Moso Bamboo Invasion Reshapes Community Structure of Denitrifying Bacteria in Rhizosphere of Alsophila spinulosa
Abstract
1. Introduction
2. Materials and Methods
2.1. Community Survey and Soil Samples Collection
2.2. Soil Chemical and Physical Properties
2.3. DNA Extraction, QnorB Gene Amplification, and MiSeq Sequencing
2.4. Data Analysis
3. Results
3.1. Effects of Invasion on Forest Structure and Soil Properties
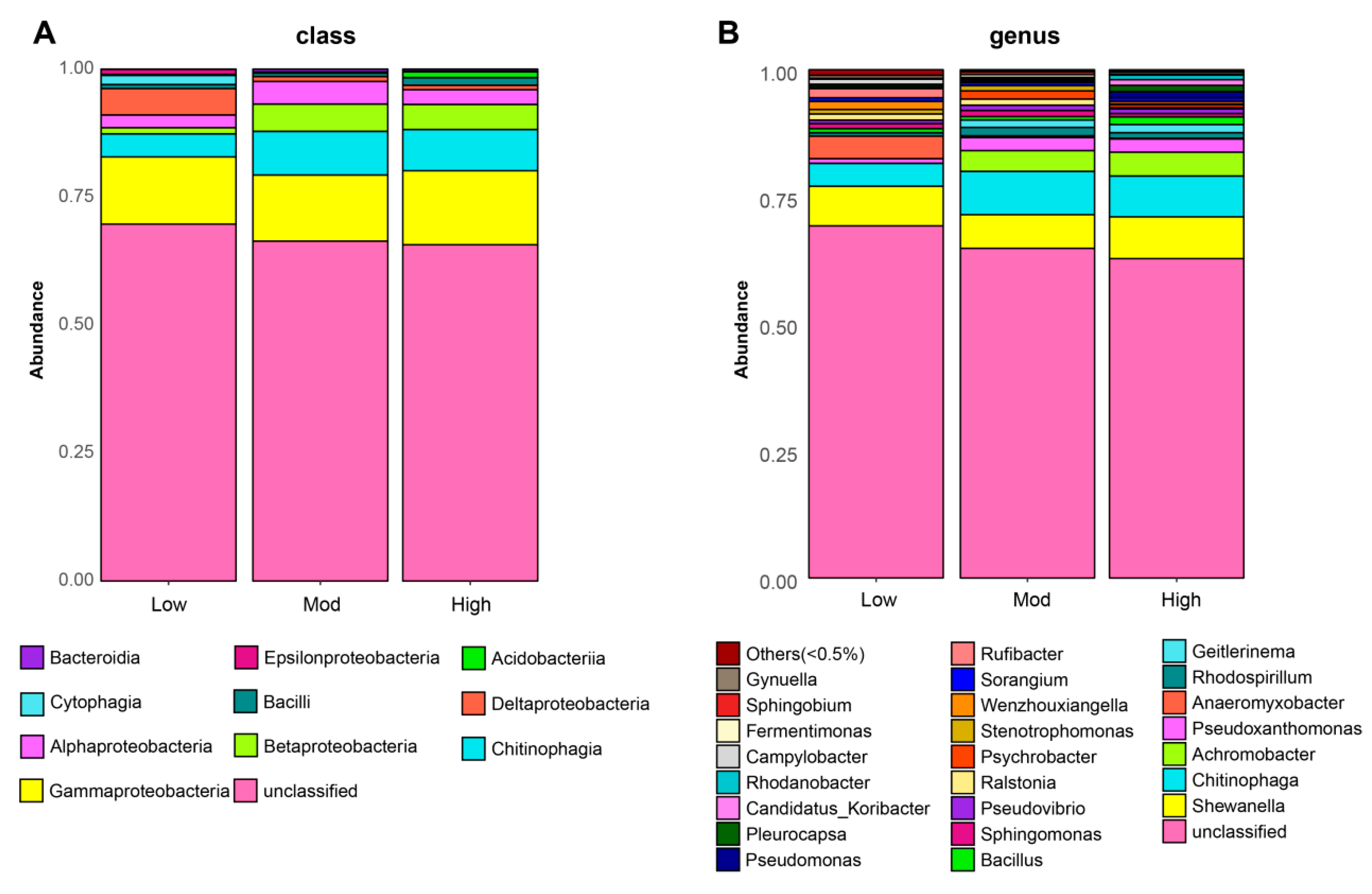
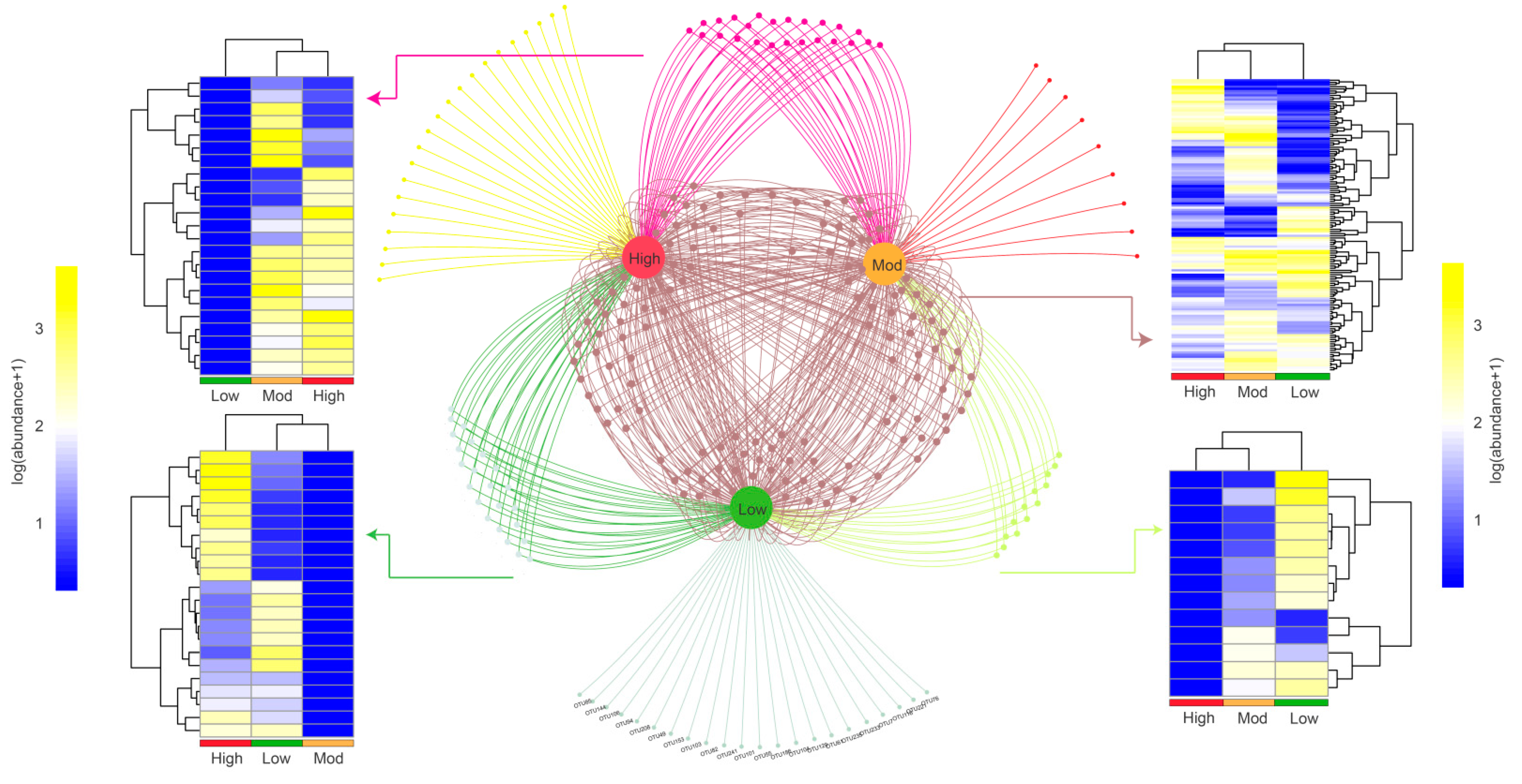
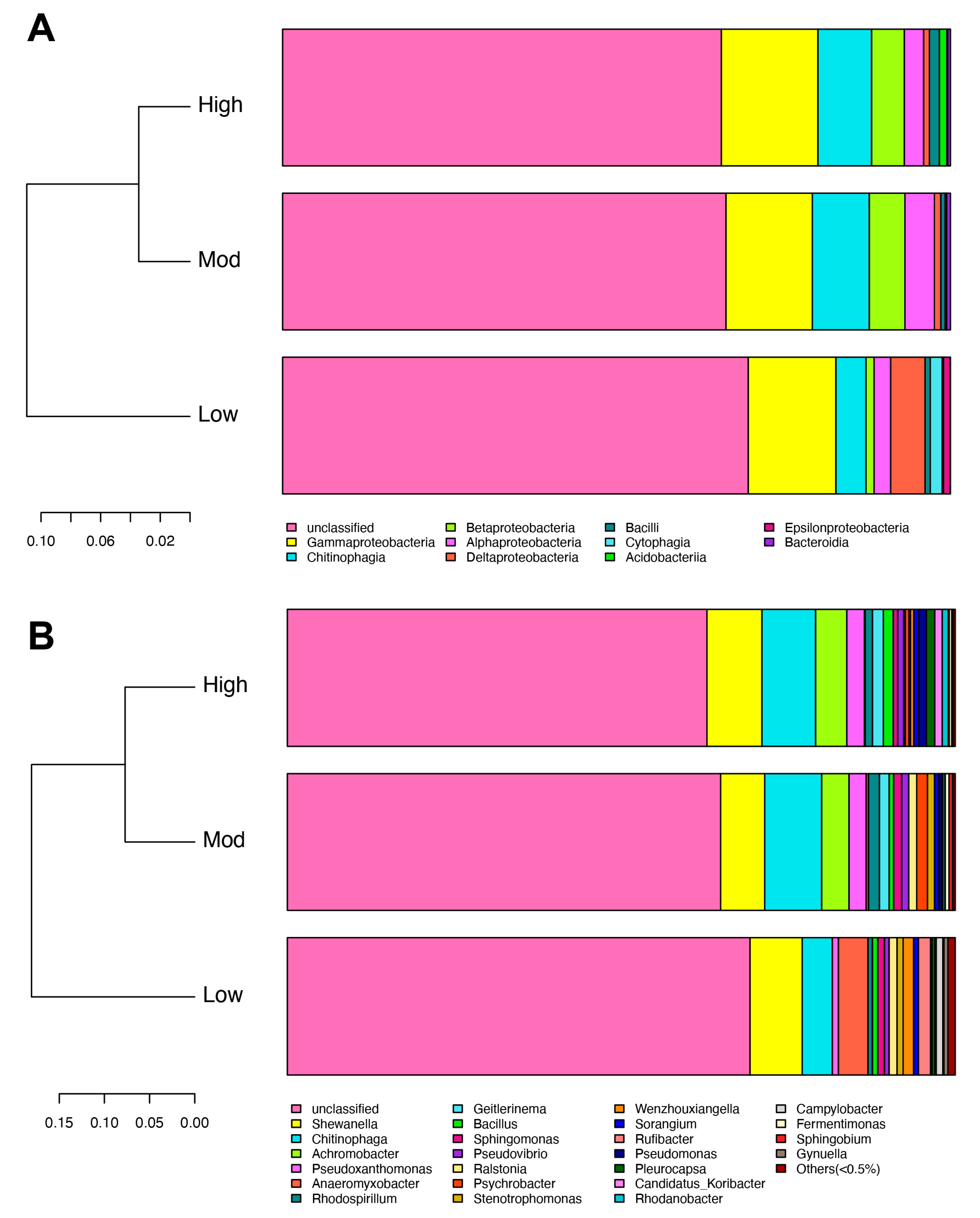
3.2. Effects of Invasion on Denitrifying Bacterial Community Composition
3.3. Effects of Invasion on Denitrifying Bacterial Community Diversity
3.4. Relationships of Denitrifying Bacteria with Soil Properties and the Growth of A. spinulosa
4. Discussion
4.1. Successful Moso Bamboo Invasion Altered Root Biomass and Soil N Content
4.2. Moso Bamboo Invasion Increased Denitrifying Bacterial Diversity and Reshaped Its Composition
4.3. Relationships of Denitrifying Bacteria with Soil Properties and the Growth of A. spinulosa
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, Q.N.; Ouyang, M.; Yang, Q.P.; Lu, H.; Yang, G.Y.; Chen, F.S.; Shi, J.M. Degradation of litter quality and decline of soil nitrogen mineralization after moso bamboo (Phyllostachys pubscens) expansion to neighboring broadleaved forest in subtropical China. Plant Soil 2016, 404, 113–124. [Google Scholar] [CrossRef]
- Song, Q.N.; Yang, Q.P.; Liu, J.; Yu, D.K.; He, Y.J. Effects of Phyllostachys edulis expansion on soil nitrogen mineralization and its availability in evergreen broadleaf forest. Chin. J. Appl. Ecol. 2013, 24, 338–344. [Google Scholar]
- Song, X.; Jiang, H.; Zhang, Z.; Zhou, G.; Zhang, S.; Peng, C. Interactive effects of elevated UV-B radiation and N deposition on decomposition of Moso bamboo litter. Soil Biol. Biochem. 2014, 69, 11–16. [Google Scholar] [CrossRef]
- Okutomi, K.; Shinoda, S.; Fukuda, H. Causal analysis of the invasion of broad-leaved forest by bamboo in Japan. J. Veg. Sci. 1996, 7, 723–728. [Google Scholar] [CrossRef]
- Larpkern, P.; Moe, S.R.; Totland, R. Bamboo dominance reduces tree regeneration in a disturbed tropical forest. Oecologia 2011, 165, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, L.; Deng, B.; Liu, Y.; Kong, F.; Huang, G.; Zou, Q.; Liu, Q.; Guo, X.; Fu, Y. Effects of moso bamboo (Phyllostachys edulis) invasions on soil nitrogen cycles depend on invasion stage and warming. Environ. Sci. Pollut. Res. 2017, 24, 24989–24999. [Google Scholar] [CrossRef]
- Zou, N.; Shi, W.; Hou, L.; Kronzucker, H.J.; Huang, L.; Gu, H.; Yang, Q.; Deng, G.; Yang, G. Superior growth, N uptake and NH4+ tolerance in the giant bamboo Phyllostachys edulis over the broad-leaved tree Castanopsis fargesii at elevated NH4+ may underlie community succession and favor the expansion of bamboo. Tree Physiol. 2020, 11, 1606–1622. [Google Scholar] [CrossRef]
- Li, Y.; Chang, Y.; Xu, S.; Guo, Q.; Gao, Z. Bamboo invasion of broadleaf forests altered soil fungal community closely linked to changes in soil organic C chemical composition and mineral N production. Plant Soil 2017, 418, 507–512. [Google Scholar] [CrossRef]
- Chang, E.H.; Chiu, C.Y. Changes in soil microbial community structure and activity in a cedar plantation invaded by moso bamboo. Appl. Soil Ecol. 2015, 91, 1–7. [Google Scholar] [CrossRef]
- Wu, J.S.; Jiang, P.K.; Wang, Z.L. The Effects of Phyllostachys pubescens Expansion on Soil Fertility in National Nature Reserve of Mount Tianmu. Acta Agric. Univ. Jiangxiensis 2008, 30, 689–692, (Chinese with English Abstract). [Google Scholar]
- Ma, Y.; Jiao, P.; Qi, Z.; Jiang, Z.; Guan, S. Characterization of the complete chloroplast genome of Alsophila spinulosa, an endangered species endemic to China. Mitochondrial DNA Part B 2020, 5, 2262–2263. [Google Scholar] [CrossRef]
- Smith, A.R.; Tryon, R.M.; Tryon, A.F. Ferns and allied plants with special reference to tropical America. Am. Fern J. 1983, 73, 94. [Google Scholar] [CrossRef]
- Wang, T.; Su, Y.; Li, Y. Population Genetic Variation in the Tree Fern Alsophila spinulosa (Cyatheaceae): Effects of Reproductive Strategy. PLoS ONE 2012, 7, e41780. [Google Scholar] [CrossRef][Green Version]
- Su, Y.J.; Wang, T.; Bo, Z.; Jiang, Y.; Chen, G.P.; Ouyang, P.Y.; Sun, Y.F. Genetic differentiation of relictual populations of Alsophila spinulosa in southern China inferred from cpDNA trnL-F noncoding sequences. Mol. Phylogenetics Evol. 2005, 34, 323–333. [Google Scholar] [CrossRef]
- Qu, H.; Deng, H.; Sheng, L. Effects of Phyllostachys heterocycla expansion on morphological plasticity of endangered plant Alsophila spinulosa root system. Acta Ecol. Sin. 2020, 40, 1219–1227, (Chinese with English Abstract). [Google Scholar]
- Brettar, I.; Christen, R.; Höfle, M. Shewanella denitrificans sp. nov., a vigorously denitrifying bacterium isolated from the oxic-anoxic interface of the Gotland Deep in the central Baltic Sea. Int. J. Syst. Evol. Microbiol. 2002, 52, 2211. [Google Scholar]
- Kathiravan, V.; Krishnani, K.K. Pseudomonas aeruginosa and Achromobacter sp.: Nitrifying aerobic denitrifiers have a plasmid encoding for denitrifying functional genes. World J. Microbiol. Biotechnol. 2014, 30, 1187–1198. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, G.; Zhong, S.; Wang, T.; Ji, M.; Wu, X.; Zhang, X. Antibiotic-induced role interchange between rare and predominant bacteria retained the function of a bacterial community for denitrifying quinoline degradation. J. Appl. Microbiol. 2020, 129, 1598–1608. [Google Scholar] [CrossRef] [PubMed]
- Velho, V.F.; Magnus, B.S.; Daudt, G.C.; Xavier, J.A.; Guimarães, L.B.; Costa, R.H.R. Effect of COD/N ratio on N2O production during nitrogen removal by aerobic granular sludge. Water Sci. Technol. 2017, 76, 3452–3460. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, Q.P.; Yu, D.K.; Song, Q.N.; Wang, B. Contribution of fine root to soil nutrient heterogeneity at two sides of the bamboo and broad-leaved forest interface. Chin. J. Plant Ecol. 2013, 37, 739–749. [Google Scholar] [CrossRef]
- Fukushima, K.; Usui, N.; Ogawa, R.; Tokuchi, N. Impacts of moso bamboo (Phyllostachys pubescens) invasion on dry matter and carbon and nitrogen stocks in a broad-leaved secondary forest located in Kyoto, western Japan. Plant Species Biol. 2015, 30, 81–95. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, F.S.; Wu, X.Q.; Luan, F.G.; Zhang, L.P.; Fang, X.M.; Wan, S.Z.; Hu, X.F.; Ye, J.R.; Daniel, C. Isolation and characterization of two phosphate-solubilizing fungi from rhizosphere soil of moso bamboo and their functional capacities when exposed to different phosphorus sources and pH environments. PLoS ONE 2018, 13, e0199625. [Google Scholar]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; Agricultural Publisher: Beijing, China, 2000; pp. 355–356. [Google Scholar]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996. [Google Scholar] [CrossRef]
- Craig, M.E.; Fraterrigo, J.M. Plant-microbial competition for nitrogen increases microbial activities and carbon loss in invaded soils. Oecologia 2017, 184, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Wang, J.; He, J.Z.; Yu, F.H.; Ge, Y. Plant diversity enhances soil fungal diversity and microbial resistance to plant invasion. Appl. Environ. Microbiol. 2021, 87, e00251-21. [Google Scholar] [CrossRef]
- Kronzucker, H.J.; Siddiqi, M.Y.; Glass, A. Conifer root discrimination against soil nitrate and the ecology of forest succession. Nature 1997, 385, 59–61. [Google Scholar] [CrossRef]
- Krozucker, H.J.; Siddiqi, M.Y.; Glass, A.D.M.; Britto, D.T. Root ammonium transport efficiency as a determinant in forest colonization patterns: An hypothesis. Physiol. Plant. 2010, 117, 164–170. [Google Scholar] [CrossRef]
- Song, Q.N.; Hui, L.; Liu, J.; Yang, J.; Yang, Q.P. Accessing the impacts of bamboo expansion on NPP and N cycling in evergreen broadleaved forest in subtropical China. Sci. Rep. 2017, 7, 40383. [Google Scholar] [CrossRef]
- Schomakers, J.; Jien, S.H.; Lee, T.Y.; Huang, J.C.; Hseu, Z.Y.; Lin, Z.L.; Lee, L.C.; Hein, T.; Mentler, A.; Zehetner, F. Soil and biomass carbon re-accumulation after landslide disturbances. Geomorphology 2019, 288, 164–174. [Google Scholar] [CrossRef]
- Kleinhenz, V.; Midmore, D.J. Aspects of bamboo agronomy. Adv. Agron. 2001, 74, 99–153. [Google Scholar]
- Fahey, C.; Koyama, A.; Antunes, P.M.; Dunfield, K.E.; Flory, L. Plant communities mediate the interactive effects of invasion and drought on soil microbial communities. ISME J. 2020, 14, 1396–1409. [Google Scholar] [CrossRef] [PubMed]
- James, A.; Estrada, S.; Luke, F. Cogongrass (Imperata cylindrica) invasions in the US: Mechanisms, impacts, and threats to biodiversity-Science Direct. Glob. Ecol. Conserv. 2015, 3, 1–10. [Google Scholar]
- Catford, J.A.; Daehler, C.C.; Murphy, H.T.; Sheppard, A.W.; Hardesty, B.D.; Westcott, D.A.; Rejmánek, M.; Bellingham, P.J.; Pergl, J.; Horvitz, C.C. The intermediate disturbance hypothesis and plant invasions: Implications for species richness and management. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 231–241. [Google Scholar] [CrossRef]
- Tremolieres, M. Plant response strategies to stress and disturbance: The case of aquatic plants. J. Biosci. 2004, 29, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Kurth, V.J.; D’Amato, A.W.; Bradford, J.B.; Palik, B.J.; Looney, C.E. Assessing the ecological impacts of biomass harvesting along a disturbance severity gradient. Ecol. Appl. 2020, 30, e02042. [Google Scholar] [CrossRef] [PubMed]
- Philippe, H.; Claude, P.; Benoît, J. Rhizosphere: A new frontier for soil biogeochemistry. J. Geochem. Explor. 2006, 88, 210–213. [Google Scholar]
- Castro-Díez, P.; Godoy, O.; Alonso, A.; Gallardo, A.; Saldaña, A. What explains variation in the impacts of exotic plant invasions on the nitrogen cycle? A meta-analysis. Ecol. Lett. 2014, 17, 1–12. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, L.; Wang, J.; Chen, W.; Chen, X.; Zhu, H. Effect of invasive degree of Rhynchelytrum repens on microorganisms in rhizosphere soil. J. Northwest A F Univ. -Nat. Sci. Ed. 2017, 45, 165–172, (Chinese with English Abstract). [Google Scholar]
- Si, C.C.; Dai, Z.C.; Lin, Y.; Qi, S.S.; Huang, P.; Miao, S.L.; Du, D.L. Local adaptation and phenotypic plasticity both occurred in Wedelia trilobata invasion across a tropical island. Biol. Invasions 2014, 16, 2323–2337. [Google Scholar] [CrossRef]
- Gordon, B.A.; Lenhart, C.; LaPara, T.M. A comparison of nitrate removal and denitrifying bacteria populations among three wetland plant communities. J. Environ. Qual. 2020, 49, 210–219. [Google Scholar] [CrossRef]
- Verbaendert, I.; De Vos, P.; Boon, N.; Heylen, K. Denitrification in Gram-positive bacteria: An underexplored trait. Biochem. Soc. Trans. 2011, 39, 254–258. [Google Scholar] [CrossRef][Green Version]
- Middleton, S.S.; Latmani, R.B.; Mackey, M.R.; Ellisman, M.H.; Tebo, B.M.; Criddle, C.S. Cometabolism of Cr(VI) by Shewanella oneidensis MR-1 produces cell-associated reduced chromium and inhibits growth. Biotechnol. Bioeng. 2003, 83, 627–637. [Google Scholar] [CrossRef]
- Qin, L.; Hongping, D.; Zongfeng, L.; Sheng, L.; Qiulin, L.; Dongping, N. Characteristics of plant community in the Guizhou Chishui Alsophila spinulata National Nature Reserve, southwestern China. J. Beijing For. Univ. 2019, 41, 19–31, (Chinese with English Abstract). [Google Scholar]
- Lian, T.X. The turn over of Photosynthetic Carbon of Soybean and Relevant Bacterial Community Characteristics in the Mollisols. Ph.D. Thesis, University of Chinese Academy of Sciences, Beijing, China, 2016. (In Chinese). [Google Scholar]
- Anjana, S.U.; Iqbal, M. Nitrate accumulation in plants, factors affecting the process, and human health implications. A Review. Agron. Sustain. Dev. 2007, 27, 45–57. [Google Scholar] [CrossRef]
- Noguchi, K. Nitrate addition alleviates ammonium toxicity without lessening ammonium accumulation, organic acid depletion and inorganic cation depletion in Arabidopsis thaliana Shoots. Plant Cell Physiol. 2012, 53, 577–591. [Google Scholar]
- Killham, K.; Amato, M.; Ladd, J.N. Effect of substrate location in soil and soil pore-water regime on carbon turnover. Soil Biol. Biochem. 1993, 25, 57–62. [Google Scholar] [CrossRef]
- Schjnning, P.; Thomsen, I.K.; Moldrup, P.; Christensen, B.T. Linking soil microbial activity to water- and air-phase contents and diffusivities. Soil Sci. Soc. Am. J. 2003, 67, 156–165. [Google Scholar] [CrossRef]
- Zhou, J.; Xia, B.; Treves, D.S.; Wu, L.Y.; Marsh, T.L.; O’Neill, R.V.; Palumbo, A.V.; Tiedje, J.M. Spatial and resource factors influencing high microbial diversity in soil. Appl. Environ. Microbiol. 2002, 68, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Biging, G.S.; Matthias, D. A comparison of distance-dependent competition measures for height and basal area growth of individual conifer trees. Forestence 1992, 38, 695–720. [Google Scholar]
- Lishawa, S.C.; Lawrence, B.A.; Albert, D.A.; Larkin, D.J.; Tuchman, N.C. Invasive species removal increases species and phylogenetic diversity of wetland plant communities. Ecol. Evol. 2019, 9, 6231–6244. [Google Scholar] [CrossRef]
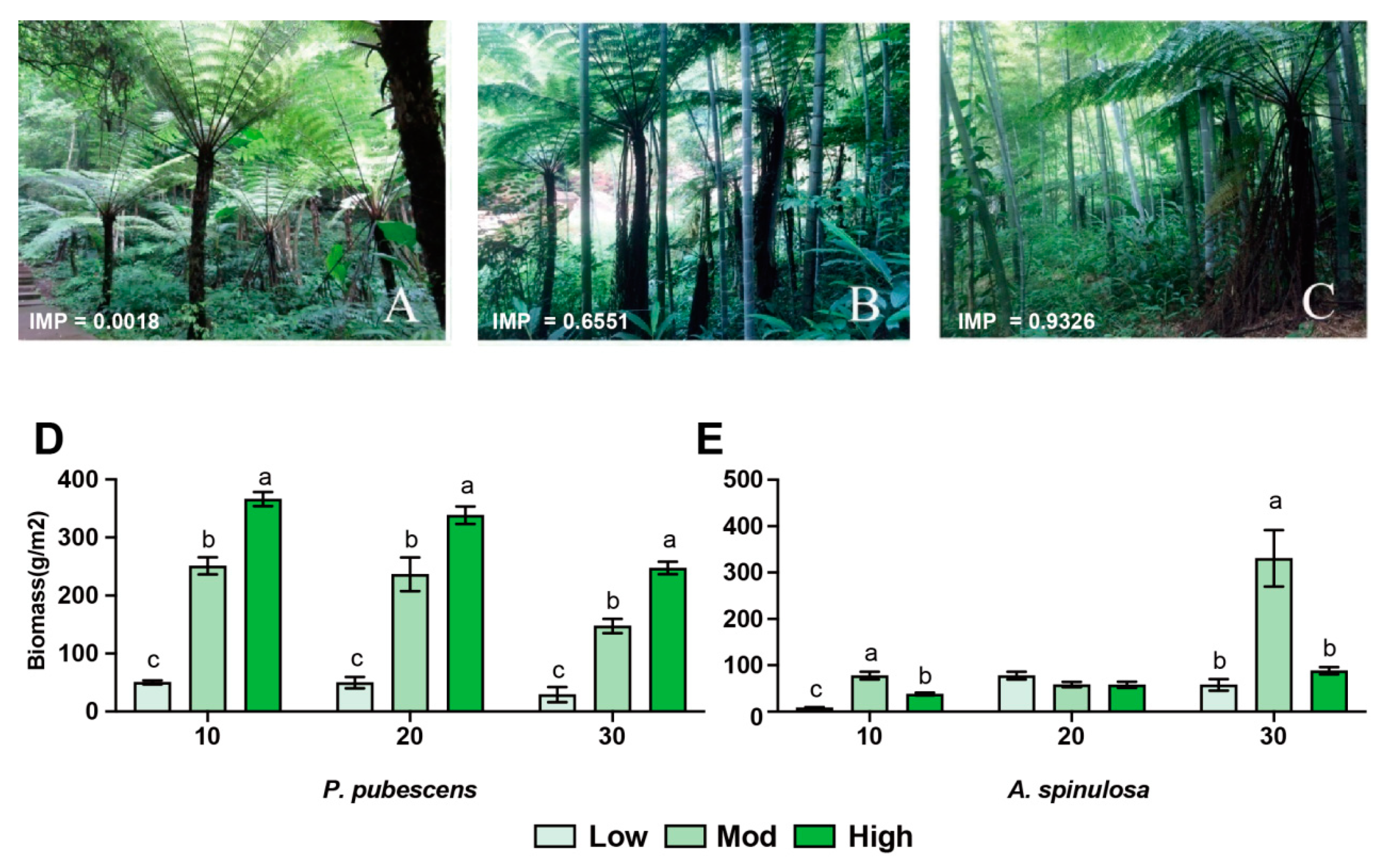

| Intensities of Disturbance | Latitude and Longitude | Elevation/m | Importance Value | Dominant Woody Species |
|---|---|---|---|---|
| high | E 106°01′38.49″ | 564 | 0.9326 | Phyllostachys heterocycla |
| N 28°28′41.89″ | ||||
| E 106°38′22.30″ | 553 | Phyllostachys heterocycla | ||
| N 28°27′23.31″ | ||||
| E 106°01′37.36″ | 562 | Phyllostachys heterocycla | ||
| N 28°28′42.81″ | ||||
| mod | E106°00′38.26″ | 547 | 0.6551 | Phyllostachys heterocycla, Alsophila spinulosa |
| N 28°27′38.00″ | ||||
| E 106°00′45.33″ | 525 | Phyllostachys heterocycla, Brassaiopsis glomerulata | ||
| N 28°19′40.14″ | ||||
| E 106°00′50.02″ | 527 | Phyllostachys heterocycla, Musa basjoo | ||
| N 28°25′36.44″ | ||||
| low | E 105°58′00.83″ | 538 | 0.0008 | Casearia balansae, Alsophila spinulosa |
| N 28°23′39.04″ | ||||
| E 105°58′02.61″ | 535 | Lasianthus chinensis, Musa basjoo | ||
| N 28°23′38.55″ | ||||
| E 105°58′03.72″ | 523 | Musa basjoo, Alsophila spinulosa | ||
| N 28°23′37.46″ |
| Type of Bacteria | Low | Moderate | High | Coverage |
|---|---|---|---|---|
| Shewanella | 0.066 | 0.078 | 0.082 | 0.076 |
| Chitinophaga | 0.045 | 0.08 | 0.085 | 0.07 |
| Achromobacter | 0 | 0.041 | 0.047 | 0.029 |
| Pseudoxanthomonas | 0.009 | 0.026 | 0.026 | 0.02 |
| Anaeromyxobacter | 0.044 | 0.003 | 0.001 | 0.016 |
| Stenotrophomonas | 0.009 | 0.002 | 0.01 | 0.007 |
| Sorangium | 0.007 | 0.007 | 0.006 | 0.007 |
| Campylobacter | 0.01 | 0.001 | 0.001 | 0.004 |
| Candidatus_Koribacter | 0.002 | 0.011 | 0.001 | 0.005 |
| Ralstonia | 0.012 | 0.002 | 0.012 | 0.009 |
| Fermentimonas | 0 | 0.004 | 0.006 | 0.003 |
| Bacillus | 0.008 | 0.015 | 0.007 | 0.01 |
| Sphingomonas | 0.01 | 0.007 | 0.012 | 0.01 |
| Rhodospirillum | 0.007 | 0.011 | 0.016 | 0.011 |
| Pseudovibrio | 0.007 | 0.009 | 0.011 | 0.009 |
| Sphingobium | 0.001 | 0.002 | 0.005 | 0.003 |
| Gynuella | 0.006 | 0 | 0.001 | 0.002 |
| Wenzhouxiangella | 0.016 | 0.005 | 0 | 0.007 |
| Intensities of Disturbance | Ace | Chao1 | Shannon | Simpson | Coverage |
|---|---|---|---|---|---|
| low | 190.18 | 191.16 | 4.15 | 0.029 | 0.99971 |
| moderate | 205.49 | 204.5 | 4.19 | 0.03 | 0.99983 |
| high | 207.69 | 205.75 | 4.29 | 0.024 | 0.99959 |
| Type of Bacteria | pH | WC | OM | AN | Diameter | Crown Width | Height |
|---|---|---|---|---|---|---|---|
| Shewanella | −0.24 | −0.99 | 1.000 * | 0.835 | 0.343 | −0.982 | −0.999 |
| Chitinophaga | −0.115 | −1.000 * | 0.991 | 0.758 | 0.221 | 0.555 | −0.984 * |
| Achromobacter | −0.117 | −1.000 * | 0.992 | 0.759 | −0.406 | −0.706 | 0.984 |
| Pseudoxanthomonas | 0 | −0.995 | 0.97 | 0.678 | 0.107 | 0.465 | 0.957 |
| Anaeromyxobacter | 0.041 | −0.998 * | 0.979 | −0.708 | −0.148 | −0.492 | −0.968 |
| Stenotrophomonas | −0.918 | 0.3 | −0.162 | 0.405 | 0.107 | 0.456 | −0.114 |
| Sorangium | 0.866 | 0.588 | 0.696 | −0.976 | −0.915 | −0.999 * | −0.73 |
| Campylobacter | 0 | −0.995 | −0.97 | 0.678 | −0.107 | −0.456 | −0.957 |
| Candidatus_Koribacter | 0.908 | −0.322 | 0.185 | −0.383 | −0.324 | −0.617 | 0.138 |
| Ralstonia | −0.866 | 0.407 | −0.274 | 0.298 | −0.871 | −0.635 | −0.228 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, Y.; Qu, H.; Xia, C.; Zhang, H.; Zhang, J.; Deng, H. Moso Bamboo Invasion Reshapes Community Structure of Denitrifying Bacteria in Rhizosphere of Alsophila spinulosa. Microorganisms 2022, 10, 180. https://doi.org/10.3390/microorganisms10010180
Zuo Y, Qu H, Xia C, Zhang H, Zhang J, Deng H. Moso Bamboo Invasion Reshapes Community Structure of Denitrifying Bacteria in Rhizosphere of Alsophila spinulosa. Microorganisms. 2022; 10(1):180. https://doi.org/10.3390/microorganisms10010180
Chicago/Turabian StyleZuo, Youwei, Huanhuan Qu, Changying Xia, Huan Zhang, Jiahui Zhang, and Hongping Deng. 2022. "Moso Bamboo Invasion Reshapes Community Structure of Denitrifying Bacteria in Rhizosphere of Alsophila spinulosa" Microorganisms 10, no. 1: 180. https://doi.org/10.3390/microorganisms10010180
APA StyleZuo, Y., Qu, H., Xia, C., Zhang, H., Zhang, J., & Deng, H. (2022). Moso Bamboo Invasion Reshapes Community Structure of Denitrifying Bacteria in Rhizosphere of Alsophila spinulosa. Microorganisms, 10(1), 180. https://doi.org/10.3390/microorganisms10010180






