficiency of water- and mineral nutrient-use [18,19,24]. In addition, heavy metals substantially reduce leaf gas exchange parameters (net photosynthesis, transpiration, stomatal conductance, etc.), thereby causing lower water flow from the soil to the leaves [18,25], which may cause water stress in seedlings [24]. On the other hand, CO2 assimilation and leaf transpiration are decreased due to reduced stomatal opening [26,27], which may be induced by direct interaction of metal ion toxicity with guard cells [19]. Furthermore, long-term exposure to high levels of toxic heavy metals is often followed by water deficits [19,28]. This leads to the appearance of physiological responses consistent with those found under drought stress, including reductions in root water uptake, leaf turgor and stomatal conductance [19,29,30]. Heavy metals, such as Pb, Zn and Cd, reduce cell membrane permeability [18,31], chlorophyll concentrations [32,33] and photosystem II activity [25], which in turn can limit photosynthesis leading to metabolic disruptions [27,34,35].
To improve the survival, growth and physiological processes of tree seedlings that are intended for reforestation and mine site restoration programs, the use of seedlings with high morphophysiological quality that are produced in modern forest nurseries is necessary [8,9,36]. In addition to the choice of local forest species that are already adapted to the interactions of different environmental stresses, improving the root system using compatible host-ectomycorrhizal fungi that are resistant to environmental stresses would improve the survival, growth and physiology of the seedlings after their installation in mining and reforestation sites. Under drought conditions, as is the case in arid and semi-arid areas of North Africa, it was shown that ectomycorrhizal seedlings with fungal genotypes that produce mycelial strands or rhizomorphs capable of transporting large amounts of water [37] can substantially improve physiological processes (gas exchange and water relations parameters, mineral nutrition and hydraulic conductivity of the roots, among others). Consequently, the drought tolerance of the seedlings is improved [9,14,38,39,40]. In contrast, it is recognized that in the presence of heavy metals, ectomycorrhizal fungi improve survival, growth and various physiological processes (transpiration, gas exchange parameters: stomatal conductance, transpiration, net photosynthesis and osmotic adjustment, among others) of forest seedlings under controlled conditions and on mining sites [11,41,42]. Despite the publication of several reviews on the water relations of different species in response to heavy metal stresses [18,19,24,43], no information has been made available, to our knowledge, regarding ectomycorrhizal effects on water relations parameters that are derived from pressure–volume curves [44,45,46] in response to heavy metal stresses (Pb, Zn and Cd). These water relations variables for ectomycorrhizal tree seedlings, include osmotic potential at saturation (Ψπ100), osmotic potential with loss of turgor (Ψπ0), relative water content at loss of turgor (RWC0), water content of the symplasm (SWC), the modulus of elasticity (εmax) and osmotic adjustment (OA). The determination of these variables that are specific to water relations is fundamental to better quantifying and understanding the effects of ectomycorrhizal fungi on the physiology of cells and tissues of forest seedlings, which allow them to survive, grow and maintain their physiological functions (net photosynthesis, transpiration, etc.) in response to multi-heavy metal stresses. In addition, the results of this study would help advance operational practices, thereby further improving the morphophysiological quality of seedlings that are produced in forest nurseries, together with their performance in mining sites that are located in arid and semi-arid regions. This research continues our recent work [47], which showed that ectomycorrhizal fungi (Rhizopogon sp.) improved growth and mineral nutrient contents of Pinus halepensis mill seedlings that were subjected to heavy metal stresses. The presence of ectomycorrhizal fungi also reduced translocation factors for Zn and Cd, and bioaccumulation factors for Pb and Cd. This study was designed to test the hypothesis that ectomycorrhizal fungi can improve the water relations and the gas exchange parameters of P. halepensis seedlings grown in response to multi-heavy metal stresses.
The objectives of this study were: (i) to compare gas exchange variables, water use-efficiency (WUE), electrolyte leakage and chlorophyll fluorescence under multiple heavy metal stresses (Pb, Zn and Cd) in ectomycorrhizal and non-ectomycorrhizal Pinus halepensis seedlings; (ii) to determine water relations parameters that were derived from pressure–volume curves (Ψπ100, Ψπ0, RWC0, SWC and εmax) of ectomycorrhizal and non-ectomycorrhizal seedlings that were grown in the absence and presence of multi-metal stresses; and (iii) to examine whether osmotic adjustment occurs as a result of long-term exposure of ectomycorrhizal seedlings to high levels of heavy metal toxicity. This evaluation of gas exchange and water relations parameters will help to understand the physiological processes relevant to the performance of plants in response to multi-heavy metal stresses.
2. Materials and Methods
2.1. Soils, Plant Material, Experimental Design and Growth Conditions
Contaminated soil samples were collected from the abandoned mine site of “Jebel Ressas” in North Tunisia (36°36′021.4″ N, 10°19′04.0″ E) to a depth of 20 cm, as described by Hachani et al. [47]. Control soil was collected from a non-contaminated area, as described by Hachani et al. [47]. Heavy metal concentrations were determined by Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES) using three soil composite samples (six soil samples per composite sample) [47]. Heavy metal concentrations were 15.587 ± 0.796 mg·g−1 (mean ± standard deviation) for Pb, 37.766 ± 3.210 mg·g−1 for Zn and 0.181 ± 0.033 mg.g−1 for Cd in contaminated soil. In control soil, metal concentrations were 0.009 ± 0.0004 mg·g−1 and 0.021 ± 0.002 mg·g−1 for Pb and Zn, respectively, while Cd concentrations were at or below detection limits [47]. The other physicochemical characteristics of the contaminated and control soils (pHwater, pHCaCl2, electrical conductivity, soil fertility, etc.) that were used in this study are described in detail in our previous study [47].
The experiment was conducted at the National Institute of Research in Rural Engineering, Water and Forests (INRGREF) in Tunis (Tunisia). Nine month old naturally ectomycorrhizal and non-ectomycorrhizal Aleppo pine (Pinus halepensis) seedlings were sampled from a modern forest nursery (Ouchtata) in northwestern Tunisia (36°57′40.3″ N, 8°59′50.0″ E). The seedlings were divided into two classes according to the degree of the surface colonization of their root plugs by the extraradical mycelium of the ectomycorrhizal fungus Rhizopogon sp., as described in our previous studies [9,47,48]. The first class included seedlings with no surface colonization (non-mycorrhizal seedlings), while the second included seedlings with more than 50% of their root plug areas that were covered by the extramatrical phase of the fungus. The initial growth variables of the different parts of the Pinus halepensis seedlings that were used in this study are described in detail by Hachani et al. [47].
The selected seedlings were transplanted into pots (volume: 2 L; height: 45 cm; diameter: 30 cm) that were filled with contaminated (C) and non-contaminated (NC) soil at the rate of 10 kg of soil/pot (one seedling/pot). The pots containing the seedlings of the four treatments were grown for 12 months and they were installed according to a 2 × 2 factorial experimental design in four complete random blocks. These four treatments include: NM-NC (non-mycorrhizal seedlings + non-contaminated soil); M-NC (mycorrhizal seedlings + non-contaminated soil); NM-C (non-mycorrhizal seedlings + contaminated soil); and M-C (mycorrhizal seedlings + contaminated soil). The treatments were randomly distributed in each block. A total of 320 seedlings were deployed at the rate of 20 seedlings/treatment/block (20 seedlings × 4 treatments × 4 blocks). For each pot, soil moisture was maintained at 78 ± 6% field capacity throughout the experiment using Time Domain Reflectometry (TDR, Trase system I, Soil Moisture Equipment Corp., Goleta, CA, USA) [49,50].
2.2. Gas Exchange, Water-Use Efficiency and Chlorophyll Fluorescence Measurements
Maximal gas exchange variables were measured on five randomly selected seedlings/treatment/block. Measurements were carried out on three dates (4, 8 and 12 months), between 09.30 and 11.30 solar time to ensure maximum photosynthetic assimilation [51]. Net photosynthesis (A), stomatal conductance (gs), transpiration (E) and intercellular CO2 concentration (Ci) were measured using an infrared analyzer (LCpro+, ADC Bio Scientifc Ltd., Hoddesdon, UK) with a cylindrical coniferous cuvette at an active photosynthetic photon flux density (PPFD) of 984 ± 56 µmol m−2 s−1, a CO2 concentration of 350 µmol mol−1 and a leaf temperature of 29 ± 4 °C. Water-use efficiency (WUE) was calculated as the ratio of net photosynthesis to transpiration [52]. The gas exchange variables were corrected by taking into account the leaf area of the needles, which were estimated using the approach described by Lamhamedi et al. [49].
Chlorophyll fluorescence was determined on the same seedlings used for gas exchange measurements using a PEA fluorometer (Plant Efficiency Analyzer, Hansatech, King’s Lynn, Norfolk, UK). Measurements of minimum (F0) and maximum (Fm) chlorophyll fluorescence were performed on attached needles after 1 h of dark adaptation. The potential quantum yield of photosystem II (PSII), which was expressed as Fv/Fm, was calculated as follows [53]:
Fv/Fm = (Fm − F0)/Fm
2.3. Water Relations Parameters
Water relations parameters were determined after 4, 8 and 12 months from pressure-volume (P-V) curves using two randomly selected seedlings per block and per treatment (32 samples). For each seedling, fresh twigs at the distal end of branches at the same height were selected to avoid possible variations due to differences in hydraulic architecture of forest trees [46]. The development stage and the orientation of the twigs were the same in each treatment. Measurements were conducted using simultaneous pressure chambers (PMS 1000, PMS Instrument Co., Corvallis, OR, USA) and a precision balance to generate the data that were needed to establish pressure–volume curves, according to the method described by Ritchie [54]. Eleven pressure levels (starting at −0.2 MPa down to −5.2 MPa) were applied and each level was maintained for 10 min, as described by Zine El Abidine et al. [46,55]. Before taking the measurements, the twigs of each seedling were placed in distilled water in the dark at 25 °C at 12.00, 14.00, 16.00 and 18.00 h. The length of the saturation period was 20 h. This rehydration until turgor is essential to standardize the relative water content of all samples [46,54]. The measurements were taken over 4 days. Within each day, eight samples were analyzed (four samples per pressure chamber) and 8 P-V curves were generated including two repetitions of each treatment. Each day, samples were chosen randomly among the treatments to avoid rehydration-time effects, as described by Zine El Abidine et al. [46]. The data for the pressure–volume curves were generated at 08.00, 10.00, 12.00 and 14.00 h.
Each pressure–volume curve permitted the estimation of a set of variables describing the water relations of the ectomycorrhizal and non-ectomycorrhizal seedlings, namely, the osmotic potential at saturation (Ψπ100), the osmotic potential with loss of turgor (Ψπ0), the relative water content at loss of turgor (RWC0), the symplastic water content (SWC), the modulus of elasticity (εmax) and the osmotic adjustment (OA). These variables (Ψπ100, Ψπ0 and RWC0) were determined from the pressure–volume curves (Figure 1), as described by Schulte and Hinckley [45] and Zine El Abidine et al. [46].
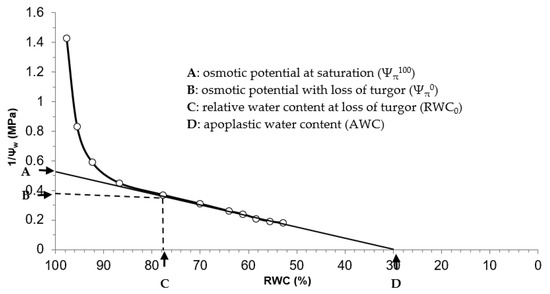
Figure 1.
Example of a pressure-volume (P-V) curve generated from different paired measurements [inverse of xylem water potential (1/Ψw) and relative water content (RWC)] indicated by circles. Different variables describing the water relations of the seedlings that were subjected to the four treatments are generated from the P-V curves (indicated by black arrows). These variables include: osmotic potential at saturation (Ψπ100) osmotic potential with loss of turgor (Ψπ0), relative water content at loss of turgor (RWC0) and apoplastic water content (AWC).
Symplastic water content (SWC) was calculated as follows:
SWC (%) = 100 − AWC
Apoplastic water content was determined from the P-V curve. The εmax was calculated as follows [56,57]:
εmax = −Ψπ100 (1 − AWC)/(1 − RWC0)
Osmotic adjustment (OA) was calculated as follows [58]:
OA = Ψπ100 (control) − Ψπ100 (treatment)
In the case of the total water potential (Ψw) of small sized plants that were used to generate the pressure–volume curves, only two major components (the osmotic potential (Ψπ) and the turgor potential (also termed pressure potential, Ψp) are included, as shown in the following equation:
Ψw = Ψπ + Ψp
Ψπ measures the concentration of the solution that is produced by dissolved solutes (always negative). Ψp represents the hydrostatic pressure that is produced by the inward pressure of cell walls in seedlings or due to water weight (always positive).
2.4. Electrolyte Leakage
Electrolyte leakage was measured according to the method described by Blum and Ebercon [59]. Measurements were taken at 4, 8 and 12 months using 5 randomly selected seedlings per block and per treatment (20 seedlings/treatment). Needles were cut into 1 cm long fragments. The fragments (20 fragments per treatment) were washed twice with distilled water, then, were soaked in sterile test tubes containing 15 mL of distilled water in the dark at 40 °C for 1 h. Free conductivity (FC) of the solution was measured after the first incubation using a conductivity meter (Cellox 325, Multiline P3 PH/LF-SET, WTW Gmbh, Weilheim, Germany). The test tubes were incubated in a water bath at 100 °C for 1 h to insure the complete electrolyte leakage. Total conductivity (TC) was measured after the solution reached 25 °C. The rate of electrolytes leakage was calculated as follows [59]:
Electrolyte leakage (%) = (FC/TC) × 100
2.5. Statistical Analyses
Analysis of variance (ANOVA) was used to test the significance of the main effect of the treatments being evaluated, the main effect of sampling date and their interactions on the various variables that were measured (gas exchanges, water relations, fluorescence and electrolyte losses, among others) using SPSS 22.0 software (IBM, Armonk, NY, USA). The assumptions of residue normality and variance uniformity for the different variables measured were verified prior to two-way ANOVA [60]. Means comparisons were performed using Tukey’s tests at a 5% significance level. Values are presented as the means ± standard deviation (SD).
3. Results
3.1. Gas Exchange, Water-Use Efficiency and Chlorophyll Fluorescence
There were significant treatment and time effects (p < 0.05) on gas exchange variables (A, gs, E and Ci) (Table 1; Figure 2). With the exception of WUE and F0, strong and significant interactions between treatment × sampling dates were observed for several physiological variables (Table 1). Under contaminated soil conditions (NM-C and M-C), P. halepensis seedlings showed a significant decrease (p < 0.05) in A, gs and E. This effect was always more pronounced in non-ectomycorrhizal seedlings (NM-C) during the experiment (Figure 2).

Table 1.
Significance levels of treatment effects (T), date effects (D) and their interaction (T × D).
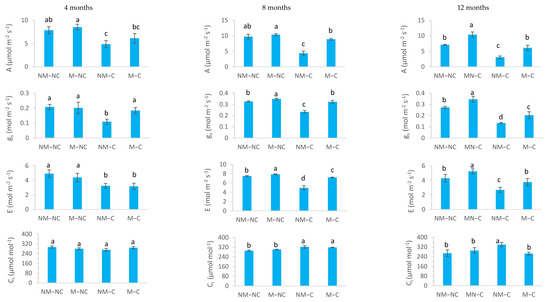
Figure 2.
Net photosynthesis (A), stomatal conductance (gs), transpiration (E) and intercellular CO2 concentration (Ci) of ectomycorrhizal (M) and non-ectomycorrhizal (NM) Pinus halepensis seedlings after 4, 8 and 12 months of growth in contaminated (C) and control or non-contaminated soil (NC). Means (±SD; n = 20) with different letters significantly differ from one another based on Tukey’s tests at p ≤ 0.05.
After 12 months of growth, the seedlings were well colonized by the ectomycorrhizal fungus Rhizopogon sp. and the extramatrical phase had explored the entire soil volume of the 2 L container. On a microscopic scale, our observations showed that the structures of ectomycorrhizae are characterized by the presence of mantle hyphae and Hartig net hyphae. However, the roots of control seedlings were not colonized by Rhizopogon and showed the absence of these microscopic structures. Contaminated soil significantly decreased net photosynthesis in NM-C seedlings after 4 months (38%, p = 0.003), 8 months (55%, p = 0.0001) and 12 months (57%, p = 0.0001), compared to the control (NM-NC). Ectomycorrhizae significantly stimulated net photosynthesis compared to non-ectomycorrhizal seedlings under both treatments (contaminated and non-contaminated soils) (Figure 2). The effect of mycorrhizae on the rate of net photosynthesis was apparent, under longer-term heavy metal stress (>8 months), given that no significant difference was noted between M-C seedlings and the control after 8 months (p = 0.153) and 12 months (p = 0.758). In addition, M-C seedlings exhibited two-fold higher photosynthesis rates than NM-C after 8 and 12 months (Figure 2).
Non-ectomycorrhizal seedlings (NM-C) had significantly lower stomatal conductance (gs) at 4 months (47%, p = 0.0001) and 8 months (28%, p = 0.0001), compared to the control. After 12 months, gs decreased significantly for both NM-C (52%, p = 0.0001) and M-C (26%, p = 0.034). This reduction was 1.5 times less pronounced in ectomycorrhizae seedlings (M-C) than in non-mycorrhizal seedlings. In uncontaminated soil, gs was significantly increased by 7% (p = 0.042) at 8 months and by 26% (p = 0.002) at 12 months for ectomycorrhizal seedlings (M-NC) compared to non-ectomycorrhizal ones (NM-NC) (Figure 2).
Under Pb, Zn and Cd stress, the transpiration rate (E) was significantly reduced for NM-C seedlings at 4 months (33%, p = 0.011), 8 months (34%, p = 0.0001) and 12 months (37%, p = 0.047). This reduction was significantly less accentuated in mycorrhizal seedlings (M-C) throughout the experiment, reaching 35% (p = 0.016) at 4 months and 4% (p = 0.041) at 8 months, compared to the control. Yet, after 12 months, this effect was not significant (p = 0.992). In control soil, the M-NC seedlings showed higher E rates reaching 5% at 8 months (p = 0.043) and 22% at 12 months (p = 0.005), compared to the control (NM-NC) (Figure 2).
The intercellular CO2 concentration (Ci) of unstressed (NM-NC and M-NC) and stressed (NM-C and M-C) seedlings varied over the experimental period. At 4 months, Ci did not exhibit any significant variations (p > 0.05) between treatments. After 8 months, Ci revealed a significant increase by 11% (p = 0.001) for NM-C and by 9% (p = 0.014) for M-C. At the end of the experiment, Ci increased significantly for NM-C seedlings (26%, p = 0.009), while no difference was noted between M-C seedlings and the control (NM-NC) (Figure 2).
For water-use efficiency (WUE), only NM-C seedlings showed a significant decrease by 32% at 8 months (p = 0.002) and by 31% at 12 months (p = 0.018), compared to the control (NM-NC) (Figure 3).
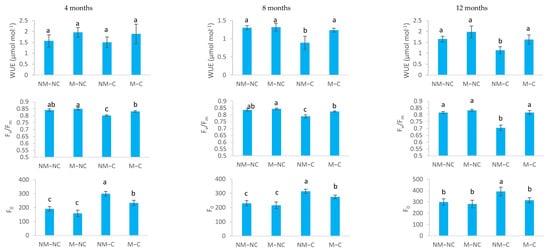
Figure 3.
Water-use efficiency (WUE), the yield of photosystem II (Fv/Fm) and minimum chlorophyll fluorescence (F0) of ectomycorrhizal (M) and non-ectomycorrhizal (NM) Pinus halepensis seedlings after 4, 8, and 12 months of growth in contaminated (C) and control soil (NC). Means (± SD; n = 20) with different letters significantly differ from one another based on Tukey’s tests at p ≤ 0.05.
Maximum quantum yield of PSII (Fv/Fm) was significantly reduced in non-ectomycorrhizal seedlings (NM-C) under Pb, Zn and Cd stress (Figure 3). Fv/Fm was reduced significantly by 7% (p = 0.001) at 4 months, 6% (p = 0.014) at 8 months and 14% (p = 0.0001) at 12 months, compared to the control (NM-NC). No significant differences in Fv/Fm were noted between mycorrhizal seedlings (M-NC and M-C) and the control (NM-NC) over time (Figure 3). In contrast, exposure to heavy metals (Pb, Zn and Cd) significantly increased minimum chlorophyll fluorescence (F0) (Figure 3). NM-C seedlings showed an increase in F0, reaching 57% (p = 0.0001) at 4 months, 36% (p = 0.001) at 8 months and 31% (p = 0.036) at 12 months, compared to the control. M-C seedlings showed a less pronounced increase of 22% (p = 0.031) and 19% (p = 0.048) at 4 and 8 months, respectively, compared to the control. Beyond this period, F0 remained constant (p = 0.997).
3.2. Water Relation Variables
Water relation variables were determined after 4, 8 and 12 months of growth in pots. However, no significant differences (p > 0.05) were observed at 4 and 8 months.
Prolonged exposure to heavy metals (Pb, Zn and Cd) during 12 months of growth affected water relations variables (p < 0.05) (Figure 4). Under heavy metal stress, osmotic potential at full turgor (Ψπ100) decreased for NM-C by 12% (p = 0.030) and for M-C by 27% (p = 0.0001), compared to the control (NM-NC) (Figure 4a). Osmotic potential at zero turgor (Ψπ0) also revealed a decrease by 25% for M-C (p = 0.0001), compared to the control (NM-NC). No difference was recorded between NM-C (p = 0.054), M-NC (p = 0.231) and the control (Figure 4b). Values of Ψπ100 and Ψπ0 of mycorrhizal contaminated seedlings (M-C) were significantly more negative than those of seedlings that were grown under the other treatments (NM-NC, M-NC and NM-C). Yet, NM-NC (control) and M-NC seedlings showed higher Ψπ100 and Ψπ0 (less negative) values (Figure 4a,b).
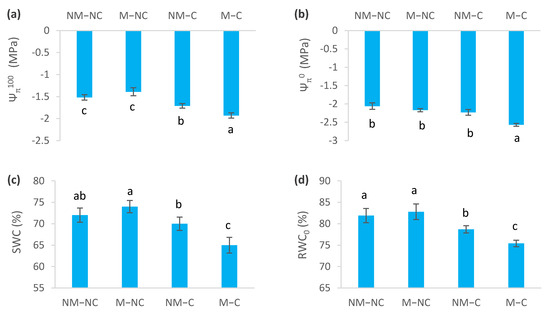
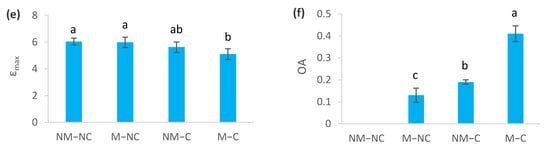
Figure 4.
Osmotic potential at saturation (Ψπ100) (a), osmotic potential with loss of turgor (Ψπ0) (b), symplastic water content (SWC) (c), relative water content at loss of turgor (RWC0) (d), modulus of elasticity (εmax) (e), and osmotic adjustment (OA) (f) of ectomycorrhizal (M) and non-ectomycorrhizal (NM) Pinus halepensis seedlings after 12 months of growth in contaminated (C) and control soil (NC). Means (± SD; n = 8), with different letters indicating significant differences from one another based upon Tukey’s tests at p ≤ 0.05.
Symplastic water content (SWC) decreased for mycorrhizal seedlings (M-C) by 10% (p = 0.001), compared to the control (NM-NC). No significant difference was observed between M-NC (p = 0.696), NM-C (p = 0.262) and the control (NM-NC) (Figure 4c). RWC0 was significantly reduced in NM-C by 4% (p = 0.031) and M-C by 8% (p = 0.001), compared to the control (Figure 4d). The modulus of elasticity (εmax) decreased by 16% for ectomycorrhizal seedlings M-C (p = 0.005), but it did not significantly differ from NM-C (Figure 4e). Mycorrhizal seedlings (M-C) showed 2.15-fold higher osmotic adjustment (OA) than non-mycorrhizal ones (NM-C), and 3.15-fold higher than M-NC (Figure 4f).
3.3. Electrolyte Leakage
The effects of heavy metals on membrane permeability varied throughout the experiment, depending upon the presence or absence of ectomycorrhizal fungi (Figure 5). The sampling date had no effect (p = 0.141) on electrolyte leakage (Table 1). In contaminated soil, electrolyte leakage was increased for both ectomycorrhizal (M-C) and non-ectomycorrhizal (NM-C) seedlings, compared to the control (NM-NC). The increase in electrolyte leakage for NM-C reached 112% (p = 0.0001) at 4 months, 102% (p = 0.0001) at 8 months and 183% (p = 0.0001) at 12 months, compared to the control (Figure 5). No difference (p = 0.753) was noted between NM-C and M-C at 4 months (Figure 5a). Compared to the control (NM-NC), ectomycorrhizal seedlings (M-C) had significantly lower electrolyte leakage rates, reaching 102% (p = 0.0001) at 4 months, 21% (p = 0.031) at 8 months and 33% (p = 0.001) at 12 months (Figure 5). In control soil (NC), leakage of electrolytes was reduced for M-NC after 8 months (31%, p = 0.003) and 12 months (34%, p = 0.0001), compared to the control. M-NC seedlings generally exhibited the lowest values of electrolyte leakage throughout the experiment (Figure 5).
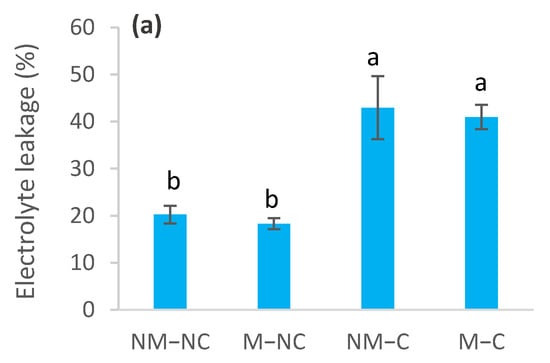
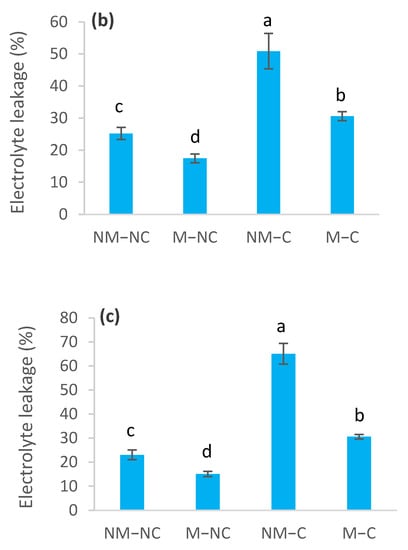
Figure 5.
Electrolyte leakage of ectomycorrhizal (M) and non-ectomycorrhizal (NM) Pinus halepensis seedlings after 4 (a), 8 (b) and 12 months (c) of growth in contaminated (C) and control soil (NC). Means (± SD; n = 4) with different letters significantly differ from one another based on Tukey’s tests at p ≤ 0.05.
4. Discussion
The use of ectomycorrhizal P. halepensis seedlings that were infected with Rhizopogon sp. in the presence of soil contaminated with heavy metals significantly improved gas exchange and water relations (Figure 2, Figure 3 and Figure 4). After 12 months of growth, the mycorrhizae also conferred on Aleppo pine seedlings an osmotic adjustment and a high elasticity allowing the plants to survive, grow and maintain various physiological processes (Figure 2, Figure 3, Figure 4 and Figure 5) despite the presence of extremely high concentrations of heavy metals in the soil (Pb, Zn and Cd). Our previous results [47] showed that after 12 months of growth in contaminated soil (NM-C) with heavy metals (Pb, Zn, and Cd), shoot and root dry masses of P. halepensis seedlings were reduced compared to the control (NM-NC), while no differences were observed for M-C compared to the control (NM-NC). Prolonged exposure to heavy metals primarily affects seedling growth and significantly decreases nutrient uptake, water-use efficiency, photosynthetic activity and cell membrane integrity [19,22,25,29]. In contrast, other studies showed that mycorrhizal fungi alleviate metal toxicity by improving physiological mechanisms and adaptation of host plants [23,61]. This observation is consistent with the current research indicating a significant increase in photosynthetic rate among ectomycorrhizal seedlings (M-C) to a level similar to seedlings under controlled conditions (NM-NC) (Figure 2). The increase in M-C was two times greater than non-ectomycorrhizal seedlings (NM-C) after 8 and 12 months of exposure to heavy metal contaminated soil (Figure 2). Other results have demonstrated an adverse effect of heavy metals on gas exchange parameters [25,62,63]. This was evaluated through analysis of maximum quantum yield of PSII photochemistry (Fv/Fm), which is considered to be a critical parameter of plant photosynthetic performance and a useful tool for evaluating plant tolerance to heavy metal toxicity. Our results revealed a significant Fv/Fm reduction under Pb, Zn and Cd toxicity, particularly in non-ectomycorrhizal seedlings (NM-C), while M-C seedlings always exhibited higher values (Figure 3). For non-ectomycorrhizal seedlings, the decline in Fv/Fm ratio suggests that photodamage and photoinhibition occurred to PSII [64,65]. This effect significantly decreases net photosynthesis as was observed in non-ectomycorrhizal seedlings (Figure 2). Under controlled conditions, net photosynthesis was positively correlated with internal CO2 concentration [66]. Yet, an increase in internal CO2 (Ci) concentrations may be associated with low net uptake of CO2. Therefore, photosynthetic limitation was posed by internal conductance to CO2 movement in seedlings that were exposed to contaminated soil (NM-C and M-C) after 8 months (Figure 2). It has been shown that Cd stress is able to increase CO2 concentrations in the intercellular spaces of the mesophyll [67]. High internal CO2 levels can be explained by the decreased capacity of chloroplasts to assimilate CO2 [68]. In contrast, ectomycorrhizae enabled the seedlings (M-C) to withstand metal toxicity and to maintain Ci concentrations that were similar to the control after 12 months of growth (Figure 2). Ectomycorrhizae also increased transpiration (E) rates in seedlings (M-C) after 8 and 12 months of exposure to excess of Pb, Zn and Cd (Figure 2). Similarly, Han et al. [42] demonstrated an increase in photosynthetic and transpiration rates in ectomycorrhizal hybrid poplar (Populus alba × tremula var. glandulosa) seedlings that had been subjected to high concentrations of Cd.
Mycorrhizal plants often exhibit higher stomatal conductance (gs) than non-mycorrhizal ones [69]. Our results showed that stomatal conductance of ectomycorrhizal seedlings (M-C) is not different to the control (NM-NC), but it is always significantly greater than the non-ectomycorrhizal seedlings (NM-C) throughout the growing period (12 months) (Figure 2). Heavy metals negatively affect absorption of mineral nutrients due, in part, to stomatal closure, which leads to the decrease in CO2 uptake and photosynthesis [33,70,71,72]. Other major positive effects of ectomycorrhizae on water relations and gas exchange parameters are associated with (i) increasing soil root exploration by extending the extraradical phase (hyphae and mycelial strands) of ectomycorrhizal fungi that are capable of penetrating soil micropores, which are not accessible to the roots, to extract water [37,39,40,73,74,75,76]. (ii) The formation of mycelial strands or rhizomorphs by certain ectomycorrhizal fungi, as is the case in our study (Rhizopogon sp.), which are capable of transporting large quantities of water [37,74], and the extraradical mycelium of ectomycorrhizal fungi, by itself (without the roots), make it possible to satisfy the moisture requirements of the seedling in order to maintain photosynthesis and transpiration [14,38]. (iii) Furthermore, water stress is reduced at the soil-root interface [39], together with (iv) improvement of soil structure and soil water reserves depending upon the density and expanse of the fungal hyphae in the soil [75,77,78]. Our previous results have shown that ectomycorrhizae significantly increase the dry root masses of P. halepensis seedlings [47]. The development of root systems in ectomycorrhizal plants was linked to higher rates of CO2 uptake and greater WUE in stone pine (Pinus pinea L.) [79]. It should be noted that ectomycorrhizal seedlings (M-C) had the most rapid and efficient recovery from the harmful effects of heavy metals compared to the non-ectomycorrhizal ones (NM-C) (Figure 2 and Figure 3). The recovery time was associated to the rapid growth of plants, which causes a reduction in internal metal concentrations due to a dilution effect [28]. Our previous results showed that ectomycorrhizal P. halepensis seedlings exhibit higher growth and lower Pb, Zn and Cd uptake than non-ectomycorrhizal seedlings [47], which demonstrates the substantial benefits of ectomycorrhizal fungi to their hosts under multi-metal stress.
Stomatal closure disrupts the flow of sap and, consequently, the water relations of the seedlings [80]. Therefore, the importance is demonstrated for osmotic adjustment (OA) as an adaptation mechanism [81]. This is consistent with our findings showing a substantial increase in OA for ectomycorrhizal (M-C) seedlings that was two-fold higher than non-ectomycorrhizal ones (NM-C) at the end of the experiment (Figure 4f). The increase in osmotic adjustment may be associated with decreases in osmotic potentials (Ψπ100 and Ψπ0) (Figure 4a,b), suggesting that mycorrhizae can give plants increased resistance to various heavy metals. A greater decline in Ψπ100 and Ψπ0 for ectomycorrhizal seedlings (M-C), together with greater osmotic adjustment (Figure 4), may grant them the ability to absorb water from contaminated soil, even when the former is hardly available due to effects of contamination [18]. As a result, M-C seedlings can maintain their turgor for a long time before reaching the loss of turgor point [46], which is consistent with lower values of RWC0 and increased elasticity of cell membranes (Figure 4). This allows gas exchange (stomata conductance and net photosynthesis) to be maintained for longer periods [52], as was observed in this study. The positive effects of these significant improvements in physiological processes were reflected on several growth parameters [47].
In contrast, Muhsin and Zwiazek [82] revealed that ectomycorrhizae increase apoplastic water transport and root hydraulic conductivity in American elm (Ulmus americana L.) seedlings, and suggested that this is related to decreased resistance to the flow of water from the apoplast through ectomycorrhizal hyphae. The increased root water flow in mycorrhizal seedlings may be related to the nutritional and metabolic effects of mycorrhizae on the activity of root water channels [82]. It has been demonstrated that ectomycorrhizal fungi improve water transport and water relations of seedlings [37,38,39] through the improvement in the mineral nutrition of plants. Phosphorus and potassium, especially, are two key elements that are involved in active adjustments [73]. Several ectomycorrhizal fungi improve the osmotic adjustment of their host seedling’s cells. This improvement came from the synthesis of organic acids and accumulation of mineral nutrients [83,84]. For example, high concentrations and contents of nitrogen, phosphorus and calcium increase the rate of net photosynthesis [48,76,85], which could increase water-use efficiency compared to non-ectomycorrhizal ones (NM-C), as was noted in the present study (Figure 3). Indeed, our recent findings revealed that ectomycorrhizal association of P. halepensis seedlings improved mineral nutrition, particularly in terms of nitrogen and calcium [47]. This last mineral nutrient (Ca) stimulates photosynthesis, cell division, cell wall rigidity and absorption of major nutrients (N, P and K) [86,87,88]. Furthermore, osmotic adjustments are known to prevent oxidative damage [26,27,89]. This effect becomes more important in the presence of mycorrhizae [90]. This may explain the low electrolyte leakage rates that were sustained by ectomycorrhizal contaminated (M-C) seedlings (Figure 5b,c). Reduction in electrolyte leakage may be associated with the cell conservation of water absorption and transport structures within the seedlings [27]. Membrane stability of ectomycorrhizal seedlings may be further linked to the decrease in the modulus of elasticity (εmax) (Figure 4e), which provides more flexibility and stability to the membranes [46]. This flexibility provides the plant with the possibility of undergoing significant variation in the water content of the apoplast without affecting the dynamic structure of the cell walls [91]. This is confirmed by the significant decrease in the symplastic water content (SWC) in ectomycorrhizal (M-C) seedlings, compared to the control (NM-NC) and the non-ectomycorrhizal ones (NM-C) (Figure 4c). As such, the influence of metal contamination on membrane stability was not associated with heavy metal exposure time, unlike the other variables (Table 1). This response is probably due to the protection that is offered by the ectomycorrhizal symbiosis, which confers greater resistance to stress, thereby lowering the occurrence of stress symptoms, such as membrane stability maintenance and decreased osmo-protectant production [92].
5. Conclusion and Research Needs
The present study emphasizes the importance of ectomycorrhizae in enhancing physiological processes in P. halepensis seedlings that are subjected to heavy metal contaminated soil, and supports the use of a mycorrhizoremediation approach in reforestation and rehabilitation of heavy metal contaminated sites. Our results, combined with our previous results [47], suggest that the use of ectomycorrhizal fungi is among the promising practices that would improve the morphophysiological quality of tree seedlings produced in forest nurseries, their performance and their tolerance to multiple heavy metal stresses (Pb, Zn and Cd). Therefore, further study is warranted regarding the combined effects of heavy metals and ectomycorrhizae on the mineral status of the seedlings using vector analysis of foliar nutrients and biomass.
Author Contributions
Conceptualization: C.H., Z.B., M.S.L. and A.Z.E.A., Supervision and project administration: Z.B., Methodology, experimental design, data collection, laboratory analyses: C.H., Z.B., M.S.L., M.A., A.Z.E.A. and D.P.K., Statistical analyses: C.H.; Writing—original draft preparation: C.H., Z.B., A.Z.E.A. and M.S.L., Writing—review and editing: C.H., Z.B., M.S.L., A.Z.E.A., D.P.K. and M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Carthage, Ministry of Higher Education and Scientific Research, Tunisia and by the National Institute of Research in Rural Engineering, Water and Forests (INRGREF), Tunisia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to Najet Hammami, technician in INRGREF, for technical assistance. We are thankful to nursery staff of INRGREF (Mohamed Trabelsi, Lazhar Ben Sadok, Badreddine Hajaij and Ridha May) for their help during the collection of soil samples and implementation of the experimental design.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses or interpretation of data, in the writing of the manuscript or in the decision to publish the results.
References
- FAO; ITPS. Status of the World’s Soil Resources (SWSR)—Main Report; Food and Agriculture Organization of the United Nations and Intergovernmental Technical Panel on Soils: Rome, Italy, 2015; p. 650. [Google Scholar]
- FAO. Soil Pollution a Hidden Reality; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018; p. 142. [Google Scholar]
- Wang, Q.; Shaheen, S.M.; Jiang, Y.; Li, R.; Slaný, M.; Abdelrahman, H.; Kwon, E.; Bolan, N.; Rinklebe, J.; Zhang, Z. Fe/Mn-and P-modified drinking water treatment residuals reduced Cu and Pb phytoavailability and uptake in a mining soil. J. Hazard. Mater. 2021, 403, 123–628. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Taabni, M.; Jihad, M.D.E. Eau et changement climatique au Maghreb: Quelles stratégies d’adaptation? Les Cahiers d’Outre-Mer. Rev. Géogr. Bordx. 2012, 65, 493–518. [Google Scholar]
- Trenberth, K.E.; Dai, A.; Van Der Schrier, G.; Jones, P.D.; Barichivich, J.; Briffa, K.R.; Sheffield, J. Global warming and changes in drought. Nat. Clim. Chang. 2014, 4, 17–22. [Google Scholar] [CrossRef]
- Schilling, J.; Hertig, E.; Tramblay, Y.; Scheffran, J. Climate change vulnerability, water resources and social implications in North Africa. Reg. Environ. Chang. 2020, 20, 15. [Google Scholar] [CrossRef]
- Lamhamedi, M.S.; Ammari, Y.; Fecteau, B.; Fortin, J.A.; Margolis, H. Problématique des pépinières forestières en Afrique du Nord et stratégies de développement. Cah. Agric. 2000, 9, 369–380. [Google Scholar]
- Lamhamedi, M.S.; Abourouh, M.; Fortin, J.A. Technological transfer: The use of ectomycorrhizal fungi in conventional and modern forest tree nurseries in northern Africa. In Advances in Mycorrhizal Science and Technology; Khasa, D., Piché, Y., Coughlan, A.P., Eds.; NRC Research Press: Ottawa, ON, Canada, 2009; pp. 139–152. [Google Scholar]
- Gaba-Chahboub, H.; Lamhamedi, M.S.; Abrous-Belbachir, O. Effet de l’inoculation ectomycorhizienne en pépinière sur la croissance et la nutrition des plants du cèdre de l’Atlas en Algérie. Bois For. Trop. 2016, 330, 57–67. [Google Scholar] [CrossRef]
- Marx, D.H.; Marrs, L.F.; Cordell, C.E. Practical use of the mycorrhizal fungal technology in forestry, reclamation, arboriculture, agriculture, and horticulture. Dendrobiology 2002, 47, 27–40. [Google Scholar]
- Otero-Blanca, A.; Folch-Mallol, J.L.; Lira-Ruan, V.; del Rayo Sánchez Carbente, M.; Batista-García, R.A. Phytoremediation and fungi: An underexplored binomial. In Approaches in Bioremediation: The New Era of Environmental Microbiology and Nanobiotechnology; Prasad, R., Aranda, E., Eds.; Springer: Cham, Switzerland, 2018; pp. 79–95. [Google Scholar]
- Assad, R.; Reshi, Z.A.; Rashid, I.; Mir, S.H. Restoration of heavy metal-contaminated environs through ectomycorrhizal symbiosis. In Bioremediation and Biotechnology; Bhat, R.A., Hakeem, K.R., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 313–330. [Google Scholar] [CrossRef]
- Lamhamedi, M.S.; Fortin, J.A.; Bernier, P.Y. La génétique de Pisolithus sp.: Une approche de biotechnologie forestière pour une meilleure survie des plants en conditions de sécheresse. Sécheresse 1991, 2, 251–258. [Google Scholar]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Cambridge, MA, USA, 2008; p. 815. [Google Scholar]
- Tang, M.; Sheng, M.; Chen, H.; Zhang, F.F. In vitro salinity resistance of three ectomycorrhizal fungi. Soil Biol. Biochem. 2009, 41, 948–953. [Google Scholar] [CrossRef]
- Guerrero-Galán, C.; Calvo-Polanco, M.; Zimmermann, S.D. Ectomycorrhizal symbiosis helps plants to challenge salt stress conditions. Mycorrhiza 2019, 29, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Barcelo, J.; Poschenrieder, C. Plant water relations as affected by heavy metal stress: A review. J. Plant. Nut. 1990, 13, 1–37. [Google Scholar] [CrossRef]
- Rucińska-Sobkowiak, R. Water relations in plants subjected to heavy metal stresses. Acta Physiol. Plant. 2016, 38, 257. [Google Scholar] [CrossRef]
- Handa, N.; Kohli, S.K.; Sharma, A.; Thukral, A.K.; Bhardwaj, R.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Selenium ameliorates chromium toxicity through modifications in pigment system, antioxidative capacity, osmotic system, and metal chelators in Brassica juncea seedlings. S. Afr. J. Bot. 2018, 119, 1–10. [Google Scholar] [CrossRef]
- Shahzad, B.; Tanveer, M.; Che, Z.; Rehman, A.; Cheema, S.A.; Sharma, A.; ur Rehman, S.; Song, H.; Zhaorong, D. Role of 24-epibrassinolide (EBL) in mediating heavy metal and pesticide induced oxidative stress in plants: A review. Ecotoxicol. Environ. Saf. 2018, 147, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Kohli, S.K.; Handa, N.; Bali, S.; Khanna, K.; Arora, S.; Sharma, A.; Bhardwaj, R. Current scenario of Pb toxicity in plants: Unraveling plethora of physiological responses. Rev. Environ. Contamin. Toxicol. 2019, 249, 153–197. [Google Scholar] [CrossRef]
- Dhalaria, R.; Kumar, D.; Kumar, H.; Nepovimova, E.; Kuča, K.; Torequl Islam, M.; Verma, R. Arbuscular mycorrhizal fungi as potential agents in ameliorating heavy metal stress in plants. Agronomy 2020, 10, 815. [Google Scholar] [CrossRef]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111–887. [Google Scholar] [CrossRef]
- Rai, R.; Agrawal, M.; Agrawal, S.B. Impact of heavy metals on physiological processes of plants: With special reference to photosynthetic system. In Plant Responses to Xenobiotics; Singh, A., Prasad, S.M., Singh, R.P., Eds.; Springer Nature: Singapore, 2016; pp. 127–140. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.S.M.A.; Fujita, D.B.S.M.A.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. In Sustainable Agriculture; Lichtfouse, E., Navarrete, M., Debaeke, P., Véronique, S., Alberola, C., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 153–188. [Google Scholar]
- Parkash, V.; Singh, S. A review on potential plant-based water stress indicators for vegetable crops. Sustainability 2020, 12, 3945. [Google Scholar] [CrossRef]
- Chmielowska-Bąk, J.; Deckert, J. Plant Recovery after Metal Stress—A Review. Plants 2021, 10, 450. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Yadav, S.K. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Przedpelska-Wasowicz, E.M.; Wierzbicka, M. Gating of aquaporins by heavy metals in Allium cepa L. epidermal cells. Protoplasma 2011, 248, 663–671. [Google Scholar] [CrossRef]
- Petrovic, D.; Krivokapic, S. The effect of Cu, Zn, Cd, and Pb accumulation on biochemical parameters (proline, chlorophyll) in the water caltrop (Trapa natans L.), Lake Skadar, Montenegro. Plant 2020, 9, 1287. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, L.; Huang, X.; Zhou, Y.; Quan, Q.; Li, Y.; Zhu, X. Response of photosynthesis to different concentrations of heavy metals in Davidia involucrata. PLoS ONE 2020, 15, e0228563. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.; Shukla, A. Acclimation and tolerance strategies of rice under drought stress. Rice Sci. 2015, 22, 147–161. [Google Scholar] [CrossRef]
- Muhammad, I.; Shalmani, A.; Ali, M.; Yang, Q.H.; Ahmad, H.; Li, F.B. Mechanisms regulating the dynamics of photosynthesis under abiotic stresses. Front. Plant Sci. 2021, 11, 2310. [Google Scholar] [CrossRef]
- Haase, D.L.; Bouzza, K.; Emerton, L.; Friday, J.B.; Lieberg, B.; Aldrete, A.; Davis, A.S. The high cost of the low-cost polybag system: A review of nursery seedling production systems. Land 2021, 10, 826. [Google Scholar] [CrossRef]
- Duddridge, J.A.; Malbari, A.; Read, D.J. Structure and function of ectomycorrhizal rhizomorphs with especial reference to their role in water transport. Nature 1980, 287, 834–836. [Google Scholar] [CrossRef]
- Read, D.J.; Boyd, R. Water relations of mycorrhizal fungi and their host plants. In Water, Fungi and Plants; Ayres, P.G., Body, L., Eds.; Cambridge University Press: Cambridge, MA, USA, 1986; pp. 287–304. [Google Scholar]
- Lamhamedi, M.S.; Bernier, P.Y.; Fortin, J.A. Hydraulic conductance and soil water potential at the soil–root interface of Pinus pinaster seedlings inoculated with different dikaryons of Pisolithus sp. Tree Physiol. 1992, 10, 231–244. [Google Scholar] [CrossRef]
- Lamhamedi, M.S.; Bernier, P.Y.; Fortin, J.A. Growth, nutrition and response to water stress of Pinus pinaster inoculated with ten dikaryotic strains of Pisolithus sp. Tree Physiol. 1992, 10, 153–167. [Google Scholar] [CrossRef]
- Marx, D.H. Forest Application of the Ectomycorrhizal Fungus Pisolithus tinctorius; The Marcus Walenberg Prize: Stockholm, Sweden, 1991. [Google Scholar]
- Han, S.H.; Kim, D.H.; Lee, J.C. Effects of the ectomycorrhizal fungus Pisolithus tinctorius and Cd on physiological properties and Cd uptake by hybrid poplar Populus alba× glandulosa. J. Ecol. Field Biol. 2011, 34, 393–400. [Google Scholar] [CrossRef][Green Version]
- Poschenrieder, C.H.; Barceló, J. Water relations in heavy metal stressed plants. In Heavy Metal Stress in Plants; Springer: Berlin/Heidelberg, Germany, 1999; pp. 207–229. [Google Scholar]
- Tyree, M.T.; Hamel, H.T. The measurements of the turgor pressure and the water relations of plants by the pressure bomb technique. J. Exp. Bot. 1972, 23, 267–282. [Google Scholar] [CrossRef]
- Schulte, P.J.; Hinckley, T.M. A comparison of pressure-volume curve data analysis techniques. J. Exp. Bot. 1985, 36, 1590–1602. [Google Scholar] [CrossRef]
- Zine El Abidine, A.; Bernier-Cardou, M.; Bernier, P.Y.; Plamondon, A.P. Control of pressure-chamber and rehydration-time effects on pressure–volume determination of water-relation parameters. Can. J. Bot. 1993, 71, 1009–1015. [Google Scholar] [CrossRef]
- Hachani, C.; Lamhamedi, M.S.; Cameselle, C.; Gouveia, S.; Zine El Abidine, A.; Khasa, D.P.; Béjaoui, Z. Effects of ectomycorrhizal fungi and heavy metals (Pb, Zn, and Cd) on growth and mineral nutrition of Pinus halepensis seedlings in North Africa. Microorganisms 2020, 8, 2033. [Google Scholar] [CrossRef] [PubMed]
- Lamhamedi, M.S.; Renaud, M.; Auger, I.; Fortin, J.A. Granular calcite stimulates natural mycorrhization and growth of white spruce seedlings in peat-based substrates in forest nursery. Microorganisms 2020, 8, 1088. [Google Scholar] [CrossRef]
- Lamhamedi, M.S.; Lambany, G.; Margolis, H.A.; Renaud, M.; Veilleux, L.; Bernier, P.Y. Growth, physiology and leachate losses in Picea glauca seedlings (1 + 0) grown in air-slit containers under different irrigation regimes. Can. J. For. Res. 2001, 31, 1968–1980. [Google Scholar] [CrossRef]
- Lamhamedi, M.S.; Labbé, L.; Margolis, H.A.; Stowe, D.C.; Blais, L.; Renaud, M. Spatial variability of substrate water content and growth of white spruce seedlings. Soil Sci. Soc. Am. J. 2006, 70, 108–120. [Google Scholar] [CrossRef]
- Béjaoui, Z. Tolérance de Divers Clones de Peuplier À l’hydromorphie: Aspects Morphologiques, Écophysiologiques et Métaboliques. Ph.D. Thesis, University of Carthage, Tunis, Tunisia, 2006. [Google Scholar]
- Zine El Abidine, A.; Stewart, J.D.; Plamondon, A.P.; Bernier, P.Y. Diurnal and seasonal variations in gas exchange and water relations of lowland and upland black spruce ecotypes. Can. J. Bot. 1995, 73, 716–722. [Google Scholar] [CrossRef]
- Krause, G.H.; Weis, E. Chlorophyll fluorescence and photosynthesis: The basics. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 313–349. [Google Scholar] [CrossRef]
- Ritchie, G.A. Assessing seedling quality. In Forest Nursery Manual: Production of Bareroot Seedlings; Duryea, M.L., Landis, T.D., Eds.; Martinus Nijhoff/Dr. Junk W. Publishers: Dordrecht, The Netherlands, 1984; pp. 243–259. [Google Scholar]
- Zine El Abidine, A.; Bernier, P.Y.; Plamondon, A.P. Water relations parameters of lowland and upland black spruce: Seasonal variations and ecotypic differences. Can. J. For. Res. 1994, 24, 587–593. [Google Scholar] [CrossRef]
- Jones, M.M.; Turner, N.C. Osmotic adjustment in expanding and fully expanded leaves of sunflower in response to water deficits. Funct. Plant Biol. 1980, 7, 181–192. [Google Scholar] [CrossRef]
- Nabil, M.; Coudret, A. Effects of sodium chloride on growth, tissue elasticity and solute adjustment in two Acacia nilotica subspecies. Physiol. Plant. 1995, 93, 217–224. [Google Scholar] [CrossRef]
- Albouchi, A.; Béjaoui, Z.; Lamhamedi, M.S.; Abassi, M.; El Aouni, M.H. Relations hydriques chez trois clones de peuplier euraméricain soumis à un gradient d’hydromorphie. Geo-Eco-Trop 2016, 40, 385–400. [Google Scholar]
- Blum, A.; Ebercon, A. Cell membrane stability as a measure of drought and heat tolerance in wheat. Crop Sci. 1981, 21, 43–47. [Google Scholar] [CrossRef]
- Steel, G.D.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed.; The McGraw-Hill Companies Inc.: New York, NY, USA, 1997. [Google Scholar]
- Zhang, Y.; Hu, J.; Bai, J.; Wang, J.; Yin, R.; Wang, J.; Lin, X. Arbuscular mycorrhizal fungi alleviate the heavy metal toxicity on sunflower (Helianthus annuus L.) plants cultivated on a heavily contaminated field soil at a WEEE-recycling site. Sci. Total Environ. 2018, 628, 282–290. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- El Rasafi, T.; Pereira, R.; Pinto, G.; Gonçalves, F.G.M.; Haddioui, A.; Ksibi, M.; Römbke, J.; Sousa, J.P.; Marques, C.R. Potential of Eucalyptus globulus for the phytoremediation of metals in a Moroccan iron mine soil—A case study. Environ. Sci. Pollut. Res. 2021, 28, 15782–15793. [Google Scholar] [CrossRef]
- Gururani, M.A.; Venkatesh, J.; Tran, L.S.P. Regulation of photosynthesis during abiotic stress-induced photoinhibition. Mol. Plant 2015, 8, 1304–1320. [Google Scholar] [CrossRef] [PubMed]
- Guidi, L.; Lo Piccolo, E.; Landi, M. Chlorophyll fluorescence, photoinhibition and abiotic stress: Does it make any difference the fact to be a C3 or C4 species? Front. Plant Sci. 2019, 10, 174. [Google Scholar] [CrossRef]
- Sengupta, U.K.; Sharma, A. Carbon dioxide enrichment effects on photosynthesis and plant growth. In Photosynthesis: Photoreactions to Plant Productivity; Abrol, Y.P., Mohanty, P., Govindjee, Eds.; Springer Science + Business Media: Dordrecht, The Netherlands, 1993. [Google Scholar]
- Tang, Y.; Bao, Q.; Tian, G.; Fu, K.; Cheng, H.; Chen, S.; Zhou, S. Heavy metal cadmium tolerance on the growth characteristics of industrial hemp (Cannabis sativa L.) in China. In Series Advances in Engineering Research, Proceedings of the international Conference on Advances in Energy, Environment and Chemical Engineering, Changsha, China, 26–27 September 2015; Atlantis Press: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Pezeshki, S.R. Differences in patterns of photosynthetic responses to hypoxia in flood tolerant and food sensitive tree species. Photosynthetica 1993, 28, 223–430. [Google Scholar]
- Augé, R.M. Arbuscular mycorrhizae and soil/plant water relations. Can. J. Soil Sci. 2004, 84, 373–381. [Google Scholar] [CrossRef]
- García-Sánchez, I.E.; Barradas, V.L.; de León Hill, C.A.P.; Esperón-Rodríguez, M.; Pérez, I.R.; Ballinas, M. Effect of heavy metals and environmental variables on the assimilation of CO2 and stomatal conductance of Ligustrum lucidum, an urban tree from Mexico City. Urban For. Urban Green. 2019, 42, 72–81. [Google Scholar] [CrossRef]
- Kozlowski, T.T. Carbohydrate sources and sinks in woody plants. Bot. Rev. 1992, 58, 107–222. [Google Scholar] [CrossRef]
- Pellicer, V.; Guehl, J.M.; Daudet, F.A.; Cazet, M.; Riviere, L.M.; Maillard, P. Carbon and nitrogen mobilization in Larix × eurolepis leafy stem cuttings assessed by dual 13C and 15N labeling: Relationships with rooting. Tree Physiol. 2000, 20, 807–814. [Google Scholar] [CrossRef][Green Version]
- Garbaye, J.; Guehl, J.M. Le Rôle des ectomycorhizes dans l’utilisation de l’eau par les arbres forestiers. Rev. For. Fr. 1997, 49, 110–120. [Google Scholar] [CrossRef]
- Brownlee, C.; Duddridge, J.A.; Malibari, A.; Read, D.J. The structure and function of mycelial system of ectomycorrhizal roots with special reference to their role in forming inter-plant connection and providing pathways for assimilate and water transport. Plant Soil 1983, 71, 433–443. [Google Scholar] [CrossRef]
- Read, D.J. The mycorrhizal mycelium. In Mycorrhizal Functioning: An Integrative Plant-Fungal Process; Allen, M., Ed.; Chapman and Hell: New York, NY, USA, 1992; pp. 102–133. [Google Scholar]
- Lehto, T.; Zwiazek, J.J. Ectomycorrhizas and water relations of trees: A review. Mycorrhiza 2011, 21, 71–90. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Mummey, D.L. Mycorrhizas and soil structure. New Phytol. 2006, 171, 41–53. [Google Scholar] [CrossRef]
- Allen, F.A. Mycorrhizal fungi: Highways for water and nutrients in arid soils. Vadose Zone J. 2007, 6, 291–297. [Google Scholar] [CrossRef]
- Guehl, J.M.; Mousain, D.; Falconnet, G.; Gruez, J. Growth, carbon dioxide assimilation capacity and water use efficiency of Pinus pinea L. seedlings inoculated with different ectomycorrhizal fungi. Ann. Sci. For. 1990, 47, 91–100. [Google Scholar] [CrossRef]
- Pirasteh-Anosheh, H.; Saed-Moucheshi, A.; Pakniyat, H.; Pessarakli, M. Stomatal responses to drought stress. In Water Stress and Crop Plants: A Sustainable Approach, 1st ed.; Parvaiz, A., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2016. [Google Scholar]
- Turner, N.C. Turgor maintenance by osmotic adjustment: 40 years of progress. J. Exp. Bot. 2018, 69, 3223–3233. [Google Scholar] [CrossRef] [PubMed]
- Muhsin, T.M.; Zwiazek, J.J. Ectomycorrhizas increase apoplastic water transport and root hydraulic conductivity in Ulmus americana seedlings. New Phytol. 2002, 153, 153–158. [Google Scholar] [CrossRef]
- Plassard, C.; Chalot, M.; Botton, B.; Martin, F. Le rôle des ectomycorhizes dans la nutrition azotée des arbres forestiers. Rev. For. Française 1997, 49, 82–98. [Google Scholar] [CrossRef]
- Gadd, G.M.; Bahri-Esfahani, J.; Li, Q.; Rhee, Y.J.; Wei, Z.; Fomina, M.; Liang, X. Oxalate production by fungi: Significance in geomycology, biodeterioration and bioremediation. Fungal Biol. Rev. 2014, 28, 36–55. [Google Scholar] [CrossRef]
- Guehl, J.M.; Garbaye, J. The effects of ectomycorrhizal status on carbon dioxide assimilation capacity, water-use efficiency and response to transplanting in seedlings of Pseudotsuga menziesii (Mirb) Franco. Ann. Sci. For. 1990, 47, 551–563. [Google Scholar] [CrossRef]
- Desilva, D.L.R.; Hetherington, A.M.; Mansfield, T.A. Synergism between calcium ions and abscisic acid in preventing stomatal opening. New Phytol. 1985, 100, 473–482. [Google Scholar]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
- Hochmal, A.K.; Shulze, S.; Trompelt, K.; Hippler, M. Calcium-dependent regulation of photosynthesis. Biochim. Biophys. Acta 2015, 1847, 993–1003. [Google Scholar] [CrossRef]
- Chaves, M.M.; Oliveira, M.M. Mechanisms underlying plant resilience to water deficits: Prospects for water-saving agriculture. J. Exp. Bot. 2004, 55, 2365–2384. [Google Scholar] [CrossRef]
- Saroy, K.; Garg, N. Relative effectiveness of arbuscular mycorrhiza and polyamines in modulating ROS generation and ascorbate-glutathione cycle in Cajanus cajan under nickel stress. Environ. Sci. Pollut. Res. 2021, 28, 48872–48889. [Google Scholar] [CrossRef] [PubMed]
- Clifford, S.C.; Arndt, S.K.; Corlett, J.E.; Joshi, S.; Sankhla, N.; Popp, M.; Jones, H.G. The role of solute accumulation, osmotic adjustment and changes in cell wall elasticity in drought tolerance in Ziziphus mauritiana (Lamk.). J. Exp. Bot. 1998, 49, 967–977. [Google Scholar] [CrossRef]
- Sebastiana, M.; da Silva, A.B.; Matos, A.R.; Alcântara, A.; Silvestre, S.; Malhó, R. Ectomycorrhizal inoculation with Pisolithus tinctorius reduces stress induced by drought in cork oak. Mycorrhiza 2018, 28, 247–258. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).